Perioperative haemodynamic optimisation is crucial to improve outcomes after surgery.1, 2 The basis of perioperative haemodynamic optimisation is cardiovascular monitoring, although the target of optimisation is tissue perfusion.3 With the advent of minimally invasive advanced cardiovascular monitoring, the concept of perioperative goal-directed therapy (GDT) has gained prominence as an individualised approach to optimising flow parameters. The basis of GDT is optimisation of flow through measurement of stroke volume (SV) after administration of i.v. fluid volume boluses to eliminate cardiac preload dependency.1, 3, 4 Volume therapy based on GDT has been shown to improve perioperative outcomes when compared with a conventional approach with haemodynamic interventions based upon HR, BP, and clinical observation.5
Different algorithms for GDT have been proposed, but the cornerstone has been the use of an i.v. fluid bolus to eliminate preload-dependency based on SV measurement or continuous assessment of stroke volume variation (SVV).1, 4 Ideally, this ensures that cardiac output is optimised, which hopefully will optimise the possibility of adequate tissue perfusion, while at the same time avoiding unnecessary fluid overload in those patients who are not fluid responsive. The paradigm is opposed to the earlier concepts of liberal or restrictive fluid therapy, where the delivered volume was not guided by individual flow response to fluids.6
The GDT concept is flow based, even though most algorithms have conceded that after flow optimisation a target MAP ≥65 mm Hg, or individualised from the preoperative baseline MAP, should be attained with supplementary vasoconstriction.7 Consequently, the strategy is to optimise flow by fluid bolus and then apply vasoconstriction to avoid hypotension once flow is optimised.8, 9
The impact of perioperative hypotension has been studied extensively in recent large studies, and there is a strong association between even short perioperative periods of hypotension and increased postoperative morbidity.7, 10, 11, 12 However, hypotension can obviously occur both in high-flow states by vasodilation and afterload reduction with preserved contractility, or in low-flow states by reduction of circulating volume, vasoplegia with or without decreased contractility.7 The causality of the association between hypotension and poor outcome has not been established; the main question to be answered is whether poor outcome is related only to hypotension induced by low-flow or also to high-flow states with hypotension. Consequently, the best treatment decision for any given clinical hypotensive state may be difficult—fluids, vasoconstriction, or inotropic support? Pragmatic GDT approaches have prioritised correction of flow by volume followed, in some studies, by inotropic therapy and then vasoconstriction if hypotension persists.8, 9 However, scientific evidence for the specific therapeutic sequence of this approach is limited.
Vasoconstriction and preload responsiveness
The current approach to vasoconstriction in GDT has been based on enhancement of afterload to attain a targeted arterial pressure after flow optimisation. General and neuraxial anaesthesia induces sympatholysis with vasodilation reducing both preload and afterload potentially inducing preload responsiveness. However, preload may also be modifiable by vasoconstriction, as demonstrated experimentally and clinically after norepinephrine and phenylephrine administration.13, 14, 15 Similarly, the ability of the cardiovascular system to modulate venous return has been shown in experimental hypovolaemia, where preload responsiveness can be masked by sympathetic vasoconstriction in the non-anaesthetised patient.16 Consequently, the overall compensatory reserve capacity may depend on adrenergic-controlled ability for central fluid recruitment.
Induction of anaesthesia produces dose-dependent vasoplegia by suppression of sympathetic tone accentuating preload responsiveness. Consequently, the clinician is faced with the choice of vasoconstriction or volume loading, which within the present paradigm of GDT is not discernible solely by looking at cardiovascular parameters. If volume loading is chosen, the patient is at risk of postanaesthetic fluid overload, and if vasoconstriction is chosen, tissue hypoxia as a result of inadequate capillary blood flow may be the consequence with an insufficient circulating intravascular volume.2, 14 The use of vasopressors is frequent, but variable in GDT studies of major surgery. In the OPTIMISE trial, vasopressor (or non-dopexamine inotrope) infusion was 28% in the GDT group, while in the POM-O trial it was reported at 19%8, 9. Other studies on GDT intervention by automated closed loop systems have reported vasoactive infusions in 55–89% of patients.17, 18 Recently, the INPRESS trial reported an incidence of norepinephrine infusion of 95% to maintain BP within 10% of resting arterial pressure after initial GDT, as compared with 26% in the control group with more conventional arterial pressure control.19 However, as the present paradigm only considers the ability of vasoconstrictors to affect afterload and BP, it assumes that after GDT-guided volume loading, the administration of vasopressors to achieve a minimum MAP threshold will be the ‘correct’ amount of vasoconstriction. As such, once vasopressor therapy is initiated, the ‘setpoint’ for preload dependency will be pushed towards a maintained preload even with less circulating volume.7, 15 Therefore, continuous infusion of vasopressors may mask preload responsiveness resulting in uncoupling of the GDT intervention by vasoconstriction.
Thus, the therapeutic concept behind a given haemodynamic strategy depends on several factors: intravascular volume, vasoconstriction/dilation and inotropic support, and the sequence and timing of these. In addition, many high-risk patients today are treated with potent vasodilators, such as angiotensin-converting-enzyme inhibitors and beta blockers, which exacerbate the risk of intraoperative vasoplegia. Also, patients treated within enhanced recovery pathways may have better preoperative hydration status, which may reduce the role of hypovolaemia as a first cause of post-induction preload responsiveness.20 Therefore, the assumption that post-induction preload dependency is primarily caused by insufficient circulating volume may be challenged. Another approach, which includes vasoconstriction, may be to target optimal intravascular volume which, combined with adequate vasoconstriction to eliminate preload responsiveness, maintains organ perfusion.15, 16 This approach changes the therapeutic concept, such that vasoconstriction will be the first choice for preload dependency followed by volume loading, inotropic drugs, or both, but only if adequate perfusion is not attained. This will both minimise unnecessary volume therapy and reduce periods of intraoperative hypotension. Such a paradigm will obviously require both monitoring of preload dependency and continuous monitoring of perfusion, which is clinically available with near infrared spectroscopy, sublingual microcirculation assessments, and the ‘peripheral perfusion index’.21, 22 However, there is currently insufficient clinical research data to support this hypothesis.
Vasoconstriction as diagnostic and therapeutic modality
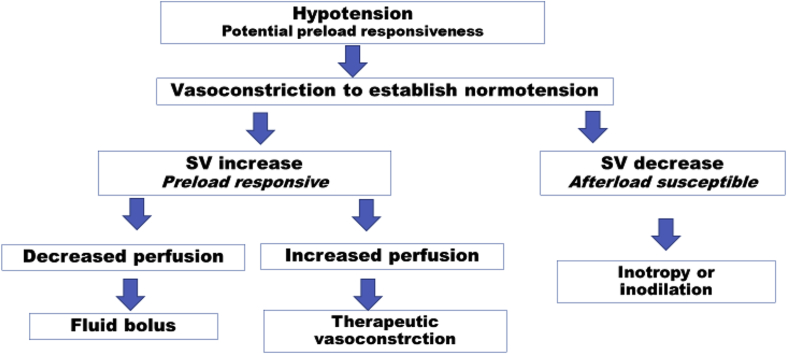
Although the diagnosis of preload responsiveness is simple, there has been little exploration in venous recruitment by vasoconstriction to test for preload responsiveness, even though vasoconstrictors can recruit preload.17 A bolus dose of a pure alpha-agonist vasoconstrictor such as phenylephrine has an extremely fast onset, a short half-life, and as such could act as a diagnostic test of the combined response to an increase in preload and afterload in hypotensive patients, especially if guided by simultaneous monitoring of perfusion. Subsequently, the decision can be made to either proceed with vasoconstrictor infusion, if perfusion is improved together with an increase in SV and BP, or, if central haemodynamics are improved without an increase in tissue perfusion, to supplement with volume therapy (Fig. 1). Obviously, vasoconstriction as a diagnostic test will increase afterload which can demonstrate ‘afterload susceptibility’ when the combined increase in preload and afterload results in a decrease in SV. The approach of using an initial vasoconstriction diagnostic test coupled with perfusion monitoring has the potential to quickly guide adequate resuscitative measures, while at the same time avoiding hypotension and vasoconstriction-induced uncoupling of microcirculation leading to tissue hypoperfusion. Consequently, the clinician can decide to use therapeutic vasoconstriction to enhance preload and thereby improve cardiac output in situations with preload dependency where appropriate.
Fig. 1.
Vasoconstriction as diagnostic/therapeutic intervention. SV, stroke volume.
Therapeutic vasoconstriction is already used in Caesarean section, where continuous phenylephrine is used routinely to attenuate vasodilation caused by neuraxial blockade, and in vasoconstriction with norepinephrine in high-risk surgery in GDT algorithms, although only guided by BP response.8, 9 Recently, improved outcome has been reported for individualised BP control with norepinephrine added to an initial GDT SV-based fluid intervention, an effect that may be based on either correction of vasoplegia and thereby preload responsiveness, increased cardiac output as a result of the inotropic effect of norepinephrine, or both.7, 19 As such, norepinephrine as a primary intervention is rational if used to decrease preload responsiveness, augment cardiac output, and maintain systemic arterial pressure while maintaining organ perfusion. However, this approach requires monitoring of both central cardiovascular parameters and central/peripheral perfusion, which is the true target of the intervention. Therefore, monitoring of both macro-circulatory parameters and measures of peripheral/central perfusion are required for the clinician to ‘squeeze safely’. The described hypothetical approach to diagnostic and therapeutic vasoconstriction needs further clinical research to investigate the effects of vasoconstriction on macro-micro circulatory interactions before pragmatic clinical trials are performed.23
Conclusions
Perioperative optimisation of haemodynamics is an object of intensive research, but the challenge for future research is how to intervene in the most rational way, and how different physiological parameters interact with our interventions. Correction of hypotension is currently based on an inadequate physiological basis within the GDT concept. We hypothesise that a rational approach and major research focus may be an initial short-acting vasoconstrictor diagnostic intervention to assess the combined macro- and micro-circulatory response to preload and afterload enhancement and its interaction with tissue perfusion followed by fluid-based GDT where appropriate. This approach will need to be investigated both in specific patient categories and with different methods of anaesthesia to develop algorithms for the timing and sequence of perioperative cardiovascular interventions to provide better patient outcomes.
Authors' contributions
Both authors contributed to the conception and design, writing, and approved the final draft.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Michard F., Giglio M.T., Brienza N. Perioperative goal-directed therapy with uncalibrated pulse contour methods: impact on fluid management and postoperative outcome. Br J Anaesth. 2017;119:22–30. doi: 10.1093/bja/aex138. [DOI] [PubMed] [Google Scholar]
- 2.Miller T.E., Myles P.S. Perioperative fluid therapy for major surgery. Anesthesiology. 2019;130:825–832. doi: 10.1097/ALN.0000000000002603. [DOI] [PubMed] [Google Scholar]
- 3.Navarro L.H.C., Bloomstone J.A., Auler J.O.C. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioper Med (Lond) 2015;4:3. doi: 10.1186/s13741-015-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecconi M., Corredor C., Arulkumaran N. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2012;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong M.A., Wang Y., Berbenetz N.M., McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? Eur J Anaesthesiol. 2018;35:1. doi: 10.1097/EJA.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 6.Myles P.S., Bellomo R., Corcoran T. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 7.McEvoy M.D., Gupta R., Koepke E.J. Perioperative Quality Initiative consensus statement on postoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:575–586. doi: 10.1016/j.bja.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Pearse R.M., Harrison D.A., MacDonald N. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 9.Ackland G.L., Iqbal S., Paredes L.G. Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: a multicentre, randomised, double-blind, controlled, mechanistic trial. Lancet Respir Med. 2015;3:33–41. doi: 10.1016/S2213-2600(14)70205-X. [DOI] [PubMed] [Google Scholar]
- 10.Sessler D.I., Meyhoff C.S., Zimmerman N.M. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327. doi: 10.1097/ALN.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 11.Sanders R.D., Hughes F., Shaw A. Perioperative Quality Initiative consensus statement on preoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:552–562. doi: 10.1016/j.bja.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Sessler D.I., Bloomstone J.A., Aronson S. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–574. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Rebet O., Andremont O., Gérard J.-L., Fellahi J.-L., Hanouz J.-L., Fischer M.-O. Preload dependency determines the effects of phenylephrine on cardiac output in anaesthetised patients. Eur J Anaesthesiol. 2016;33:638–644. doi: 10.1097/EJA.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 14.Nouira S., Elatrous S., Dimassi S. Effects of norepinephrine on static and dynamic preload indicators in experimental hemorrhagic shock. Crit Care Med. 2005;33:2339–2343. doi: 10.1097/01.ccm.0000182801.48137.13. [DOI] [PubMed] [Google Scholar]
- 15.Kalmar A.F., Allaert S., Pletinckx P. Phenylephrine increases cardiac output by raising cardiac preload in patients with anesthesia induced hypotension. J Clin Monit Comput. 2018;32:969–976. doi: 10.1007/s10877-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Genderen M.E., Bartels S.A., Lima A. Peripheral perfusion index as an early predictor for central hypovolemia in awake healthy volunteers. Anesth Analg. 2013;116:351–356. doi: 10.1213/ANE.0b013e318274e151. [DOI] [PubMed] [Google Scholar]
- 17.Joosten A., Coeckelenbergh S., Delaporte A. Implementation of closed-loop-assisted intra-operative goal-directed fluid therapy during major abdominal surgery: a case-control study with propensity matching. Eur J Anaesthesiol. 2018;35:650–658. doi: 10.1097/EJA.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 18.Joosten A., Delaporte A., Ickx B. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system. Anesthesiology. 2018;128:55–66. doi: 10.1097/ALN.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 19.Futier E., Lefrant J.-Y., Guinot P.-G. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery. JAMA. 2017;318:1346. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bundgaard-Nielsen M., Jørgensen C.C., Secher N.H., Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand. 2010;54:464–469. doi: 10.1111/j.1399-6576.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- 21.Lima A., van Bommel J., Sikorska K. The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Crit Care Med. 2011;39:1649–1654. doi: 10.1097/CCM.0b013e3182186675. [DOI] [PubMed] [Google Scholar]
- 22.van Genderen M.E., Paauwe J., de Jonge J. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: a prospective observational study in adults. Crit Care. 2014;18:R114. doi: 10.1186/cc13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackland G.L., Brudney C.S., Cecconi M. Perioperative Quality Initiative consensus statement on the physiology of arterial blood pressure control in perioperative medicine. Br J Anaesth. 2019;122:542–551. doi: 10.1016/j.bja.2019.01.011. [DOI] [PubMed] [Google Scholar]