Abstract
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder characterized by hyperandrogenism and chronic anovulation. Depending on diagnostic criteria, 6% to 20% of reproductive aged women are affected. Symptoms of PCOS arise during the early pubertal years. Both normal female pubertal development and PCOS are characterized by irregular menstrual cycles, anovulation, and acne. Owing to the complicated interwoven pathophysiology, discerning the inciting causes is challenging. Most available clinical data communicate findings and outcomes in adult women. Whereas the Rotterdam criteria are accepted for adult women, different diagnostic criteria for PCOS in adolescent girls have been delineated. Diagnostic features for adolescent girls are menstrual irregularity, clinical hyperandrogenism, and/or hyperandrogenemia. Pelvic ultrasound findings are not needed for the diagnosis of PCOS in adolescent girls. Even before definitive diagnosis of PCOS, adolescents with clinical signs of androgen excess and oligomenorrhea/amenorrhea, features of PCOS, can be regarded as being “at risk for PCOS.” Management of both those at risk for PCOS and those with a confirmed PCOS diagnosis includes education, healthy lifestyle interventions, and therapeutic interventions targeting their symptoms. Interventions can include metformin, combined oral contraceptive pills, spironolactone, and local treatments for hirsutism and acne. In addition to ascertaining for associated comorbidities, management should also include regular follow-up visits and planned transition to adult care providers. Comprehensive knowledge regarding the pathogenesis of PCOS will enable earlier identification of girls with high propensity to develop PCOS. Timely implementation of individualized therapeutic interventions will improve overall management of PCOS during adolescence, prevent associated comorbidities, and improve quality of life.
Keywords: adolescence, diagnosis, hyperandrogenism, polycystic ovary syndrome, neuroendocrinology, treatment
The female hypothalamic–pituitary–ovarian (HPO) axis is a meticulously synchronized and tightly regulated network ultimately responsible for reproductive competence and survival of the species. The HPO axis responds to internal signals (i.e., hormonal and neuronal) and external factors (i.e., environment influences). Beginning during gestation, these factors impact future generations through epigenetic factors affecting the brain and the developing germ cells [1].
Polycystic ovary syndrome (PCOS), a disorder primarily characterized by signs and symptoms of androgen excess and ovulatory dysfunction, disrupts HPO axis function. Depending on diagnostic criteria, this disorder affects ∼6% to 20% of reproductive aged women [2, 3]. Typical clinical features include hirsutism, irregular menses, chronic anovulation, and infertility. The persistent hyperandrogenism is associated with impaired hypothalamic–pituitary feedback, LH hypersecretion, premature granulosa cell luteinization, aberrant oocyte maturation, and premature arrest of activated primary follicles [4].
1. Pathophysiology
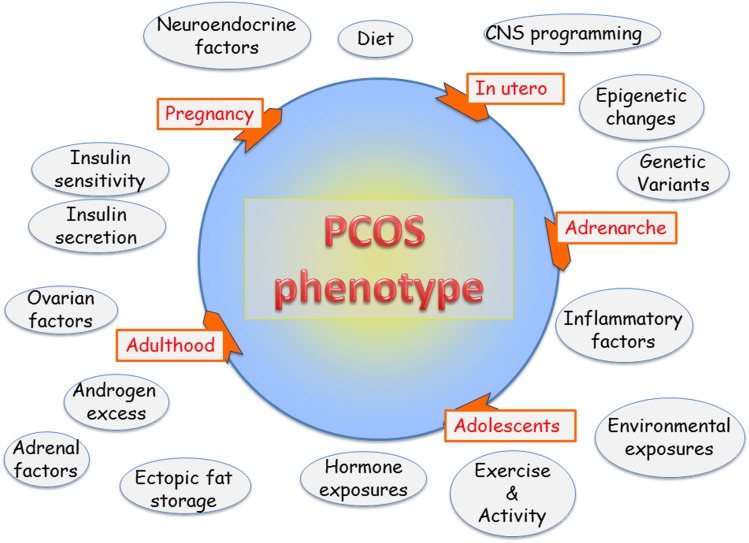
By the time the diagnosis is established, PCOS presents as a phenotype reflecting a self-perpetuating vicious cycle involving neuroendocrine, metabolic, and ovarian dysfunction. Over the years, numerous hypotheses have been proposed regarding the proximate physiologic origins for PCOS. PCOS reflects the interactions among multiple proteins and genes influenced by epigenetic and environmental factors (Fig. 1) [5]. Specific sections of this article deconstruct the factors contributing to the development of PCOS in humans and preclinical models. Clinical and biochemical hyperandrogenism are major features of PCOS.
Figure 1.
Factors contributing to PCOS phenotype. PCOS encompasses a woman’s life cycle. Factors potentially impacting the pathophysiology of PCOS are shown in circles. Not all factors affect each individual. PCOS epitomizes a biologic network of interacting neuroendocrine, hormonal, metabolic, genetic, and environmental influences.
PCOS develops during the early pubertal years [6]. However, most relevant information has been accrued through clinical studies involving adult women in which referral bias focuses on investigation of the more severe phenotypes [7]. Preclinical models involving animal and in vitro studies supplement clinical investigation and benefit from other approaches to study this complex disorder. Recent clinical, experimental, and genetic data emphasize neuroendocrine involvement in the pathophysiology of PCOS.
A. Ovary, Adrenal, and Androgen Excess
PCOS is characterized by excessive ovarian and/or adrenal androgen secretion. Intrinsic ovarian factors such as altered steroidogenesis and factors external to the ovary such as hyperinsulinemia contribute to the excessive ovarian androgen production. Characteristic features include more growing follicles in women with PCOS compared with normal controls with premature growth arrest of antral follicles at 5 to 8 mm. The classic ovarian phenotype of enlarged ovaries with string-of-pearl morphology and theca interstitial hyperplasia reflects androgen exposure; this morphology has also been observed in women with congenital adrenal hyperplasia (CAH) and female-to-male transgender individuals [8]. Distorted interactions among the endocrine, paracrine, and autocrine factors responsible for follicular maturation may contribute to ovarian dysregulation in PCOS.
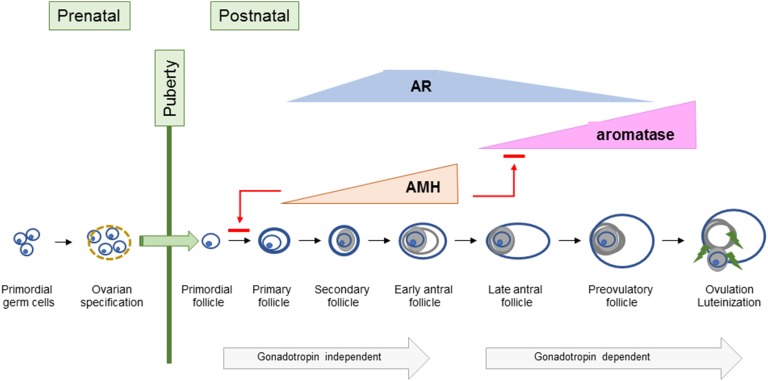
The stages of follicular maturation are briefly reviewed (Fig. 2). Developing during gestation, primordial follicles are comprised of meiotically arrested oocytes surrounded by pregranulosa cells. Hence, a woman’s ovaries have been exposed to the ambient maternal environment during gestation. Ovaries are relatively quiescent until the onset of puberty. Detailed knowledge regarding follicular morphology in prepubertal and early pubertal ovaries is lacking. Ovarian tissue obtained from prepubertal and early pubertal girls shows differences in follicle morphology and growth potential. Specifically, prepubertal ovaries contain a high proportion of abnormal nongrowing follicles, which are not found in pubertal ovaries [9]. The physiologic relevance of this finding is unclear.
Figure 2.
Ovarian follicle development. This illustration shows ovarian follicular development during developmental periods.
The precise signaling mechanisms initiating follicular activation are poorly understood. Presumably a balance of factors influences the options—continuation in a resting state or activation. One such factor appears to be follicle density [10]. Following activation from the resting pool, initial follicular growth is gonadotropin-independent until the antral stage.
Anti-Müllerian hormone (AMH), a glycoprotein secreted by granulosa cells, inhibits initial follicular recruitment and indicates follicular reserve. In contrast to mice where AMH inhibits preantral follicle growth and antral follicle maturation, AMH appears to promote growth of preantral follicles to the antral stage in nonhuman primate (NHP) ovaries [11, 12]. Peak AMH concentrations are found in antral follicles. Once FSH-stimulated granulosa cell estradiol concentrations achieve the necessary threshold, estradiol suppresses AMH expression [13].
Despite prior assumptions that androgens negatively impact follicles, androgens synthesized in preantral follicle theca cells promote growth of preantral and antral follicles and induce granulosa cell FSH receptor (FSHR) expression in early antral follicles [14]. Androgens promote aromatase expression and, ultimately, LH/chorionic gonadotropin receptor (LHCGR) expression in granulosa cells. As a follicle matures, androgens appear to inhibit proliferation and promote apoptosis. This biphasic androgen action was initially demonstrated in an NHP, the marmoset; androgens augmented FSH action in small antral follicles but had an inhibitory effect in larger follicles [15].
Androgen actions are mediated by androgen receptors (ARs), which are expressed in theca cells, granulosa cells, oocytes, and stromal cells [16]. Both canonical androgen signaling where AR functions as ligand-dependent transcription factor and nongenomic signaling occur. Peak AR gene expression occurs in small antral follicles (∼6 mm in diameter) and decreases in antral and preovulatory follicles [17].
Typically, one follicle is “selected” as the dominant follicle [18]. With increasing estrogen secretion, pituitary FSH secretion declines due to negative feedback. The dominant follicle compensates for this loss of FSH stimulation through increased LHCGR expression and increased responsiveness to LH stimulation. Subordinate follicles undergo atresia, presumably due to relative FSH deficiency and androgen excess. Upon achieving a sufficient estradiol concentration, neuroendocrine mechanisms trigger the LH surge to induce ovulation.
Under normal circumstances, the ovarian stroma provides a structural framework undergoing dynamic changes to support follicular growth. However, the ovarian stroma from women with PCOS tends to be more rigid. The developing oocyte and its surrounding scaffolding rely on endocrine, paracrine, and autocrine signaling mechanisms to maintain cell-to-cell communication and assure synchronized developmental progression. Aberrant development during these earliest stages of follicular growth likely contributes to the ovarian aspects of PCOS [19]. Another feature of PCOS ovaries is accelerated transition from primordial to growing follicles with increased numbers of 2- to 3-mm and 3- to 4-mm follicles [20, 21]. AMH concentrations correlate with the number of these small antral follicles [22]. The growing follicle is exposed to an atypical environment with increased LH, insulin, androgen, and AMH concentrations accompanied by insufficient FSH concentrations [19]. Additional differences in PCOS ovaries include factors impacting vascular function and immune responsiveness [23].
Additionally, intrinsic alterations in ovarian steroidogenesis likely contribute to excessive ovarian androgen production. Available data document constitutively increased androgen production and CYP17A1 expression in cultured theca cells isolated from PCOS ovaries [24–26]. Steroidogenesis in the ovary involves both theca and granulosa cells. The theca cells produce ovarian androgens, which are converted to estrogens in the granulosa cell due to the actions of FSH-stimulated aromatase.
One interesting locus identified through genetic studies is DENND1A (see “H. Genetics” below). Overexpression of the alternative spliced variant, DENND1A.V2, of this gene recapitulated a PCOS phenotype in cultured theca cells obtained from normal women, indicating a role for this variant in the excessive theca cell androgen production [27]. Overexpression of DENND1A.V2 in an adrenal cell line led to increased expression of the mRNAs for CYP17A1 and CYP11A1. However, the mechanisms responsible for increased expression of this alternatively spliced variant of DENND1A remain to be elucidated [28].
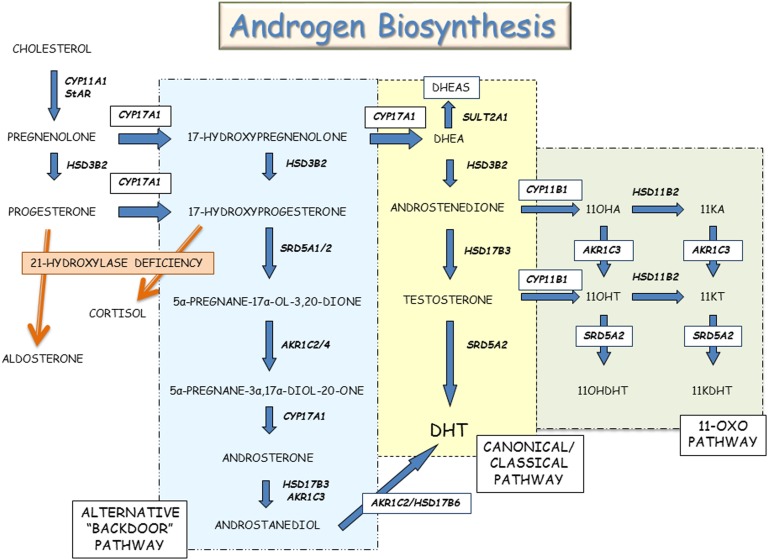
The adrenal zona reticularis is responsible for biosynthesis of the C-19 adrenal androgens, including dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), androstenedione, and testosterone. At least three distinct adrenal pathways contribute to androgen synthesis: (i) canonical/classical, (ii) “alternative backdoor,” and (iii) 11-oxo-androgens (Fig. 3). In the canonical/classical pathway, progesterone is successively transformed by the enzyme 17α-hydroxylase/17,20-lyase (P450c17) to DHEA, which is subsequently converted by 3β-hydroxysteroid dehydrogenase type 2 to androstenedione. The alternative backdoor pathway bypasses the usual steroid hormone intermediates, DHEA, androstenedione, and testosterone, to produce dihydrotestosterone [29]. This pathway likely contributes to virilization of the external genitalia among girls with classical CAH and normal male external genital development [30, 31]. The extent of the contribution of the alternative backdoor pathway to adrenal and ovarian steroid biosynthesis in PCOS is unclear [32].
Figure 3.
Androgen biosynthesis. This illustration shows the classical/canonical, alternative backdoor, and 11-oxo-steroid pathways for androgen biosynthesis.
The adrenal steroidogenic enzyme, 11β-hydroxylase (P450c11B1), encoded by CYP11B1, is expressed in both zona fasciculata and zona reticularis; this enzyme converts androstenedione and testosterone to their respective 11β-hydroxyl derivatives, 11β-hydroxy-androstenedione and 11β-hydroxy-testosterone. Both 11β-hydroxy-androstenedione and 11β-hydroxy-testosterone can be converted to their 11-keto counterparts, 11-ketoandrostenedione and 11-ketotestosterone. Testosterone, DHT, 11-ketotestosterone, and 11-ketodihydrotestosterone bind to the human AR and promote AR-regulated gene expression [33]. The concentrations of these 11-oxygenated steroids were reported to be higher in women with PCOS than among healthy premenopausal women [34]. Urinary steroid profiling using 24-hour urine collections in a relatively small number of women with PCOS and controls found androstanediol concentrations to discriminate between PCOS and controls; the overall pattern of steroid hormone excretion indicated enhanced androgen biosynthesis via canonical/classical, alternative backdoor, and 11-oxygenated steroid pathways rather than a specific steroid enzyme disorder [35].
A-1. Preclinical models
One unresolved conundrum regarding folliculogenesis is the regulation and interrelationships between androgens and AMH. Androgens and AMH are essential for normal cyclic ovulation. Using short hairpin RNAs to decrease AMH expression in macaques, preantral follicle growth and survival were reduced. Cotreatment with supplemental AMH overcame these effects. These results emphasize the important role of AMH as a critical factor to promote preantral follicle survival and growth in primates [36]. AMH treatment of cultured antral stage rhesus macaque follicles decreased estradiol production compared with untreated follicles despite similar follicle size [12]. Hence, AMH appears to have a dual role: whereas AMH promotes preantral follicle survival, it negatively impacts later stages of antral follicle maturation [12].
In a series of in vivo and in vitro experiments, a subset of hypothalamic GnRH neurons, both mouse and human, were shown to express AMR receptors; AMH treatment increased GnRH-dependent LH pulsatility and secretion [37]. Using a mouse model, AMH treatment of pregnant mice was associated with diminished placental metabolism of testosterone to estradiol, decreased aromatase expression, masculinization of exposed female offspring, estrus cycle disturbances, increased LH pulse frequency, brain masculinization, and infertility compared with unexposed mice; postnatal GnRH antagonist treatment reversed this PCOS-like phenotype [38]. These data suggest that excessive prenatal AMH exposure could promote the aberrant neuroendocrine function typical of PCOS and that AMH can modulate GnRH neuron function [37, 38].
Knockout mice have been used to explore consequences of gene deletions. Using a postnatal androgen PCOS model, global AR knockout mice were protected from DHT-induced PCOS-like features. Curiously, neuron-specific AR knockout mice were protected from DHT-induced ovarian dysfunction and several metabolic traits, reinforcing a role for extraovarian tissues in the pathophysiology of PCOS [39]. Hence, intricate interrelationships exist between androgens, AMH, follicle growth, metabolism, and neuroendocrine factors in PCOS.
Recently, a group of naturally hyperandrogenic female rhesus monkeys have been described. The high testosterone animals had increased LH, AMH, and androstenedione concentrations. Additionally, five of the six high-testosterone monkeys had no live offspring [40]. Future study of these animals will provide insight into the pathogenesis of PCOS.
B. Neuroendocrine Factors
Increased LH pulse frequency, LH pulse amplitude, and increased LH/FSH ratios are described in women with PCOS. The initial features of PCOS emerge during the early pubertal years, concomitant with reactivation of the hypothalamic GnRH pulse generator, increased gonadotropin secretion, and subsequent increased ovarian estrogen production. Loci identified in the genome-wide association studies (GWASs) studies include LHCGR, FSHR, and FSH-β polypeptide (FSHB) genes, emphasizing neuroendocrine contributions to PCOS pathophysiology (see H. Genetics below).
Hypothalamic neurons in the arcuate nucleus secrete kisspeptin, neurokinin B, and dynorphin. These neurons, labeled as the KNDy neurons, are the leading contenders for the hypothalamic GnRH pulse generator because of the colocalization of these three peptides and their roles in episodic GnRH secretion [41]. Rather than initiating puberty, the GnRH pulse generator and GnRH neurons represent downstream nodes modulated by other hormones and neurosecretory factors [42]. In other words, activation of excitatory inputs and inactivation of inhibitory inputs moderated by multiple influences regulate the output of the GnRH pulse generator to govern the timing of puberty [43–45]. This process culminates in increased GnRH and gonadotropin secretion.
The hypothalamic GnRH neurons secrete GnRH in discrete pulses that travel through the median eminence to the pituitary gonadotrophs, resulting in pulsatile LH and FSH secretion [46]. LH and FSH pulse frequencies are modulated by GnRH pulse frequency. Increased GnRH pulse frequency increases LH pulse frequency and decreases FSH pulse frequency [47]. The GnRH neurons integrate diverse influences, decode metabolic signals, and serve as the output “managers” of the HPO axis [48, 49].
Increased LH pulse amplitude and pulse frequency observed in PCOS are likely driven by increased pulsatile GnRH secretion. Manipulation of the hypothalamic kisspeptin–neurokinin B–GnRH pathway with an NK3 receptor antagonist, AZD4901, reduced serum LH pulse frequency and, subsequently, serum LH and testosterone concentrations. These data suggest the possibility of targeting neuroendocrine pathophysiology to treat HPO axis dysfunction in PCOS [50].
GnRH neurons express estrogen receptor-β, but they do not express AR, progesterone receptor, or estrogen receptor-α. Hence, steroid-mediated negative feedback is indirect and is mediated through the hypothalamic neuronal network upstream of the GnRH neuron. This negative feedback mechanism is impaired in some women with PCOS who appear to require higher progesterone and estradiol concentrations. This effect can be abrogated with androgen antagonist treatment [51].
One conundrum is that LH hypersecretion is less obvious in women with obesity with PCOS. Although GnRH and LH pulses generally exhibit a 1:1 ratio, preclinical data exist suggesting that a faster GnRH pulse frequency may be associated with decreased LH secretion [52]. Potential explanations for this mismatch between GnRH and LH pulses include the longer half-life of LH obscuring pulse detection, exhaustion of the pituitary pool of readily releasable LH, or lower amplitude GnRH pulses [53]. Measurement of circulating kisspeptin and LH concentrations showed temporal kisspeptin–LH pulse coupling in eumenorrheic women with PCOS; however, a greater frequency of kisspeptin pulses was associated with a loss of temporal coupling in women with oligomenorrhea with PCOS [54]. This study identified dissociated coupling of kisspeptin and LH pulses in women with oligomenorrhea with PCOS.
Tanycytes are specialized nonciliated cells lining the floor of the third ventricle. These polarized cells contribute to regulation of reproduction and metabolism in the median eminence. Specifically, tanycytes affect GnRH secretion, generate active forms of thyroid hormone, and influence exchange of signaling factors such as leptin between the blood and hypothalamic extracellular fluid [55]. Dynamic structural remodeling of tanycytes modulates GnRH neuron access to the pituitary portal system. Leptin and ghrelin enter the hypothalamus through the tanycytes [56]. Astrocytes, located at the interface between blood vessels and neurons, can function as metabolic sensors. This physical location enables them to modulate glucose fluxes between the periphery and the central nervous system [57]. Hence, dynamic tanycyte–neuron interactions and astrocytes orchestrate the ongoing communication between the neuroendocrine axis and the periphery [58]. Whereas the precise role of tanycytes in PCOS is indeterminate, these cells likely allow leptin, ghrelin, and AMH access to GnRH neurons.
B-1. Preclinical models
Numerous studies have described the development of neuroendocrine features reminiscent of PCOS following prenatal androgen exposure in rodents, sheep, and rhesus macaques [59–61]. Prenatal androgen exposure during early gestation (late first to second trimester) increased LH and androgen secretion in female rhesus monkeys [59]. Prenatally androgenized (PNA) female mice showed increased γ-aminobutyric acid (GABA)ergic transmission to GnRH neurons by 3 weeks of age, suggesting that prenatal androgen treatment affected neuronal development [62]. Questions regarding prenatal imprinting of the neuroendocrine components of the HPO axis persist.
C. Valproate and HPO Axis Function
Valproic acid (VPA), a branched short-chain fatty acid derived from valeric acid, is used to treat epilepsy, bipolar disorders, and prevent migraine headaches. VPA increases GABA levels by interfering with GABA degradation pathways [63]. GnRH neurons express both GABAA and GABAB receptors, implicating GABA signaling in the regulation of GnRH secretion. Signaling through the GABAA receptor can elicit an excitatory effect on GnRH neurons [64].
Women treated with VPA can develop PCOS-like symptoms. Lean women with PCOS had significantly higher CSF GABA concentrations compared with eumenorrheic lean control women; the women with PCOS also demonstrated increased LH pulse amplitude and LH pulse frequency on frequent blood sampling [65]. These clinical observations suggest that GABA signaling could influence the neuroendocrine changes associated with PCOS such as LH pulse frequency.
C-1. Preclinical models
PNA mice models have enabled investigation regarding the consequences of prenatal androgen exposure. In an elegant series of experiments, Silva et al. [66] demonstrated increased GABA synaptic input in prepubertal PNA mice. Their observations suggest that prenatal androgenization is associated with prenatal enhanced GABAergic structural wiring input onto GnRH neurons, that these changes are reversible with long term antiandrogen treatment, and that these structural changes precede postpubertal development of PCOS features [66].
D. Insulin Resistance, Hyperinsulinemia, and the β-Cell
The phenotype of female patients with insulin receptor gene mutations includes insulin resistance (IR), compensatory hyperinsulinemia, and hyperandrogenism [67]. Although IR and hyperinsulinemia are commonly detected in women with PCOS, insulin receptor gene mutations are extremely rare among women with PCOS.
Women with PCOS have intrinsic IR independent of the extent of obesity and magnitude of androgen concentrations [68]. Even lean women with PCOS manifest IR; increasing body mass index (BMI) exacerbates IR [69]. Normal-weight adolescent girls with PCOS have peripheral IR, increased liver fat, and muscle mitochondrial dysfunction compared with normal-weight girls [70].
Insulin is the hormone primarily responsible for glucose homeostasis and lipogenesis. In addition to its effects on carbohydrate, fat, and protein metabolism, insulin functions as a mitogenic hormone. Insulin actions are mediated by insulin receptors, which are found in numerous tissues of the HPO axis. In steroidogenic tissues such as the ovary and the adrenal cortex, insulin potentiates the cognate trophic hormones to promote steroidogenesis. The compensatory hyperinsulinemia associated with IR provokes excessive ovarian/adrenal androgen secretion and decreases hepatic SHBG synthesis with the net result of increasing circulating testosterone concentrations. This leads to the paradox of insulin signaling in PCOS; liver, skeletal muscle, and adipose tissue exhibit IR, whereas steroid-producing tissues and the pituitary retain insulin sensitivity [71, 72]. This paradox is illustrated by differences in insulin actions in granulosa–lutein cells obtained from women with anovulation with PCOS; insulin-stimulated glucose uptake is impaired whereas insulin-stimulated progesterone production is preserved [73].
The central role of compensatory hyperinsulinemia has been established by improved clinical features with insulin-sensitizing medications and weight loss. The transient IR and hyperinsulinemia typical of early puberty may kindle the factors associated with development of PCOS [74, 75].
The prevalence of the metabolic syndrome defined as obesity, hypertension, dyslipidemia, and hyperglycemia is approximately threefold higher in women with PCOS [76]. Although a consensus definition of metabolic syndrome in adolescents is lacking, published pediatric criteria are based on adult criteria and include a combination of elevated triglyceride concentration, elevated low high-density lipoprotein cholesterol concentration, fasting blood glucose ≥110 mg/dL, increased waist circumference, and hypertension for age [77]. A meta-analysis suggested that although IR is likely a common factor linking the metabolic and reproductive features of PCOS, the metabolic and reproductive features develop through independent mechanisms [78]. One relatively consistent finding is that obesity exacerbates the symptoms of PCOS, especially regarding the risk for development of T2D and the metabolic syndrome [76].
Primary hyperinsulinemia can precede the development of peripheral tissue IR. It is beyond the scope of this review to discuss arguments supporting the opposing viewpoints, that is, primary IR vs primary hyperinsulinemia [79]. Importantly, numerous genetic and epigenetic factors, nonheritable prenatal and extrauterine environmental influences, and varying adaptations to nutrient excess likely contribute to the development of IR and hyperinsulinemia.
D-1. Preclinical models
Preclinical data show β-cell dysfunction associated with hyperinsulinemia in monkeys and sheep prenatally exposed to androgens [80, 81].
E. Obesity, the Adipocyte, and Nutrient Excess
Overweight and obesity are common among adolescent girls and adult women with PCOS. In response to nutrient excess, adipocytes can enlarge (hypertrophy) or form new adipocytes (hyperplasia). According to the adipose tissue expandability hypothesis, adipocyte hypertrophy establishes a microenvironment characterized by hypoxia, proinflammatory cytokine secretion, free fatty acid “spillover,” macrophage invasion, and IR [82]. IR decreases suppression of adipocyte lipolysis, resulting in increased serum free fatty acids and triglycerides, ultimately leading to increased hepatic de novo lipogenesis and hyperlipidemia [83]. Another consequence is increased fat storage in skeletal muscle, liver, and pancreas because the adipose tissue capacity to store lipid is exceeded. In the liver, ectopic fat storage is labeled hepatic steatosis, which can develop into nonalcoholic fatty liver disease [84].
White adipose tissue has several distinct locations, that is, visceral and subcutaneous. Partitioning of fat among different storage sites influences metabolic consequences: increased abdominal fat is associated with greater risk for dysglycemia and cardiovascular disease. Investigation of normal-weight women with PCOS showed increased total abdominal fat mass due to preferential deposition of intra-abdominal fat with an increased population of small subcutaneous abdominal adipocytes [85]. In a pilot study involving normal-weight women with PCOS, subcutaneous adipose IR correlated with serum androgen concentrations and the percentage of small subcutaneous abdominal adipocytes. These data support the hypothesis that expansibility of the subcutaneous abdominal adipose depot is limited and unable to expand sufficiently to meet the metabolic needs for most normal-weight women with PCOS [86]. Emerging pilot data in adolescent girls with PCOS showed that reduction of visceral fat improved menstrual irregularity [87].
In a small cross-sectional study, girls related to women with PCOS showed higher 17-hydroxyprogesterone concentrations, decreased insulin sensitivity, and decreased insulin-induced suppression of nonesterified fatty acid concentrations compared with healthy control girls. These findings suggest onset of adipocyte dysfunction, IR, and possible lipotoxicity among girls aged ∼9 to 15 years [88]. In another small study using frequently sampled IV glucose tolerance tests, the authors reported early β-cell dysfunction in first-degree female relatives with overweight/obesity of women with PCOS compared with control girls with overweight/obesity [89]. Small sample sizes limit the conclusions that can be drawn from these studies. Nevertheless, the studies hint that β-cell function and insulin sensitivity may differ beginning in childhood and early adolescent years among girls “destined” to develop PCOS.
Mismatches between prenatal and postnatal weights have led to the advance of the developmental origins of disease hypothesis [90]. The longitudinal prospective population-based study (Northern Finland Birth Cohort Study) found that women with PCOS had lower birth weights, experienced adiposity rebounds at younger ages, and had higher subsequent BMI values [91]. These findings are consistent with the concept that a mismatch between prenatal weight and postnatal weight gain is associated with increased risk for PCOS, ectopic fat storage, and hepatic steatosis [92–94].
Adipose tissue expresses enzymes that activate and inactivate androgen precursors. The enzyme aldo-ketoreductase type 1C, encoded by the AKR1C3 gene, is expressed in adipose tissue and converts the preandrogen androstenedione to testosterone. Additionally, the enzyme 5α-reductase type 1, encoded by the SRD5A1 gene, converts testosterone to DHT and is expressed in adipose tissue. A deep in vivo metabolic phenotyping study showed increased AKR1C3 and decreased SRD5A1 mRNA expression in subcutaneous fat of women with PCOS [95]. Activation appears to regulate adipocyte proliferation and differentiation, insulin sensitivity, adipokine signaling, and lipid metabolism [96]. Using a human preadipocyte cell line, both testosterone and DHT increased de novo lipogenesis in the absence of insulin [95], whereas pharmacologic inhibition of AKR1C3 activity prevented androgen-mediated adverse effects on adipocyte lipogenesis. Using this model system, insulin increased AKR1C3 expression. Based on these data, O’Reilly et al. [95] proposed the existence of a vicious cycle linking adipocyte androgen biosynthesis and adipocyte lipid accumulation to IR and hyperinsulinemia.
Another situation demonstrating androgen effects on lipid metabolism was described in girls with obesity with and without PCOS. Girls with obesity with PCOS compared with those without PCOS demonstrated decreased lipid mobilization, diminished fat oxidation, and impaired ability to switch from lipid to carbohydrate oxidation during insulin stimulation (metabolic inflexibility) [97].
E-1. Preclinical models
In a treatment paradigm comparing a high-fat diet with/or without testosterone treatment in rhesus monkeys, the combination of a high-fat diet and testosterone treatment accelerated development of white adipose tissue dysfunction [98].
F. Developmental Hypothesis/Fetal Origins
The developmental theory of PCOS proposes that exposure of the female fetus to elevated androgen concentrations contributes to the development of PCOS. Potential mechanisms include effects on steroidogenesis, insulin signaling, pancreatic β-cell function, hypothalamic–pituitary organization, neuroendocrine secretory patterns, and epigenetic modifications [99].
Fetal, neonatal, prepubertal, and/or pubertal ovaries may be genetically predisposed to increased androgen secretion [100, 101]. Women with classical CAH often develop a secondary PCOS phenotype; it is unclear whether this reflects prenatal imprinting of the hypothalamus and GnRH pulse generator or androgen effects on the ovary [102]. Available data support the hypothesis that prenatal androgen exposure programs the neuroendocrine, metabolic, and reproductive manifestations of PCOS [103]. Women with PCOS typically have higher androgen concentrations than do women without PCOS. One report involving 23 mothers self-reporting PCOS and 277 women reporting no PCOS indicated increased anogenital differences, a marker of prenatal androgen exposure, in daughters of women with PCOS [104]. How the fetus is exposed to androgen excess when placental aromatase and maternal SHBG limit fetal exposure to maternal androgens remains an enigma.
F-1. Preclinical models
Preclinical models involving androgen exposure in rodents, sheep, and NHPs recapitulate features of PCOS. Impaired adipocyte differentiation has been demonstrated in NHP models [105]. Among prenatally androgenized NHPs, when the capacity of subcutaneous adipocytes to store fat is exceeded, excess free fatty acids may be deposited in ectopic locations such a liver and muscle; consequences of ectopic fat deposition may include impaired tissue hypoxia, inflammation, and IR [106]. Curiously, transient pancreatic dysfunction manifested by hypoglycemia, an increased number of β-cells, small islets, and relative hyperinsulinemia have been observed in this NHP model of early gestational androgen exposure [80]. Early pubertal NHP treated with testosterone and a “Western style diet” with increased fat content showed increased larger visceral adipocytes, greater IR, and ectopic fat storage [106].
G. Microbiome
Bacteria, archaea, fungi, and viruses comprise the microbial community or microbiome of the gastrointestinal tract. These organisms play roles in fermentation of dietary fiber, bile acid metabolism, host defense, and modulation of metabolism. It has been suggested that the gut microbiome influences development of nonalcoholic fatty liver disease and is associated with insulin sensitivity [107, 108]. Sex and sex steroids modulate the composition of the gut microbiome. Women are reported to show greater α-diversity. α-Diversity represents the number of species, and β-diversity indicates similarity between samples. Decreased α-diversity has been described in women with PCOS [109, 110]. Numerous questions remain to be answered regarding the functional relationships, if any, between sex steroids, metabolic dysregulation, and the gut microbiome [111]. To the best of our knowledge, no data for adolescents are available.
H. Genetics
Twin studies suggest that the hereditability is ∼70% [112]. The few identified genetic loci explain only a modest proportion of estimated hereditability. GWASs involving women of Han Chinese and European origins have identified at least 16 susceptibility loci for PCOS [113–116]. Several genetic variants are similar in both Han Chinese and European populations, implying that PCOS is an ancient disease [117]. Several novel loci have recently been identified [118]. A meta-analysis showed that identified loci are linked to genes plausibly associated with the metabolic and reproductive characteristics of PCOS [118]. Linkage disequilibrium score regression analysis demonstrated genetic correlations with metabolic traits, that is, fasting insulin, lipid levels, and PCOS. With the exception of the GATA4/NEIL2 locus, the genetic architecture did not differ whether National Institutes of Health or Rotterdam criteria were used to diagnose PCOS [118]. Genes involved in HPO axis function, that is, LHCGR, FSHR, and FSHB, were identified in these GWASs implicating gonadotropins in the pathophysiology of PCOS [115]. Using family-based quantitative trait meta-analysis, rare DENND1A variants were associated with metabolic and reproductive traits in PCOS families; these data are consistent with the hypothesis that complex disorders such as PCOS are associated with genetic variations in noncoding regions [119]. Epigenetic modifications such as changes in methylation and miRNAs offer another level of regulation affecting the PCOS phenotype. Epigenetic variants have been reported for adipose tissue and muscle [120, 121].
2. Diagnosis of PCOS
The classic features of PCOS include clinical or biochemical hyperandrogenism, oligomenorrhea or amenorrhea associated with chronic anovulation, and polycystic ovary syndrome morphology [122]. The current consensus is that use of the Rotterdam criteria is appropriate for adult women. For diagnosis of PCOS, women must fulfill two of the three characteristics: oligo-ovulation or anovulation, clinical and/or biochemical hyperandrogenism, or polycystic ovary morphology on ultrasound with exclusion of other disorders. The 2012 National Institutes of Health–sponsored Evidence-Based Methodology PCOS Workshop categorized PCOS into four phenotypes as follows: phenotype A, hyperandrogenism, ovulatory dysfunction, and polycystic ovary morphology; phenotype B, hyperandrogenism and ovulatory dysfunction; phenotype C, hyperandrogenism and polycystic ovary morphology; and phenotype D, ovulatory dysfunction and polycystic ovary morphology [123, 124].
However, delineating appropriate diagnostic criteria for PCOS among adolescent girls has been problematic because irregular menses, cystic acne, mild hyperandrogenism, and multifollicular ovarian morphology occur during normal pubertal maturation. These similarities between normal pubertal development and the clinical features associated with PCOS confound the diagnosis in adolescent girls (Table 1) [125–127]. Similar to the evaluation of adult women, other disorders associated with irregular menses and/or hyperandrogenism need to be excluded. These disorders include CAH, typically nonclassic 21-hydroxylase deficiency, androgen-secreting tumors, thyroid dysfunction, hyperprolactinemia, Cushing syndrome, exogenous use of steroid hormones/androgens, or severe IR syndrome [128, 129].
Table 1.
Definition of Irregular Menses in Adolescent Girls
| • Normal during the first year postmenarche |
| • From 1 to 3 y postmenarche, <21 d or >45 d |
| • From 3 y postmenarche to perimenopause, <21 d or >35 d or fewer than eight cycles per year |
| • From 1 y postmenarche, >90 d for any one cycle |
| • Primary amenorrhea by age 15 y or >3 y after thelarche |
[Adapted from: Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110(3):364–379.].
A. Menses
With reactivation of the GnRH pulse generator, increased gonadotropin secretion stimulates ovarian estrogen secretion and follicular development. Estrogen promotes uterine growth and endometrial proliferation; endometrial estrogen exposure eventually culminates in vaginal withdrawal bleeding and menarche. A longitudinal study found that the median age at menarche for American girls was 12.25 years, with lower menarcheal ages in black and Hispanic girls compared with white and Asian girls [130]. By age 15 years, 98% of girls will have experienced menarche [131].
Contemporary understanding is that it takes 3 to 4 years postmenarche for adult menstrual cyclicity to mature. By the third year after menarche, 10 or more menses occur annually in 90% of adolescent girls [132]. Approximately 41% of girls have achieved ovulatory cycles by the fourth gynecologic year [133]. Importantly, ovulation may occur despite irregular menses [134].
Currently, evidence-based data regarding the first gynecologic year are limited and are largely derived from studies published prior to 2000. A 2018 systemic review of menstrual patterns during the first gynecologic year concluded that menstrual and ovulatory patterns are diverse during this time period. In 22 studies involving >2000 adolescents, frequent menstrual bleeding (<21 days) occurred in 23% and prolonged menstrual bleeding (>30 to 45 days) occurred in at least 33% [135]. A pilot study entailing serial hormone concentrations and ultrasound studies in ovulatory postmenarcheal girls revealed lower steroid (estrogen and progesterone) concentrations, slower dominant follicle growth rate, and longer follicular phases compared with adult women; these data suggest that coordinated development of all components of the HPO axis may take up to 5 years postmenarche [136, 137].
Oligomenorrheic adolescents tend to have persistent oligomenorrhea [138, 139]. Secondary amenorrhea for >90 days is uncommon and warrants additional consideration. Girls presenting with primary amenorrhea at ages 15 to 16 years merit further evaluation.
B. Hyperandrogenism
Hirsutism, defined as excessive terminal hair growth in male pattern distribution in women, is the primary clinical sign of hyperandrogenism. The modified semisubjective Ferriman–Gallwey scoring system is one widely used approach [140, 141]. The extent of the clinical features of hyperandrogenism represents the interactions between circulating androgen concentrations, local androgen concentrations, and sensitivity of the pilosebaceous unit/hair follicle to androgens. The severity of hirsutism does not correlate with circulating androgen concentrations. Ethnic and genetic variations influence the development of hirsutism [141]. Depending on ethnicity, a modified Ferriman–Gallwey score ≥4 to 6 indicates hirsutism [125]. Other cutaneous signs of androgen excess include severe cystic acne and male pattern baldness.
Biochemical hyperandrogenism is confirmed by documentation of elevated serum androgen concentrations. One caveat is the importance of measuring androgens using high-quality assays such as liquid chromatography–tandem mass spectrometry or extraction/chromatography immunoassays [142]. Calculated free testosterone, free androgen index, calculated bioavailable testosterone, androstenedione, and DHEAS may provide helpful information. Testosterone determinations are confounded by several problems, including inadequate assay sensitivity to accurately measure low concentrations, limited evidence-based normal ranges, assay interference due to other steroid molecules or SHBG, and technical aspects of the assay methodology. In view of these constraints, the Canadian Laboratory Initiative in Pediatric Reference Intervals (CALIPER) project has developed sensitive and accurate liquid chromatography–tandem mass spectrometry methodology to simultaneously measure eight steroids [143]. Measuring 11-oxo-androgens shows promise as a method to assess for hyperandrogenism [144, 145].
C. Polycystic Ovary Morphology
Polycystic ovary morphology (PCOM) is defined as enlarged ovaries with increased stroma and more small peripheral cysts. The Androgen Excess–PCOS Society Task Force recommended that PCOM is defined as ≥20 follicles per ovary using a transvaginal probe and high-resolution technology (transducer frequency ≥8 MHz) [146]. However, assessment of ovarian morphology is difficult in the adolescent girl because the increased gonadotropin stimulation leads to increased ovarian volume and follicular growth, giving rise to the appearance of multifollicular ovaries in adolescent girls. Additionally, use of transvaginal probes are problematic in adolescent girls. PCOM is an inconsistent finding in adolescent girls and is not associated with anovulation or metabolic abnormalities [147]. Hence, ovarian ultrasounds are unnecessary in adolescent girls.
D. Evaluation and Diagnosis
The approach to the evaluation of a girl with signs and symptoms suggestive of PCOS begins with a thorough history, including detailed family history and complete physical examination. The individualized laboratory evaluation typically includes thyroid function studies as well as the determination of prolactin, total testosterone, androstenedione, SHBG, DHEAS, and 17-hydroxyprogesterone concentrations. Direct free testosterone assays should be avoided due to inadequate sensitivity, accuracy, and reproducibility of available assays. Fasting glucose, HbA1c, and lipid concentrations should be determined. Ideally, the blood sample should be obtained prior to 8:30 am. If CAH is a diagnostic possibility, an ACTH stimulation test can be obtained. The cut point of a basal 17-hydroxyprogesterone >200 ng/dL has been suggested as the threshold for performing ACTH stimulation tests [148]. Nevertheless, when the clinical picture is highly suggestive of a steroidogenic enzyme deficiency, an ACTH stimulation test might be warranted. Adrenal and pelvic imaging may be considered depending on the clinical information, physical examination, and initial laboratory data.
AMH concentrations are often elevated in women with PCOS. AMH concentrations reflect ovarian reserve and are correlated with the number of growing follicles [149]. Although it is premature to use AMH concentrations to diagnose PCOS, AMH concentrations have been found to be elevated in nonobese girls with PCOS [150, 151]. AMH concentrations were found to be higher in girls with obesity with PCOS compared to girls with obesity without PCOS of comparable age and pubertal status [152].
Insulin resistance, hyperinsulinemia, and obesity are commonly identified in women with PCOS. However, with the exception of a single publication, none of the current definitions, recommendations, or guidelines includes IR and/or hyperinsulinemia as a diagnostic feature [153].
Hence, the diagnosis of PCOS can be considered for the adolescent girl with persistence of oligoamenorrhea for 3 to 4 years postmenarche with clinical and/or biochemical hyperandrogenism after exclusion of other disorders associated with irregular menses or hyperandrogenism. When oligomenorrhea has not persisted for >2 years, these girls can be considered to be “at risk” for PCOS and require longitudinal evaluation to assess for ongoing features of PCOS. Deferred diagnosis attempts to avoid overdiagnosis with its potential for premature labeling, anxiety, and unnecessary interventions. Nevertheless, diagnostic labeling needs to be balanced with the patient’s desire for a diagnosis and specific therapeutic interventions [125–127, 154, 155].
3. Treatment of Adolescent PCOS
Adolescents presenting with PCOS features, before the diagnosis is confirmed, often require management of their symptoms [125–127]. The management of adolescents with a clear diagnosis of PCOS should include education about the condition and lifestyle interventions. The interventions can be individualized to target the foremost complaints and symptoms. Interventions include metformin, combined oral contraceptive pills (COCPs), spironolactone, and local treatments for hirsutism and acne. Management should also include management of comorbidities, regular follow-up, and a plan for transition to adult care providers.
A. Education and Counseling
Education and counseling about the condition is very important. The explanation and discussion of PCOS should be culturally sensitive as well as appropriate, comprehensive, and tailored to the individual [125]. This discussion should use an empathetic approach, promote self-care, and highlight peer support groups, which are available in multiple countries (www.pcoschallenge.org/, www.verity-pcos.org.uk/, and www.facebook.com/PCOSAustralia/). Counseling about fertility concerns is important, as adolescents with PCOS are more concerned than theirs peers about future fertility after diagnosis [156].
B. Lifestyle Interventions
Healthy lifestyle interventions must be incorporated in the management plan of all adolescents with PCOS [125–127] because a large proportion of these adolescents are overweight/obese or are at risk for gaining excessive weight [157]. Lifestyle interventions comprise multiple components, including healthy diets, physical activity, decreased sedentary behaviors, and behavioral strategies [158]. The interventions should also include the family, as parents’ involvement and their readiness to change affect adolescent outcomes [159, 160]. Engagement and adherence to lifestyle interventions can be improved by management of psychological factors such as anxiety, body image concerns, and disordered eating, which are common in adolescents [125, 161]. Two systematic reviews of lifestyle interventions in women with PCOS show improvements in weight, hyperandrogenism, and IR [162, 163]. Lifestyle interventions in adolescents with PCOS have shown additional improvements in quality of life [160, 164, 165].
Limited data are available regarding the specific type of diet to achieve weight loss in PCOS [125]. Five randomized controlled trials (RCTs) have evaluated diets in the management of adolescents with overweight/obesity with PCOS, with only three that evaluated diet as a single intervention [160, 164, 166–168]. A low-carbohydrate diet (20 to 40 g/d) and a hypocaloric diet (<40 g of fat per day) during 12 weeks improved weight and menstrual irregularities with no difference between the diets. Similarly, both low–glycemic load and low-fat diets during 6 months improved weight with no difference between diets [168]. A low-energy diet compared with a healthy diet for 6 months was associated with weight loss, more regular menses, and decreased hirsutism [167]. Nutrition education in addition to exercise training and behavioral therapy for 12 months resulted in weight loss, as well as improvement of menstrual irregularities and androgen levels in adolescents with obesity and PCOS [160].
Physical activity of longer duration, frequency, and intensity results in better maintenance of health. Importantly, moderate to vigorous physical activity for at least 60 minutes per day is associated with better physical and psychosocial health in children and adolescents [169]. Sixty minutes of moderate to vigorous physical activity at least 3 times a week should be encouraged for the prevention of weight gain and maintenance of health in PCOS [125, 170]. Exercise interventions can also improve cardiometabolic risk factors in women with PCOS [171]. Alternative exercise activities such as yoga for 12 weeks can also improve PCOS symptoms during adolescence [172]. Limiting sedentary behaviors such as watching television and the use of tablets, computers, and/or mobile phones to 2 h/d is advised for adolescents and relates to better health [173].
Data regarding behavioral interventions in adolescents with PCOS are limited [125]. However, family therapy in addition to other lifestyle interventions show beneficial effects on adolescent PCOS symptoms, and a small open trial shows that cognitive behavior therapy improved depressive symptoms [160, 174].
Prevention of weight gain and effective weight management is important in adolescent PCOS, as obesity exacerbates metabolic and psychological comorbidities of PCOS [175, 176]. Additionally, weight loss strategies up to 7% of body weight have resulted in improving menstrual irregularity and testosterone levels [164, 167]. There are limited data for the use of weight loss medications in adolescents.
C. Metformin
Metformin is the single most studied insulin sensitizer in PCOS. It is commonly used in adolescents 15 to 19 years of age despite being “off label” for this indication [177]. Additionally, according to the recent international evidence-based guidelines for assessment and management of PCOS, “The use of metformin in addition to lifestyle could be considered in adolescents with a clear diagnosis of PCOS or with symptoms of PCOS before the diagnosis is made” [125].
A meta-analysis of metformin use with and without lifestyle changes in PCOS (including two RCTs in adolescents [164, 178]) showed beneficial effects on BMI and menstrual cycles [164, 178, 179]. There have been multiple observational studies and six RCTs evaluating the effect of metformin on a total of 275 adolescents with PCOS. These studies have demonstrated short-term beneficial effects mostly in adolescents with overweight/obesity [164, 180–183]. Metformin doses used ranged from 1000 to 2000 mg daily with the major side effect being mild gastrointestinal distress. Limitations are that the frequency of side effects and adherence to medications have not been fully reported. Side effects can be reduced by starting metformin at a lower dose with slow increments and the use of extended release preparations. RCTs were mostly of 6-month duration; only one study lasted 24 months, and no longer-term studies have been reported.
Metformin at a dose of 1700 to 2000 mg/d is associated with greater improvement of BMI, and COCPs are associated with improvement in menstrual irregularity and acne according to a meta-analysis of metformin vs oral contraceptives in adolescents with PCOS and including four RCTs (170 adolescents) [164, 180–184]. Both metformin and oral contraceptives had similar beneficial effects on hirsutism, triglycerides, and high-density lipoprotein cholesterol, but the estimates of effect were derived from low-quality evidence involving small studies [184]. Meta-analyses including larger number of RCTs in women with PCOS showed limited or no benefit of insulin sensitizers on hirsutism [185, 186].
Metformin also can be used in addition to COCPs, especially in adolescents with PCOS and BMI ≥25 kg/m2, as well as high–metabolic risk groups such as certain ethnicities and individuals at increased risk of type 2 diabetes [125].
D. COCPs
COCPs (estrogen and progestin preparations) should be considered for management of menstrual irregularity and/or clinical hyperandrogenism in adolescents with a clear diagnosis of PCOS and in adolescents at risk of PCOS before the diagnosis is confirmed according to the recent international evidence-based guidelines [125]. There are limited evidence-based data regarding specific types or doses of progestins, estrogens, or combinations of COCPs for management of PCOS in adolescents and women, but the lowest effective estrogen dose (20 to 30 µg of ethinylestradiol) should be considered [125]. Contraindications such as thromboembolism risk should be assessed when prescribing COCPs by obtaining thorough medical histories of the patient and her family. In most instances, 35 µg of ethinylestradiol plus cyproterone acetate preparations should not be considered first line in PCOS [125, 187]. Duration of treatment has not been evaluated beyond 24 months in adolescents with PCOS. However, COCPs have been used for contraception in longer periods of time.
COCPs improve menstrual irregularity in adolescents with PCOS [164, 180–184]. COCPs should be also offered when contraception is required and/or medical treatment of hirsutism or acne is needed [184]. When no contraception is required, menstrual irregularity alone can also be managed with cyclical medroxyprogesterone acetate (10 mg per day for 10 days) [188, 189]. This can be offered when there is a desire to have fewer menstrual cycles and/or a preference for not taking daily medications or being on COCPs due to cultural reasons.
E. Management of Hirsutism
Acknowledgment of the significance of the hirsutism, irrespective of the severity, for a particular adolescent is important when offering treatment options as well as understanding expectations of the treatment [125]. Long-term commitment is required for any topical and/or medical interventions. More severe hirsutism may require a combination of strategies. Current available therapies have been mostly evaluated in women and include physical hair removal methods, topical medications, light-based therapies, COCPs, and antiandrogens [190–192].
Physical hair removal methods include waxing, shaving, chemical epilation, plucking, bleaching, and electrolysis. All but electrolysis are temporary hair removal methods, easily available and commonly used by adolescents even before they are evaluated for PCOS. There have been no RCTs evaluating these methods. Electrolysis is a permanent hair removal method, as it causes destruction of hair bulb, but it requires an experienced technician and can cause scaring and pigmentation changes [193].
Topical medications such as 13.9% eflornithine cream, an irreversible inhibitor of ornithine decarboxylase, affects hair follicle growth and differentiation and can improve mild facial hirsutism in women with mild skin irritation [194, 195].
Professional light-based therapies include lasers (alexandrite, diode, and neodymium-doped yttrium aluminum) and intense pulsed light. These light therapies provide wavelengths of 600 to 1100 nm that are absorbed by the melanin in the hair and destroy the hair. This approach provides a prolonged solution for hirsutism after multiple treatments. The light can also be absorbed by epidermal melanin, which is greater in darker skinned individuals, increasing the risk of blisters, dyspigmentation, and scarring [196]. The neodymium-doped yttrium laser has longer wavelengths, which is less absorbed by epidermal melanin of darker skinned individuals, decreasing side effects. Light-based therapies should be the first line of treatment of localized hirsutism [125, 126]. Laser treatment was associated with a 50% reduction of hair at 6 months after treatment with mild side effects such as pain, skin redness, and perifollicular edema [197]. Uncommon side effects include burns, blisters, hyperpigmentation/hypopigmentation, and scarring that can be reduced by topical anesthetic creams prior to treatment and by cooling mechanisms after treatment. Sun exposure should be avoided before and after treatment. Improvement of hirsutism with laser has been associated with improvement in quality of life, anxiety, and depression in young women with PCOS [198, 199].
Light-based home-use devices are also available and approved by the US Food and Drug Administration. These devices provide less optical energy and should be carefully used to avoid injuries to skin and eyes [200, 201]. Fewer RCTs have evaluated the efficacy of these devices [202].
Hormonal therapies should be considered in moderate or severe forms of hirsutism and include COCPs and antiandrogens [192]. COCPs alone improve hirsutism in adolescents with PCOS [184]. Estrogens in the COCPs decrease free androgens by increasing hepatic production of SHBG and decrease ovarian and adrenal androgen production by suppressing LH levels [203]. Progestins in the COCPs also have some antiandrogenic properties by blocking the AR and inhibiting 5α-reductase activity. A small RCT involving adolescents with PCOS showed no difference in hirsutism improvement when two COCPs were compared during 12 months (30 µg of ethinyl estradiol and 0.15 mg of desogestrel vs 35 µg of ethinyl estradiol and 2 mg of cyproterone acetate) [204]. However, cyproterone acetate is not available in the United States.
Spironolactone, cyproterone acetate (which can be part of COCPs), and flutamide are antiandrogens that have been evaluated and used to treat hirsutism in women [191]. Spironolactone is an aldosterone antagonist that blocks the AR. It should be used after 6 months of COCPs; monitoring for side effects such as volume depletion and electrolyte disturbances should be explained and performed [125, 205]. The starting dose for spironolactone is ∼25 mg/d. Subsequently, doses can range from 100 to 200 mg/d divided in two doses. Flutamide at a dose of 250 to 500 mg/d divided in two doses during 12 months has shown beneficial effects on hirsutism in women, but there are no RCTs evaluating the effect of flutamide alone or in combination with COCPs in adolescents. Low doses of flutamide (125 mg/d) in combination with metformin have been used in adolescents with ovarian hyperandrogenism [206]. Flutamide has been associated with severe side effects such as liver toxicity [191, 207]. Finasteride is a topical medication that inhibits 5α-reductase that should be avoided in adolescents, as data are very limited even among adult women [190].
Antiandrogens alone could be considered to treat hirsutism or alopecia when COCPs are contraindicated or poorly tolerated. However, antiandrogens must be used with effective contraception in sexually active adolescents to avoid fetal undervirilization [125, 126]. The combination of COCPs and antiandrogens is superior for management of hirsutism [186].
F. Management of Acne
Treatment will be guided by severity of acne with the following goals of treatment: reduction of sebum production, prevention of formation of microcomedones, suppression of Propionibacterium acnes, and reduction of inflammation to prevent scaring [208]. Mild acne can be managed initially with over-the-counter topical treatments such as benzoyl peroxide 0.1%/2.5% (Epiduo gel) or topical retinoids or the combination of the two agents as well as appropriate skin care. Moderate and severe forms of acne require the addition of systemic antibiotics (macrolides) for 3 or 4 months but discontinuation after new inflammatory lesions have stopped appearing [208, 209]. COCPs can also be added for management of moderate to severe acne in adolescents [184]. Timely referral to a dermatologist should be considered when the response is poor or in severe cases, as acne has a major negative impact on adolescent psychosocial well-being.
G. Screening of Other Comorbidities
Additional comorbidities can occur in adolescents with PCOS that might be independent of overweight status. These comorbidities include impaired glucose tolerance and type 2 diabetes [125, 126, 210, 211]. Additional comorbidities include decreased quality of life, depression, anxiety, eating disorders and disordered eating, and altered body image [192, 212–214]. Identification of IR, hyperinsulinemia, and obesity galvanizes efforts to investigate and initiate treatment of associated comorbidities such as impaired glucose tolerance, type 2 diabetes mellitus, dyslipidemia, and sleep apnea. Adequate screening for comorbidities should be guided by symptoms, clinical examination, and specific personal and family risks factors. This should be followed by appropriate management to avoid further complications [125, 215, 216].
As per management of any adolescent, the HEEADDSS screening tool should be used (that is, H, home environment; E, education and employment; E, exercise and healthy eating; A, activity and peers; D, drugs, smoking, and alcohol; D, depression and suicide ideation; S, sexuality and sexual health; S, sleep) [217]. Prompt referral to social work, psychology, and counseling in the presence of psychosocial comorbidities is necessary, as these comorbidities will affect adherence to any interventions. Self-management strategies such as mindfulness and yoga in PCOS are emerging and require more research [172, 218]. Contraception should be discussed in sexually active adolescents with PCOS who are not taking COCPs for PCOS.
H. Transition to Adult Care Providers
Preparation for transition to adult care will require reinforcement of education about PCOS, its comorbidities, lifestyle interventions, medical treatment, and the need of long-term follow-up [125, 126, 219]. Women with PCOS are best managed by multidisciplinary health care teams comprised of endocrinologists, general physicians, gynecologists, family doctors, or general practitioners. Therapeutic options should be discussed with the adolescent or emerging adult. The selection of an appropriate specialist for adult care should be based on adolescent preferences and major complaints, local availability of health care professionals or specialized clinics, health care insurance, and the possible need of fertility management in the near future.
4. Summary
PCOS is a complex disorder involving multiple organ systems with onset during the early pubertal years (Fig. 1). The list of factors involved in the pathophysiology continues to expand, with accruing evidence indicating that hyperandrogenism is a pivotal factor affecting multiple tissues [220, 221]. GWASs have identified genes common to both Han Chinese and white populations that are involved in neuroendocrine, metabolic, and reproductive pathways [118]. Data obtained from animal models have consistently implicated testosterone as an important factor in the pathogenesis of PCOS. The important contributions of ectopic fat storage and adipocyte androgen biosynthesis are emerging. Promising clinical and preclinical data point toward neuroendocrine involvement with supporting roles for GABA signaling and neuronal ARs.
At this time, an individualized treatment plan can be developed for the adolescent girl with features of PCOS. Attention to the history, physical examination, and laboratory data is important to identify adolescent girls at risk to develop PCOS. Whereas deferring diagnostic labeling may be appropriate, treatment of clinical features and comorbidities is vital to the health and self-esteem of these patients. One future goal includes prevention through timely identification of at-risk prepubertal and early pubertal girls through lifestyle interventions.
Acknowledgments
Financial Support: This work was supported in part by the Australian National Health and Medical Research Council Centre for Research Excellence scheme in the origins, outcomes, and optimal management of PCOS (Grant APP1078444 to A.S.P.).
Glossary
Abbreviations:
- AMH
anti-Müllerian hormone
- AR
androgen receptor
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- COCP
combined oral contraceptive pill
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- FSHR
FSH receptor
- GABA
γ-aminobutyric acid
- GWAS
genome-wide association study
- HPO
hypothalamic–pituitary–ovarian
- IR
insulin resistance
- LHCGR
LH/chorionic gonadotropin receptor
- NHP
nonhuman primate
- PCOM
polycystic ovary morphology
- PCOS
polycystic ovary syndrome
- PNA
prenatally androgenized
- RCT
randomized controlled trial
- VPA
valproic acid
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References and Notes
- 1. Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Boileau P, Le Bouc Y, Deal CL, Lillycrop K, Scharfmann R, Sheppard A, Skinner M, Szyf M, Waterland RA, Waxman DJ, Whitelaw E, Ong K, Albertsson-Wikland K. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011;32(2):159–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. [DOI] [PubMed] [Google Scholar]
- 4. Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–198. [DOI] [PubMed] [Google Scholar]
- 5. Mohamed-Hussein ZA, Harun S. Construction of a polycystic ovarian syndrome (PCOS) pathway based on the interactions of PCOS-related proteins retrieved from bibliomic data. Theor Biol Med Model. 2009;6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apter D, Bützow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79(1):119–125. [DOI] [PubMed] [Google Scholar]
- 7. Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP, Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil Steril. 2016;106(6):1510–1520.e2. [DOI] [PubMed] [Google Scholar]
- 8. Pache TD, Fauser BC. Polycystic ovaries in female-to-male transsexuals. Clin Endocrinol (Oxf). 1993;39(6):702–703. [PubMed] [Google Scholar]
- 9. Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaytan F, Morales C, Leon S, Garcia-Galiano D, Roa J, Tena-Sempere M. Crowding and follicular fate: spatial determinants of follicular reserve and activation of follicular growth in the mammalian ovary. PLoS One. 2015;10(12):e0144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31(7):1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumont A, Robin G, Dewailly D. Anti-Müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):377–384. [DOI] [PubMed] [Google Scholar]
- 14. Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83(7):2479–2485. [DOI] [PubMed] [Google Scholar]
- 15. Harlow CR, Shaw HJ, Hillier SG, Hodges JK. Factors influencing follicle-stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular maturity. Endocrinology. 1988;122(6):2780–2787. [DOI] [PubMed] [Google Scholar]
- 16. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97(8):E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, Ernst E, Macklon KT, Andersen CY. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol (Lausanne). 2018;8:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franks S, Hardy K. Androgen action in the ovary. Front Endocrinol (Lausanne). 2018;9:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. [DOI] [PubMed] [Google Scholar]
- 21. Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321–5327. [DOI] [PubMed] [Google Scholar]
- 22. Homer MV, Toloubeydokhti T, Lawson MA, Garzo G, Duleba AJ, Chang RJ. Individual 17-hydroxyprogesterone responses to hCG are not correlated with follicle size in polycystic ovary syndrome. J Endocr Soc. 2019;3(4):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev. 2018;39(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158–1165. [DOI] [PubMed] [Google Scholar]
- 25. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf). 1997;47(1):93–99. [DOI] [PubMed] [Google Scholar]
- 26. Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 27. McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF III. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, Strauss JF III, Miller WL. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016;434:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–438. [DOI] [PubMed] [Google Scholar]
- 30. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367–E375. [DOI] [PubMed] [Google Scholar]
- 31. Miller WL, Auchus RJ. The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biol. 2019;17(4):e3000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marti N, Galván JA, Pandey AV, Trippel M, Tapia C, Müller M, Perren A, Flück CE. Genes and proteins of the alternative steroid backdoor pathway for dihydrotestosterone synthesis are expressed in the human ovary and seem enhanced in the polycystic ovary syndrome. Mol Cell Endocrinol. 2017;441:116–123. [DOI] [PubMed] [Google Scholar]
- 33. Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One. 2016;11(7):e0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhayat NA, Marti N, Kollmann Z, Troendle A, Bally L, Escher G, Grössl M, Ackermann D, Ponte B, Pruijm M, Müller M, Vogt B, Birkhäuser MH, Bochud M, Flück CE; members of the SKIPOGH Study Group. Urinary steroid profiling in women hints at a diagnostic signature of the polycystic ovary syndrome: a pilot study considering neglected steroid metabolites. PLoS One. 2018;13(10):e0203903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Xu F, Lawson MS, Tkachenko OY, Ting AY, Kahl CA, Park BS, Stouffer RR, Bishop CV. Anti-Müllerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol Reprod. 2018;98(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, Giacobini P. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7(1):10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tata B, Mimouni NE, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caldwell AS, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114(16):E3334–E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abbott DH, Rayome BH, Dumesic DA, Lewis KC, Edwards AK, Wallen K, Wilson ME, Appt SE, Levine JE. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017;32(4):923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messina A, Langlet F, Chachlaki K, Roa J, Rasika S, Jouy N, Gallet S, Gaytan F, Parkash J, Tena-Sempere M, Giacobini P, Prevot V. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty [published correction appears in Nat Neurosci. 2016;19(8):1115]. Nat Neurosci. 2016;19(6):835–844. [DOI] [PubMed] [Google Scholar]
- 45. Lomniczi A, Ojeda SR. The emerging role of epigenetics in the regulation of female puberty. Endocr Dev. 2016;29:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Casoni F, Malone SA, Belle M, Luzzati F, Collier F, Allet C, Hrabovszky E, Rasika S, Prevot V, Chédotal A, Giacobini P. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969–3981. [DOI] [PubMed] [Google Scholar]
- 47. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 48. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452–466. [DOI] [PubMed] [Google Scholar]
- 49. Hill JW, Elias CF. Neuroanatomical framework of the metabolic control of reproduction. Physiol Rev. 2018;98(4):2349–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–4321. [DOI] [PubMed] [Google Scholar]
- 51. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12(4):351–361. [DOI] [PubMed] [Google Scholar]
- 52. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moenter SM. Leap of faith: does serum luteinizing hormone always accurately reflect central reproductive neuroendocrine activity? Neuroendocrinology. 2015;102(4):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine. 2018;61(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22(1):7–14. [DOI] [PubMed] [Google Scholar]
- 56. Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166(4):867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev. 2018;39(3):333–368. [DOI] [PubMed] [Google Scholar]
- 59. Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144(4):1426–1434. [DOI] [PubMed] [Google Scholar]
- 60. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71(9):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Berg T, Silveira MA, Moenter SM. Prepubertal development of GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J Neurosci. 2018;38(9):2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9(2):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872–2891. [DOI] [PubMed] [Google Scholar]
- 65. Kawwass JF, Sanders KM, Loucks TL, Rohan LC, Berga SL. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum Reprod. 2017;32(7):1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight. 2018;3(7):99405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moller DE, Flier JS. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988;319(23):1526–1529. [DOI] [PubMed] [Google Scholar]
- 68. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165–1174. [DOI] [PubMed] [Google Scholar]
- 69. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–784. [DOI] [PubMed] [Google Scholar]
- 70. Cree-Green M, Rahat H, Newcomer BR, Bergman BC, Brown MS, Coe GV, Newnes L, Garcia-Reyes Y, Bacon S, Thurston JE, Pyle L, Scherzinger A, Nadeau KJ. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1(7):931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Geffner ME, Golde DW. Selective insulin action on skin, ovary, and heart in insulin-resistant states. Diabetes Care. 1988;11(6):500–505. [DOI] [PubMed] [Google Scholar]
- 72. Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012;61(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20(2):373–381. [DOI] [PubMed] [Google Scholar]
- 74. Caprio S. Insulin: the other anabolic hormone of puberty. Acta Paediatr Suppl. 1999;88(433):84–87. [DOI] [PubMed] [Google Scholar]
- 75. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60(6):759–763. [DOI] [PubMed] [Google Scholar]
- 76. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 77. de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110(16):2494–2497. [DOI] [PubMed] [Google Scholar]
- 78. Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. 2019;20(2):339–352. [DOI] [PubMed] [Google Scholar]
- 79. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nicol LE, O’Brien TD, Dumesic DA, Grogan T, Tarantal AF, Abbott DH. Abnormal infant islet morphology precedes insulin resistance in PCOS-like monkeys. PLoS One. 2014;9(9):e106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ramaswamy S, Grace C, Mattei AA, Siemienowicz K, Brownlee W, MacCallum J, McNeilly AS, Duncan WC, Rae MT. Developmental programming of polycystic ovary syndrome (PCOS): prenatal androgens establish pancreatic islet α/β cell ratio and subsequent insulin secretion. Sci Rep. 2016;6(1):27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–349. [DOI] [PubMed] [Google Scholar]
- 83. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grundy SM. Overnutrition, ectopic lipid and the metabolic syndrome. J Investig Med. 2016;64(6):1082–1086. [DOI] [PubMed] [Google Scholar]
- 85. Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab. 2016;101(11):4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(6):2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ibáñez L, Del Río L, Díaz M, Sebastiani G, Pozo ÓJ, López-Bermejo A, de Zegher F. Normalizing ovulation rate by preferential reduction of hepato-visceral fat in adolescent girls with polycystic ovary syndrome. J Adolesc Health. 2017;61(4):446–453. [DOI] [PubMed] [Google Scholar]
- 88. Trottier A, Battista MC, Geller DH, Moreau B, Carpentier AC, Simoneau-Roy J, Baillargeon JP. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertil Steril. 2012;98(6):1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Torchen LC, Fogel NR, Brickman WJ, Paparodis R, Dunaif A. Persistent apparent pancreatic β-cell defects in premenarchal PCOS relatives. J Clin Endocrinol Metab. 2014;99(10):3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Koivuaho E, Laru J, Ojaniemi M, Puukka K, Kettunen J, Tapanainen JS, Franks S, Järvelin MR, Morin-Papunen L, Sebert S, Piltonen TT. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS [published online ahead of print 4 February 2019]. Int J Obes (Lond). 2019;43(7):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ibáñez L, Ong KK, López-Bermejo A, Dunger DB, de Zegher F. Hyperinsulinaemic androgen excess in adolescent girls [published correction appears in Nat Rev Endocrinol. 2014;10:578]. Nat Rev Endocrinol. 2014;10(8):499–508. [DOI] [PubMed] [Google Scholar]
- 93. de Zegher F, López-Bermejo A, Ibáñez L. Central obesity, faster maturation, and “PCOS” in girls. Trends Endocrinol Metab. 2018;29(12):815–818. [DOI] [PubMed] [Google Scholar]
- 94. de Zegher F, Reinehr T, Malpique R, Darendeliler F, López-Bermejo A, Ibáñez L. Reduced prenatal weight gain and/or augmented postnatal weight gain precedes polycystic ovary syndrome in adolescent girls. Obesity (Silver Spring). 2017;25(9):1486–1489. [DOI] [PubMed] [Google Scholar]
- 95. O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–284. [DOI] [PubMed] [Google Scholar]
- 97. Kim JY, Tfayli H, Michaliszyn SF, Arslanian S. Impaired lipolysis, diminished fat oxidation, and metabolic inflexibility in obese girls with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103(2):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Varlamov O, Bishop CV, Handu M, Takahashi D, Srinivasan S, White A, Roberts CT Jr. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum Reprod. 2017;32(9):1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23(4):421–432. [DOI] [PubMed] [Google Scholar]
- 100. Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol. 2002;174(1):1–5. [DOI] [PubMed] [Google Scholar]
- 101. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 102. Witchel SF. Congenital adrenal hyperplasia. J Pediatr Adolesc Gynecol. 2017;30(5):520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abbott DH, Bacha F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil Steril. 2013;100(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barrett ES, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN, Swan SH. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018;9(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155(7):2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. True C, Abbott DH, Roberts CT Jr, Varlamov O. Sex differences in androgen regulation of metabolism in nonhuman primates. Adv Exp Med Biol. 2017;1043:559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sharpton SR, Ajmera V, Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol. 2019;17(2):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O; MetaHIT Consortium. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. [DOI] [PubMed] [Google Scholar]
- 109. Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. [DOI] [PubMed] [Google Scholar]
- 110. Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, Thackray VG. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Thackray VG. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019;30(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 113. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 114. Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. [DOI] [PubMed] [Google Scholar]
- 115. Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations [published correction appears in Nat Commun. 2016;7:10762]. Nat Commun. 2015;6(1):7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JS, Ong KK, Perry JR. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6(1):8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fessler DM, Natterson-Horowitz B, Azziz R. Evolutionary determinants of polycystic ovary syndrome: part 2. Fertil Steril. 2016;106(1):42–47. [DOI] [PubMed] [Google Scholar]
- 118. Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, Magi R, Saxena R, Laisk T, Urbanek M, Hayes MG, Thorleifsson G, Fernandez-Tajes J, Mahajan A, Mullin BH, Stuckey BGA, Spector TD, Wilson SG, Goodarzi MO, Davis L, Obermayer-Pietsch B, Uitterlinden AG, Anttila V, Neale BM, Jarvelin MR, Fauser B, Kowalska I, Visser JA, Andersen M, Ong K, Stener-Victorin E, Ehrmann D, Legro RS, Salumets A, McCarthy MI, Morin-Papunen L, Thorsteinsdottir U, Stefansson K, Styrkarsdottir U, Perry JRB, Dunaif A, Laven J, Franks S, Lindgren CM, Welt CK; 23andMe Research Team. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome [published online ahead of print 30 April 2019]. J Clin Endocrinol Metab. doi: 10.1210/jc.2018-02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kokosar M, Benrick A, Perfilyev A, Fornes R, Nilsson E, Maliqueo M, Behre CJ, Sazonova A, Ohlsson C, Ling C, Stener-Victorin E. Epigenetic and transcriptional alterations in human adipose tissue of polycystic ovary syndrome [published correction appears in Sci Rep. 2016;6:25321]. Sci Rep. 2016;6(1):22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nilsson E, Benrick A, Kokosar M, Krook A, Lindgren E, Källman T, Martis MM, Højlund K, Ling C, Stener-Victorin E. Transcriptional and epigenetic changes influencing skeletal muscle metabolism in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103(12):4465–4477. [DOI] [PubMed] [Google Scholar]
- 122. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Johnson T, Kaplan L, Ouyang P, Rizza P. National Institutes of Health Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome. NIH EbMW Reports. Bethesda, MD: National Institutes of Health, 2012;1:1–14. Available at: https://prevention.nih.gov/sites/default/files/2018-06/FinalReport.pdf. Accessed 17 February 2019.
- 124. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. [DOI] [PubMed] [Google Scholar]
- 125. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, López-Bermejo A, Ong K, Peña AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, Lee PA. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. 2017;88(6):371–395. [DOI] [PubMed] [Google Scholar]
- 127. Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D, Tfayli H, Arslanian S, Dabadghao P, Garcia Rudaz C, Lee PA. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–389. [DOI] [PubMed] [Google Scholar]
- 128. Trapp CM, Oberfield SE. Recommendations for treatment of nonclassic congenital adrenal hyperplasia (NCCAH): an update. Steroids. 2012;77(4):342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pall M, Azziz R, Beires J, Pignatelli D. The phenotype of hirsute women: a comparison of polycystic ovary syndrome and 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 2010;94(2):684–689. [DOI] [PubMed] [Google Scholar]
- 130. Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, Greenspan LC, Kushi LH, Teitelbaum SL. Age of menarche in a longitudinal US cohort. J Pediatr Adolesc Gynecol. 2018;31(4):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, Sun SS. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):110–113. [DOI] [PubMed] [Google Scholar]
- 132. Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85(3):1021–1025. [DOI] [PubMed] [Google Scholar]
- 133. Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20(1):231–236. [DOI] [PubMed] [Google Scholar]
- 134. Peña AS, Doherty DA, Atkinson HC, Hickey M, Norman RJ, Hart R. The majority of irregular menstrual cycles in adolescence are ovulatory: results of a prospective study. Arch Dis Child. 2018;103(3):235–239. [DOI] [PubMed] [Google Scholar]
- 135. Gunn HM, Tsai MC, McRae A, Steinbeck KS. Menstrual patterns in the first gynecological year: a systematic review. J Pediatr Adolesc Gynecol. 2018;31(6):557–565.e6. [DOI] [PubMed] [Google Scholar]
- 136. Sun BZ, Kangarloo T, Adams JM, Sluss PM, Welt CK, Chandler DW, Zava DT, McGrath JA, Umbach DM, Hall JE, Shaw ND. Healthy post-menarchal adolescent girls demonstrate multi-level reproductive axis immaturity. J Clin Endocrinol Metab. 2019;104(2):613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 138. van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19(2):383–392. [DOI] [PubMed] [Google Scholar]
- 139. Southam AL, Richart RM. The prognosis for adolescents with menstrual abnormalities. Am J Obstet Gynecol. 1966;94(5):637–645. [DOI] [PubMed] [Google Scholar]
- 140. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21(11):1440–1447. [DOI] [PubMed] [Google Scholar]
- 141. Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rosner W, Vesper H; Endocrine Society; American Association for Clinical Chemistry; American Association of Clinical Endocrinologists; Androgen Excess/PCOS Society; American Society for Bone and Mineral Research; American Society for Reproductive Medicine; American Urological Association; Association of Public Health Laboratories; Endocrine Society;Laboratory Corporation of America; North American Menopause Society; Pediatric Endocrine Society. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95(10):4542–4548. [DOI] [PubMed] [Google Scholar]
- 143. Kyriakopoulou L, Yazdanpanah M, Colantonio DA, Chan MK, Daly CH, Adeli K. A sensitive and rapid mass spectrometric method for the simultaneous measurement of eight steroid hormones and CALIPER pediatric reference intervals. Clin Biochem. 2013;46(7-8):642–651. [DOI] [PubMed] [Google Scholar]
- 144. Turcu AF, Auchus RJ. Clinical significance of 11-oxygenated androgens. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pretorius E, Arlt W, Storbeck KH. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 2017;441:76–85. [DOI] [PubMed] [Google Scholar]
- 146. Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20(3):334–352. [DOI] [PubMed] [Google Scholar]
- 147. Codner E, Villarroel C, Eyzaguirre FC, López P, Merino PM, Pérez-Bravo F, Iñiguez G, Cassorla F. Polycystic ovarian morphology in postmenarchal adolescents. Fertil Steril. 2011;95(2):702–706.e1–2. [DOI] [PubMed] [Google Scholar]
- 148. Armengaud JB, Charkaluk ML, Trivin C, Tardy V, Bréart G, Brauner R, Chalumeau M. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94(8):2835–2840. [DOI] [PubMed] [Google Scholar]
- 149. Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. [DOI] [PubMed] [Google Scholar]
- 150. Tokmak A, Timur H, Aksoy RT, Çınar M, Yılmaz N. Is anti-Mullerian hormone a good diagnostic marker for adolescent and young adult patients with Polycystic ovary syndrome? Turk J Obstet Gynecol. 2015;12(4):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sopher AB, Grigoriev G, Laura D, Cameo T, Lerner JP, Chang RJ, McMahon DJ, Oberfield SE. Anti-Mullerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J Pediatr Endocrinol Metab. 2014;27(11–12):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kim JY, Tfayli H, Michaliszyn SF, Lee S, Nasr A, Arslanian S. Anti-Müllerian hormone in obese adolescent girls with polycystic ovary syndrome. J Adolesc Health. 2017;60(3):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sultan C, Paris F. Clinical expression of polycystic ovary syndrome in adolescent girls. Fertil Steril. 2006;86(Suppl 1):S6. [DOI] [PubMed] [Google Scholar]
- 154. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cree-Green M. Worldwide dissatisfaction with the diagnostic process and initial treatment of PCOS. J Clin Endocrinol Metab. 2017;102(2):375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Trent ME, Rich M, Austin SB, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome: implications for quality of life. J Pediatr Adolesc Gynecol. 2003;16(1):33–37. [DOI] [PubMed] [Google Scholar]
- 157. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. [DOI] [PubMed] [Google Scholar]
- 158. Ells LJ, Rees K, Brown T, Mead E, Al-Khudairy L, Azevedo L, McGeechan GJ, Baur L, Loveman E, Clements H, Rayco-Solon P, Farpour-Lambert N, Demaio A. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes. 2018;42(11):1823–1833. [DOI] [PubMed] [Google Scholar]
- 159. Jakubowski KP, Black JJ, El Nokali NE, Belendiuk KA, Hannon TS, Arslanian SA, Rofey DL. Parents’ readiness to change affects BMI reduction outcomes in adolescents with polycystic ovary syndrome. j obes. 2012;2012:298067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96(11):3533–3540. [DOI] [PubMed] [Google Scholar]
- 161. Kaczmarek C, Haller DM, Yaron M. Health-related quality of life in adolescents and young adults with polycystic ovary syndrome: a systematic review. J Pediatr Adolesc Gynecol. 2016;29(6):551–557. [DOI] [PubMed] [Google Scholar]
- 162. Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011; (7):CD007506. [DOI] [PubMed] [Google Scholar]
- 163. Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, Phung OJ, Wang A, Hoeger K, Pasquali R, Erwin P, Bodde A, Montori VM, Murad MH. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4655–4663. [DOI] [PubMed] [Google Scholar]
- 164. Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Harris-Glocker M, Davidson K, Kochman L, Guzick D, Hoeger K. Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertil Steril. 2010;93(3):1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ornstein RM, Copperman NM, Jacobson MS. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2011;24(3):161–165. [DOI] [PubMed] [Google Scholar]
- 167. Marzouk TM, Sayed Ahmed WA. Effect of dietary weight loss on menstrual regularity in obese young adult women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2015;28(6):457–461. [DOI] [PubMed] [Google Scholar]
- 168. Wong JM, Gallagher M, Gooding H, Feldman HA, Gordon CM, Ludwig DS, Ebbeling CB. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr Obes. 2016;11(3):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Poitras VJ, Gray CE, Borghese MM, Carson V, Chaput JP, Janssen I, Katzmarzyk PT, Pate RR, Connor Gorber S, Kho ME, Sampson M, Tremblay MS. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(6Suppl 3):S197–S239. [DOI] [PubMed] [Google Scholar]
- 170. Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2011;17(2):171–183. [DOI] [PubMed] [Google Scholar]
- 171. Benham JL, Yamamoto JM, Friedenreich CM, Rabi DM, Sigal RJ. Role of exercise training in polycystic ovary syndrome: a systematic review and meta-analysis. Clin Obes. 2018;8(4):275–284. [DOI] [PubMed] [Google Scholar]
- 172. Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Effect of holistic yoga program on anxiety symptoms in adolescent girls with polycystic ovarian syndrome: a randomized control trial. Int J Yoga. 2012;5(2):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Carson V, Hunter S, Kuzik N, Gray CE, Poitras VJ, Chaput JP, Saunders TJ, Katzmarzyk PT, Okely AD, Connor Gorber S, Kho ME, Sampson M, Lee H, Tremblay MS. Systematic review of sedentary behaviour and health indicators in school-aged children and youth: an update. Appl Physiol Nutr Metab. 2016;41(6Suppl 3):S240–S265. [DOI] [PubMed] [Google Scholar]
- 174. Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive–behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: a pilot study. J Pediatr Psychol. 2009;34(2):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Li L, Feng Q, Ye M, He Y, Yao A, Shi K. Metabolic effect of obesity on polycystic ovary syndrome in adolescents: a meta-analysis. J Obstet Gynaecol. 2017;37(8):1036–1047. [DOI] [PubMed] [Google Scholar]
- 176. Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5(2):107–111. [DOI] [PubMed] [Google Scholar]
- 177. Wang T, McNeill AM, Chen Y, Senderak M, Shankar RR. Metformin prescription patterns among US adolescents aged 10–19 years: 2009–2013. J Clin Pharm Ther. 2016;41(2):229–236. [DOI] [PubMed] [Google Scholar]
- 178. Ladson G, Dodson WC, Sweet SD, Archibong AE, Kunselman AR, Demers LM, Lee PA, Williams NI, Coney P, Legro RS. Effects of metformin in adolescents with polycystic ovary syndrome undertaking lifestyle therapy: a pilot randomized double-blind study. Fertil Steril. 2011. Jun 30;95(8):2595–2598.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–574. [DOI] [PubMed] [Google Scholar]
- 180. Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L, Reiter EO. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18(8):761–768. [DOI] [PubMed] [Google Scholar]
- 181. Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160(3):241–246. [DOI] [PubMed] [Google Scholar]
- 182. Al-Zubeidi H, Klein KO. Randomized clinical trial evaluating metformin versus oral contraceptive pills in the treatment of adolescents with polycystic ovarian syndrome. J Pediatr Endocrinol Metab. 2015;28(7-8):853–858. [DOI] [PubMed] [Google Scholar]
- 183. El Maghraby HA, Nafee T, Guiziry D, Elnashar A. Randomized controlled trial of the effects of metformin versus combined oral contraceptives in adolescent PCOS women through a 24 month follow up period. Middle East Fertil Soc J. 2015;20(3):131–137. [Google Scholar]
- 184. Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E. Metformin or oral contraceptives for adolescents with polycystic ovarian syndrome: a meta-analysis. Pediatrics. 2016;137(5):e20154089. [DOI] [PubMed] [Google Scholar]
- 185. Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, Elamin MB, Erwin PJ, Montori VM. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 186. Barrionuevo P, Nabhan M, Altayar O, Wang Z, Erwin PJ, Asi N, Martin KA, Murad MH. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103(4):1258–1264. [DOI] [PubMed] [Google Scholar]
- 187. Bird ST, Hartzema AG, Brophy JM, Etminan M, Delaney JA. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ. 2013;185(2):E115–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ozdemir S, Görkemli H, Gezginç K, Ozdemir M, Kiyici A. Clinical and metabolic effects of medroxyprogesterone acetate and ethinyl estradiol plus drospirenone in women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;103(1):44–49. [DOI] [PubMed] [Google Scholar]
- 189. Chung JP, Yiu AK, Chung TK, Chan SS. A randomized crossover study of medroxyprogesterone acetate and Diane-35 in adolescent girls with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2014;27(3):166–171. [DOI] [PubMed] [Google Scholar]
- 190. Pasquali R, Gambineri A. Therapy in endocrine disease: treatment of hirsutism in the polycystic ovary syndrome. Eur J Endocrinol. 2013;170(2):R75–R90. [DOI] [PubMed] [Google Scholar]
- 191. Somani N, Turvy D. Hirsutism: an evidence-based treatment update. Am J Clin Dermatol. 2014;15(3):247–266. [DOI] [PubMed] [Google Scholar]
- 192. van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45–61. [DOI] [PubMed] [Google Scholar]
- 193. Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12(3):441–449. [DOI] [PubMed] [Google Scholar]
- 194. Jackson J, Caro JJ, Caro G, Garfield F, Huber F, Zhou W, Lin CS, Shander D, Schrode K; Eflornithine HCl Study Group. The effect of eflornithine 13.9% cream on the bother and discomfort due to hirsutism. Int J Dermatol. 2007;46(9):976–981. [DOI] [PubMed] [Google Scholar]
- 195. Wolf JE Jr, Shander D, Huber F, Jackson J, Lin CS, Mathes BM, Schrode K; Eflornithine HCl Study Group. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46(1):94–98. [DOI] [PubMed] [Google Scholar]
- 196. Gan SD, Graber EM. Laser hair removal: a review. Dermatol Surg. 2013;39(6):823–838. [DOI] [PubMed] [Google Scholar]
- 197. Haedersdal M, Gøtzsche PC. Laser and photoepilation for unwanted hair growth. Cochrane Database Syst Rev. 2006; (4):CD004684. [DOI] [PubMed] [Google Scholar]
- 198. Maziar A, Farsi N, Mandegarfard M, Babakoohi S, Gorouhi F, Dowlati Y, Firooz A. Unwanted facial hair removal with laser treatment improves quality of life of patients. J Cosmet Laser Ther. 2010;12(1):7–9. [DOI] [PubMed] [Google Scholar]
- 199. Clayton WJ, Lipton M, Elford J, Rustin M, Sherr L. A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br J Dermatol. 2005;152(5):986–992. [DOI] [PubMed] [Google Scholar]
- 200. Town G, Ash C, Dierickx C, Fritz K, Bjerring P, Haedersdal M. Guidelines on the safety of light-based home-use hair removal devices from the European Society for Laser Dermatology. J Eur Acad Dermatol Venereol. 2012;26(7):799–811. [DOI] [PubMed] [Google Scholar]
- 201. Town G, Botchkareva NV, Uzunbajakava NE, Nuijs T, van Vlimmeren M, Ash C, Dierickx C. Light-based home-use devices for hair removal: why do they work and how effective they are? [published online ahead of print 25 January 2019]. Lasers Surg Med. 2019;51(6):481-490. [DOI] [PubMed] [Google Scholar]
- 202. Thaysen-Petersen D, Bjerring P, Dierickx C, Nash JF, Town G, Haedersdal M. A systematic review of light-based home-use devices for hair removal and considerations on human safety. J Eur Acad Dermatol Venereol. 2012;26(5):545–553. [DOI] [PubMed] [Google Scholar]
- 203. Creatsas G, Hassan E, Deligeoroglou E, Tolis G, Aravantinos D. Treatment of polycystic ovarian disease during adolescence with ethinylestradiol/cyproterone acetate versus a D-Tr-6-LHRH analog. Int J Gynaecol Obstet. 1993;42(2):147–153. [DOI] [PubMed] [Google Scholar]
- 204. Mastorakos G, Koliopoulos C, Creatsas G. Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives. Fertil Steril. 2002;77(5):919–927. [DOI] [PubMed] [Google Scholar]
- 205. Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, Shapiro J, Montori VM, Swiglo BA. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(4):1105–1120. [DOI] [PubMed] [Google Scholar]
- 206. Ibáñez L, Ong K, Ferrer A, Amin R, Dunger D, de Zegher F. Low-dose flutamide-metformin therapy reverses insulin resistance and reduces fat mass in nonobese adolescents with ovarian hyperandrogenism. J Clin Endocrinol Metab. 2003;88(6):2600–2606. [DOI] [PubMed] [Google Scholar]
- 207. Domecq JP, Prutsky G, Mullan RJ, Sundaresh V, Wang AT, Erwin PJ, Welt C, Ehrmann D, Montori VM, Murad MH. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Eichenfield LF, Krakowski AC, Piggott C, Del Rosso J, Baldwin H, Friedlander SF, Levy M, Lucky A, Mancini AJ, Orlow SJ, Yan AC, Vaux KK, Webster G, Zaenglein AL, Thiboutot DM; American Acne and Rosacea Society. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(Suppl 3):S163–S186. [DOI] [PubMed] [Google Scholar]
- 209. Mwanthi M, Zaenglein AL. Update in the management of acne in adolescence. Curr Opin Pediatr. 2018;30(4):492–498. [DOI] [PubMed] [Google Scholar]
- 210. Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1017–1023. [DOI] [PubMed] [Google Scholar]
- 211. Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–467. [DOI] [PubMed] [Google Scholar]
- 212. Trent ME, Rich M, Austin SB, Gordon CM. Quality of life in adolescent girls with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2002;156(6):556–560. [DOI] [PubMed] [Google Scholar]
- 213. Jones GL, Hall JM, Lashen HL, Balen AH, Ledger WL. Health-related quality of life among adolescents with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2011;40(5):577–588. [DOI] [PubMed] [Google Scholar]
- 214. Guidi J, Gambineri A, Zanotti L, Fanelli F, Fava GA, Pasquali R. Psychological aspects of hyperandrogenic states in late adolescent and young women. Clin Endocrinol (Oxf). 2015;83(6):872–878. [DOI] [PubMed] [Google Scholar]
- 215. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Goldenring J, Cohen E. Getting into adolescents HEADS. Contemp Pediatr. 1988;5(7):75–90. [Google Scholar]
- 218. Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, Kunselman AR, Reibel DK, Legro RS. Mindfulness-based stress reduction in women with overweight or obesity: a randomized clinical trial. Obesity (Silver Spring). 2017;25(8):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Dokras A, Witchel SF. Are young adult women with polycystic ovary syndrome slipping through the healthcare cracks? J Clin Endocrinol Metab. 2014;99(5):1583–1585. [DOI] [PubMed] [Google Scholar]
- 220. Abbott DH. Neuronal androgen receptor: molecular gateway to polycystic ovary syndrome? Proc Natl Acad Sci USA. 2017;114(16):4045–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Puttabyatappa M, Padmanabhan V. Ovarian and extra-ovarian mediators in the development of polycystic ovary syndrome. J Mol Endocrinol. 2018;61(4):R161–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]