Abstract
Context
Information about adrenal medullary hyperplasia (AMH) is scarce.
Objective
To study a large cohort of AMHs.
Design, Setting, and Participants
Nineteen AMH cases were compared with 95 pheochromocytomas (PCCs) without AMH. AMH without (n = 7) and with PCC (n = 12) were analyzed separately.
Results
Of 936 adrenalectomies, 2.1% had AMH. Mean age was 47.2 ± 15.1 years. Only two (11%) AMHs had no concurrent PCC or adrenocortical adenoma. In AMHs, a genetic syndrome was present in 58% vs 4% in PCCs (P < 0.001). The noradrenaline/metanephrine levels were lower in AMHs, whereas suppression of dexamethasone was less than in PCCs. Cushing syndrome was found in 11% of AMHs. More AMHs were found during screening and less as incidentalomas. PCC symptoms were less prevalent in AMHs. Surgical management was similar; however, fewer of the AMHs were pretreated with alpha-blockers. Adrenalectomy improved blood pressure slightly less in AMHs. The disappearance of glycemic disturbances was similar to the PPCs. During a period of 11.2 ± 9.4 years, a new PCC developed in 32% of patients with AMH, 11% died, but no PCC metastasis occurred (PCCs: 4%, P < 0.001; 14% and 5%). AMHs without PCC had milder symptoms but more often Cushing disease than patients with PCC, whereas AMH with PCC more often displayed a familiar syndrome with more PCC recurrences.
Conclusion
A total of 2.1% of all adrenalectomies displayed AMH. AMH seemed to be a PCC precursor. The symptoms and signs were milder than PCCs. AMHs were mainly found due to screening. Outcomes seemed favorable, but new PCCs developed in many during follow-up.
Keywords: presentation, blood pressure, diabetes, mortality, surgery, genetic syndrome
Pheochromocytomas (PCCs) are rare catecholamine-producing neuroendocrine tumors arising from the adrenal medulla [1]. Adrenal medullary hyperplasia (AMH) is considered a precursor of PCC and is even rarer [2]. AMH can be difficult to diagnose but can be suspected if the adrenal medulla is more than a third of the adrenal thickness, in the absence of cortical atrophy [3, 4]. Adrenal medullary lesions <1 cm are considered to be AMH and, if larger, a PCC [2], even though the term micro-PCC has been suggested [5]. AMH has been thought to be diagnostic of MEN2 syndrome [3]; however, NF1 [6], SDHB [7], and MAX mutations [8] have all been associated with AMH. AMH and PCC have been reported to coexist in the same patient [2, 5, 8]. There is limited literature about AMH with only case reports or small case series, and sporadic AMH has only occasionally been published [2, 3, 5–11]. AMH has been claimed to mimic PCC clinically with symptoms and signs of catecholamine excess [9–11], but no formal comparisons have been made.
The aims of the current study were to investigate in a large cohort of AMHs, found on adrenalectomies, the presentation, management, histology, short-term (surgical complications, hypertension, and diabetes), and long-term outcomes (recurrence, metastasis, and mortality) and compare with PCCs. Moreover, we also wanted to explore if AMH without a concomitant PCC differed from AMH lesions arising in the setting of a synchronous PCC.
1. Materials and Methods
The histopathology database at the Department of Pathology and Cytology, Karolinska University Hospital, Stockholm, Sweden, was used to identify AMH cases from 1992 to 2018. Moreover, to identify PCCs, all patients with an International Classification of Diseases (ICD) 10th revision code of E27.5 (adrenomedullary hyperfunction) and/or C74.1 (malignant neoplasm of medulla of adrenal gland) being admitted and/or attending the outpatient clinic between 2000 and 2018 at the Department of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Stockholm, Sweden, were screened. The patients’ electronic medical files and histology were reviewed, and if an AMH and/or a PCC could not be confirmed, the case was excluded. Moreover, the AMH group was also divided into patients with AMH without a concomitant PCC and AMH with a concomitant PCC. The National Population Register was consulted to confirm if the patient was dead or alive, and the date of death was retrieved if applicable [12]. All hospital admissions and outpatient visits are coded in Sweden by the attending physician with ICD 10th revision codes, and these are stored in both local and national databases [13]. The mode of presentation [incidentaloma (defined as a patient having imaging for a cause unrelated to the adrenals and not part of staging for cancer), suspicion of PCC (defined as a patient suspected to have a PCC due to symptoms and signs before biochemical confirmation and imaging), screening (defined as a known genetic condition with increased occurrence of PCC and therefore screened with catecholamines on a yearly basis)], concomitant tumor size, perioperative management (surgical complications was defined to occur within 30 days of surgery), length of stay (from the day of surgery to discharge), histology, blood pressure, glucose abnormalities (both at the time of presentation and at the first endocrine review 6 to 12 months after surgery), genetic results (RET mutations, for MEN2 syndrome, were tested in all, but other genetic tests, most notably NF1 and VHL, were tested depending on what methodology was available at the time of diagnosis), follow-up time, mortality including cause of death, metastasis from the PCC, and recurrence were noted. Catecholamine levels were measured using HPLC for 24-hour urinary adrenaline and noradrenaline (normal <80 and <400 nmol/24 h, respectively) and liquid chromatography–tandem mass spectrometry for plasma metanephrine and normetanephrine (normal <0.3 and <0.6 nmol/L, respectively). As not all individuals had both tests performed, the highest urine or plasma level was divided with the upper level of normal and noted. Morning serum cortisol was measured after an overnight 1-mg dexamethasone suppression test (DST) as described previously [14]. Blood pressures were measured with an appropriate-sized blood pressure cuff on two occasions at rest, mostly seated in an office or occasionally on the ward. A reduction of systolic and diastolic blood pressure of at least 10 mm Hg and/or reduction in blood pressure medications postsurgery were defined as improvement of blood pressure. An HbA1c 42 to 47 mmol/mol and/or fasting plasma glucose 6 to 6.9 mmol/L and/or random plasma glucose 7.8 to 11 mmol/L was defined as prediabetes; levels above these were defined as diabetes. Parts of this PCC cohort, but also one patient with AMH, have been reported in previous publications [14–18].
The Regional Ethical Review Board in Stockholm, Sweden, approved the study, and, as this was a retrospective study, formal consent was not required.
A. Statistical Analysis
If normally distributed, mean ± SD was used; otherwise, median and interquartile range (25% to 75%) was used. Continuous variables and two groups were analyzed with the unpaired t test if normally distributed or otherwise Mann–Whitney rank-sum test. The χ2 or Fisher exact test were used in frequency table calculation. A P value <0.05 was considered significant. SigmaStat 3.0 for Windows (Systat Software Inc., San Jose, CA) was used for all computations.
2. Results
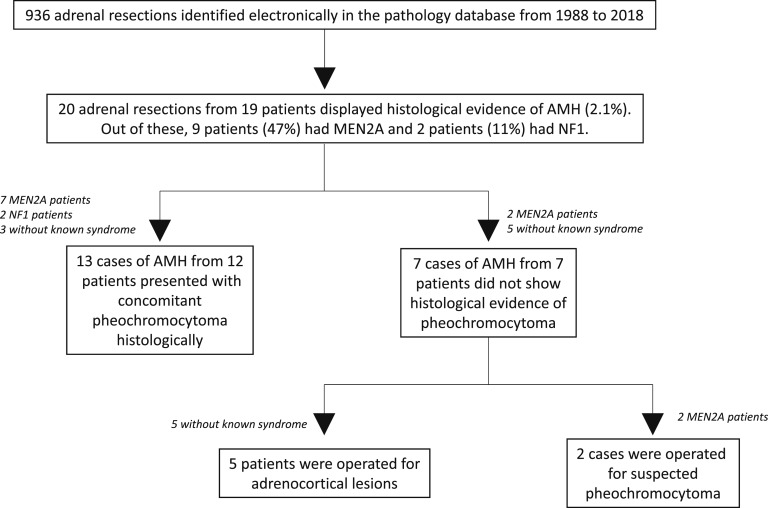
In total, 19 individuals with AMH (with 20 adrenalectomies) were identified out of 936 adrenalectomies (i.e., a frequency of 2.1%) (Fig. 1). Seven patients had AMH without concurrent PCC, and 12 had AMH with concurrent PCC. As controls, 95 patients with PCC without AMH were identified; however, two patients with adrenocortical cancers and concomitant catecholamine excess but without an eligible ICD code (E275 or C741) were known to us as well, were not included, but have been presented elsewhere [19]. Thus, 19 cases of AMH and 95 cases of PCCs were included in the analysis.
Figure 1.
Schematic representation of our AMH cohort.
A. Presenting Characteristics of Patients With an AMH Compared With Patients With a PCC Without AMH
A-1. Entire AMH cohort
In patients with AMH, the mean age at presentation was 47.2 ± 15.1 years, which was a tendency (P = 0.058) to be lower than in patients with PCC only (Table 1). Of the patients with AMH, 63% were female, which was similar to the patients with PCC. Almost two-thirds of patients with AMH had a concurrent PCC, and a quarter had a concurrent cortical adenoma. A genetic syndrome was present in 58% of patients with AMH (mainly MEN2A but also NF1), which was more than in patients with PCC. The largest concomitant tumor size (i.e., the size of the concomitant PCC or adrenocortical adenoma in the patients with AMH) was 21.5 mm compared with 49 mm in the PCCs. No concomitant tumor was only found in 11% of AMHs (two patients, both with known MEN2A syndrome, displayed increased catecholamine levels during annual screening and unilateral adrenal uptake on the iodine-123 meta-iodobenzylguanidine scan, raising the suspicion of PCC). The urinary noradrenalin and/or plasma metanephrine levels were lower, and the DST was less suppressed in AMHs than in PCCs (Table 1).
Table 1.
Presenting Characteristics, Preoperative Management, Surgery, and Long-Term Outcomes in Adult Patients With AMH and its Subgroups Compared With Adult Patients With PCCs Without AMH
| All AMHs (n = 19) | P Value vs PCCs | AMHs Without PCC (n = 7) | P Value vs PCCs | AMHs With PCC (n = 12) | P Value vs PCCs | PCCs Without AMH (n = 95) | |
|---|---|---|---|---|---|---|---|
| Age at presentation, y | 47.2 ± 15.1 | 0.058 | 52.9 ± 8.6 | 0.713 | 43.9 ± 17.4 | 0.032 | 55.2 ± 17.0 |
| Females, n (%) | 12 (63) | 0.450 | 5 (71) | 0.499 | 7 (58) | 0.762 | 48 (51) |
| PCC, n (%) | 12 (63) | <0.001 | 0 (0) | <0.001 | 12 (100) | 1 | 95 (100) |
| Concurrent cortical adenoma, n (%) | 5 (26) | <0.001 | 5 (71) | <0.001 | 12 (100) | 1 | 0 (0) |
| No adenoma, n (%) | 2 (11) | 0.026 | 2 (29) | <0.001 | 0 (0) | 1 | 0 (0) |
| MEN2A/NF1, n (%) | 11 (9/2, 58) | <0.001 | 2 (2/0, 29) | 0.070 | 9 (7/2, 75) | <0.001 | 4 (1/3, 4) |
| Tumor size,a mm | 21.5 (20–40) | <0.001 | 28 (16–45) | 0.074 | 21.4 (20–40) | 0.002 | 49 (35–63) |
| U-A/P-MNE, × ULN | 2.3 (0.9–6.4) | 0.220 | 0.7 (0.4–1.2) | <0.001 | 4.1 (2.5–16.8) | 0.331 | 3.4 (1.3–15.0) |
| U-NA/P-NMNE, × ULN | 1.2 (0.7–3.1) | <0.001 | 1.1 (0.5–1.3) | <0.001 | 1.5 (0.9–3.6) | <0.001 | 7.9 (3.2–18.3) |
| Highest level, × ULN | 2.5 (1.4–7.0) | <0.001 | 1.2 (0.7–1.8) | <0.001 | 4.1 (2.0–16.8) | 0.039 | 13.5 (5.1–26.8) |
| Post-DST, mmol/L | 59 (30–440) | 0.048 | 70 (44–539) | 0.045 | 32 (30–40) | 0.943 | 30 (27–37) |
| Doxazosin dose preoperative, mg | 12 (0–23) | 0.021 | 0 (0–0) | <0.001 | 22 (20–32) | 0.956 | 24 (16–36) |
| Phenoxybenzamine dose preoperative, mg | 80 (50–88) | 0.158 | 50 (50–50) | 0.823 | 80 (73–148) | 0.055 | 50 (40–68) |
| No alpha-blockage, n (%) | 5 (26) | 0.006 | 5 (71) | <0.001 | 0 (0) | 1 | 4 (4) |
| Days on alpha-blockage prior to surgery | 30 (0–60) | 0.004 | 0 (0–5) | <0.001 | 37 (30–62) | 0.232 | 60 (33–90) |
| Surgery, n (%) | 19 (100) | 1 | 7 (100) | 0.495 | 12 (100) | 1 | 92 (97)b |
| Laparoscopic, n (%) | 13 (68) | 0.990 | 5 (71) | 1 | 8 (67) | 0.740 | 66/92 (72) |
| Converted to open, n (%) | 2/13 (15) | 0.637 | 1/5 (20) | 0.499 | 1/8 (13) | 1 | 7/59 (12) |
| Complications, n (%) | 1 (5) | 0.187 | 0 (0) | 0.338 | 1 (8) | 1 | 18/92 (20) |
| LOS postoperative, d | 5 (2–8) | 0.824 | 5 (2–7) | 0.418 | 5.5 (2.5–9.5) | 0.757 | 4 (3–8) |
| Follow-up, y | 11.2 ± 9.4 | 0.437 | 11.5 ± 10.5 | 0.553 | 11.1 ± 9.2 | 0.556 | 9.7 ± 7.1 |
| New PCC, n (%) | 6 (32) | <0.001 | 0 (0) | 0.649 | 6 (50) | <0.001 | 4 (4) |
| Metastasis, n (%) | 0 (0) | 0.588 | 0 (0) | 1 | 0 (0) | 1 | 5 (5) |
| Dead, n (%) | 2 (11) | 1 | 0 (0) | 0.645 | 2 (17) | 1 | 13 (14) |
Boldface indicates P < 0.05; italics indicate P = 0.05–0.09.
Abbreviations: Highest level, highest U-adrenaline/P-metanephrine/U-noradrenaline/P-normetanephrine level divided the ULN; LOS postoperative, number of days admitted in hospital after surgery; NA, not applicable; P, plasma; Post-DST, serum cortisol level post 1-mg DST (i.e., at 8 AM the next morning); U, urinary; U-A/P-MNE, highest U-adrenaline or P-metanephrine level divided the upper level of normal (ULN); U-NA/P-NMNE, highest U-noradrenaline or P-normetanephrine level divided the ULN.
Tumor size of the PCC or concurrent cortical tumor.
One patient declined due to old age, one patient due to separate extensive adenocarcinoma, and one patient died prior to surgery due to multiple myeloma.
A-2. AMHs with and without concomitant PCC
Patients with AMH and concomitant PCC were younger than patients with PCC, but sex was equally distributed between the groups (Table 1). Concurrent adrenocortical adenoma was very common in patients with AMH lacking PCC (71%) but was not present in any of the patients with PCC. A genetic syndrome was present in 29% of patients with AMH without PCC (all MEN2A cases) and in 75% of patients with AMH with PCC. Tumor size was smaller in the AMH group with PCCs compared with the tumor size of patients with PCCs only. No concurrent tumor was found in 29% of patients with AMH without PCCs. The catecholamine levels were lower in both AMH subgroups, but the DST was less suppressed in only the patients with AMH without PCCs compared with patients with PCCs only.
B. Presentation and Symptoms of Patients With an AMH Compared With Patients With a PCC Without AMH
B-1. Entire AMH cohort
Of the AMHs, 37% were found serendipitously (i.e., presented as an incidentaloma) (all had had a CT scan), which was much less than in patients with PCC (Table 2). However, 42% of AMH cases were found due to screening of a genetic syndrome. Only a fifth of the AMH cases was suspected to have a PCC before biochemical testing or imaging. The AMH group had less PCC symptoms than the PCC group; however, of all individual symptoms, only orthostatic symptoms were significantly lower. Cushing-related symptoms were more often present in the AMH group (11% vs 1%; all were diagnosed biochemically with Cushing syndrome), but this did not reach statistical significance (Table 2).
Table 2.
Presentation and Symptoms of Adult Patients With AMH and its Subgroups Compared With Adult Patients With PCCs Without AMH
| All AMHs (n = 19) | P Value vs PCCs | AMHs Without PCC (n = 7) | P Value vs PCCs | AMHs With PCC (n = 12) | P Value vs PCCs | PCCs Without AMH (n = 95) | |
|---|---|---|---|---|---|---|---|
| Presentation | |||||||
| Incidentaloma, n (%) | 7 (37) | 0.025 | 3 (43) | 0.365 | 4 (33) | 0.021 | 64 (67) |
| Suspicion of PCC, n (%) | 4 (21) | 0.522 | 2 (29) | 0.798 | 2 (17) | 0.288 | 30 (32) |
| Screening, n (%) | 8 (42) | <0.001 | 2 (29) | 0.003 | 8 (67) | <0.001 | 1 (1) |
| PCC symptoms | |||||||
| Paroxysmal symptoms, n (%) | 9 (47) | 0.395 | 2 (29) | 0.198 | 7 (58) | 1 | 58 (61) |
| Headaches, n (%) | 3 (16) | 0.081 | 0 (0) | 0.088 | 3 (25) | 0.365 | 38 (40) |
| Palpitations, n (%) | 7 (37) | 0.175 | 1 (14) | 0.041 | 6 (50) | 0.761 | 54 (57) |
| Sweating, n (%) | 5 (26) | 0.234 | 3 (43) | 0.745 | 2 (17) | 0.117 | 42 (44) |
| Pallor, n (%) | 2 (11) | 0.777 | 0 (0) | 0.871 | 2 (17) | 0.357 | 9 (9) |
| Anxiety, n (%) | 8 (42) | 0.867 | 2 (29) | 0.569 | 6 (50) | 1 | 45 (47) |
| Feeling warm/flush, n (%) | 2 (11) | 0.311 | 2 (29) | 0.844 | 0 (0) | 0.066 | 23 (24) |
| Nausea, n (%) | 1 (5) | 0.226 | 1 (14) | 0.900 | 0 (0) | 0.119 | 19 (20) |
| Weight loss, n (%) | 1 (5) | 0.399 | 0 (0) | 0.588 | 1 (8) | 0.689 | 15 (16) |
| Tiredness, n (%) | 5 (26) | 0.926 | 3 (43) | 0.705 | 2 (17) | 0.507 | 27 (28) |
| Tremor, n (%) | 1 (5) | 0.457 | 0 (0) | 0.600 | 1 (8) | 0.547 | 14 (15) |
| Orthostatic symptoms, n (%) | 0 (0) | 0.037 | 0 (0) | 0.312 | 0 (0) | 0.066 | 23 (24) |
| Number of PCC symptoms | 2.0 (0.3–3.0) | 0.031 | 1.0 (0–3.0) | 0.049 | 2.0 (1.0–3.5) | 0.267 | 3.0 (1.0–5.0) |
| Classic triad, n (%) | 1 (5) | 0.195 | 0 (0) | 0.389 | 1 (8) | 0.453 | 20 (21) |
| Cushing symptoms,a n (%) | 2 (11) | 0.114 | 2 (29) | 0.003 | 0 (0) | 1 | 1 (1) |
| Asymptomatic, n (%) | 5 (26) | 0.097 | 3 (43) | 0.042 | 1 (8) | 1 | 9 (9) |
The patients screened due to familiar disease had a previously known RET mutation (MEN2A). The classic triad with paroxysmal symptoms was defined as headaches, sweating, and palpitation. Boldface indicates P < 0.05; italics indicate P = 0.05–0.09.
Symptoms that could indicate Cushing syndrome (e.g., moon face, fat deposition at the supraclavicular fossa/between the shoulders/abdomen, acne, skin atrophy, depression, fatigue, insomnia, concentration difficulties, easy bruising, proximal muscle weakness, muscle and joint pain, and amenorrhea).
B-2. AMHs with and without concomitant PCC
More AMHs, both with and without concomitant PCC, were found due to screening due to a genetic syndrome compared with the PCC group (Table 2). Less PCC symptoms were found in the AMH group without PCC than in the PCC controls; however, of all individual symptoms, only palpitations were significantly lower. More patients had Cushing symptoms, and more patients were asymptomatic in the AMH group without PCC compared with the PCC controls. However, patients with AMH with PCC had similar symptoms as patients with PCC without AMH.
C. Blood Pressure, Glycemic Disturbances, Preoperative Management, Surgery, and Outcomes in Patients With AMH Compared With Patients With PCCs Without AMH
C-1. Entire AMH cohort
At presentation, doxazosin was initiated and uptitrated in six patients (32%), phenoxybenzamine in eight (42%), and no alpha-blockade in five (26%), the latter a significantly higher percentage than in PCCs. The majority of patients with AMH had laparoscopic surgery (68%), of which 15% were converted to open surgery (Table 1), one patient (5%) had complications of surgery (pneumonia), and the length of stay after surgery was 5 days, all similar to PCCs. Four patients with AMH did not have increased catecholamine levels preoperatively, but one was started on alpha-blockers anyway because he also had suspected von Recklinghausen disease (but no NF1 mutation was found). The three others had no alpha-blockers, but one developed severe hypertension (blood pressure 250/120) during surgery; thus, surgery had to be temporally paused, and intravenous alpha-blocker in addition to intravenous adenosine were given during the rest of the operative procedure. No other complications were seen in these three cases.
At presentation, blood pressure in patients with AMH was similar to PCC controls; however, the number of antihypertensive medications was less in the AMH group (Table 3). After adrenalectomy, both systolic and diastolic blood pressure, as well as the number of blood pressure medications, decreased similar to PCCs, though the combined outcome blood pressure improvement was slightly lower in the AMH group. Diabetes had a tendency to be less common in patients with AMH prior to surgery compared with PCC controls, while prediabetes was similar as well as the disappearance of the glycemic disturbances postsurgery. The mean follow-up time was 11.2 ± 9.4 years in the AMH group, which did not differ from the PCCs (Table 1). At first presentation or at follow-up, none of the included patients with AMH had metastasis, while the development of a new PCC during follow-up was more common in AMHs than in PCCs (32% vs 4%; P < 0.001). Only two (11%) died in the AMH group, which was similar to PCCs (Table 1). Both deaths in the AMH group were considered to be unrelated to the adrenals (one alcohol-related death 12 years and one heart valve–related death 5 years, respectively, after adrenalectomy).
Table 3.
Blood Pressure and Glycemic Abnormalities in Adult Patients With AMH and its Subgroups Compared With Adult Patients With PCC Without AMH, at Diagnosis and at the First Endocrine Follow-up Outpatient Visit After Surgery
| All AMHs (n = 19) | AMHs Without PCC (n = 7) | AMHs With PCC (n = 12) | PCCs Without AMH (n = 95) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Presentation | Postoperative Visit | P Value | At Presentation | Postoperative Visit | P Value | At Presentation | Postoperative Visit | P Value | At Presentation | Postoperative Visit | P Value | |
| Systolic BP (mm Hg) | 151 ± 32 | 126 ± 14 | 0.003 | 152 ± 25 | 129 ± 19 | 0.083 | 151 ± 37 | 123 ± 11 | 0.019 | 155 ± 30 | 124 ± 14 | <0.001 |
| Diastolic BP (mm Hg) | 90 ± 16 | 78 ± 10 | 0.011 | 89 ± 7 | 83 ± 9a | 0.164 | 90 ± 19 | 76 ± 10 | 0.031 | 89 ± 14 | 76 ± 9 | <0.001 |
| BP medication | 1 (0–1)b | 0 (0–0) | 0.006 | 1 (0–1) | 0 (0–1) | 0.318 | 1 (0.5–2)c | 0 (0–0) | 0.010 | 2 (1–2) | 0 (0–1) | <0.001 |
| Improvement BP | 14 (74)a | 6 (86) | 8 (67)a | 89 (94) | ||||||||
| Diabetes, n (%) | 1 (5)c | 0 (0) | 1 | 0 (0) | 0 (0) | 1 | 1 (8) | 0 (0) | 1 | 27 (28) | 9 (9) | 0.002 |
| Diet only, n (%) | 1/1 (100) | 0 (0) | 1 | 11/27 (41) | 0/9 (0) | 0.034 | ||||||
| Prediabetes, n (%) | 5 (26) | 0 (0) | 0.046 | 1 (14) | 0 (0) | 1 | 4 (33) | 0 (0) | 0.093 | 22 (23) | 0 (0) | <0.001 |
| Any glycemic disturbance, n (%) | 6 (32) | 0 (0) | 0.020 | 1 (14) | 0 (0) | 1 | 5 (42) | 0 (0) | 0.037 | 49 (52) | 9 (9) | <0.001 |
Prediabetes was defined as HbA1c 42–47 mmol/mol and/or fasting plasma glucose 6–6.9 mmol/L and/or random plasma glucose 7.8–11 mmol/L. Boldface indicates P < 0.05; italics indicate P = 0.05–0.09.
Abbreviations: BP, blood pressure; Improvement BP, defined as reduction of systolic and diastolic BP at least 10 mm Hg together and/or reduction in BP medications.
Compared with PCCs without AMH:
P < 0.05;
P < 0.01;
P = 0.05–0.09.
C-2. AMHs with and without concomitant PCC
No alpha-blockade preoperatively was significantly more common and the number of days on alpha-blocker less in patients with AMH without PCCs (Table 1). Laparoscopic surgery, conversion to open surgery, complications, and the length of stay after surgery was similar in all groups. After adrenalectomy, improvements in blood pressure were less obvious in patients with AMH without and with PCC than in PCCs (Table 3). Mean follow-up time was similar in all groups (Table 1). Half of the patients with AMHs and concurrent PCCs had a PCC recurrence during follow-up.
D. Histological Aspects
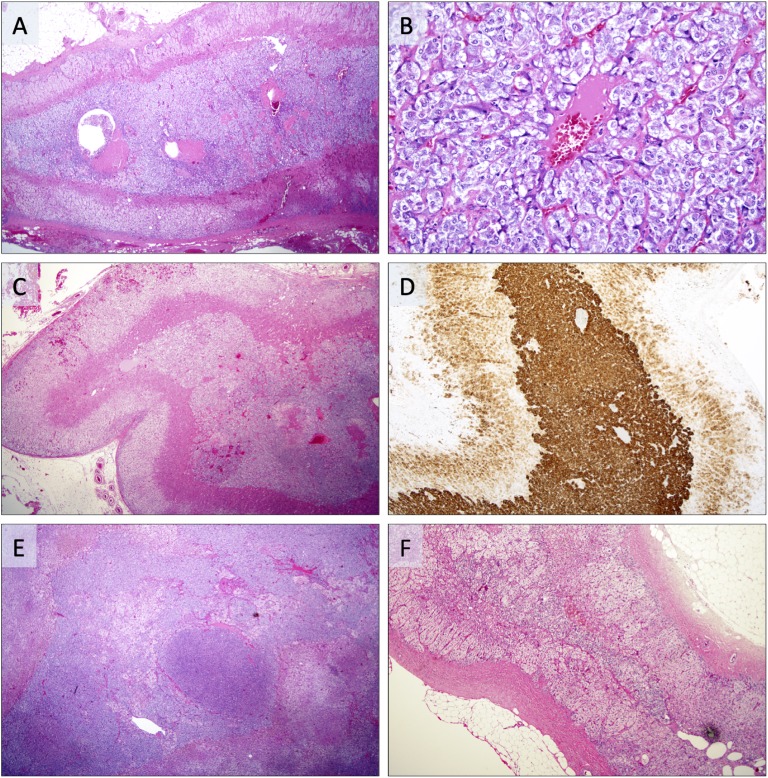
All 19 AMH cases had previously been assessed by an experienced endocrine pathologist. The histological definition of AMH has varied somewhat over the years, with an original definition as a nodular or diffuse adrenomedullary lesion measuring <1 cm in diameter, whereas larger lesions were considered a PCC [2, 3]. The current definition is based on the recently updated World Health Organization classification of endocrine tumors from 2017 [4], in which AMH is suggested when the medulla is more than a third of the adrenal thickness, in the absence of cortical atrophy. In our study, 15 patients (79%) were diagnosed prior to 2017 and therefore classified according to the older definition, whereas 4 patients (21%) were diagnosed according to the novel criteria. In one of the patients with Cushing-related symptoms, the AMH was stained for ACTH and found to be positive (histology has been previously presented [14]). Examples of histological AMH features are illustrated in Fig. 2.
Figure 2.
Photomicrographs of adrenal specimen with AMH. (A) AMH represented by a substantial widening of the adrenal medulla in a patient with synchronous PCC. (B) Same case magnified ×200, demonstrating chromaffin cells without pleomorphism arranged in small lobules. (C) Patient with MEN2A and a germline RET mutation, displaying AMH with concurrent PCC. Note the augmented widening of the medulla. (D) Same case positive for synaptophysin, with strong cytoplasmic immunoreactivity in the AMH and weaker expression in the adjacent adrenal cortex. (E) Patient with synchronous PCC displaying AMH with distinct micronodular arrangements. (F) Unrelated reference case without AMH. Note the width of the adrenal medulla compared with photomicrographs A–E.
3. Discussion
This is the largest study of AMH ever reported. In this study, we explore the frequency and compare characteristics, treatment, and outcomes for patients with AMH compared with PCC controls. The study reinforces some results of previous case reports and series but also demonstrates other findings, including mode of presentation, details on symptoms, and outcomes. Generally, there were many similarities between patients with PCCs and AMH, most probably because most patients with AMH also exhibited a concurrent PCC. Even so, several differences between these groups were detected. In general, patients with AMH were more often found due to screening of catecholamines than patients with PCC. Moreover, the levels of noradrenaline/normetanephrine were lower, while DST was less suppressed, less symptoms were present, concurrent tumors were smaller than the size of tumors in the PCC control group, fewer had been pretreated with α-blocker before adrenalectomy, improvement in blood pressure postsurgery was less, and more patients developed a new PCC during follow-up. Of the deaths recorded in the AMH group, none was adrenal related.
The average age of the patients presenting with an AMH was 47 years and in those with AMH plus PCC 44 years, which was 8 and 11 years earlier than in the AMH-devoid PCC control group. Also, other AMH cohorts have had similar age at presentation [5, 9, 11], which seems lower than the age of presentation in sporadic PCC [1]. The probable reason is that AMHs are more frequently associated with hereditary conditions, mainly MEN2 [3], which was also shown in our study. However, 20% (2 out of 11) of our hereditary AMH cases were associated with NF1 mutations, which has rarely been described previously [6]. We even had another case of suspected von Recklinghausen disease, but because no NF1 mutations could be confirmed, this case was considered a sporadic case. Establishing the diagnosis of NF1 is challenging with ∼50% having de novo mutations and complicated genetic testing [18, 20]. This may explain why previous AMH case series have not found an NF1 association similar to the results in this study.
Most of our patients with AMH were found due to screening because they exhibited a genetic syndrome. The second most common route to diagnose was in the workup of an adrenal incidentaloma and only a fifth due to symptoms of catecholamine excess. Nowadays, most PCCs are found in the workup of an incidentaloma, secondly to PCC-related symptoms and lastly due to screening [15, 16, 21, 22]. The number of different PCC-related symptoms was less in patients with AMH than in PCC controls. However, the patients with AMH with PCC had similar numbers of symptoms as the PCC group. Screening probably explains why our entire AMH group had less PCC-related symptoms than AMH-devoid patients with PCC. Moreover, the catecholamine levels, especially noradrenaline/norepinephrine, were lower than in PCCs. The catecholamine levels were particularly low among AMH patients without PCC. Others have found more elevated levels in AMHs compared with us [8–11], probably explained by the fact that their cases seemed to have been detected due to overt PCC symptoms. Of note, two of our AMH cases (11%) had symptoms and biochemical evidence of Cushing syndrome, and the DST was generally less suppressed in the AMH group compared with PCC controls. However, only the two cases with Cushing syndrome had clearly unsuppressed DST, and the others were either suppressed or slightly unsuppressed, interpreted to be falsely positive. Ectopic ACTH production from a PCC is rare, with only 1.3% of PCCs giving rise to Cushing syndrome [14], but the current study indicates that it may be more common in AMH. ACTH immunohistochemistry was performed in only one single case, displaying positive staining [14]. Especially in patients with AMH without PCC, excessive cortisol excretion seemed increased. However, this needs to be confirmed in future studies.
Alpha-blockers are the preferred choice to reduce perioperative complications in PCCs [23], and they were used in the majority of patients with AMH with good results but with lower doses and shorter duration. However, 26% of patients with AMH were not pretreated with alpha-blockers, all of these being AMHs without PCC, because AMHs was not suspected preoperatively, as the adrenalectomy was performed for an adrenocortical adenoma. Similar to patients with PCC, laparoscopic surgery was the preferred method for adrenalectomy in patients with AMH, with similar numbers requiring conversion to open surgery and having complications in addition to similar length of hospital stay postadrenalectomy. Both the case series of 7 patients with AMH by Marín et al. [9] and of 12 patients with AMH by Yang et al. [11] appeared to have comparable peri- and postoperative management as our cohort; however, surgical complications were not detailed. Thus, peri- and postoperative outcomes seem good in AMH, but if suspected preoperatively, AMH should have similar pre- and postoperative management as PCC including a laparoscopic approach.
Of all adrenalectomies performed at our center, 2.1% had a histological confirmed AMH. How common it is in other populations is unclear, but probably very rare in line with the limited number of AMH cases published. AMH was not suspected prior to surgery; the finding was always accidental because a PCC or an adrenocortical adenoma were the reasons for adrenalectomy. In the case of PCC, this may not be an issue because the perioperative management should be the same. However, in the case of concurrent adrenocortical adenoma, it can be problematic because a catecholamine-induced hypertensive crisis could occur in the absence of pretreatment with alpha-blockers and if the anesthetist is not prepared. Thus, all adrenal hormones should be investigated in all adrenal tumors to avoid any perioperative surprises; however, sometimes the hormonal investigations are not conclusive [24]. As seen in the current study, one case of catecholamine excess was not being picked up preoperatively, which led to hypertensive crisis during surgery. Moreover, if the DST is not suppressed in the preoperative investigation as discussed previously, postoperative weaning doses of hydrocortisone should be used to avoid adrenal insufficiency and adrenal crisis [25].
Hypertension, both sustained and paroxysmal, is common in patients with PCC, with up to 90% affected at diagnosis [1, 15, 21]. Among patients with AMH, the blood pressure seemed to be similarly affected, but the number of antihypertensive medications was lower at presentation compared with PCCs. Postadrenalectomy, the blood pressure improved in all patients with AMH but the composite outcome improvement of blood pressure was less compared with PCC controls; however, the number of antihypertensive medications was also less among patients with AMH preoperatively. The AMH subgroup devoid of PCC appeared to have less blood pressure improvement than those with AMH with PCC or the pure PCC control group, but the numbers were small. In our 6- to 12-month follow-up data of hypertension, the majority were normotensive, suggesting that for patients with both AMH and PCC, adrenalectomy will cure hypertension in most patients nowadays, at least short term. Others have also shown increased blood pressure at presentation in patients with AMH [9, 11], with almost normalized blood pressure during follow-up [11].
Glycemic disturbances are common at PCC presentation [1, 16, 21, 26, 27], with dramatic improvements in insulin sensitivity by adrenalectomy [16, 26, 27]. Occasional case reports have described diabetes in patients with AMH [9, 10], but with unclear improvements in glycemic control postadrenalectomy. We had one case of diet-controlled diabetes in our AMH cohort, and surgery cured the condition. Moreover, a quarter of all patients with AMH, and less in the AMH subgroup without PCC, had prediabetes, and all were cured.
The histological discrimination between benign and malignant tumors is challenging in PCC, with metastasis being the definition of a malignant PCC according to the World Health Organization [4]. By this definition, 5% of our PCC controls were malignant, but none were in the AMH group. AMH is considered a benign condition [3], and the current study confirmed this. However, most of our AMH cases had a concurrent PCC or adrenocortical tumor, which potentially could evolve into a malignancy.
Of all patients with AMH, 26% (all having a genetic syndrome) subsequently developed PCCs during follow-up, which was much more frequent than in the PCC control group. The prevalence of a family syndrome explains this difference. Two deaths (11%), both unrelated to adrenal disorders, were found in our AMH cohort. The death rates in our PCC controls were similar (14%), but the PCC-related mortality was prevalent in that group [16]. In the studies by Marín et al. [9] and Yang et al. [11], no death was reported during a follow-up of 15 months to 5 years and 6.5 years, respectively.
Like all retrospective studies, there are limitations, specifically that of ascertainment bias. Not all AMH cases had had a genetic evaluation for all new mutations that are constantly being reported in the field of PCCs [1]. However, all had a MEN2 evaluation, which is the predominant hereditary syndrome in AMH [3]. While this is the largest study of AMH, the number of cases was still quite small, especially in the subgroups; thus, the results must be interpreted with caution. Furthermore, we were not able to standardize the measurements and follow-up due to the retrospective nature of the study, but the follow-up of mortality was complete thanks to the all-inclusive National Population Register.
In conclusion, 2.1% of all adrenalectomies displayed AMH. AMH appeared mainly to be a precursor lesion of PCC, and many patients with AMH had concurrent PCCs or adrenocortical adenomas. The symptoms and signs were milder than in PCC controls, and the AMHs were predominately found due to screening or serendipitously. Short- and long-term outcomes seem favorable, but AMH needs life-long follow-up similar to PCCs [28].
Acknowledgments
Financial Support: This was an academic investigation with funding from the Magnus Bergvall Foundation.
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- AMH
adrenal medullary hyperplasia
- DST
dexamethasone suppression test
- ICD
International Classification of Diseases
- PCC
pheochromocytoma
- WHO
World Health Organization
References and Notes
- 1. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–675. [DOI] [PubMed] [Google Scholar]
- 2. Carney JA, Sizemore GW, Sheps SG. Adrenal medullary disease in multiple endocrine neoplasia, type 2: pheochromocytoma and its precursors. Am J Clin Pathol. 1976;66(2):279–290. [DOI] [PubMed] [Google Scholar]
- 3. Mete O, Asa SL. Precursor lesions of endocrine system neoplasms. Pathology. 2013;45(3):316–330. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd RV, Osamura R.Y., Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Vol 10 4th ed Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 5. Korpershoek E, Petri BJ, Post E, van Eijck CH, Oldenburg RA, Belt EJ, de Herder WW, de Krijger RR, Dinjens WN. Adrenal medullary hyperplasia is a precursor lesion for pheochromocytoma in MEN2 syndrome. Neoplasia. 2014;16(10):868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshida A, Hatanaka S, Ohi Y, Umekita Y, Yoshida H. von Recklinghausen’s disease associated with somatostatin-rich duodenal carcinoid (somatostatinoma), medullary thyroid carcinoma and diffuse adrenal medullary hyperplasia. Acta Pathol Jpn. 1991;41(11):847–856. [DOI] [PubMed] [Google Scholar]
- 7. Grogan RH, Pacak K, Pasche L, Huynh TT, Greco RS. Bilateral adrenal medullary hyperplasia associated with an SDHB mutation. J Clin Oncol. 2011;29(8):e200–e202. [DOI] [PubMed] [Google Scholar]
- 8. Romanet P, Guerin C, Pedini P, Essamet W, Castinetti F, Sebag F, Roche P, Cascon A, Tischler AS, Pacak K, Barlier A, Taïeb D. Pathological and genetic characterization of bilateral adrenomedullary hyperplasia in a patient with germline MAX mutation. Endocr Pathol. 2017;28(4):302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marín MR, Arenas MF, Valverde FM, Garaulet ET, Maderuelo MM, Avilés AM, Quirante FP, Blázquez AA. Laparoscopic adrenalectomy for nonfamilial adrenal medullary hyperplasia. JSLS. 2013;17(3):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta L, Bhatt AS, Mallya V, Khurana N, Lal P. Adrenal medullary hyperplasia with coexistent cerebral angiomas. Indian J Pathol Microbiol. 2018;61(4):587–589. [DOI] [PubMed] [Google Scholar]
- 11. Yang L, Gao L, Lv X, Qian S, Bu S, Wei Q, Yuan J, Fan T. Diagnosis and treatment of adrenal medullary hyperplasia: experience from 12 cases. Int J Endocrinol. 2014;2014:752410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017;58(2):267–275. [DOI] [PubMed] [Google Scholar]
- 13. Falhammar H, Frisén L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjöld A, Nordenström A. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520–3528. [DOI] [PubMed] [Google Scholar]
- 14. Falhammar H, Calissendorff J, Höybye C. Frequency of Cushing’s syndrome due to ACTH-secreting adrenal medullary lesions: a retrospective study over 10 years from a single center. Endocrine. 2017;55(1):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falhammar H, Kjellman M, Calissendorff J. Initial clinical presentation and spectrum of pheochromocytoma: a study of 94 cases from a single center. Endocr Connect. 2018;7(1):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falhammar H, Kjellman M, Calissendorff J. Treatment and outcomes in pheochromocytomas and paragangliomas: a study of 110 cases from a single center. Endocrine. 2018;62(3):566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stenman A, Svahn F, Hojjat-Farsangi M, Zedenius J, Söderkvist P, Gimm O, Larsson C, Juhlin CC. Molecular profiling of pheochromocytoma and abdominal paraganglioma stratified by the PASS algorithm reveals chromogranin B as associated with histologic prediction of malignant behavior. Am J Surg Pathol. 2019;43(3):409–421. [DOI] [PubMed] [Google Scholar]
- 18. Welander J, Andreasson A, Juhlin CC, Wiseman RW, Bäckdahl M, Höög A, Larsson C, Gimm O, Söderkvist P. Rare germline mutations identified by targeted next-generation sequencing of susceptibility genes in pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2014;99(7):E1352–E1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calissendorff J, Calissendorff F, Falhammar H. Adrenocortical cancer: mortality, hormone secretion, proliferation and urine steroids - experience from a single centre spanning three decades. BMC Endocr Disord. 2016;16(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longo JF, Weber SM, Turner-Ivey BP, Carroll SL. Recent advances in the diagnosis and pathogenesis of neurofibromatosis type 1 (NF1)-associated peripheral nervous system neoplasms. Adv Anat Pathol. 2018;25(5):353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110–2116. [DOI] [PubMed] [Google Scholar]
- 22. Gruber LM, Hartman RP, Thompson GB, McKenzie TJ, Lyden ML, Dy BM, Young WF Jr, Bancos I. Pheochromocytoma characteristics and behavior differ depending on method of discovery. J Clin Endocrinol Metab. 2019;104(5):1386–1393. [DOI] [PubMed] [Google Scholar]
- 23. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 24. Patrova J, Jarocka I, Wahrenberg H, Falhammar H. Clinical outcomes in adrenal incidentaloma: experience from One Center. Endocr Pract. 2015;21(8):870–877. [DOI] [PubMed] [Google Scholar]
- 25. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises: perspectives and research directions. Endocrine. 2017;55(2):336–345. [DOI] [PubMed] [Google Scholar]
- 26. Wiesner TD, Blüher M, Windgassen M, Paschke R. Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. J Clin Endocrinol Metab. 2003;88(8):3632–3636. [DOI] [PubMed] [Google Scholar]
- 27. Komada H, Hirota Y, So A, Nakamura T, Okuno Y, Fukuoka H, Iguchi G, Takahashi Y, Sakaguchi K, Ogawa W. Insulin secretion and insulin sensitivity before and after surgical treatment of pheochromocytoma or paraganglioma. J Clin Endocrinol Metab. 2017;102(9):3400–3405. [DOI] [PubMed] [Google Scholar]
- 28. Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O; Guideline Working Group. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. European journal of endocrinology / European Federation of Endocrine Societies. 2016;174(5):G1–G10. [DOI] [PubMed] [Google Scholar]