Abstract
Background
Emerging data highlights the potential role of cyclooxygenase (COX) inhibitors in the primary prevention of malignancy, reducing metastatic spread and improving overall mortality. Despite nonsteroidal anti-inflammatory drugs (NSAIDs) forming a key component of the WHO analgesic ladder, their use in cancer pain management remains relatively low. This review re-appraises the current evidence regarding the efficacy of COX inhibitors as analgesics in cancer pain, providing a succinct resource to aid clinicians’ decision making when determining treatment strategies.
Methods
Medline® and Embase® databases were searched for publications up to November 2018. Randomised controlled trials (RCTs) and double-blind controlled studies considering the use of NSAIDs for management of cancer-related pain in adults were included. Animal studies, case reports, and retrospective observational data were excluded.
Results
Thirty studies investigating the use of NSAIDs in cancer pain management were identified. There is a lack of high-quality evidence regarding the analgesic efficacy of NSAIDs in cancer pain, with short study durations and heterogeneity in outcome measures limiting the ability to draw meaningful conclusions.
Conclusions
Despite the renewed interest in these cost-effective, well-established medications in cancer treatment outcomes, there is a paucity of data from the past 15 yr regarding their efficacy in cancer pain management. However, when analgesic strategies in the cancer population are being formulated, it is important that the potential benefits of this class of drug are considered. Further work investigating the role of NSAIDs in cancer pain management is undoubtedly warranted.
Keywords: analgesia, anti-inflammatory agents, non-steroidal, cancer pain, cyclooxygenase, neoplasms, pain management
The broad therapeutic effects of nonsteroidal anti-inflammatory drugs (NSAIDs) have been exploited for centuries, with reports of the use of willow tree extracts for pain and inflammatory remedies dating back to the time of Hippocrates (ca. 400 b.c.).1 The wide-scale production of salicylic acid and aspirin began in the late 1800s,1, 2 yet it was only discovered in 1971 that their mechanism of action related to inhibition of prostaglandin synthesis,3 with award of the Nobel prize for Physiology or Medicine in 1982 to Bergström, Samuelsson, and Vane for this discovery.4 Although it was postulated that isoenzymes of cyclooxygenase (COX) existed, it was not until 1989 that a second distinct protein with COX activity was isolated.5 The identification of a constitutively expressed enzyme in almost all human tissues (COX-1) and an alternative, tightly regulated enzyme that is predominantly expressed in states of inflammation and tumourigenesis (COX-2) led to the recognition that inhibition of COX-1 was responsible for the many side-effects associated with NSAIDs.6 Development of COX-2 specific inhibitors, and the prospect of anti-inflammatory effects with fewer adverse effects,7, 8 was heralded with enthusiasm amongst clinicians and patients alike.9 The popularity of these agents surged rapidly, with annual sales of rofecoxib (the COX-2 selective inhibitor, Vioxx) exceeding US $2.5 billion.10 However, in 2004, a little more than 5 yr after its licence was granted, rofecoxib was voluntarily withdrawn from the market because of an association with excess relative risk of cardiovascular and cerebrovascular events.11 The withdrawal of valdecoxib, another COX-2 inhibitor, soon followed.12
NSAIDs form a key component of the WHO analgesic ladder and have been advocated as a useful adjunct for management of cancer pain.13 Despite initial widespread popularity, prescription patterns in certain patient groups have demonstrated a decline in the use of NSAIDs over recent years, and a rapid decrease of COX-2 inhibitor use, since 2004.14 It is difficult to identify specific publications that have considered prescribing patterns of NSAIDs for cancer pain over time. A cross-sectional study of 2282 patients conducted across Europe in 2014 reported that 29.9% of those with moderate-to-severe pain, receiving a mean oral morphine equivalent dose of 230 ± 457 mg day−1, were also taking NSAIDs.15 When dipyrone (metamizole) is excluded from this data, 19.4% were using NSAIDs. Smaller-scale publications across Europe, Canada, and Australia investigating the use of analgesic agents in certain populations with cancer pain report even lower usage of NSAIDs (4–13.2%).16, 17, 18
A small number of systematic reviews on non-opioid analgesics for the management of cancer pain have been published.19, 20, 21, 22 Of these four, one has subsequently been withdrawn.23 One review considers non-opioids in a broader palliative care setting,22 and is therefore not necessarily specific to NSAIDs in the setting of cancer pain. Another paper reviews non-opioid analgesics in addition to opioids for the management of advanced cancer pain,20 neglecting the growing population of cancer survivors, many of whom experience pain.24 The fourth systematic review identified only 11 relevant studies involving 949 participants,21 perhaps because of certain inclusion criteria, namely duration of study and oral route of administration. This review did not include any studies comparing NSAIDs with placebo; and if studies relating to dipyrone (whose clinical use is no longer permitted in many countries including the UK) are removed, this review would include nine studies and 778 participants.
Despite the prominence of NSAIDs in the WHO analgesic ladder, their overall usage in cancer pain management appears to be low. Conversely, data regarding the impact of COX inhibitors on primary prevention, metastatic spread, and mortality have led to a resurgence in interest in NSAIDs (especially aspirin) and COX-2 inhibitors as anti-cancer treatments. This review aims to present a systematically conducted review of analgesic efficacy of COX inhibitors for patients with cancer pain, providing a catalogued and critical overview of pertinent data on the use of NSAIDs for cancer pain management.
Methods
Literature searches were conducted of the Medline® (via PubMed®) and Embase® databases from inception to November 2018. Searches of titles, keywords, and abstracts were conducted, using a combination of controlled vocabulary (e.g. Medical Subject Headings) and individual searches. The search strategy aimed to identify papers that combined two subject matters within them, firstly cancer pain and secondly NSAIDs. Variations for terms considering ‘cancer pain’, included variations on the term ‘cancer’ (including neoplastic, neoplasm, oncology, oncological, malignant, malignancy, tumour, and tumor) combined with variations on the term ‘pain’ (including related pain, associated pain and analgesia). NSAID-related terms were searched using drug class, individual drug names, common acronyms, and suffixes. Comprehensive details of the search strategy used can be found within the appendix.
After removal of duplicates, the abstracts of identified articles were reviewed for relevance and to select those for full-text screening. Inclusion criteria for analgesic efficacy were randomised controlled trials (RCTs) and double-blind controlled studies considering the use of NSAIDs for management of cancer-related pain. Only studies considering adult patients were included. Papers that were not available in English or no longer readily available from their publisher were excluded. Studies investigating the use of NSAIDs during the perioperative period were also excluded. Review articles that considered NSAIDs in the management of cancer pain, either as a subsection or as the basis for the publication, were evaluated for any original data or relevant references for inclusion. The references of publications included were also searched to identify any additional studies for inclusion.
Publications identified for analgesic efficacy were grouped based upon the drug classification of comparator arm of the studies (e.g. placebo, other NSAIDs, opioid analgesics). For the purposes of this review, the term NSAID includes all agents that inhibit COX. When more specific details are appropriate or required, drugs may be named individually. At times it may be important to distinguish between agents with different mechanisms of action. For instance, although a non-selective COX inhibitor, aspirin irreversibly inhibits COX enzymes and is reported to have COX-independent mechanisms of action. These unique mechanisms may warrant distinction, in which case, aspirin will be discussed separately from ‘traditional NSAIDs’, which reversibly inhibit COX enzymes. COX-2 inhibitors selectively inhibit COX-2 and therefore may be considered separately from traditional NSAIDs.
Results
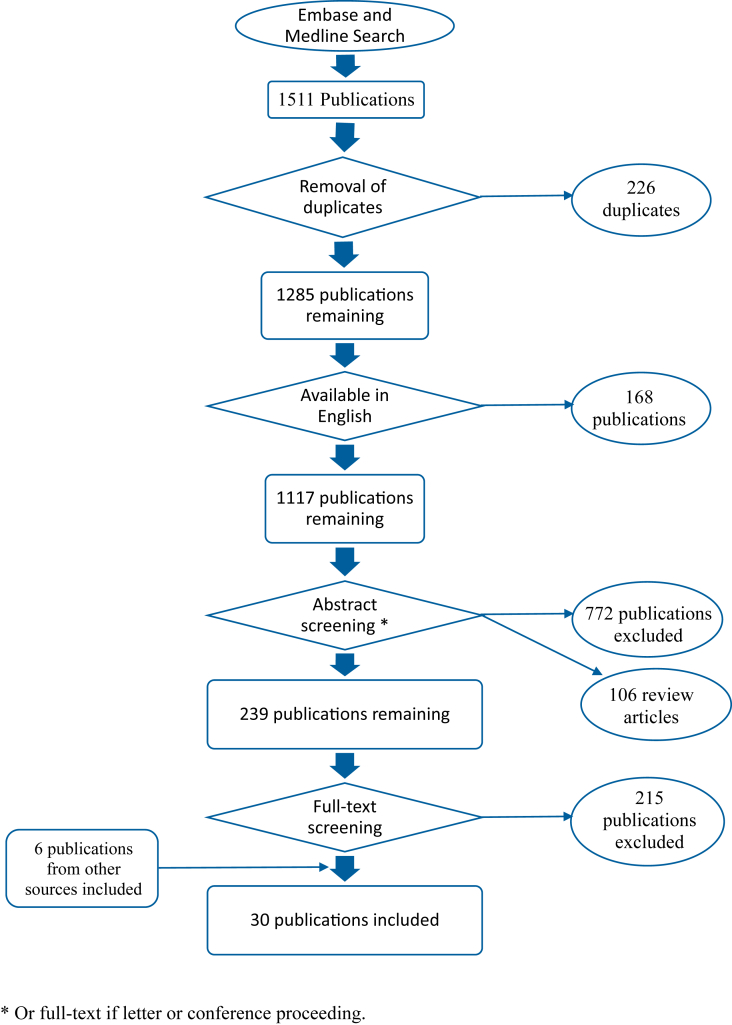
A total of 1511 publications were identified by the literature search. After removal of duplicates, 1285 publications remained. Figure 1 summarises the literature search and selection process of relevant publications.
Fig 1.
Summary of literature search.
Thirty-seven publications regarding analgesic efficacy in cancer pain were identified. Five were excluded because the NSAIDs studied are no longer commercially available.25, 26, 27, 28, 29, 30 Although frequently described as an NSAID, dipyrone's (metamizole's) mechanism of action continues to be investigated many years after its introduction to clinical practice.31, 32 Furthermore, its use is not permitted in many countries, including both the UK and the USA. For these reasons, studies, or arms of studies, considering the use of dipyrone were also excluded.33, 34 As a consequence, 30 studies were included for analysis.
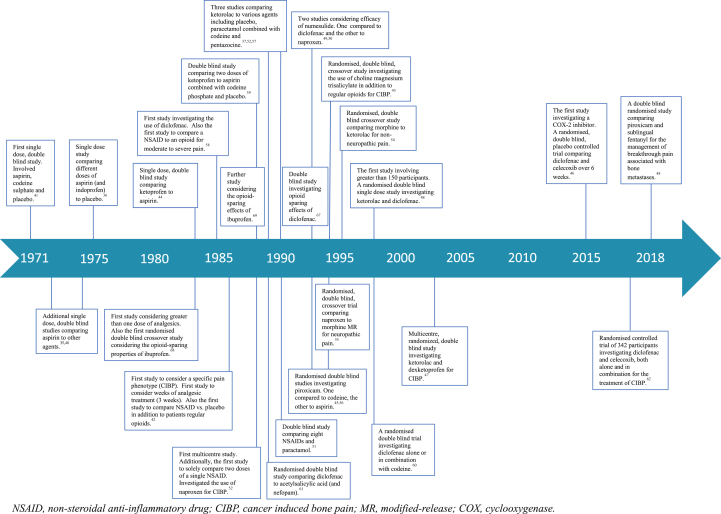
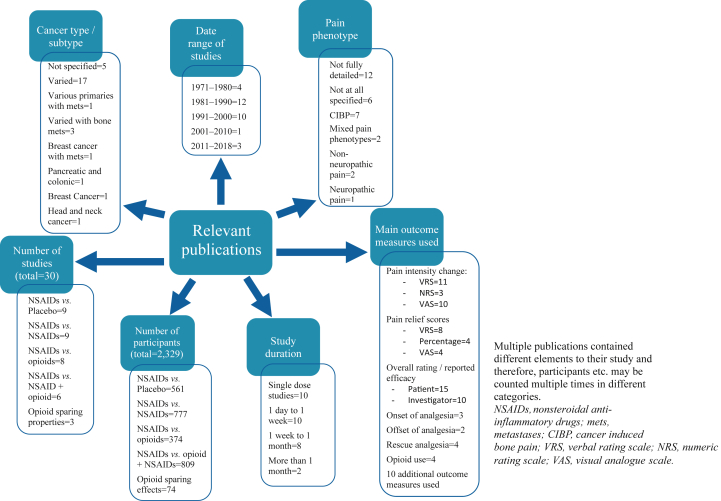
Study designs, durations of treatment, durations of follow-up, dosing regimens, routes of administration, underlying cancer diagnoses, and pain phenotypes all varied considerably. Fig 2, Fig 3 provide an overview of these elements. To facilitate consideration, studies are summarised in the supplementary tables based upon the comparator drug in the study. Elements of studies pertaining to NSAIDs no longer commercially available, are not detailed but the remaining arm(s) of the study are described.
Fig 2.
Timeline of relevant studies.
Fig 3.
Breakdown of relevant studies by number, number of participants, date of publication, cancer type, pain phenotype, study duration, and main outcome measures utilised.
NSAIDs vs placebo
Supplementary Table S1 details the studies identified comparing NSAIDs vs placebo. A total of seven studies enrolling 509 participants are detailed in this table.35, 36, 37, 38, 39, 40, 41 All studies considered aspirin or other traditional NSAIDs. There were no studies relating to COX-2 inhibitors. All publications studied single doses of analgesic agents and were performed before 1991. All studies demonstrated analgesic superiority of NSAIDs when compared with placebo (one study only showed advantage with higher doses of aspirin). NSAIDs and doses that demonstrated superior outcomes to placebo were ketorolac 10 mg p.o., ketorolac 10 mg i.m., ketorolac 30 mg i.m., ketorolac 90 mg i.m., ketoprofen 100 mg p.o., ketoprofen 300 mg p.o., aspirin 1000 mg p.o., aspirin 650 mg p.o., and mefenamic acid 250 mg p.o. Adverse effects appeared comparable between NSAID and placebo groups.
Despite the reported superiority of these agents over placebo, outcome measures utilised varied considerably between the publications. The most common outcome measures used for analysis were the mean summed pain intensity difference (SPID), mean total pain relief (TOPAR), and proportion of participants reporting greater than 50% pain relief. One study made specific reference to the reported analgesic efficacy of placebo, stating that 21% reported greater than 50% reduction in pain.35 Although not specifically commented upon, the two other studies using the same outcome measure had similar findings.40, 41
Six of the seven studies specifically commented on the fact that no opioid analgesia was permitted during the study period; this was not specified in the remaining publication.36 Supplementary Table S1 therefore details the use of NSAIDs alone in the management of cancer pain compared with placebo. Two additional studies identified NSAID use compared with placebo in addition to the participants' usual background opioid analgesia42, 43; consequently, direct comparison with the seven studies detailed in Supplementary Table S1 is not possible. Both studies enrolled 26 participants with cancer-induced bone pain (CIBP), each investigating a different NSAID (choline magnesium trisalicylate43 and flurbiprofen42) compared with placebo. They report lower pain intensity scores in the NSAID groups that do not reach statistical significance, but conclude that their sample sizes are most likely underpowered for their primary outcome measure.
NSAIDs vs other NSAIDs
Supplementary Table S2 details publications identified comparing different NSAIDs; only two were published in the past 20 yr.44, 45, 46, 47, 48, 49, 50, 51, 52 A single study related to COX-2 inhibitors was identified. Most findings reported no significant differences amongst the NSAIDs investigated; however, the largest sample size used, involved 60 participants in each arm, raising the question of whether these studies are powered appropriately to detect efficacy differences between drugs with the same mechanism of action. The one study that reported a difference in pain relief and patient preference, found ketoprofen 400 mg significantly superior to both ketoprofen 100 mg and aspirin 1 g. However, the maximum licenced daily dose for ketoprofen is 300 mg, making the clinical relevance of this finding uncertain.44 Most publications failed to identify a significant difference between adverse events associated with different NSAIDs. One study found significantly greater gastrointestinal side-effects requiring antacid therapy in those taking aspirin compared with piroxicam.45
NSAIDs vs opioids
Supplementary Table S3 details studies identified comparing NSAIDs with opioids.37, 41, 53, 54, 55, 56, 57, 58 Sample sizes were again small (most n=100). Only one study conducted in the past 20 yr was identified. Facilitating interpretation by combining data is extremely difficult. Although oral morphine equivalence would theoretically allow such comparisons, other factors—not least heterogeneity in study design, duration of follow-up, and outcome measures—render this a challenging and potentially futile exercise.
The findings of these studies vary considerably. Of the eight studies detailed in Supplementary Table S3, outcome measures favoured the opioid treatment arm in two studies, the NSAID treatment arm in three studies, and no significant difference in the remaining three. No two studies compared the same two agents. A total of five NSAIDs and four opioid analgesics were utilised in these eight studies. One additional publication mentions unpublished data, for which the methodology and specifics cannot be fully interrogated.59 It reports termination of a study because of poor enrolment, yet concludes ketoprofen administered orally is equal or more effective than morphine administered parenterally.59
NSAIDs with opioids
A total of five identified studies compared NSAIDs against NSAIDs combined with an opioid, and are detailed in Supplementary Table S4.39, 40, 56, 60, 61 None of these studies involved COX-2 inhibitors, nor were conducted in the past 20 yr. Only two of these studies considered use of these agents beyond a single dose. Two publications reported that the combination of NSAID plus opioid resulted in either superior analgesic efficacy or fewer patients withdrawing because of inadequate analgesia. However, only one of these studies report that their findings were statistically significant. The remaining three studies failed to show any significant difference in analgesic efficacy between the two groups. Although three studies report a greater incidence of adverse effects associated with the combination treatments, only one performed statistical analysis and concluded that the difference was not significant. One further publication details a randomised controlled trial of 342 patients assigned to three treatment arms (diclofenac with morphine, celecoxib with morphine, and both diclofenac and celecoxib with morphine).62 The dose of NSAID remained constant, whereas opioid dose was titrated over the four week study period. A 50% reduction in visual analogue scale (VAS) scores across all three groups was reported. This reduction was greater in the group taking both diclofenac and celecoxib in combination with morphine (P<0.05).62
Opioid-sparing effects of NSAIDs
Three studies considered the opioid-sparing effects of NSAIDs. Although these effects are well documented in postoperative care and postoperative cancer care,63, 64 with demonstrable reductions in opioid adverse effects,65, 66 no large studies appear to have been performed with respect to non-postoperative cancer pain management. A study of 16 patients demonstrated the addition of diclofenac 50 mg suppositories reduced morphine patient-controlled analgesia (PCA) usage but was perhaps underpowered to detect significance in altered pain intensity.67 A randomised double-blind crossover study of 28 participants with moderate to severe persistent pain related to their cancer reported that addition of ibuprofen 600 mg to two different doses of methadone significantly reduced pain intensity and improved pain relief scores without an increase in side-effects.68 A further randomised, double-blind study of 30 participants regularly using a combination of paracetamol and oxycodone showed that ibuprofen 600 mg was superior to placebo in daily pain intensity, pain relief, and opioid-sparing properties.69
Discussion
Given the high prevalence of pain at all stages of cancer and the relative lack of efficacious treatments for this pain, it is surprising that there is such a paucity of research into the use of NSAIDs in cancer pain. The age of many of the published papers (>20 yr) is also notable, as there have been a number of developments in this area such as the introduction of COX-2 antagonists and the potential influence of NSAIDs on the oncogenic process. The therapeutic landscape of cancer continues to shift at a remarkable pace, outpacing the few reviews in this area that have been published. Novel treatments are being introduced to clinical practice at a high rate, which is altering the natural history of many tumour types and bolstering the numbers of cancer survivors, the net outcome of which is more patients experiencing pain.
Our search identified 106 review articles that considered use of NSAIDs in cancer pain management. Despite numerous review articles, only 30 randomised controlled trials or double-blind controlled studies were identified for inclusion. The results of these publications suggest that NSAIDs are more effective than placebo in reducing pain intensity and providing pain relief in cancer pain. The overwhelming majority of data supporting this is based upon single dose studies. Longer-term studies, randomising the use of NSAIDs or placebo alone, for pain management could be ethically challenging. A smaller number of studies considered the use of NSAIDs in participants additionally receiving opioid analgesia. These studies also demonstrated superiority of NSAIDs over placebo (greater reduction in pain intensity, enhanced pain relief, and opioid-sparing effects). Although a total of nine publications, involving 777 participants were identified, there are insufficient data available to suggest that any NSAID is superior to another. None of these comparative studies considered more than 60 patients in each arm when comparing efficacy; therefore, it is possible they were underpowered to adequately address this topic. Eight publications comparing the analgesic efficacy of NSAIDs to opioids were identified; however, heterogeneity and small sample sizes make conclusions difficult to derive. Because of the small sample sizes throughout all publications, adverse effects are difficult to quantify. It is important to note when considering the data presented that the findings outlined above represent the conclusions of the authors based upon the limited and varied data available. Caution is therefore required when attempting to extrapolate these data.
Attempts to consider analgesic strategies based upon underlying pain mechanisms and phenotype are appealing. However, of the 30 studies included, 18 lacked full details regarding pain phenotype, whereas two studies included a broad mix of different pain phenotypes. Of the remaining studies, seven considered CIBP, two investigated non-neuropathic pain, and one studied neuropathic pain alone. Drawing meaningful conclusions on the efficacy of NSAIDs based upon pain phenotype is therefore challenging. With respect to the single most represented phenotype, CIBP (which in itself is often considered to include both neuropathic and non-neuropathic features), each of the seven publications investigated the use of different NSAIDs. No study investigated the use of NSAIDs compared with placebo alone for management of CIBP. Although all seven studies suggest an analgesic benefit regarding NSAID administration in CIBP, translating these findings into clinical recommendations is hindered by disparate study designs and the diversity of outcome measures utilised.
There are a number of obstacles to developing meaningful conclusions regarding the efficacy of NSAIDs in cancer pain management. Firstly, 10 of the total 30 studies were single dose studies, where patients were followed up for up to a maximal time point of 6 h after the single dose. Although eight of the studies were conducted over more than 7 days, only six studied the use of analgesic agents for longer than one week. The longest study duration was 6 weeks. Given the short duration of many of the studies in question, it is conceivable that their results are not transferable to longer-term use. The relative analgesic efficacy of all agents considered could be over- or underestimated. Furthermore, the significance of adverse events is challenging to extrapolate. It is plausible that repeated exposure cumulatively increases the incidence of certain side-effects (e.g. ulcers of the gastrointestinal tract), potentially making single dose studies falsely reassuring.
Another barrier to wider scale interpretation relates to heterogeneity of the study designs. Variation in drugs used, doses, frequency, routes of administration, and outcome measures make combined data analysis difficult and inadvisable. The choice of outcome measure in evaluation of pain management has been shown to have a greater influence on measured pain relief than the analgesic agent itself.70 This highlights the difficulty and conceivable inaccuracy associated with interpretation of multiple studies with heterogeneous outcome measures.
We failed to identify any studies that specifically aimed to quantify the risk associated with short-, medium-, or long-term NSAID use in the cancer pain population. However, other considerations identified by the literature search that are not directly related to the primary aim of this study warrant discussion, namely the use of NSAIDs in the perioperative period and their potential role in oncogenesis.
Although NSAIDs are not used universally for perioperative analgesia, their efficacy has been demonstrated in numerous meta-analyses for a number of surgical procedures,71, 72, 73 and their effects on reducing opioid requirements and related side-effects are well documented.65, 66 With emerging evidence for the benefits of enhanced recovery after surgery (ERAS) pathways and the shift towards multi-modal analgesia, NSAIDs are currently recommended in an array of pathways and protocols.74, 75 A systematic review investigating the specific role NSAIDs have in both pre-emptive and preventative analgesia for all types of surgery is currently being conducted,76 the results of which are keenly anticipated. Perhaps the predominant factors in the underutilisation of NSAIDs perioperatively relate to concerns about potential adverse consequences: risk of increased blood loss, acute kidney injury, anastomotic leak, and deleterious effects on bone healing. The absolute significance of these risks is difficult to quantify because of the multifactorial nature of such complications, and it is therefore unsurprising that studies have resulted in conflicting conclusions on the impact that NSAIDs have on such outcomes.77, 78
There is a growing body of evidence to suggest that the perioperative period and anaesthetic techniques play a significant role in the outcome of patients undergoing tumour resections.79, 80 With respect to NSAIDs, there are two main considerations: direct mechanisms of NSAIDs and their effects on opioid use. Direct mechanisms include their anti-inflammatory effects and direct influences on oncogenesis. The oncogenic considerations are not confined to the perioperative period as discussed in greater detail below.
Inflammation is a key component of the metastatic process, and targeting this element is considered to have a critical role in preventing metastasis.81 The anti-inflammatory effects of NSAIDs are well known, with studies confirming reduced circulating inflammatory mediators associated with administration of traditional NSAIDs and COX-2 inhibitors perioperatively.82, 83 Furthermore, concerns exist regarding the immunosuppressive properties of opioids,84 thereby potentially affecting cancer recurrence. The opioid-sparing properties of NSAIDs may be utilised to minimise these immunosuppressive effects. Despite this concern, a large prospective cohort study involving 34 188 patients found no clinically relevant evidence of an association between opioids and breast cancer recurrence.85 A recent systematic review considering the perioperative use of NSAIDs on long-term survival after cancer surgery concluded that studies (predominantly retrospective and observational) have produced conflicting results, but a number of ongoing RCTs aim to provide much-needed clarity on the subject.78
A further perioperative consideration is the potential opioid-sparing properties of NSAIDs, a major area of interest when placed in the context of the recent ‘opioid crisis’. It is reported that 3–8% of opioid-naïve individuals undergoing elective surgery progress to persistent opioid use when followed up many months after their surgical procedure.86, 87 These rates seem consistent irrespective of whether the surgical procedure is considered to be minor or major.88 Analgesic strategies that limit the use of opioids in the perioperative setting may reduce these numbers, easing the health and economic burden associated with aberrant opioid usage.
The direct effects of NSAIDs on oncogenesis raise intriguing considerations for their use in cancer pain management. The first association between long-term aspirin use and reduced incidence of colorectal cancer was reported in the 1980s.89 Subsequently, more epidemiological data emerged, indicating the potential role of aspirin and other NSAIDs in the prevention of colorectal carcinoma.90, 91, 92 The design of appropriate randomised, placebo-controlled trials are fraught with challenges, not least the large sample sizes and duration of enrolment required. Randomised placebo-controlled trials have therefore been conducted in groups at higher risk of developing colorectal cancer, such as those with previous colorectal cancer, familial adenomatous polyposis, or adenomas. Although not unanimous, studies have been published with positive results for aspirin, other NSAIDs, and COX-2 inhibitors.93, 94, 95 The evidence base for other tumour types is predominantly for the use of aspirin and based upon epidemiological studies. Such studies have reported a reduced risk overall of multiple cancer types, including lung, breast, and oesophageal.96, 97 The reported positive effects on cancer risk are not uniform. NSAID use has been reported to both increase and decrease the risk of prostate cancer,98, 99 and various studies have failed to show any benefit.100, 101
With respect to adjuvant therapy, a systematic review and meta-analysis has reported that low-dose aspirin is associated with reduced mortality, metastatic spread, and thrombo-embolic events.102 The heterogeneity of the available studies, however, confirms the need for adequately powered randomised placebo-controlled trials. As such, the Add-Aspirin Trial currently continues to recruit, aiming for 11 000 participants to determine whether regular aspirin after early-stage cancer treatment improves mortality and recurrence rates for numerous different tumour types.103
Despite the early promise of a potential role in prevention of carcinoma formation, a significant barrier to the selection and interrogation of appropriate candidates for such therapy remains the lack of identification of the precise mechanism by which these anti-tumour effects may be exhibited. Proposed mechanisms include inhibiting the over-expression of COX-2 and increased prostanoid levels in cancerous cells,104 reducing prostaglandin-mediated angiogenesis105 and induction of apoptosis.106 COX-2 expression has been demonstrated in a broad range of malignant cells.107, 108 The tumour micro-environment and neovasculature also have a role in COX-2 expression.109 COX-2 expression is associated with increased prostanoid levels,110 which have in turn been associated with tumourigenesis.111 Additionally, COX-2 promotes release of angiogenic peptides including vascular endothelial growth factor (VEGF), endothelin-1, and prostaglandin E2.105, 112 Inhibition of these processes would account for therapeutic actions in oncogenesis. Furthermore, inhibition of COX-2 has been demonstrated to induce apoptosis113 although the mechanism for this remains unclear.106
The lack of high-quality evidence regarding the analgesic efficacy of NSAIDs in cancer pain is a situation that has existed for years. A comprehensive systematic review conducted in 2005 concluded that ‘there remains a need for a substantial increase in the number of high-quality trials of NSAIDs in patients with cancer’.19 Despite this ‘call to arms’, we identified only three studies performed subsequent to this publication. It is important to appreciate that a lack of high-quality evidence for an agent does not equate to lack of efficacy. Although concerns exist regarding the well-established adverse effects associated with NSAID use, we were unable to identify studies that are designed appropriately to quantify these events within the context of cancer pain management (a population of patients who are often frail and susceptible to such adverse events occurring). Emerging potential benefits for NSAIDs within the context of influencing oncogenesis should now be considered in the risk:benefit analysis for the use of NSAIDs in cancer pain management.
Authors' contributions
Concept: all authors.
Content: DJM, SJ, PF-S, MRDB.
Authorship and review of manuscript: DJM, SJ, PF-S, MRDB.
Mentorship: GP.
Declarations of interest
PF-S has previously undertaken paid consultancy work for Astellas, Pfizer, Grunenthal, and Kyowa Kirin. MRDB undertakes paid consultancy work for Sativa Investments Ltd (UK). The other authors have no conflicts to declare.
Funding
NHS funding to the Royal Marsden/Institute of Cancer Research NIHR Biomedical Research Centre.
Handling editor: H.C. Hemmings Jr
Editorial decision date: 28 February 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.02.028.
Appendix.
Medline search strategy
(*"CANCER PAIN"/OR (Cancer Pain).ti,ab OR (Cancer associated pain).ti,ab OR (Cancer-associated pain).ti,ab OR (Cancer-related pain).ti,ab OR (Cancer related pain).ti,ab OR (Neoplasm associated pain).ti,ab OR (Neoplasm-associated pain).ti,ab OR (Neoplasm related pain).ti,ab OR (Neoplasm-related pain).ti,ab OR (oncological pain).ti,ab OR (oncology pain).ti,ab OR (tumor associated pain).ti,ab OR (tumor-associated pain).ti,ab OR (tumour-associated pain).ti,ab OR (tumour associated pain).ti,ab OR (tumour related pain).ti,ab OR (tumour-related pain).ti,ab OR (tumor-related pain).ti,ab OR (tumor related pain).ti,ab) AND (*"ANTI-INFLAMMATORY AGENTS, NON-STEROIDAL"/OR *"CYCLOOXYGENASE 2 INHIBITORS"/OR (NSAIDs).ti,ab OR (Non steroidal anti-inflammatory drugs).ti,ab OR (Non steroidal anti inflammatory drugs).ti,ab OR (Non steroidal anti inflammatory agents).ti,ab OR (Non steroidal anti-inflammatory agents).ti,ab OR (Non steroidals).ti,ab OR (Non-steroidals).ti,ab OR (Non-steroidal anti-inflammatory drugs).ti,ab OR (Non-steroidal anti inflammatory drugs).ti,ab OR (Non-steroidal anti inflammatory agents).ti,ab OR (Non-steroidal anti-inflammatory agents).ti,ab OR (Aspirin).ti,ab OR ("profen").ti,ab OR (ibuprofen).ti,ab OR (diclofenac).ti,ab OR ("coxib").ti,ab OR (salicylate).ti,ab OR ("bufen").ti,ab OR ("brufen").ti,ab OR (naproxen).ti,ab OR (ketorolac).ti,ab OR (COX-2 inhibitors).ti,ab OR (COX 2 inhibitors).ti,ab OR (Cyclooxygenase inhibitors).ti,ab OR (Cyclo-oxygenase inhibitors).ti,ab)
Embase search strategy:
(*"CANCER PAIN"/OR (Cancer Pain).ti,ab OR (Cancer associated pain).ti,ab OR (Cancer-associated pain).ti,ab OR (Cancer-related pain).ti,ab OR (Cancer related pain).ti,ab OR (Neoplasm associated pain).ti,ab OR (Neoplasm-associated pain).ti,ab OR (Neoplasm related pain).ti,ab OR (Neoplasm-related pain).ti,ab OR (oncological pain).ti,ab OR (oncology pain).ti,ab OR (tumor associated pain).ti,ab OR (tumor-associated pain).ti,ab OR (tumour-associated pain).ti,ab OR (tumour associated pain).ti,ab OR (tumour related pain).ti,ab OR (tumour-related pain).ti,ab OR (tumor-related pain).ti,ab OR (tumor related pain).ti,ab) AND (*"NONSTEROID ANTIINFLAMMATORY AGENT"/OR *"CYCLOOXYGENASE 2 INHIBITOR"/OR (NSAIDs).ti,ab OR (Non steroidal anti-inflammatory drugs).ti,ab OR (Non steroidal anti inflammatory drugs).ti,ab OR (Non steroidal anti inflammatory agents).ti,ab OR (Non steroidal anti-inflammatory agents).ti,ab OR (Non steroidals).ti,ab OR (Non-steroidals).ti,ab OR (Non-steroidal anti-inflammatory drugs).ti,ab OR (Non-steroidal anti inflammatory drugs).ti,ab OR (Non-steroidal anti inflammatory agents).ti,ab OR (Non-steroidal anti-inflammatory agents).ti,ab OR (Aspirin).ti,ab OR ("profen").ti,ab OR (ibuprofen).ti,ab OR (diclofenac).ti,ab OR ("coxib").ti,ab OR (salicylate).ti,ab OR ("bufen").ti,ab OR ("brufen").ti,ab OR (naproxen).ti,ab OR (ketorolac).ti,ab OR (COX-2 inhibitors).ti,ab OR (COX 2 inhibitors).ti,ab OR (Cyclooxygenase inhibitors).ti,ab OR (Cyclo-oxygenase inhibitors).ti,ab)
Appendix ASupplementary data
The following is/are the supplementary data to this article:
References
- 1.Praveen Rao P.N., Knaus E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 2.Desborough M.J.R., Keeling D.M. The aspirin story – from willow to wonder drug. Br J Haematol. 2017;177:674–683. doi: 10.1111/bjh.14520. [DOI] [PubMed] [Google Scholar]
- 3.Vane J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:237–239. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 4.Oates J.A. The 1982 Nobel prize in physiology or medicine. Science. 1982;218:765–768. doi: 10.1126/science.6753151. [DOI] [PubMed] [Google Scholar]
- 5.Rosen G.D., Birkenmeier T.M., Raz A., Holtzman M.J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem Biophys Res Commun. 1989;164:1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- 6.Cashman J.N. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52:13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bombardier C., Laine L., Reicin A. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein F.E., Faich G., Goldstein J.L. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 9.Halpern G.M. COX-2 inhibitors: a story of greed, deception and death. Inflammopharmacology. 2005;13:419–425. doi: 10.1163/156856005774415574. [DOI] [PubMed] [Google Scholar]
- 10.Topol E.J. Failing the public health — Rofecoxib, Merck, and the FDA. N Engl J Med. 2004;351:1707–1709. doi: 10.1056/NEJMp048286. [DOI] [PubMed] [Google Scholar]
- 11.Merck Merck announces voluntary worldwide withdrawal of VIOXX®. 2018. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/vioxx/DearHealthcareProfessional.pdf Available from:
- 12.Sun S.X., Lee K.Y., Bertram C.T., Goldstein J.L. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin. 2007;23:1859–1866. doi: 10.1185/030079907X210561. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation (WHO) WHO; Geneva: 1986. Cancer pain relief. [Google Scholar]
- 14.Chen Y., Bedson J., Hayward R.A., Jordan K.P. Trends in prescribing of non-steroidal anti-inflammatory drugs in patients with cardiovascular disease: influence of national guidelines in UK primary care. Fam Pract. 2018;35:426–432. doi: 10.1093/fampra/cmx142. [DOI] [PubMed] [Google Scholar]
- 15.Kotlinska-Lemieszek A., Paulsen O., Kaasa S., Klepstad P. Polypharmacy in patients with advanced cancer and pain: a European cross-sectional study of 2282 patients. J Pain Symptom Manage. 2014;48:1145–1159. doi: 10.1016/j.jpainsymman.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Puzzoni M., Massa E., Demurtas L., Pusole G., Cubeddu A., Scartozzi M. N11 A single institution survey on prevalence and management of severe cancer pain in patients with cancer of different sites. Ann Oncol. 2015;26 vi. [Google Scholar]
- 17.Bolzacchini E., Tuzi A., Vallini I., Pinotti G. R12 Survey on prevalence and management of breakthrough cancer pain: a single center experience. Ann Oncol. 2016;27 iv104. [Google Scholar]
- 18.Lopez Ramirez E., Munoz Carmona D.M., Contreras Martinez J., De La Torre-Luque A. Tapentadol in the management of opioid-naive patients with cancer pain. Rev Soc Esp Dolor. 2016;23:32–38. [Google Scholar]
- 19.McNicol E., Strassels S.A., Goudas L., Lau J., Carr D.B. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev. 2005:CD005180. doi: 10.1002/14651858.CD005180. [DOI] [PubMed] [Google Scholar]
- 20.Nabal M., Librada S., Redondo M.J., Pigni A., Brunelli C., Caraceni A. The role of paracetamol and nonsteroidal anti-inflammatory drugs in addition to WHO Step III opioids in the control of pain in advanced cancer. A systematic review of the literature. Palliat Med. 2012;26:305–312. doi: 10.1177/0269216311428528. [DOI] [PubMed] [Google Scholar]
- 21.Derry S., Wiffen P.J., Moore R.A. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) for cancer pain in adults. Cochrane Database Syst Rev. 2017:CD012638. doi: 10.1002/14651858.CD012638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schüchen R.H., Mücke M., Marinova M. Systematic review and meta-analysis on non-opioid analgesics in palliative medicine. J Cachexia Sarcopenia Muscle. 2018;9:1235–1254. doi: 10.1002/jcsm.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNicol E.D., Strassels S., Goudas L., Lau J., Carr D.B. WITHDRAWN: NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD005180.pub2. CD005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown M., Farquhar-Smith P. Pain in cancer survivors; filling in the gaps. Br J Anaesth. 2017;119:723–736. doi: 10.1093/bja/aex202. [DOI] [PubMed] [Google Scholar]
- 25.Fuccella L.M., Conti F., Corvi G., Mandelli V., Randelli M., Stefaneili G. Double-blind study of the analgesic effect of indoprofen (K 4277) Clin Pharmacol Ther. 1975;17:277–283. doi: 10.1002/cpt1975173277. [DOI] [PubMed] [Google Scholar]
- 26.Martino G., Emanueli A., Mandelli V., Ventafridda V. A controlled study of the analgetic effect of two non-steroidal anti-inflammatory drugs in cancer pain. Arzneimittel-Forschung/Drug Res. 1978;28:1657–1659. [PubMed] [Google Scholar]
- 27.Stambaugh J.E., Tejada F., Trudnowski R.J. Double-blind comparisons of zomepirac and oxycodone with APC in cancer pain. J Clin Pharmacol. 1980;20:261–270. doi: 10.1002/j.1552-4604.1980.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 28.Stambaugh J.E., Sarajian C. Analgesic Efficacy of zomepirac sodium in patients with pain due to cancer. J Clin Pharmacol. 1981;21:501–507. doi: 10.1002/j.1552-4604.1981.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 29.Stambaugh J.E. Analgesic efficacy, safety and acceptibility of zomepirac sodium in comparison to morphine sulphate in the treatment of pain secondary to malignancy. Curr Ther Res Clin Exp. 1982;31:922–929. [Google Scholar]
- 30.Pellegrini A., Massidda B., Pellegrini P. Effect of i.v. indoprofen on cancer pain and serum prolactin and growth hormone levels. A controlled pharmacologic study vs i.m. morphine and placebo. Int J Clin Pharmacol Ther Toxicol. 1983;21:483–486. [PubMed] [Google Scholar]
- 31.Shimada S.G., Otterness I.G., Stitt J.T. A study of the mechanism of action of the mild analgesic dipyrone. Agents Actions. 1994;41:188–192. doi: 10.1007/BF02001915. [DOI] [PubMed] [Google Scholar]
- 32.Rogosch T., Sinning C., Podlewski A. Novel bioactive metabolites of dipyrone (metamizol) Bioorganic Med Chem. 2012;20:101–107. doi: 10.1016/j.bmc.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez M., Barutell C., Rull M. Efficacy and tolerance of oral dipyrone versus oral morphine for cancer pain. Eur J Cancer. 1994;30:584–587. doi: 10.1016/0959-8049(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 34.Yalçin S., Güllü I.H., Tekuzman G., Savaş C., Firat D. A comparison of two nonsteroidal antiinflammatory drugs (diflunisal versus dipyrone) in the treatment of moderate to severe cancer pain: a randomized crossover study. Am J Clin Oncol. 1998;21:185–188. doi: 10.1097/00000421-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Moertel C.G., Ahmann D.L., Taylor W.F., Schwartau N. A comparative evaluation of marketed analgesic drugs. N Engl J Med. 1972;286:813–815. doi: 10.1056/NEJM197204132861504. [DOI] [PubMed] [Google Scholar]
- 36.Ventafridda V., Martino G., Mandelli V. Indoprofen, a new analgesic and anti-inflammatory drug in cancer pain. Clin Pharmacol Ther. 1975;17:284–289. doi: 10.1002/cpt1975173284. [DOI] [PubMed] [Google Scholar]
- 37.Carlson R.W., Borrison R.A., Sher H.B. A multiinstitutional evaluation of the analgesic efficacy and safety of ketorolac tromethamine, acetaminophen plus codeine, and placebo in cancer pain. Pharmacotherapy. 1990;10:211–216. [PubMed] [Google Scholar]
- 38.Staquet M.J. A double-blind study with placebo control of intramuscular ketorolac tromethamine in the treatment of cancer pain. J Clin Pharmacol. 1989;29:1031–1036. doi: 10.1002/j.1552-4604.1989.tb03274.x. [DOI] [PubMed] [Google Scholar]
- 39.Stambaugh J., Drew J. A double-blind parallel evaluation of the efficacy and safety of a single dose of ketoprofen in cancer pain. J Clin Pharmacol. 1988;28:s34–s39. doi: 10.1002/j.1552-4604.1988.tb05975.x. [DOI] [PubMed] [Google Scholar]
- 40.Moertel C.G., Ahmann D.L., Taylor W.F. Relief of pain by oral medications: a controlled evaluation of analgesic combinations. JAMA. 1974;229:55–59. [PubMed] [Google Scholar]
- 41.Moertel C.G., Ahmann D.L., Taylor W.F., Schwartau N. Aspirin and pancreatic cancer pain. Gastroenterology. 1971;60:552–553. [PubMed] [Google Scholar]
- 42.Lomen P.L., Samal B.A., Lamborn K.R., Sattler L.P., Crampton S.L. Flurbiprofen for the treatment of bone pain in patients with metastatic breast cancer. Am J Med. 1986;80:83–87. doi: 10.1016/0002-9343(86)90118-x. [DOI] [PubMed] [Google Scholar]
- 43.Johnson J.R., Miller A.J. The efficacy of choline magnesium trisalicylate (CMT) in the management of metastatic bone pain: a pilot study. Palliat Med. 1994;8:129–135. doi: 10.1177/026921639400800206. [DOI] [PubMed] [Google Scholar]
- 44.Sacchetti G., Camera P., Paolo Rossi A. Injectable ketoprofen vs. acetylsalicylic acid for the relief of severe cancer pain: a double-blind, crossover trial. Drug Intell Clin Pharm. 1984;18:403–406. doi: 10.1177/106002808401800510. [DOI] [PubMed] [Google Scholar]
- 45.Saxena A., Andley M., Gnanasekaran N. Comparison of piroxicam and acetylsalicylic acid for pain in head and neck cancers: a double-blind study. Palliat Med. 1994;8:223–229. doi: 10.1177/026921639400800306. [DOI] [PubMed] [Google Scholar]
- 46.Mohammadinejad P., Arya P., Esfandbod M. Celecoxib versus diclofenac in mild to moderate depression management among breast cancer patients: a double-blind, placebo-controlled, randomized trial. Ann Pharmacother. 2015;49:953–961. doi: 10.1177/1060028015592215. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez M.J., Contreras D., Gálvez R. Double-blind evaluation of short-term analgesic efficacy of orally administered dexketoprofen trometamol and ketorolac in bone cancer pain. Pain. 2003;104:103–110. doi: 10.1016/s0304-3959(02)00470-0. [DOI] [PubMed] [Google Scholar]
- 48.Minotti V., Betti M., Ciccarese G., Fumi G., Tonato M., Favero A Del. A double-blind study comparing two single-dose regimens of ketorolac with diclofenac in pain due to cancer. Pharmacotherapy. 1998;18:504–508. [PubMed] [Google Scholar]
- 49.Corli O., Cozzolino A., Scaricabarozzi I. Nimesulide and diclofenac in the control of cancer-related pain: comparison between oral and rectal administration. Drugs. 1993;46:152–155. doi: 10.2165/00003495-199300461-00036. [DOI] [PubMed] [Google Scholar]
- 50.Toscani F., Gallucci M., Scaricabarozzi I. Nimesulide in the treatment of advanced cancer pain. Double-blind comparison with naproxen. Drugs. 1993;46:156–158. doi: 10.2165/00003495-199300461-00037. [DOI] [PubMed] [Google Scholar]
- 51.Ventafridda V., De Conno F., Panerai A.E., Maresca V., Monza G.C., Ripamonti C. Non-steroidal anti-inflammatory drugs as the first step in cancer pain therapy: double-blind, within-patient study comparing nine drugs. J Int Med Res. 1990;18:21–29. doi: 10.1177/030006059001800104. [DOI] [PubMed] [Google Scholar]
- 52.Levick S., Jacobs C., Loukas D.F., Gordon D.H., Meyskens F.L., Uhm K. Naproxen sodium in treatment of bone pain due to metastatic cancer. Pain. 1988;35:253–258. doi: 10.1016/0304-3959(88)90134-0. [DOI] [PubMed] [Google Scholar]
- 53.Yousef A.A., Alzeftawy A.E. The efficacy of oral piroxicam fast-dissolving tablets versus sublingual fentanyl in incident breakthrough pain due to bone metastases: a double-blinded randomized study. Support Care Cancer Advance access. 2018 doi: 10.1007/s00520-018-4469-6. published on October 10. [DOI] [PubMed] [Google Scholar]
- 54.Jameel A., Stein R.C., Rawson N.S.B., Hedley A., Stuttaford J., Coombes R.C. Comparative study of intramuscular ketorolac tromethamine and morphine in patients experiencing cancer pain. Int J Oncol. 1995;6:1307–1311. doi: 10.3892/ijo.6.6.1307. [DOI] [PubMed] [Google Scholar]
- 55.Dellemijn P.L.I., Verbiest H.B.C., Van Vliet J.J., Roos P.J., Vecht C.J. Medical therapy of malignant nerve pain. A randomised double-blind explanatory trial with naproxen versus slow-release morphine. Eur J Cancer. 1994;30:1244–1250. doi: 10.1016/0959-8049(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 56.Staquet M., Renaud A. Double-blind, randomized trial of piroxicam and codeine in cancer pain. Curr Ther Res. 1993;53:435–440. [Google Scholar]
- 57.Estapé J., Viñolas N., González B. Ketorolac, a new non-opioid analgesic: a double-blind trial versus pentazocine in cancer pain. J Int Med Res. 1990;18:298–304. doi: 10.1177/030006059001800406. [DOI] [PubMed] [Google Scholar]
- 58.Tonachella R., Curcio C.G., Grossi E. Diclofenac sodium in cancer pain: a double-blind within-patients comparison with pentazocine. Curr Ther Res Clin Exp. 1985;37:1130–1133. [Google Scholar]
- 59.Sunshine A., Olson N.Z. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. J Clin Pharmacol. 1988;28:S49–S54. doi: 10.1002/j.1552-4604.1988.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 60.Minotti V., De Angelis V., Righetti E. Double-blind evaluation of short-term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain. 1998;74:133–137. doi: 10.1016/s0304-3959(97)00157-7. [DOI] [PubMed] [Google Scholar]
- 61.Minotti V., Patoia L., Roila F. Double-blind evaluation of analgesic efficacy of orally administered diclofenac, nefopam, and acetylsalicylic acid (ASA) plus codeine in chronic cancer pain. Pain. 1989;36:177–183. doi: 10.1016/0304-3959(89)90021-3. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z., Xu Y., Liu Z.L., Tian Y.Z., Shen X.H. Combined application of diclofenac and celecoxib with an opioid yields superior efficacy in metastatic bone cancer pain: a randomized controlled trial. Int J Clin Oncol. 2017;22:980–985. doi: 10.1007/s10147-017-1133-y. [DOI] [PubMed] [Google Scholar]
- 63.Legeby M., Sandelin K., Wickman M., Olofsson C. Analgesic efficacy of diclofenac in combination with morphine and paracetamol after mastectomy and immediate breast reconstruction. Acta Anaesthesiol Scand. 2005;49:1360–1366. doi: 10.1111/j.1399-6576.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 64.Lohsiriwat V. Opioid-sparing effect of selective cyclooxygenase-2 inhibitors on surgical outcomes after open colorectal surgery within an enhanced recovery after surgery protocol. World J Gastrointest Oncol. 2016;8:543–549. doi: 10.4251/wjgo.v8.i7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marret E., Kurdi O., Zufferey P., Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 66.Maund E., McDaid C., Rice S., Wright K., Jenkins B., Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106:292–297. doi: 10.1093/bja/aeq406. [DOI] [PubMed] [Google Scholar]
- 67.Björkman R., Ullman A., Hedner J. Morphine-sparing effect of diclofenac in cancer pain. Eur J Clin Pharmacol. 1993;44:1–5. doi: 10.1007/BF00315271. [DOI] [PubMed] [Google Scholar]
- 68.Ferrer-Brechner T., Ganz P. Combination therapy with ibuprofen and methadone for chronic cancer pain. Am J Med. 1984;77:78–83. doi: 10.1016/s0002-9343(84)80023-6. [DOI] [PubMed] [Google Scholar]
- 69.Stambaugh J.E., Drew J. The combination of ibuprofen and oxycodone/acetaminophen in the management of chronic cancer pain. Clin Pharmacol Ther. 1988;44:665–669. doi: 10.1038/clpt.1988.209. [DOI] [PubMed] [Google Scholar]
- 70.de Wit R., van Dam F., Abu-Saad H.H. Empirical comparison of commonly used measures to evaluate pain treatment in cancer patients with chronic pain. J Clin Oncol. 1999;17:1280. doi: 10.1200/JCO.1999.17.4.1280. [DOI] [PubMed] [Google Scholar]
- 71.Zeng A.M., Nami N.F., Wu C.L., Murphy J.D. The analgesic efficacy of nonsteroidal anti-inflammatory agents (NSAIDs) in patients undergoing Cesarean deliveries: a meta-analysis. Reg Anesth Pain Med. 2016;41:763–772. doi: 10.1097/AAP.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z., Xu H., Zhang Y. Nonsteroidal anti-inflammatory drugs for postoperative pain control after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Clin Anesth. 2017;43:84–89. doi: 10.1016/j.jclinane.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Shirvani A., Shamszadeh S., Eghbal M.J., Asgary S. The efficacy of non-narcotic analgesics on post-operative endodontic pain: a systematic review and meta-analysis. J Oral Rehabil. 2017;44:709–721. doi: 10.1111/joor.12519. [DOI] [PubMed] [Google Scholar]
- 74.Soffin E.M., Yadeau J.T. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117 doi: 10.1093/bja/aew362. iii62–72. [DOI] [PubMed] [Google Scholar]
- 75.Wahab T.A., Uwakwe H., Jumah M., Aransi R., Khan H.K. A modified enhanced recovery after surgery (ERAS): use and surgical outcome in breast cancer patients. J Biosci Med. 2018;6:15–25. [Google Scholar]
- 76.Doleman B., Leonardi-Bee J., Heinink T.P., Lund J., Williams J.P. Pre-emptive and preventive NSAIDs for postoperative pain in adults undergoing all types of surgery. Cochrane Database Syst Rev. 2018:CD012978. doi: 10.1002/14651858.CD012978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pountos I., Georgouli T., Calori G.M., Giannoudis P.V. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. Sci World J. 2012;2012:606404. doi: 10.1100/2012/606404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cata J.P., Guerra C.E., Chang G.J., Gottumukkala V., Joshi G.P. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. Br J Anaesth. 2017;119:750–764. doi: 10.1093/bja/aex225. [DOI] [PubMed] [Google Scholar]
- 79.Forget P., Vandenhende J., Berliere M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 80.Wigmore T.J., Mohammed K., Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y., Zhou B.P. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wen Y., Wang M., Yang J. A comparison of fentanyl and flurbiprofen axetil on serum VEGF-C, TNF-α, and IL-1β concentrations in women undergoing surgery for breast cancer. Pain Pract. 2015;15:530–537. doi: 10.1111/papr.12206. [DOI] [PubMed] [Google Scholar]
- 83.Hiller J.G., Sampurno S., Millen R. Impact of celecoxib on inflammation during cancer surgery: a randomized clinical trial. Can J Anesth. 2017;64:497–505. doi: 10.1007/s12630-017-0818-z. [DOI] [PubMed] [Google Scholar]
- 84.Al-Hashimi M., Scott S.W.M., Thompson J.P., Lambert D.G. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111:80–88. doi: 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 85.Cronin-Fenton D.P., Heide-Jørgensen U., Ahern T.P. Opioids and breast cancer recurrence: a Danish population-based cohort study. Cancer. 2015;121:3507–3514. doi: 10.1002/cncr.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alam A., Gomes T., Zheng H., Mamdani M.M., Juurlink D.N., Bell C.M. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172:425–430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 87.Clarke H., Soneji N., Ko D.T., Yun L., Wijeysundera D.N. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brummett C.M., Waljee J.F., Goesling J. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152:e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kune G.A., Kune S., Watson L.F. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399–4404. [PubMed] [Google Scholar]
- 90.Thun M.J., Namboodiri M.M., Heath C.W. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg L., Palmer J.R., Zauber A.G., Warshauer M.E., Stolley P.D., Shapiro S. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991;83:355–358. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- 92.Collet J.P., Sharpe C., Belzile E., Boivin J.F., Hanley J., Abenhalm L. Colorectal cancer prevention by non-steroidal anti-inflammatory drugs: effects of dosage and timing. Br J Cancer. 1999;81:62–68. doi: 10.1038/sj.bjc.6690651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandler R.S., Halabi S., Baron J.A. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 94.Nugent K.P., Farmer K.C.R., Spigelman A.D., Williams C.B., Phillips R.K.S. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 95.Steinbach G., Lynch P.M., Phillips R.K.S. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 96.Schreinemachers D.M., Everson R.B. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Bosetti C., Gallus S., La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control. 2006;17:871–888. doi: 10.1007/s10552-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 98.Sarre S., Määttänen L., Tammela T.L.J., Auvinen A., Murtola T.J. Postscreening follow-up of the Finnish Prostate Cancer Screening Trial on putative prostate cancer risk factors: vitamin and mineral use, male pattern baldness, pubertal development and non-steroidal anti-inflammatory drug use. Scand J Urol. 2016;50:267–273. doi: 10.3109/21681805.2016.1145734. [DOI] [PubMed] [Google Scholar]
- 99.Doat S., Cénée S., Trétarre B. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: results from the EPICAP study. Cancer Med. 2017;5:2461–2470. doi: 10.1002/cam4.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walter R.B., Milano F., Brasky T.M., White E. Long-term use of acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of hematologic malignancies: results from the prospective vitamins and lifestyle (VITAL) study. J Clin Oncol. 2011;29:2424–2431. doi: 10.1200/JCO.2011.34.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grabosch S.M., Shariff O.M., Helm C.W. Non-steroidal anti-inflammatory agents to induce regression and prevent the progression of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2018:CD004121. doi: 10.1002/14651858.CD004121.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elwood P.C., Morgan G., Pickering J.E. Aspirin in the treatment of cancer: Reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016;11:e0152402. doi: 10.1371/journal.pone.0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coyle C., Cafferty F.H., Rowley S. ADD-ASPIRIN: A phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp Clin Trials. 2016;51:56–64. doi: 10.1016/j.cct.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang V.W., Shields J.M., Hamilton S.R. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–1753. [PubMed] [Google Scholar]
- 105.Fukuda R., Kelly B., Semenza G.L. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–2334. [PubMed] [Google Scholar]
- 106.Hsu A.L., Ching T.T., Wang D.S., Song X., Rangnekar V.M., Chen C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 107.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 108.Koki A.T., Khan N.K., Woerner B.M. Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers. Prostaglandins Leukot Essent Fat Acids. 2002;66:13–18. doi: 10.1054/plef.2001.0335. [DOI] [PubMed] [Google Scholar]
- 109.Masferrer J.L., Leahy K.M., Koki A.T. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 110.Chinery R., Beauchamp R.D., Shyr Y., Kirkland S.C., Coffey R.J., Morrow J.D. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res. 1998;58:2323–2327. [PubMed] [Google Scholar]
- 111.Zweifel B.S., Davis T.W., Ornberg R.L., Masferrer J.L. Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res. 2002;62:6706–6711. [PubMed] [Google Scholar]
- 112.Tsujii M., Kawano S., Tsuji S., Sawaoka H., Hori M., DuBois R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 113.Liu X.H., Yao S., Kirschenbaum A., Levine A.C. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.