Abstract
Background
Percutaneous nephrolithotomy (PNL) is associated with severe postoperative pain. The current study aimed to investigate the analgesic efficacy of transmuscular quadratus lumborum (TQL) block for patients undergoing PNL surgery.
Methods
Sixty patients were enrolled in this single centre study. The multimodal analgesic regime consisted of oral paracetamol 1 g and i.v. dexamethasone 4 mg before surgery and i.v. sufentanil 0.25 μg kg−1 30 min before emergence. After operation, patients received paracetamol 1 g regularly at 6 h intervals. Subjects were allocated to receive a preoperative TQL block with either ropivacaine 0.75%, 30 ml (intervention) or saline 30 ml (control). Primary outcome was oral morphine equivalent (OME) consumption 0–6 h after surgery. Secondary outcomes were OME consumption up to 24 h, pain scores, time to first opioid, time to first ambulation, and hospital length of stay. Results were reported as mean (standard deviation) or median (inter-quartile range).
Results
Morphine consumption was lower in the intervention group at 6 h after surgery (7.2 [8.7] vs 90.6 [69.9] mg OME, P<0.001) and at 24 h (54.0 [36.7] vs 126.2 [85.5] mg OME, P<0.001). Time to first opioid use was prolonged in the intervention group (678 [285–1020] vs 36 [19–55] min, P<0.0001). Both the time to ambulation (302 [238–475] vs 595 [345–925] min, P<0.004) and length of stay (2.0 [0.8] vs 3.0 [1.2] days, P≤0.001) were shorter in the intervention group.
Conclusions
This is the first blinded, RCT that confirms that unilateral TQL block reduces postoperative opioid consumption and hospital length of stay. Further study is required for confirmation and dose optimisation.
Clinical trial registration
Keywords: local anaesthetic, length of hospital stay, multimodal analgesia, nerve block, postoperative pain, quadratus lumborum block, ultrasonography
Editor's key points.
-
•
The analgesic efficacy and impact on outcomes of transmuscular quadratus lumborum (TQL) block for patients undergoing percutaneous nephrolithotomy surgery were studied in a single centre randomised controlled trial.
-
•
In the context of an institutional multimodal analgesia protocol, a unilateral ultrasound-guided TQL block with ropivacaine performed before surgery reduced opioid consumption, time to ambulation, and hospital length of stay.
-
•
This regional analgesia technique leads to both opioid-sparing and functional improvements in a painful surgical procedure beyond multimodal analgesia.
Unilateral percutaneous nephrolithotomy (PNL) is the surgical procedure of choice for large, multiple or complex kidney stones.1, 2, 3 It is often associated with severe postoperative somatosensory and visceral pain, necessitating a substantial requirement for postoperative opioid consumption.4, 5, 6, 7, 8 Regional analgesia is a well-established opioid-sparing analgesic component of enhanced recovery after surgery protocols.9, 10, 11 Ultrasound-guided transmuscular quadratus lumborum (TQL) block is a regional analgesic technique that provides postoperative analgesia of the segmental innervation from T4 to L1.12, 13, 14 The TQL block is based on cephalad spread of local anaesthetic (LA) from the lumbar paravertebral region (site of injection) into the thoracic paravertebral space (TPVS), thereby anaesthetising the thoracic spinal nerves and segmental parts of the thoracic sympathetic trunk thereby ameliorating both somatic and visceral pain.14 The TQL block also reaches the subcostal, ilioinguinal, and iliohypogastric nerves, but not the femoral nerve, obturator nerve, or the lumbar sympathetic trunk.14 Although the analgesic efficacy of the TQL block has been described in case reports,15, 16, 17 the current study is one of the first blinded, RCTs to investigate this block in a clinical setting.
The aim was to investigate the analgesic efficacy of preoperative TQL block in patients undergoing PNL. We hypothesised that ultrasound-guided unilateral TQL block would reduce oral morphine equivalent (OME) consumption by 50% in the first 6 h period after PNL.
Methods
Study design and setting
This prospective, single centre, block-randomised, controlled and double-blind study was approved by The Regional Ethics Committee (SJ-469), The Danish Medicines Agency EudraCT (2015-004770-16), and the Danish Data Protection Agency, and was registered at ClinicalTrials.gov (NCT02818140). The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement,18 the Helsinki Declaration, and monitored by the Good Clinical Practice Unit at Copenhagen University Hospital affiliated to the Danish Health Authority in compliance with the rules set out by the Danish Medicines Agency. After written informed consent, 60 subjects were enrolled at Zealand University Hospital, Denmark from August 2016 to December 2017.
Subjects
Eligible patients were >18 yr old with ASA physical status 1–3 and scheduled for elective PNL. Exclusion criteria were inability to cooperate, dementia, allergy to LA and opioids, daily intake of opioids, known abuse of alcohol or medication, local infection at the site of injection or systemic infection, pregnancy, or inability to understand written or spoken Danish. All subjects received standardised general anaesthesia with propofol, remifentanil, and a multimodal analgesic regime consisting of oral paracetamol 1 g and i.v. dexamethasone 4 mg before surgery and i.v. sufentanil 0.25 μg kg−1 30 min before emergence. After operation all subjects received paracetamol 1 g every 6 h.
Study interventions
A computer-generated block randomisation list in blocks of 10 with a 1:1 ratio was prepared and placed in sealed opaque envelopes that were consecutively numbered from 1 to 60. Before surgery, subjects were randomly assigned to receive a unilateral ultrasound-guided TQL block with either ropivacaine 0.75%, 30 ml (intervention group) or isotonic saline 30 ml (control group). The dosage of ropivacaine was chosen according to the Danish Medicines Agency accepted dosage of ropivacaine for a single shot block19 and pharmacokinetic studies regarding the dosage of ropivacaine.20, 21, 22, 23, 24 Two anaesthesia nurses opened the randomisation envelope and then prepared a 30 ml syringe according to the allocation specified in the sealed envelope. All other investigators, staff, and patients were blinded to group allocation.
Subjects were monitored with three-lead electrocardiography, pulse oximetry, and noninvasive BP and had two i.v. lines. About 30 min before surgery, subjects were placed in the lateral decubitus position with the surgical side upwards. Skin was prepared twice with application of chlorhexidine 0.5% in ethanol 82%. The TQL block procedure was performed under ultrasound guidance (X-porte ultrasound system, Fujifilm Sonosite, Bothell, Washington, USA) with a curvilinear transducer (5–2 MHz, C60xp) covered by a sterile transparent plastic sheath (Safersonic sterile sonography cover; Safersonic Medizinprodukte Handels, Ybbs, Austria). A 21-gauge, 100-mm needle (Polymedic ultrasound Evolution needle with 30-degree bevel; Temena SAS, Carrières-sur-Seine, France) and the Shamrock technique were used for all TQL blocks.25 The transducer was placed transverse immediately cranial to the iliac crest and at the level of the posterior axillary line.12, 13, 14 The needle was then inserted in-plane from the lateral edge of the transducer and advanced through the quadratus lumborum (QL) muscle until the needle tip penetrated the epimysium of the anterior part of the QL muscle. A total of ropivacaine 0.75%, 30 ml or saline 30 ml was injected incrementally after repeated negative aspiration tests for blood in the fascial interspace between the QL and psoas major (PM) muscles posterior to the transversalis fascia.14 Successful injectate spread was confirmed by turning the transducer 90 degrees into the longitudinal sagittal plane to see separation of the QL and PM muscles in the longitudinal sagittal plane and cephalad injectate spread from the iliac crest towards the openings in the diaphragm.26 All block procedures were performed by an experienced anaesthesiologist (MD, CKH, TDP, or JB) with >200 TQL block procedures. About 30 min after block completion, subjects walked from the block procedure room to the operating theatre.
Anaesthesia
All subjects received standardised general anaesthesia as follows: induction with propofol 1–2 mg kg−1, remifentanil 0.4–0.6 μg kg−1 min−1 and rocuronium 0.6 mg kg−1; tracheal intubation; maintenance of anaesthesia with propofol 0.5–0.75 mg kg−1 h−1, remifentanil 30–45 μg kg−1 h−1; and positive pressure ventilation with oxygen 30–40%. Approximately 30 min before emergence, subjects received i.v. sufentanil 0.25 μg kg−1 and i.v. ondansetron 4 mg. No other long-acting opioids were allowed intraoperatively.
Surgical procedure
Subjects were placed in Galdakao or modified Valdivia position, with a body tilt of ∼30° ipsilateral to the operative field. Puncture was performed with an 18 G nephrostomy needle. This allowed the transpapillary route of the percutaneous tract, a basic condition for prevention of bleeding. After withdrawal of the puncture needle and urine drainage, a flexible guide wire was inserted and advanced to the upper calyx or ureter. The tract was dilated using coaxial dilators over the guide wire into the collecting system. Once in place, an Amplatz Super Stiff guide wire (AGA Medical, Plymouth, MN, USA) was placed as a working wire. Once access was obtained, nephroscopy and nephrolithotomy were performed with a variety of energy sources for stone fragmentation including ultrasonic lithotripsy and electrohydraulic lithotripsy. Larger stone fragments were extracted with a stone extractor. Postoperative stenting, placement of a nephrostomy tube, or both, were subject and perioperative status dependent. Both the nephrostomy catheter and ureteral catheter were removed on the first postoperative hospital day.
Outcomes
The primary outcome measure was 6 h OME consumption after surgery. Secondary outcomes were: (1) cumulative OME consumption during the first 24 h after surgery (mg), (2) OME consumption at 6 h intervals (0–6, 6–12, 12–18, and 18–24 h) after surgery (mg), (3) numeric rating pain scores (NRS 0–10/10) at rest (supine position) and during activity (defined as changing position from prone to sitting position), (4) pain related to the block procedure (NRS 0–10/10), (5) nausea and vomiting (yes/no), (6) time to first analgesic demand (time from arrival in the postoperative care unit (PACU) to the first patient-controlled analgesia (PCA) opioid bolus (min), (7) time from arrival in the PACU until first ambulation, that is, until able to stand up and walk unassisted (min), and (8) length of hospital stay (LOS) (days).
Assessment of outcomes
Arrival time in the PACU was defined as time zero. When subjects entered the PACU, a protocol trained nurse immediately connected the PCA pump (Rythmic Evolution pump; Micrel Medical, Athens, Greece) to one of the two i.v. lines. Before operation, subjects were instructed only to use the pump if the NRS score was >3. The PCA pump was programmed to deliver a standard bolus of morphine 5 mg only on patient demand. There was a 20-min lockout time and a maximum of eight boluses per 4 h. Before the PCA pump would deliver the bolus, subjects were asked to enter the NRS score (0–10/10) on the pump display.
All supplemental administrations of opioids were registered in the subject's electronic files, and opioid consumption from the PCA pumps and electronic files were tallied to form the total postoperative opioid consumption. Morphine i.v. was adjusted to OME in the ratio of 1:3. NRS scores at rest and activity were assessed at predefined time points from T0 to T24 h after surgery. Time to first opioid was defined as time from T0 to first PCA bolus. Time to first ambulation was defined as time from T0 until the subject was able to stand up and walk without support. Subjects stayed in the PACU until standard discharge criteria were fulfilled according to the Danish national guidelines.27 The surgical team decided the time of discharge from the hospital and recorded this in the subjects' electronic files.
Statistics and sample size
Our 1-yr retrospective study of 110 patients who had PNL surgery revealed a mean (standard deviation [SD]) OME consumption of 72.3 (44.4) mg during the first 6 h after surgery. We wanted to detect at least a 50% reduction in OME with a level of significance (α)=0.05 and power 80% (1-β) for intervention vs control unilateral TQL block. The required sample size was 48 subjects. In order to avoid decreased power as a result of potential dropouts, we enrolled 60 subjects.
Data were analysed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA 2018). Continuous data with normal distribution are presented as mean (SD), count (%) and range; time-to-event data were presented using median (inter-quartile range). Group differences were tested with t-tests, t-test using 0-censored Gaussian distributions and log-rank tests as appropriate. Measurements were split into 6 h time intervals from which we calculated the mean value for all scales, transforming ordinal measures to continuous measures. Time-to-event data were analysed using Kaplan–Meier curves and log-rank tests. Bonferroni correction was used to accommodate multiple testing.
Results
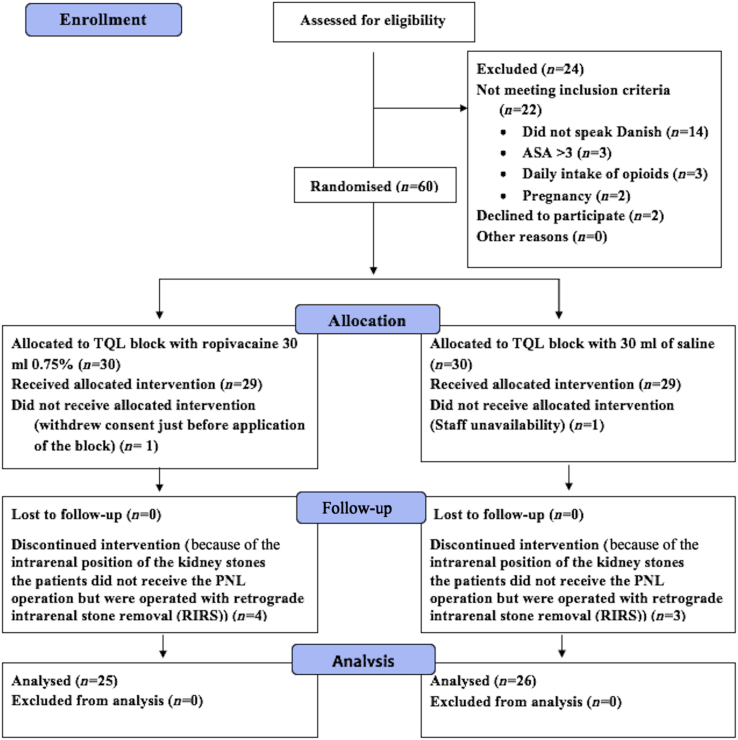
Eighty-four subjects were screened for eligibility from August 2016 to December 2017 (Fig. 1) and 60 were included and randomised (Fig. 1). One subject had the surgical procedure cancelled after randomisation (because of a labour dispute amongst auxiliary staff), and another withdrew consent just before block application. In addition, seven subjects had the surgical procedure changed from PNL to retrograde internal stone removal after randomisation and block application but before surgery. Fifty-one subjects completed the study as per-protocol and were included in the per-protocol analysis (i.e. 25 in the intervention group and 26 in the control group). There were no signs of LA systemic toxicity or other complications (Fig. 1). There were no differences in subject characteristics between the groups (Table 1).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. PNL, percutaneous nephrolithotomy; TQL, transmuscular quadratus lumborum.
Table 1.
Subject characteristics, primary outcome, and secondary outcomes. NRS, numeric rating pain scores; ns, not significant
| Intervention (n=25) | Control (n=26) | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Sex (female/male) | 12/13 | 14/12 | |
| Age (yr), mean (range) | 58.5 (29–82) | 61.7 (34–83) | |
| BMI (kg m−2) | 26.4 (4.2) | 26.6 (5.9) | |
| Weight (kg) | 78.4 | 81.2 | |
| ASA score physical status 1/2/3 | 5/15/5 | 5/17/4 | |
| Surgery time (min) | 97.6 (25.0) | 101.1 (34.3) | |
| Nephrostomy (n) | 18 | 20 | |
| Time to removal of nephrostomy (days) | 1.2 (0.5) | 1.3 (0.7) | |
| Primary outcome | |||
| Oral morphine equivalents 0–6 h (mg) | 7.2 (8.7) | 90.6 (69.9) | <0.001 |
| Secondary outcomes | |||
| Oral morphine equivalents 6–12 h (mg) | 5.4 (9.6) | 14.42 (13.74) | <0.009* |
| Oral morphine equivalents 12–18 h (mg) | 16.8 (11.72) | 10.38 (10.19) | 0.04* |
| Oral morphine equivalents 18–24 h (mg) | 24.6 (20.71) | 10.38 (9.27) | <0.004* |
| Total oral morphine equivalents 0–24 h (mg) | 54.0 (36.74) | 126.15 (85.53) | <0.0011* |
| Time to removal of nephrostomy (days) | 1.2 (0.5) | 1.3 (0.7) | ns |
| Length of stay (days) | 2.0 (0.78) | 3.03 (1.25) | <0.001 |
| Time to first opioid (min) | 678.0 (285.0–1025.0) | 36 (19.0–55) | <0.0001 |
| Nausea/vomiting (n) | 4/0 | 7/0 | ns |
| NRS score (0–10/10), block procedural pain (0–10/10) | 1.36 (1.2) | 1.85 (1.35) | <0.2 |
∗ When adjusting the P-value with a factor 4 because of repeated measures (Bonferroni correction) in the 6 h intervals, differences between the groups remained statistically significant.
OME consumption in the first 6 h interval after surgery was lower in the intervention group compared with the control group (7.2 [8.7] vs 90.6 [69.9] mg, P<0.001). However, OME consumption in the control group was reduced during the 12–24 h period compared with the intervention group. In addition, total OME consumption during the first 24 h after surgery was lower in the intervention group compared with the control group (54.0 [36.74] vs 126.2 [85.5] mg, P<0.001). When adjusting the P-value with a factor 4 because of repeated measures (Bonferroni correction) in the 6 h intervals, differences between the groups remained statistically significant. Two subjects in each group did not use any opioids at all during the study period. Results from the 6 h intervals are presented in Table 1.
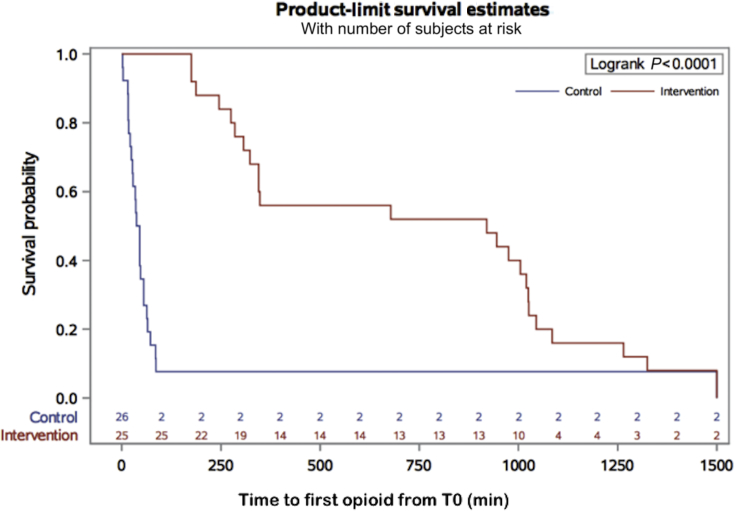
The intervention group had a longer time to first opioid demand compared with the control group (678 [285–1020] vs 36 [19–55] min, P<0.0001 (Fig. 2).
Fig. 2.
Kaplan–Meier survival plot. Time to first opioid from arrival at PACU (T0). Kaplan–Meier survival plot of time to first opioid (min), defined as time from T0 until first request for opioid.
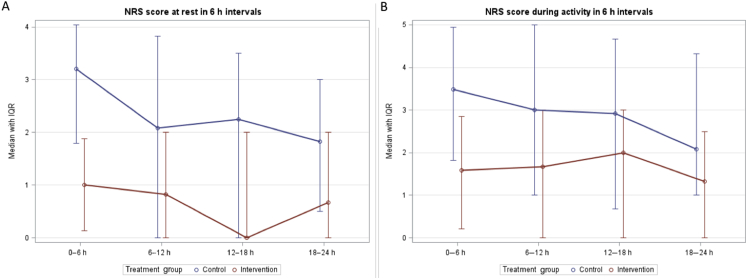
Both the overall and 6 h after surgery NRS pain scores at rest were lower in the intervention group compared with the control group (i.e. P<0.02 and P<0.0004, respectively) (Fig. 3). The NRS pain score during activity was also lower during the first 6 h after surgery in the intervention group (P<0.01). However, the overall NRS score during activity showed no significant differences between the two groups. When adjusting the P-value as a result of repeated measures (Bonferroni correction) in the 6 h intervals, differences between the groups with regard to NRS pain scores during the first 6 h intervals remained statistically significant, but there were no longer statistically significant differences in the overall NRS score at rest.
Fig. 3.
Numeric Rating Scale (NRS) scores at rest and at activity. Median NRS pain scores 0–10/10 with inter-quartile range (IQR). Both figure parts show the median (IQR) NRS scores at the intervals between 0–6, 6–12, 12–18, 18–24 h. (A) median (IQR) NRS score at rest, defined as lying in bed; (B) median (IQR) NRS score with activity.
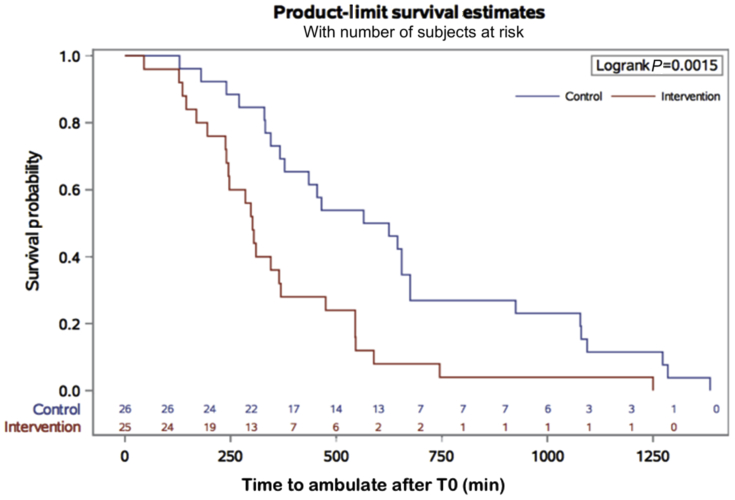
The time to first ambulation was also shorter in the intervention group compared with the control group (302 [238–475] vs 595 [345–925] min, P<0.004) (Fig. 4). Hospital LOS was shorter in the intervention group compared with the control group (1.9 [0.8] vs 3.1 [1.3] days, P<0.001).
Fig. 4.
Kaplan–Meier survival plot. Time to first ambulation from arrival at PACU. Kaplan–Meier survival plot of time to first ambulation (min), defined as time from PACU (T0) until the subject was able to stand up and walk unassisted.
Regarding the block procedural pain, there was no significant difference in NRS pain score, and there were no significant differences between the groups regarding nausea and vomiting (Table 1).
Discussion
The major finding of the current study is the significant opioid-sparing analgesic effect of unilateral TQL block after PNL surgery at both 6 h and 24 h after surgery. In addition, TQL block significantly prolonged the time to first opioid demand and reduced both the time to ambulation and time to hospital discharge.
Significant differences in NRS pain scores between groups at rest and with activity were observed during the first 6 h interval, but not subsequently. The clinical relevance of a difference in NRS pain scores at rest and with activity during the first 6 h interval is debatable considering both groups had NRS<4. We believe that the subjects in the intervention group had a low NRS score because of the fact that they had a working TQL block in contrast to the control group who consumed significantly more OME to maintain a low NRS pain score.
Good pain relief and low opioid consumption most likely contributed to earlier ambulation and expedited hospital discharge in the TGL block group. This effect is well recognised and consistent with previous results.9 10 As mentioned in the results section, there were no differences between groups in vomiting and nausea, probably because of the prophylactic antiemetic treatment.
Our results suggest that a single shot unilateral TQL block with plain ropivacaine 0.75% provides effective analgesia up to 15 h, as the block was administered 30 min before surgery or ∼3 h before T0. Such prolonged analgesic duration seems to exceed that expected for ropivacaine 0.75% in peripheral nerve blockade.28 Similar block efficacy and duration has also been reported for thoracic paravertebral block. The proposed mechanism of analgesic action is combined somatic and sympathetic blockade (i.e. blockade of the ventral rami of the spinal nerves and the sympathetic trunk in the lower thoracic segments).29
Other studies have examined thoracic paravertebral block efficacy with regard to postoperative pain after PNL,30, 31 also reporting significant reduction in postoperative opioid consumption. Thoracic paravertebral block is well known to provide visceral pain relief and somatosensory analgesia extending up to five dermatomes after a single injection of LA.31 While the risk for pneumothorax is quite low when performing ultrasound-guided thoracic paravertebral block,32 TQL block entails no risk of pneumothorax. Furthermore, there is a high incidence of unintended epidural spread when performing thoracic paravertebral block.33 We have had no indications of epidural spread associated with TQL block. A future study comparing TQL and thoracic paravertebral block for PNL is recommended.
The TQL block is a relatively deep block demanding expert ultrasound-guided peripheral nerve block proficiency, with needle visibility at all times to direct the needle towards the intended endpoint in the fascial plane between the QL and PM muscles. The needle and needle tip should always be observed throughout the procedure to avoid unintentional puncture of internal organs such as the kidney, which is extremely unlikely with the TQL technique. The PM muscle acts as a safety backstop, which potentially enhances the safety aspect of this TQL approach. Block failure may result if the LA is not injected into the correct interfascial plane between the QL and PM muscles posterior to the transversalis fascia. This might explain the unsuccessful attempt of a TQL block by Kumar and colleagues34 who utilised continuous fluoroscopic imaging of contrast injected in their version of the TQL block in one fresh tissue cadaver. They found that radiopaque dye spared the TPVS. We believe this likely results from injection into the pararenal fat compartment (Fig. 5).26 When LA is injected inside the pararenal fat compartment anterior to the transversalis fascia, this no longer represents a TQL block but rather a lateral QL block (i.e. a QL1 block or transversalis fascia plane block)35 (Fig. 5), and thus will fail to reach the TPVS. It is important to emphasise that a successful TQL block injection will show separation of the QL and PM muscles and cephalad spread of the injectate towards the diaphragm and beyond when scanning in the longitudinal plane.26
Fig. 5.
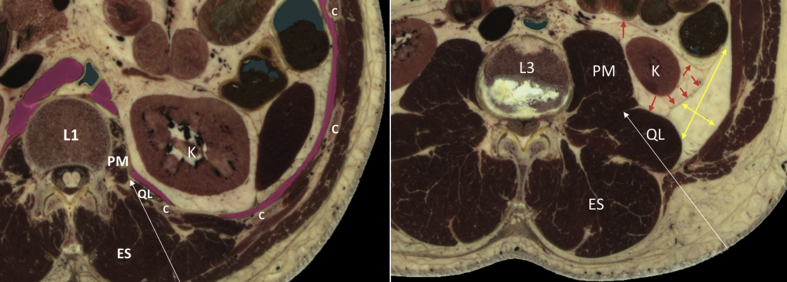
Anatomical details at vertebral levels L1 and L3. At level L1 (left), the psoas major (PM) and quadratus lumborum (QL) muscles are visible postero-medial to the diaphragm (indicated by pink colour). At level L3 (right), the perirenal and pararenal fat compartments are distinct and clearly visible. Modified excerpt from VH Dissector with permission from Touch of Life Technologies Inc (www.toltech.net). Built on real anatomy from the National Library of Medicine Visible Human Project. Red arrows indicate the perirenal fat compartment, yellow arrows indicate the pararenal fat compartment, white arrows indicate the needle pathway. C, costae; ES, erector spinae muscle; K, kidney.
All TQL blocks were performed in awake subjects and were well-tolerated. Procedure-related pain was reported as mild without the need of opioids or sedatives. If the block is not performed correctly (e.g. if injection is i.m.), patients usually report moderate to severe pain because of the high volume injected, thereby alerting the anaesthesiologist of an erroneous point of injection. We therefore recommend performing the TQL block before operation in awake patients. Hydro-dissecting the interfascial plane between the QL and PM muscles with saline also facilitates correct deposition of LA and enhances block success.
All subjects were able to walk from the block procedure room to the operating theatre without support. This is consistent with our cadaveric study that found no dye staining of the femoral or obturator nerves after dye injections using the TQL block technique.14
With all regional anaesthesia techniques, the risk of LA toxicity should always be considered. We used a total dosage of ropivacaine 225 mg which is in compliance with other studies and the Danish Medicine Agency.19, 20, 21, 22, 23, 24 A Danish Anaesthesia textbook suggests ropivacaine 4 mg kg−1 for single shot blocks.20 Further, in several studies the pharmacokinetic profile resulting from a total dose of ropivacaine 150–400 mg in single shot blocks or local infiltration revealed that serum ropivacaine concentrations remained below assumed systemic toxic thresholds.21, 22, 23, 24 We observed no signs of LA systemic toxicity at this dose. Future studies should investigate the minimal dosage of LA possible with the maximum beneficial clinical efficacy to minimise the risk of toxicity.
There are some limitations to our study. Firstly, we did not evaluate block success (dermatomes and myotomes affected) because of the risk of unblinding subjects to block allocation. Secondly, optimal concentration and volume for ropivacaine for unilateral TQL block are still unknown, and our study cannot give information regarding the adequate (or minimal effective) dose or volume. Thirdly, we cannot rule out the possibility of systemic LA absorption adding to the overall analgesic efficacy of the TQL block. Fourthly, we did not test the quadriceps strength before and after TQL block with a dynamometer; this needs to be evaluated in a future randomised controlled trial. Finally, all patients had a urinary bladder catheter inserted after operation, but we did not monitor the time for removal or need for re-catheterisation.
Conclusions
Our study is the first blinded, randomised and controlled trial to show that unilateral TQL block administered before operation provides significant and clinically relevant reduced postoperative opioid consumption, prolonged time to first opioid, reduced time to ambulation, and earlier discharge from the hospital in patients undergoing PNL surgery.
Authors' contributions
Study design/planning: MD, JB, TFB, GL, NHW, MW, VC.
Study conduct: MD, CKH, TDP, JB.
Data analysis: GL, MD, JB.
Writing paper: MD, JB.
Revising paper: MD, JB, TFB, GL, NHW, CKH, TDP, MW, VC.
All authors read approved the final version of the manuscript.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
The Research Foundation of Region Zealand.
Handling editor: H.C. Hemmings Jr
Editorial decision: 17 April 2019
References
- 1.Moe O.W. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 2.Preminger G.M., Assimos D.G., Lingeman J.E., Nakada S.Y., Pearle M.S., Wolf J.S., Jr. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173:1991–2000. doi: 10.1097/01.ju.0000161171.67806.2a. [DOI] [PubMed] [Google Scholar]
- 3.Miller N.L., Lingeman J.E. Management of kidney stones. BMJ. 2007;334:468–472. doi: 10.1136/bmj.39113.480185.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.H., Wang C.J., Huang S.W. Totally tubeless percutaneous nephrolithotomy: a prospective randomized controlled study. Urol Res. 2011;39:459–465. doi: 10.1007/s00240-011-0363-0. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal M.S., Sharma M., Agarwal K. Tubeless percutaneous nephrolithotomy using antegrade tether: a randomized study. J Endourol. 2014;28:644–648. doi: 10.1089/end.2013.0693. [DOI] [PubMed] [Google Scholar]
- 6.Karacalar S., Bilen C.Y., Sarihasan B., Sarikaya S. Spinal-epidural anesthesia versus general anesthesia in the management of percutaneous nephrolithotripsy. J Endourol. 2009;23:1591–1597. doi: 10.1089/end.2009.0224. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Zhou Z., Sun W., Zhao T., Wang H. Minimally invasive percutaneous nephrolihotomy under peritubal local infiltration anesthesia. World J Urol. 2011;29:773–777. doi: 10.1007/s00345-011-0730-z. [DOI] [PubMed] [Google Scholar]
- 8.Aravantinos E., Kalogeras N., Stamatiou G. Percutaneous nephrolithotomy under a multimodal analgesia regime. J Endourol. 2009;23:853–856. doi: 10.1089/end.2008.0448. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H., Dahl J.B. The value of ‘multimodal’ or ‘balanced analgesia’ in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Joshi G.P., Stephan A., Schug S.A., Kehlet H. Procedure-specific pain management and outcome strategies. Clin Anaesth. 2014;28:191–201. doi: 10.1016/j.bpa.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 11.McHugh G.A. The management of pain following day-case surgery. Anesthesiology. 2002;57:270–275. doi: 10.1046/j.1365-2044.2002.2366_2.x. [DOI] [PubMed] [Google Scholar]
- 12.Børglum J., Moriggl B., Jensen K. Letter to the editor. Ultrasound-guided transmuscular quadratus lumborum blockade. Br J Anaesth. 2013;111 http://academic.oup.com/bja/article/doi/10.1093/bja/el_9919/2451466 eLetters Suppl (Available at: Accessed September 18) [Google Scholar]
- 13.Hansen C.K., Dam M., Bendtsen T.F., Børglum J. Ultrasound-guided quadratus lumborum blocks: definition of the clinical relevant endpoint of injection and the safest approach. A A Case Rep. 2016;6:39. doi: 10.1213/XAA.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 14.Dam M., Moriggl B., Hansen C.K., Hoermann R., Bendtsen T.F., Børglum J. The pathway of injectate spread with the transmuscular quadratus lumborum block: a cadaver study. Anesth Analg. 2018;125:303–312. doi: 10.1213/ANE.0000000000001922. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty Arunangshu, Goswami Jyotsna. Ultrasound-guided continuous quadratus lumborum block for postoperative analgesia in a pediatric patient. A A Case Rep. 2015;4:34–36. doi: 10.1213/XAA.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 16.Mænchen N., Hansen C.K., Dam M., Børglum J. Ultrasound-guided transmuscular quadratus lumborum (TQL) block for pain management after Caesarean section. Int J Anesth Anesthesiol. 2016;3:048. [Google Scholar]
- 17.Warusawitharana C., Basar S.H.M.A., Jackson B.L., Niraj G. Ultrasound guided continuous transmuscular quadratus lumborum analgesia for open renal surgery: a case series. J Clin Anesth. 2017;42:100–101. doi: 10.1016/j.jclinane.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Schulz K.F., Altman D.G., Moher D., Consort Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. [Google Scholar]
- 19.Danish Medicines Agency. Available from http://produktresume.dk/AppBuilder/search?utf8=%E2%9C%93&id=&type=&q=ropivacain&button=S%C3%B8g (accessed 12 March 2019)
- 20.Bendtsen T.F., Kølsen J.A.P. Regional analgesi. In: Rasmussen L.S., Steinmetz J., editors. Anæstesi. 4th Edn. FADL’s Forlag; København: 2014. pp. 143–172. [Google Scholar]
- 21.Rosenberg P.H., Veering B.T., Urmey W.F. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–575. doi: 10.1016/j.rapm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Børglum J., Jensen K., Christensen A.F. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transverse abdominis plane block. Reg Anesth Pain Med. 2012;37:294–301. doi: 10.1097/AAP.0b013e31824c20a9. [DOI] [PubMed] [Google Scholar]
- 23.Murouchi T., Iwasaki S., Yamakage M. Quadratus lumborum block. Analgesic effect and chronological ropivacaine concentrations after laparoscopic surgery. Reg Anesth Pain Med. 2016;41:146–150. doi: 10.1097/AAP.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 24.Bakker S.M.K., Fenten M.G.E., Touw D.J. Pharmokinetics of 400 mg locally infiltrated ropivacaine after total knee arthroplasty without perioperative tourniquet use. Reg Anesth Pain Med. 2018;43:699–704. doi: 10.1097/AAP.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen M.V., Bendtsen T.F., Børglum J. Superiority of ultrasound-guided Shamrock lumbar plexus block. Minerva Anestesiol. 2018;84:115–121. doi: 10.23736/S0375-9393.17.11783-9. [DOI] [PubMed] [Google Scholar]
- 26.Dam M., Hansen C.K., Moriggl B., Hoermann R., Bendtsen T.F., Børglum J. In Response. Anesth Analg. 2017;125:709–710. doi: 10.1213/ANE.0000000000002244. [DOI] [PubMed] [Google Scholar]
- 27.DASAIM (Dansk Selskab for Anæstesi og Intensiv Medicin): Danish national guidelines for discharge criteria from recovery. Available from http://www.dasaim.dk/wp-content/uploads/2016/03/Udskrivningskriterier-2016-1.pdf (accessed 18 July 2018)
- 28.Dansk Laegemiddel Information A/S (DK). Ropivacain. Available from https://pro.medicin.dk/Medicin/Praeparater/5981 (accessed 18 July 2019)
- 29.McDonnell J.G., Finnerty O., Laffey J.G. Stellate ganglion blockade for analgesia following upper limb surgery. Anaesthesia. 2011;66:611–614. doi: 10.1111/j.1365-2044.2011.06626.x. [DOI] [PubMed] [Google Scholar]
- 30.Hatipoglu Z., Gulec E., Turktan M. Comparative study of ultrasound-guided paravertebral block versus intravenous tramadol for postoperative pain control in percutaneous nephrolithotomy. BMC Anesthesiol. 2018;18:24. doi: 10.1186/s12871-018-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ak K., Gursoy S., Duger Thoracic paravertebral block for postoperative pain management in percutaneous nephrolithotomy patients: a randomized controlled clinical trial. Med Princ Pract. 2013;22:229–233. doi: 10.1159/000345381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schanbel A., Reichl S.U., Kranke P., Pogatzki-Zahn E.M., Zahn P.K. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842–852. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 33.Richardson J., Lönnqvist P.A., Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth. 2011;106:164–171. doi: 10.1093/bja/aeq378. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A., Sadeghi N., Wahal C., Gadsden J., Grant S.A. Quadratus lumborum spares paravertebral space in fresh cadaver injection. Anesth Analg. 2017;125:708–709. doi: 10.1213/ANE.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 35.Hebbard P.D. Transversalis fascia plane block, a novel ultrasound-guided abdominal wall nerve block. Can J Anaesth. 2009;56:618–620. doi: 10.1007/s12630-009-9110-1. [DOI] [PubMed] [Google Scholar]