Abstract
Context
Can injectable testosterone undecanoate (TU) be administered effectively and acceptably by the subcutaneous (SC) route?
Objective
To investigate the acceptability and pharmacokinetics (PK) of SC injection of TU.
Design
Randomized sequence, crossover clinical study of SC vs IM TU injections.
Setting
Ambulatory clinic of an academic andrology center.
Participants
Twenty men (11 hypogonadal, 9 transgender men) who were long-term users of TU. injections.
Intervention: Injection of 1000 mg TU (in 4 mL castor oil vehicle) by SC or IM route.
Main Outcome Measures: Patient-reported pain, acceptability, and preference scales. PK by measurement of serum testosterone, dihydrotestosterone (DHT), and estradiol (E2) concentrations with application of population PK methods and dried blood spot (DBS) sampling.
Results
Pain was greater after SC compared with IM injection 24 hours (but not immediately) after injection but both routes were equally acceptable. Ultimately 11 preferred IM, 6 preferred SC, and 3 had no preference. The DBS-based PK analysis of serum testosterone revealed a later time of peak testosterone concentration after SC vs IM injection (8.0 vs 3.3 days) but no significant route differences in model-predicted peak testosterone concentration (8.4 vs 9.6 ng/mL) or mean resident time (183 vs 110 days). The PK of venous serum testosterone, DHT, and E2 did not differ according to route of injection.
Conclusions
We conclude that SC TU injection is acceptable but produces greater pain 24 hours after injection that may contribute to the overall majority preference for the IM injection. The PK of testosterone, DHT, or E2 did not differ substantially between SC and IM routes. Hence whereas further studies are required, the SC route represents an alternative to IM injections without a need to change dose for men for whom IM injection is not desired or recommended.
Keywords: testosterone, pharmacology, subcutaneous, pharmacokinetics, quality of life
Testosterone replacement therapy requires long-term treatment of lifelong, irreversible reproductive disorders so that ongoing compliance relies on acceptable products with convenient delivery. Although testosterone has been administered via almost every feasible route, since the 1950s IM injection of testosterone esters in an oil vehicle [1] has been the most widely used and cost-effective modality for testosterone replacement. Testosterone esters incorporated into these products requiring frequent injections (from twice weekly to every 2 weeks) include propionate, enanthate, cypionate, or a mixed-ester preparation are marketed in small volumes (1 mL) of oil vehicle designed solely for IM injection [2]. These oil-based IM injections are usually given deep into the upper outer quadrant of the buttocks by trained medical personnel although some patients self-inject into the quadriceps or deltoid muscles, mainly to obviate frequent medical visits. More recently, long-acting depot injectable testosterone undecanoate (TU) in 3 to 4 mL oil vehicle for injection at 10- to 12-week intervals has been marketed for deep IM injection. The limitations of this product include risks of injection site pain [3], pulmonary oil microembolism [4], and the need for trained medical personnel to administer the larger volume of oil vehicle by deep IM injection. Because the volume is too large for other muscular sites, self-injection is usually not considered feasible. Furthermore, men with bleeding disorders or using anticoagulant or antiplatelet drugs could theoretically experience serious bleeding or bruising from a large-volume deep IM injection, although the actual risk seems low [4]. Hence, the option to administer TU by subcutaneous (SC) injection might be useful. We [5] and others [6–8] have shown that SC injection of small volumes (up to 1 mL) of oil vehicle-based testosterone esters is a feasible and acceptable alternative for hypogonadal [8, 9] and transgender men [7, 10, 11], including some with strong preferences for self-administered SC injection [11]. There is also anecdotal evidence from androgen abusers who regularly self-inject SC large volumes of oil-based androgen esters (including testosterone, nandrolone, and synthetic androgen esters). However, the large volume of the injectable TU (4 mL) poses questions about its acceptability and pharmacokinetics (PK) profile. Hence, the hypothesis of this study was that SC injection of TU was acceptable and has equivalent PK with the IM route. We investigated this in a randomized sequence, crossover design allowing for each patient to serve as their own control. Furthermore, we applied population PK analysis using home-collected dried blood spot (DBS) to increase the number of samples available for PK analysis while also reducing the frequency of clinic visits for blood sampling.
1. Materials and Methods
A. Study Design
This investigator-initiated, single-center, open label clinical trial of injectable TU (1000 mg in 4 mL castor oil vehicle; Reandron, Bayer Australia) involved a randomized sequence, crossover between SC and IM injections. The study was conducted in an ambulatory clinic of an academic andrology center (Andrology Department, Concord Hospital) between 2014 and 2018 without external funding. The participants were recruited from regular clinic patients with satisfactory long-term experience with IM injection who were willing to participate in a research study without any need to change injection route. They comprised 11 participants who were men with proven hypogonadism (7 primary and 4 secondary) from disease of the hypothalamic-pituitary-testicular axis and 9 transgender men (female to male transgender). The drug was supplied by the Concord Hospital pharmacy within the national health scheme. The study had ethics approval from the Sydney Local Health District Human Research Ethics Committee and was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12613000101785).
B. Randomization
Eligible consenting participants were randomized in a 1:1 ratio to start with SC or IM injection. Randomization was achieved by participants being issued sequential, unique (never reused) study identification numbers with the computer-generated randomization code supplied in opaque sealed envelopes marked on the outside by the study identification. The randomization code envelope was opened after a participant consented to enter the study.
C. Study Amendment
There was one study amendment to the blood sampling schedule. Initially the study had a schedule of intensive home-based DBS sampling that was later changed to less frequent, clinic-based venous blood sampling. In a previous study we verified that clinic-based DBS sampling provided virtually identical serum testosterone measurements to simultaneous venous blood sampling [5]. Therefore, this study initially aimed to obtain intensive blood sampling without frequent clinic visits by using home-based DBS collections. After 9 participants completed the home-based DBS collections, an interim analysis revealed an adequate PK profile of serum testosterone despite some (∼5%) DBS samples being unquantifiable for steroid liquid chromatography-mass spectrometry analysis as a result of interference from manual handling by participants of blood spots on the DBS cards. Despite instruction to use a no-touch technique when spotting blood onto the cards, some participants handled the cards in the process of milking the finger-pricked blood onto the cards to fill the spots. To reduce participant burden from intensive home-based DBS monitoring and to widen the PK profile to include testosterone’s bioactive metabolites, DHT and estradiol (E2), the study sampling reverted to less frequent clinic-based venous blood sampling for the remaining 11 participants.
D. Recruitment and Entry Criteria
Participants were recruited from men undergoing regular TU injection treatment in the andrology department. The only additional entry criterion was providing written consent to complete the study. No payment was offered to participants. Exclusion criteria were (i) major psychiatric disease or psychological condition that limited understanding and/or compliance with study requirements, (ii) regular medications that interfere with testosterone absorption, metabolism, or action that might be subject to altered dosage during the study, and (iii) having bleeding disorders or taking antiplatelet or anticoagulant therapy.
E. Study Procedures
After randomization to start with IM or SC injection, consenting participants were administered a single injection of TU and were followed for 12 weeks after each injection before crossover without any washout period. In the original study design DBS samples were collected before injection and then daily for the first week (days 1, 2, 3, 4, 5, 6, 7), followed by three times weekly for the remainder of the first month (days 12, 14, 16, 19, 21, 23, 28, 30), and then on days 35, 37, 43, 44, 51, 58, 66, 70 days. For each of the intended 24 DBS samples, the participants recorded the exact time and date of finger-prick spotting onto the DBS cards below that blood spot. After an interim analysis of the DBS samples from nine participants, the blood sampling schedule was simplified to clinic-based venous blood samples before and then at 1, 4, 8, and 12 weeks after injection. All injections were given by an experienced nurse. The IM injections were given slowly over two to three minutes by a deep IM injection using a 21-gauge 38 mm needle into the upper outer quadrant of the buttocks aiming for the gluteal muscle. The SC injections were given slowly over two to three minutes into the SC tissues of the abdomen using a 21-gauge 25 mm needle.
F. Patient Reported Outcomes
The acceptability scale was depicted as a vertical thermometer scale and was administered twice for each injection, immediately after and then again at the end of each 12-week injection cycle. The acceptability scores range from 10 (very acceptable, willing to have regularly) to 0 (unacceptable, not willing to have regularly) with 5 meaning acceptable.
The pain scale was a vertical, color-coded (red, yellow, and green in a continuous shading continuum) scale [3] that was administered twice for each injection, immediately after and 24 hours after each injection. The 24-hour score was completed by the participant at home on the printed scale and returned at next visit. The pain scores ranging from 10 (red, unbearable distress, agonizing), 8 (horrible), 6 (dreadful), 4 (uncomfortable), 2 (annoying), to 0 (green, no distress) as used previously [3].
The preference scale was administered once for each participant at the end of the study. It was a horizontal 20-point scale with a 0 in the center (do not care which, equally bad or good) and with the ratings of their preference outward from the center to 10 in either direction to express a quantitative preference (increasing numbers according to strength of preference) for IM injections (to the left) or SC injections (to the right).
G. Steroid Measurement
Serum testosterone, DHT, E2, and estrone (E1) were measured in a single batch from solvent extracts (methyl tert-butyl ether) of serum by liquid chromatography-tandem mass spectrometry as described in detail elsewhere [5, 12]. DBS were extracted for measurement of whole blood testosterone that was converted to serum testosterone after accounting for blood volume and hematocrit as described [5]. Hematocrit at entry to, at midpoint (6 weeks) of each arm of study, and at end of study did not differ (overall mean 0.48, P = 0.30) and the average hematocrit was used for each individual participant. The limits of detection and quantification with coefficients of variation (range for three quality control samples run in triplicate in each run) were 10 pg/mL, 25 pg/mL, and 3% to 6% for testosterone; 50 pg/mL, 100 pg/mL, and 9% to 11% for DHT; 1 pg/mL, 5 pg/mL, and 7% to 13% for E2; and 1 pg/mL, 5 pg/mL, and 4% to 9% for E1. Reference ranges for circulating steroid concentrations were 1.8 to 7.8 ng/mL for testosterone, 0.07 to 0.64 ng/mL for DHT and 15 to 68 pg/mL for E2, and 7.2 to 38.4 pg/mL for E1 based on 95% CI determined from a study of 382 healthy young men aged around 20 years old from a population-based birth cohort study [13].
H. Data Analysis
In the analysis of DBS samples, the serum testosterone concentrations were fitted to a biexponential equation by nonlinear curve fitting of concentrations (C) as a function of time (T) since testosterone injection according to Eq. 1
Adopting a population PK approach [14], the time since injection was defined as the exact time (as recorded by the participant at the time of finger-prick blood spotting onto DBS card) rather than the notional time of intended blood sampling. Noncompartmental PK variables were estimated from the fitted concentration-time curves for each route [15, 16]. Peak time was estimated as ln(-Cd/Ab)/(d-b), area under the curve (AUC) as A/b + C/d, first moment of the AUC (AUMC) as A/b2 + C/d2 and the mean residence time as first moment of the AUMC/AUC. Peak concentration was defined as the concentration at the time of peak for that route with 95% CI for model-based estimates of peak concentration, AUC, and mean residence time was derived from at least 1000 bootstrap estimates. The duration of action of the TU injections was estimated as the time taken for the predicted serum testosterone concentration to return to preinjection baseline.
In the analysis of venous serum, the time-course of testosterone, DHT, E2, and E1 concentrations were analyzed by a mixed-model linear analysis for repeated (within-subject) measures utilizing restricted maximum likelihood minimization with a diagonal variance-covariance structure. The analysis included the main effects of route and time with sequence, age, body mass index (BMI), and diagnosis as covariates. All data analysis used NCSS 12 Statistical Software (NCSS, LLC., Kaysville, UT; see www.ncss.com) and calculations according to standard PK methods [15].
The quality of life scales (acceptability, pain) and the hormonal variables were analyzed by standard crossover analysis using mixed model linear analysis with main effects of treatment (route of administration), sequence (testosterone first or second), or carryover (first or second stage of study) with serial measurements over time as the within factor and age, obesity (BMI), sequence, and diagnosis as covariates using NCSS software. The analysis included first-order terms fitted by restricted maximum likelihood solved using the Newton-Raphson algorithm with a diagonal variance-covariance matrix structure without interaction terms.
2. Results
The anthropometric characteristics of the participants are listed in Table 1. The participants included 7 with primary and 4 with secondary hypogonadism as well as 9 female-to-male transgenders. All 20 completed the quality of life scales. The PK analyses were completed for the first 9 by intensive home-based DBS blood sampling whereas the remaining 11 provided clinic-based venous blood sampling.
Table 1.
Participant Characteristics
| Mean | SE | Minimum | Q1 | Q2 | Q3 | Maximum | |
|---|---|---|---|---|---|---|---|
| Age, y | 47.6 | 3.29 | 20.8 | 34.0 | 48.9 | 61.0 | 68.4 |
| Height, cm | 175 | 2.3 | 158 | 169 | 175 | 183 | 195 |
| Weight, kg | 92.6 | 5.09 | 47.5 | 83.7 | 92.4 | 112.9 | 127.3 |
| BMI, kg/m2 | 30.1 | 1.62 | 17.9 | 24.8 | 30.0 | 34.0 | 48.4 |
| BSA, m2 | 2.13 | 0.07 | 1.45 | 2.02 | 2.16 | 2.39 | 2.53 |
Abbreviation: BSA, body surface area.
A. Pain Score
Overall the pain scores were mostly low [3] (Table 2). The immediate postinjection pain score did not differ according to route of administration (P = 0.31) after adjustment for age, BMI, and diagnosis (all P > 0.38) without sequence/carryover effects (P = 0.29).
Table 2.
Pain and Acceptability Scores
| Route | Count | Mean | SE | Range | Median (IQR) | |
|---|---|---|---|---|---|---|
| Paina | ||||||
| Immediate | IM | 20 | 2.5 | 0.41 | 0 –8 | 2 (1, 3) |
| SC | 20 | 3.25 | 0.58 | 0–10 | 2.5 (1, 4.75) | |
| 24-h postinjection | IM | 18 | 1 | 0.28 | 0–4 | 1 (0, 2) |
| SC | 19 | 2.26 | 0.47 | 0–5 | 2 (0, 5) | |
| Acceptabilityb | ||||||
| Immediately after injection | IM | 18 | 7.94 | 0.49 | 3–10 | 8 (7, 10) |
| SC | 19 | 6.21 | 0.67 | 0–10 | 6 (4, 9) | |
| 12 wk after injection | IM | 19 | 8 | 0.59 | 2–10 | 9 (8, 10) |
| SC | 18 | 6.33 | 0.75 | 0–10 | 8 (3, 0) |
Higher scores indicate more pain.
Higher scores indicate greater acceptability.
The 24-hour postinjection pain score was significantly higher after SC compared with IM injection (1.25 ± 0.57, P = 0.036) after adjustment for age, BMI, and diagnosis (all P > 0.70) without any sequence/carryover effects (P = 0.44)
B. Acceptability Scale
The immediate postinjection acceptability scores (Table 2) were not significantly different between IM and SC injection before (P = 0.064) and after adjustment for age, BMI, and diagnosis (P > 0.50) without any sequence/carryover effects (P = 0.51).
The 12-week postinjection acceptability score was not significantly different for IM compared with SC injection (P = 0.095) after adjustment for age, BMI, and diagnosis (all P > 0.40) without any sequence/carryover effects (P = 0.24).
C. Preference
After completing exposure to both injections, 11 participants preferred IM injection [rating mean 7.2 ± 3.3; median 8, interquartile range (IQR) 4 to 10), 6 preferred SC injection (rating 5.8 ± 2.9; median 5.5, IQR 5 to 8), and 3 did not have a clear route preference. There was no significant difference in preference according to diagnosis. Two participants chose to continue long-term treatment with SC injections whereas the remainder continued with IM injections.
D. Pharmacokinetics
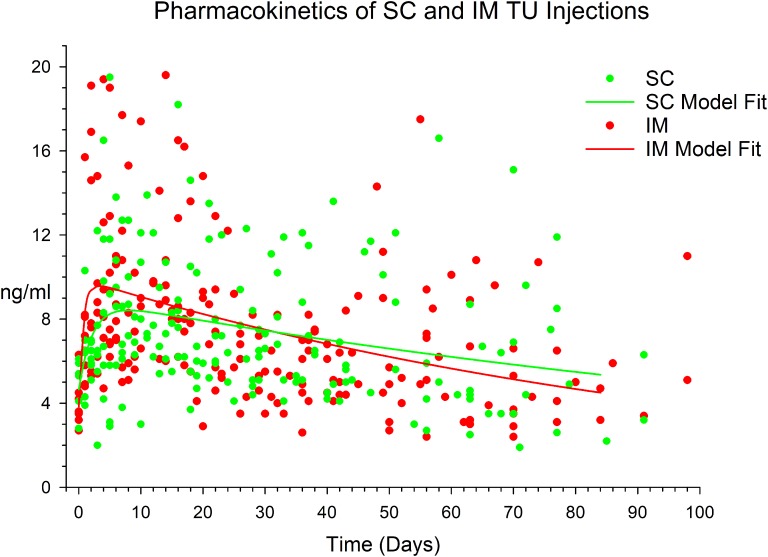
From the DBS analysis (Fig. 1), the participants provided 406 samples (94% of the 432 samples expected), each providing a median of 42 (range, 29 to 54) samples, of which 21 (5.2%) were excluded from analysis as a result of interference in the liquid chromatography-mass spectrometry analysis steroid analysis from manual handling of DBS cards. From analysis of 385 serum testosterone samples fitted to the biexponential curves, the serum testosterone profile after SC injection displayed a later time of peak (8.0 vs 3.3 days) with no significant differences compared with the IM route in model-predicted (bootstrapped) peak concentration (8.4 [95% CI 2.3 to 14.6 ng/mL] vs 9.6 ng/mL [2.9 to 16.3 ng/mL], AUC (1471 ng.day/mL [912 to 3971 ng.day/mL] vs 1059 ng.day/mL [776 to 1655 ng.day/mL], or mean resident time (183 days [101 to 434 days] vs 110 days [78 to 161 days]), respectively. The wide CIs reflect significant heterogeneity between and within individual in DBS-derived serum testosterone concentrations after TU injection. The duration of action was 104 days for the SC and 101 days for IM route. At 84 days after injection (the approved nominal duration of action of injectable TU), predicted serum testosterone was 18% above baseline for IM injection and 14% above baseline for SC injection.
Figure 1.
Plot of DBS analysis of serum testosterone after SC (green dots) and IM (red dots) injection of 1000 mg TU in 4 mL castor oil vehicle and a model of the SC (green line) and IM (red line) data fitted to a biexponential curve. For further details see Methods and Results.
All venous blood samples expected for the PK analysis were collected.
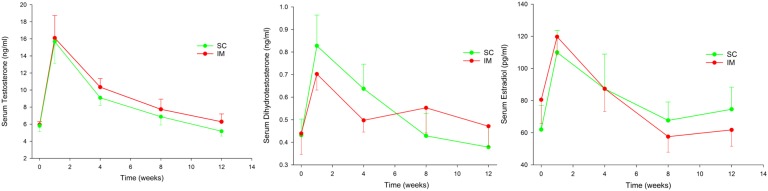
Serum testosterone concentrations (Fig. 2) did not differ according to route of administration (P = 0.54) after adjustment for age (P = 0.86), BMI (P = 0.06), and diagnosis (P = 0.83) without sequence/carryover effects (P = 0.19).
Figure 2.
Serum testosterone, DHT, and E2 after SC and IM injections of 1000 mg TU in 4 mL castor oil vehicle. Data are mean and standard error of the mean.
Serum DHT concentrations (Fig. 2) did not differ according to route of administration (P = 0.98) but were higher with older age (0.44 ± 0.19 ng/mL per kg/m2, P < 0.001) and not 0different according to BMI (P = 0.41) or diagnosis (P = 0.35). There was a significant sequence effect with lower serum DHT overall when SC injection was first (−0.25 ± 0.1 ng/mL, P = 0.008).
Serum E2 concentrations (Fig. 2) did not differ according to route of administration (P = 0.99) but were lower with older age (−0.77 ± 0.38 pg/mL per year, P = 0.044) but not affected by BMI (P = 0.40) or diagnosis (P = 0.67) without sequence/carryover effects (P = 0.96).
Serum E1 concentrations did not differ according to route of administration (P = 0.58) or age (P = 0.19) but were higher with increasing BMI (1.65 ± 0.41 pg/mL per year, P < 0.001) and with transgender diagnosis (P = 0.009). There was a significant sequence effect with higher serum E1 overall when SC injection was injected first (16 ± 8 pg/mL, P = 0.039).
3. Discussion
The current study indicates that SC injection of TU is feasible and acceptable to some hypogonadal or transgender men. Nevertheless, patients with satisfactory long-term prior use of IM injections who had a single SC injection preferred IM to SC injections, possibly related to the higher 24-hour postinjection pain score. Based on our findings, the option of SC injection could be considered a reasonable choice for some patients or an alternative when deep IM injection is undesirable or not feasible. Most likely in practice this would be based on dividing the 4 mL into two 2-mL injections administered on either side of the abdomen. This is likely to have no substantial effect on testosterone PK because the findings with transdermal testosterone indicate that dividing a single dose into four sites rather than one site does not materially change testosterone PK profile [17].
This study investigates the feasibility of SC injection of the large vehicle volume (4 mL) required for injectable TU. In this study, the acceptability of SC injection is less than reported by a recent study of transgender men [11] in an uncontrolled observational trial of SC injections of a lower volume (<1 mL) of an oil-based testosterone ester injection. These differences are most likely because of the larger volume injection in this study with the crossover design allowing participants to compare the SC and IM injections for themselves. Because this study cohort included more than half of transgender men and the lack of difference in route preference according to diagnosis, it is unlikely that the type of patient explained the different finding consistent with other PK studies of hypogonadal and transgender men [18]. Other potentially important differences were that our study provided injections without cost to patients already receiving regular TU treatment compared with newly treated patients self-selected to try SC injections and undergo uptitration to the minimum dose according to efficacy criteria, e.g., menstrual suppression [10], that may not be comparable with the virilization required for hypogonadal men.
The PK profile of serum testosterone and its two bioactive metabolites, DHT and E2 in this study indicate that the SC injections of TU displayed no significant differences from the IM route. This is consistent with PK studies of other steroids such as cortisol [19] and hydroxyprogesterone caproate [20] whereas in other studies the SC route displayed a lower and later peak serum concentration of progesterone [21] or medroxyprogesterone acetate [22]. Although Food and Drug Administration-defined bioequivalence was not formally tested, the two routes can be considered clinically comparable without the need for route-based dose adjustment. This interpretation differs from previous studies of SC injections which inferred a reduced testosterone dosage requirement by the SC route compared with the IM route [7, 10, 11] based on noncomparative studies of transgender men that did not investigate IM injections contemporaneously and relied upon historical data from other studies. Those studies used inconsistent, mostly single, sampling time points with the testosterone dosage being up-titrated according to clinical efficacy criteria, e.g., menstrual suppression [10], that may not represent effective virilization dosage for hypogonadal men [11].
Among this study’s strengths are its investigation of the target population and the crossover design allowing participants to judge acceptability for themselves as well as evaluating potential route-dependent dose requirement according to PK. Furthermore, it introduces population PK using DBS sampling that has the potential to simultaneously relax the requirement for frequent clinic visits for blood sampling while at the same time increasing the availability of blood samples for analysis via home-collected DBS samples. Among its limitations are its relatively small sample size, its inability to evaluate whether self-injection is feasible in practice, or whether the risk of pulmonary oil microembolism may be reduced by SC injection. It is possible that the relatively limited size of this study may have underestimated small but possibly clinically significant route-dependent differences that would become more evident in a large pharmacological study and/or from clinical differences in interinjection intervals in the dose-titration regimens of TU injections. Another limitation is that the acceptability, pain, and preference scales were only used for within-person route comparisons and these data cannot necessarily be extrapolated quantitatively to other settings. Furthermore, this study was based on men having regular IM TU injections at individually titrated 12-week intervals and this facile switchover without dose modification may not apply to men having shorter or longer injection intervals without further investigation.
We conclude that with clinic-based delivery SC injection of TU is effective with similar PK parameters to IM TU so not requiring dose modification. It is acceptable but generally less preferable compared with IM TU for men. Therefore, SC TU injection constitutes an alternative delivery route in circumstances in which IM injection is not desired, feasible, or recommended. Despite causing slightly more injection site pain 24 hours after injection, SC injection was generally acceptable but not preferred over IM injection by most participants in this study who were long-term satisfied users of IM TU. These preliminary findings indicate that SC injection may prove a viable alternative delivery route where required. Whether self-injection of either the 4 mL or two 2-mL injections is feasible or acceptable remains to be determined.
Acknowledgments
Clinical Trial Registration: Trial # ACTRN12613000101785, Australia and New Zealand Clinical Trials Registry (registered 25 January 2013).
Disclosure Summary: D.J.H. has received institutional grants for other investigator-initiated androgen pharmacology studies (Lawley, Besins Healthcare) but no personal income and has provided expert testimony to antidoping and professional standards tribunals and in testosterone litigation. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- BMI
body mass index
- DBS
dried-blood spot
- DHT
dihydrotestosterone
- E1
estrone
- E2
estradiol
- PK
pharmacokinetics
- SC
subcutaneous
- TU
testosterone undecanoate
References and Notes
- 1. Junkmann K. Long-acting steroids in reproduction. Recent Prog Horm Res. 1957;13:389–419, discussion 419–428. [PubMed] [Google Scholar]
- 2. Handelsman DJ. Androgen physiology, pharmacology and abuse. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 7th edPhiladelphia, PA: Elsevier Saunders; 2015:2368–2393. [Google Scholar]
- 3. Sartorius G, Fennell C, Spasevska S, Turner L, Conway AJ, Handelsman DJ. Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate. Asian J Androl. 2010;12(2):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Middleton T, Turner L, Fennell C, Savkovic S, Jayadev V, Conway AJ, Handelsman DJ. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172(5):511–517. [DOI] [PubMed] [Google Scholar]
- 5. Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ. Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays. J Clin Endocrinol Metab. 2014;99(7):2592–2598. [DOI] [PubMed] [Google Scholar]
- 6. Kaminetsky J, Jaffe JS, Swerdloff RS. Pharmacokinetic Profile of Subcutaneous Testosterone Enanthate Delivered via a Novel, Prefilled Single-Use Autoinjector: A Phase II Study. Sex Med. 2015;3(4):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson DM, Kiang TKL, Ensom MHH. Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: a pilot study. Am J Health Syst Pharm. 2018;75(6):351–358. [DOI] [PubMed] [Google Scholar]
- 8. Kaminetsky JC, McCullough A, Hwang K, Jaffe JS, Wang C, Swerdloff RS. 2018. A 52-week study of dose-adjusted subcutaneous testosterone enanthate in oil self-administered via disposable auto-injector. J Urol. 2019;201(3):587–594. [DOI] [PubMed] [Google Scholar]
- 9. Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D. Subcutaneous administration of testosterone. A pilot study report. Saudi Med J. 2006;27(12):1843–1846. [PubMed] [Google Scholar]
- 10. Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M. Subcutaneous testosterone: an effective delivery mechanism for masculinizing young transgender men. LGBT Health. 2014;1(3):165–167. [DOI] [PubMed] [Google Scholar]
- 11. Spratt DI, Stewart II, Savage C, Craig W, Spack NP, Chandler DW, Spratt LV, Eimicke T, Olshan JS. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: demonstration in female-to-male transgender patients. J Clin Endocrinol Metab. 2017;102(7):2349–2355. [DOI] [PubMed] [Google Scholar]
- 12. Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1-2):78–84. [DOI] [PubMed] [Google Scholar]
- 13. Hart RJ, Doherty DA, McLachlan RI, Walls ML, Keelan JA, Dickinson JE, Skakkebaek NE, Norman RJ, Handelsman DJ. Testicular function in a birth cohort of young men. Hum Reprod. 2015;30(12):2713–2724. [DOI] [PubMed] [Google Scholar]
- 14. Kiang TK, Sherwin CM, Spigarelli MG, Ensom MH. Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet. 2012;51(8):515–525. [DOI] [PubMed] [Google Scholar]
- 15. Gibaldi M, Perrier D. Pharmacokinetics, 2nd edNew York: Marcel Dekker; 1982 [Google Scholar]
- 16. Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 4th edBaltimore, MD: Wolters Kluwer Lippincott; 2011:717–721. [Google Scholar]
- 17. Wang C, Berman N, Longstreth JA, Chuapoco B, Hull L, Steiner B, Faulkner S, Dudley RE, Swerdloff RS. Pharmacokinetics of transdermal testosterone gel in hypogonadal men: application of gel at one site versus four sites: a General Clinical Research Center Study. J Clin Endocrinol Metab. 2000;85(3):964–969. [DOI] [PubMed] [Google Scholar]
- 18. Savkovic S, Lim S, Jayadev V, Conway A, Turner L, Curtis D, Goebel C, Handelsman DJ. Urine and serum sex steroid profile in testosterone-treated transgender and hypogonadal and healthy control men. J Clin Endocrinol Metab. 2018;103(6):2277–2283. [DOI] [PubMed] [Google Scholar]
- 19. Hahner S, Burger-Stritt S, Allolio B. Subcutaneous hydrocortisone administration for emergency use in adrenal insufficiency. Eur J Endocrinol. 2013;169(2):147–154. [DOI] [PubMed] [Google Scholar]
- 20. Krop J, Kramer WG. Comparative bioavailability of hydroxyprogesterone caproate administered via intramuscular injection or subcutaneous autoinjector in healthy postmenopausal women: a randomized, parallel group, open-label study. Clin Ther. 2017;39(12):2345–2354. [DOI] [PubMed] [Google Scholar]
- 21. Sator M, Radicioni M, Cometti B, Loprete L, Leuratti C, Schmidl D, Garhöfer G. Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the s.c. route. Gynecol Endocrinol. 2013;29(3):205–208. [DOI] [PubMed] [Google Scholar]
- 22. Shelton JD, Halpern V. Subcutaneous DMPA: a better lower dose approach. Contraception. 2014;89(5):341–343. [DOI] [PubMed] [Google Scholar]