Abstract
Context
Studies suggest that menopausal hormone therapy (MHT) prevents type 2 diabetes (T2D). The combination of conjugated estrogens (CE) with the selective estrogen receptor modulator bazedoxifene (BZA) is an MHT that improves obesity and T2D in preclinical models of menopausal metabolic syndrome. The effect of CE/BZA on adiposity and glucose homeostasis in obese postmenopausal women is unknown.
Objective
To investigate the effect of CE/BZA on body composition, glucose homeostasis, and markers of inflammation in obese postmenopausal women.
Research Design, Intervention, and Participants
Randomized, double-blind, placebo-controlled pilot trial of 12 obese menopausal women assigned to 12-week treatment with CE 0.45 mg/BZA 20 mg (n = 7) or placebo (n = 5). At baseline and after 12 weeks, we assessed body composition (dual-energy X-ray absorptiometry), glucose homeostasis (IV glucose tolerance test), and inflammation biomarkers.
Results
Women treated with CE/BZA exhibited increased β cell function using homeostatic model assessment-B [median (interquartile range) CE/BZA vs placebo: 18.5 (−0.9 to 320.6) μU/mM vs −25.5 (−39.9 to −0.1) μU/mM; P = 0.045], and decreased basal glucose concentrations (Gb) [−5.2 (−9.2 to −1.7) mg/dL vs 2.7 (0.9 to 4.9) mg/dL; P = 0.029]. Insulin sensitivity was higher in the placebo arm [1.35 (1.12 to 1.82) (μU/mL) min−1 vs −0.24 (−1.50 to 0.19) (μU/mL) min−1; P = 0.029]. No changes between treatment groups were observed for the acute insulin response to glucose (AIRg), the disposition index (DI), body composition, and inflammatory biomarkers.
Conclusions
A 12-week treatment of obese postmenopausal women with CEs/BZA improves fasting β cell function and glucose concentrations without change in AIRg, HOMA-IR, DI, body composition, or markers of inflammation.
Keywords: menopause, estrogens, bazedoxifene, β cell function, diabetes
Observational studies and large randomized controlled trials suggest that menopausal hormone therapy (MHT) reduces adiposity, improves insulin resistance (IR), and delays the incidence of type 2 diabetes (T2D) [1–6]. However, using general estrogen therapy to prevent diabetes in women is neither recommended nor approved by the Food and Drug Administration (FDA). Therefore, treatments that provide the beneficial effects of estrogens on glucose homeostasis without adverse effects are needed. Selective estrogen receptor modulators (SERMs) are compounds that exert tissue-selective estrogen receptor agonist or antagonist activity. For example, bazedoxifene (BZA) is a SERM that exhibits estrogen agonist activity in bone but estrogen antagonist activity in breast and uterus. The combination of conjugated estrogens (CE) with BZA is approved by the FDA for treatment of postmenopausal vasomotor symptoms and prevention of osteoporosis [7, 8]. The combination CE and BZA (CE/BZA) provides the benefits of estrogen therapy such as reducing hot flashes and vulvar–vaginal atrophy, preventing menopausal osteoporosis while simultaneously protecting the endometrium and breast from estrogen stimulation and without the need of a progestin [9–15]. Using a mouse model of postmenopausal metabolic syndrome, we reported that CE/BZA prevents estrogen deficiency-induced obesity, T2D, and nonalcoholic fatty liver disease as efficiently as CE alone [16]. We found that CE/BZA increased fat oxidation and energy expenditure, thus preventing ectopic fat accumulation in liver and skeletal muscle and improving IR and glucose intolerance. In addition, in female diabetic mouse models of insulin deficiency, CE/BZA prevents β cell failure and delays diabetes [17]. The current randomized, double-blind, placebo-controlled pilot trial was designed to assess the effect of a 12-week treatment with CE/BZA vs placebo on body composition, glucose homeostasis, and markers of inflammation in obese postmenopausal women.
1. Participants and Methods
A. Study Population
Participants were overweight or obese postmenopausal women 50 to 60 years of age (n = 12), symptomatic (hot flashes, vaginal dryness) or asymptomatic, with fasting glucose <125 mg/dL, and a normal mammogram within the past 12 months. Ten women had spontaneous menopause and 2 had surgical menopause. Key exclusion criteria were recent weight changes, current use of drugs that promote weight changes, MHT use within 3 months, contraindications to estrogens (history of thromboembolic disorder, coronary artery or cerebrovascular disease, clotting disorder, chronic liver disease, history of breast or uterine cancer, or unexplained vaginal bleeding). Menopause was defined as either (i) women with intact uterus and last menstrual period >1 year but <5 years or (ii) women with partial or complete hysterectomy with menopausal symptoms for >1 year and <5 years. Anthropometric data were measured at baseline and after 4 weeks, 8 weeks, and 12 weeks of treatment. Although 18 women were enrolled in the study, 4 withdrew from the study and 2 did not return to their planned study visit, resulting in 12 women completing the study. All volunteers gave their informed written consent to participate in the study. The Tulane University’s Institutional Review Board reviewed and approved the protocols. Investigational New Drug exemption was granted by the FDA to use CE/BZA in women with history of hysterectomy.
B. Randomization, Intervention, and Study Calendar
An independent biostatistician provided a random number table to the unblinded pharmacist for randomization. The random number table was generated based on the total number of subjects to be enrolled in a 1:1 ratio for placebo vs CE/BZA. The unblinded pharmacist used the random number table in sequential order for randomization assignment as subjects were enrolled. The research team remained blinded to the randomization assignment throughout the study. Treatments were CE 0.45 mg/BZA 20 mg, vs placebo. Drug was supplied by Pfizer Inc. Study design and visit schedule are shown in Figure 1.
Figure 1.
Visit schedule. *, Visit 1 and 2 can be combined if participant has had recent labs.
C. Measurement of Body Composition
Bone density, body composition, adipose, and lean mass indices were measured with a dual-energy X-ray absorptiometry (DXA) apparatus (Hologic Inc., Bedford, MA) by trained and certified research staff. The machine was calibrated daily, and software and hardware were kept up to date during the data collection process.
D. IV Glucose Tolerance Test
All women underwent an IV glucose tolerance test (IVGTT) at baseline and 12 weeks after treatment. After a 12-hour fast, blood was sampled from the antecubital vein at −15, −5, −1, 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 20, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 minutes for measurement of serum glucose, insulin, and C-peptide concentrations (33 blood samples for glucose and insulin measurements over 3.5 hours and 16 samples for C-peptide measurement over the first 35 minutes). A bolus of dextrose was given at 11.4 g/m2 body surface area over 1 minute into the antecubital vein (at time zero) followed by a 30-second insulin infusion given at 0.03 U/kg body weight (at 20 minutes postdextrose injection). Note that for technical reasons, the IVGTT serum was processed in only 11 of 12 subjects (CE/BZA n = 6).
E. Laboratory Measurements
Plasma glucose was analyzed using an automated glucose analyzer (Quest Diagnostics Laboratory, Irving, TX). Insulin and C-peptide concentrations were quantified by ELISA (EMD Millipore Corporation, Billerica, MA) [18, 19]. Serum markers of IR and inflammation were determined using commercially available ELISA kits: fibroblast growth factor 21 (FGF-21) (R&D Systems, Inc., Minneapolis, MN) [20], leptin, plasminogen activator inhibitor-1 (PAI-1), retinol binding protein 4 (RBP4), and lipocalin 2 (LCN2) (Boster Biological Technology, Pleasanton, CA) [21–24], adiponectin (Crystal Chem, Elk Grove Village, IL) [25], and C-reactive protein (CRP) (Thermo Fisher Scientific Inc., Carlsbad, CA) [26], Gla-type and Glu-type Osteocalcin (Takara Bio Inc., Mountain View, CA) [27, 28], and Intact Osteocalcin (Alpco, Salem, NH) [29]. Quantitative determination of thiobarbituric acid reactive substances (TBARS) was done using a TBARS assay kit (ZeptoMetrix Corporation, Buffalo, NY). All tests were run in the same assay in a batch other than insulin and C-peptide. Because of large numbers of insulin and C-peptide samples, we those combining samples before treatment and 12 weeks after treatment in one assay. All samples were processed blindly to the clinical status of the participants.
F. Data Analysis
Measures of insulin secretion, elimination, and sensitivity were derived by mathematical modeling analysis of the IVGTT glucose and insulin concentration profiles using the minimal model (MINMOD Millennium, Pasadena, CA, version 6.02) as previously described [30]. Four major independent parameters were derived from the analysis by the minimal model: the insulin sensitivity (SI) index, the acute insulin response to glucose (AIRg), the disposition index (DI), and glucose effectiveness (SG). Homeostatic model assessment (HOMA)-B and HOMA-IR were derived from the HOMA model as described by Matthews et al. [31]. HOMA-β cell function [HOMA-B = 20 × fasting insulin/(fasting glucose/18–3.5)] and HOMA-IR [HOMA-IR = fasting glucose × fasting insulin/405]. HOMA2-β and HOMA2-IR (using fasting C-peptide and glucose) were calculated with a HOMA2 calculator software (version 2.2.3) released by the Diabetes Trials Unit, University of Oxford (see www.dtu.ox.ac.uk/homacalculator/download.php). The measurement of insulin secretion, fasting insulin clearance (FIC), and glucose-stimulated insulin clearance (GSIC) over time zero to 20 minutes of the IVGTT were calculated by C-peptide area under the curve (AUC20), molar ratio of fasting C-peptide to insulin, and molar ratio of C-peptide to insulin AUC20, respectively. Fasting insulin, glucose, and C-peptide were calculated as an average of three values drawn 5 minutes apart, at −15, −5, and −1 minute.
F-1. Statistical analyses
Medians and interquartile ranges (IQRs) of baseline characteristics were calculated; differences in baseline characteristics between groups were tested using Wilcoxon rank sum tests. Differences in outcomes between baseline and 12 weeks were calculated; medians and IQRs of baseline, 12-week, and 12-week minus baseline outcome data are presented. Shapiro-Wilk tests were used to test for normality of all outcome variables. Because the null hypothesis of normality was rejected for some outcomes, Wilcoxon rank sum tests were used to test for differences between groups for all outcomes. Wilcoxon rank sum tests were also used to test for differences in baseline hormones by baseline metabolic parameters. All analyses were conducted using Stata 14.2 (StataCorp, College Station, TX).
2. Results
Twelve women received medication and completed the 12-week study between October 2015 and March 2018. There were no differences between the two groups at baseline (Table 1). Treatment with CE/BZA did not produce any change in body composition as assessed by anthropometric measures [weight, body mass index (BMI), waist circumference, and waist-to-hip ratio] or DXA compared with placebo (Table 2) [32]. No change in total fat mass or visceral fat mass was observed. As previously reported, women assigned to CE/BZA exhibited a trend toward increase in bone mineral density (P = 0.067), t score and z score [33] compared with the placebo arm [32]. Women assigned to CE/BZA exhibited a decrease in Gb and an increase in β cell function assessed by HOMA-B, which utilizes insulin, associated with a trend toward increase in β cell function assessed by HOMA2-B (P = 0.068), which utilizes C-peptide (Table 3;Fig. 2). There was no difference between the two groups in IR assessed by HOMA-IR or HOMA2-IR. Glucose homeostasis was further assessed using an IVGTT followed by mathematical modeling using the MINMOD Millennium software. Unlike results obtained by HOMA-B, and HOMA2-B, the AIRg was not different between the CE/BZA arm and the placebo [CE/BZA vs placebo: 189 (−31 to 444) vs −25 (−47 to −9), P = 0.273] (Table 3). However, unlike results obtained with HOMA-IR and HOMA2-IR, SI increased in the placebo arm. No changes were seen between groups in DI, SG, the basal insulin concentration, the removal rate of insulin from the interstitial space, the movement of circulating insulin to the interstitial space, glucose at time zero, and SG at zero insulin. FIC [CE/BZA vs placebo: −3.50 (−11.56 to 0.78) vs −0.25 (−0.70 to −0.0), P = 0.715] and GSIC [CE/BZA vs placebo: −32.8 (−123.6 to 6.9) vs −1.85 (−20.5 to 25.8), P = 0.201] were not different in the CE/BZA arm compared with placebo (Table 3). The effect of the treatment-related differences on serum markers of IR and inflammation are shown in Table 4. There were no differences between the two groups in systemic markers of IR (leptin, adiponectin, osteocalcin, RBP4, FGF-21), inflammation (LCN2, PAI-1, CRP), or oxidative stress (TBARS).
Table 1.
Baseline Characteristics
| Treatment | ||||
| Overall | Placebo (n = 5) | CE/BZA (n = 7) | P a | |
| Race, n, Caucasian/black | 10/2 | 5/0 | 5/2 | 0.470 |
| Age, y | 54.5 (53, 56.5) | 55 (54, 58) | 54 (52, 56) | 0.188 |
| Years since LMP | 2.7 (1.7, 4.4) | 4.5 (2.1, 4.8) | 2.0 (1.4, 3.8) | 0.123 |
| Waist-to-hip ratio | 0.85 (0.82, 0.94) | 0.84 (0.82, 0.85) | 0.9 (0.82, 0.95) | 0.290 |
| BMI, kg/m2 | 32.4 (30.7, 37.8) | 31.2 (30.7, 38.6) | 33 (30.6, 37) | 0.935 |
| WC, cm | 108.0 (91.7, 115.6) | 106.7 (92, 116.8) | 109.2 (91.4, 114.3) | 0.808 |
| Fasting glucose, mg/dL | 93.5 (83.5, 108) | 88 (84, 98) | 94 (83, 114) | 0.465 |
| TC, mg/dL | 211.5 (190.5, 231.5) | 216 (202, 245) | 208 (167, 218) | 0.291 |
| HDL, mg/dL | 63 (49.5, 71) | 66 (58, 73) | 60 (45, 69) | 0.370 |
| LDL, mg/dL | 124 (111.5, 147.5) | 128 (115, 147) | 120 (99, 148) | 0.465 |
| Triglycerides, mg/dL | 113.5 (82.5, 128.5) | 103 (102, 125) | 124 (81, 131) | 0.935 |
Data are expressed as median (IQR).
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LMP, last menstrual period; TC, total cholesterol; WC, waist circumference.
From Wilcoxon rank sum test.
Table 2.
Effect of CE/BZA on Body Composition
| Baseline | Week 12 | Change from Baseline to Week 12 | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 5) | CE/BZA (n = 7) | Placebo (n = 5) | CE/BZA (n = 7) | Placebo (n = 5) | CE/BZA (n = 7) | P a | |
| Anthropometric measurements | |||||||
| WC, cm | 106 (93.5, 114.3) | 109.2 (91.4, 116.8) | 107.4 (91, 111.8) | 106 (86.4, 112) | −2.5 (−2.5, 0.8) | −4.2 (−7.6, 0) | 0.290 |
| Weight, kg | 83.8 (75.8, 105.9) | 90.5 (79.5, 104.9) | 77.6 (75.6, 104.5) | 92.3 (78.2, 103.8) | −0.9 (−1.4, −0.2) | −0.4 (−1.4, −0.5) | 0.372 |
| WHR | 0.87 (0.80, 0.90) | 0.92 (0.8, 0.96) | 0.82 (0.8, 0.92) | 0.86 (0.8, 0.97) | −0.00 (−0.02, 0.00) | −0.05 (−0.06, 0.03) | 0.465 |
| BMI, kg/m2 | 31.6 (30.4, 38.9) | 32.6 (31.1, 37.0) | 31.0 (30.1, 38.4) | 32.9 (30.5, 36.8) | −0.5 (−0.6, −0.3) | −0.2 (−0.5, 0.3) | 0.223 |
| DXA | |||||||
| Body composition | |||||||
| Total fat mass, g | 33,163 (31,749, 49,295) | 36,918 (33,221, 38,895) | 33,630 (31,199, 47,887) | 36,864 (34,883, 39,869) | −1408 (−1932, 467) | 273 (−571, 974) | 0.361 |
| Total mass in g | 84,587 (75,177, 105,711) | 9,3007 (78,332, 104,466) | 78,073 (75,141, 105,095) | 93979 (78,280, 104,520) | −616 (−670, −36) | 75 (−52, 282) | 0.273 |
| Total % fat | 44.1 (41.4, 46.6) | 41.2 (37.8, 45.3) | 44.8 (42.3, 45.6) | 41.1 (38.8, 44.6) | −1 (−1.3, 0.7) | 0 (−0.7, 0.9) | 0.361 |
| A fat mass, g | 3227 (2596, 4608) | 3276 (2473, 4199) | 3096 (2804, 4522) | 3631 (3084, 3975) | −131 (−452, −86) | 1.5 (−226, 611) | 0.465 |
| A total mass, g | 6821 (6307, 9303) | 6965 (6065, 10375) | 6725 (6515, 9079) | 8855 (6622, 9276) | −224 (−445, −96) | 280 (−74, 1183) | 0.201 |
| A % fat | 47.3 (42.6, 47.5) | 42.6 (39.4, 45.5) | 45.3 (43, 46) | 42 (40.9, 47.3) | −1.3 (−2.2, 0.3) | 1.1 (−3.5, 3.5) | 0.234 |
| G fat mass, g | 6098 (5620, 7879) | 5959 (5567, 6916) | 5648 (5464, 7701) | 5505 (5461, 6370) | −178 (−189, −116) | −26 (−550, 279) | 0.361 |
| G total mass, g | 14926 (12,485, 17,238) | 14896 (12,570, 16,043) | 13780 (12,121, 17,181) | 13901 (12,762, 16,668) | −57 (−364, −45) | 89 (−1134, 403) | 0.584 |
| G % fat | 45.7 (45, 48.1) | 42.8 (38, 50) | 46.6 (44.8, 47.3) | 42.3 (38.2, 49.7) | −0.90 (−1.30, −0.80) | 0.35 (−0.30, 2) | 0.144 |
| Adipose indices | |||||||
| Total body % fat | 44.1 (41.4, 46.6) | 43.2 (39.1, 45.3) | 44.8 (42.3, 45.6) | 40.8 (38.8, 44.6) | −1 (−1.3, 0.70) | −0.30 (−0.70, 0.30) | 0.602 |
| A/G ratio | 0.95 (0.95, 0.98) | 1.04 (0.97, 1.16) | 0.93 (0.92, 0.97) | 1.06 (0.98, 1.07) | −0.02 (−0.03, −0.01) | 0.01 (−0.05, 0.02) | 0.602 |
| VAT mass, g | 731 (332, 947) | 584 (539, 629) | 708 (285, 813) | 630 (543, 680) | −47 (−134, −23) | 46 (−63, 118) | 0.289 |
| VAT volume, cm3 | 790 (359, 1023) | 631 (583, 680) | 766 (308, 879) | 681 (587, 735) | −51 (−144, −24) | 50 (−68, 127) | 0.289 |
| VAT area, cm2 | 152 (69, 196) | 121 (112, 131) | 147 (59, 169) | 131 (113, 141) | −10 (−27, −5) | 10 (−13, 25) | 0.289 |
Data are expressed as median (IQR).
Abbreviations: A, android; BMI, body mass index; G, gynoid; VAT, visceral adipose tissue; WC, waist circumference; WHR, waist-to-hip ratio.
From Wilcoxon rank sum test.
Table 3.
Effect of CE/BZA on Glucose Homeostasis
| Baseline | Week 12 | Change from Baseline to Week 12 | |||||
| Placebo (n = 5) | CE/BZA (n = 6) | Placebo (n = 5) | CE/BZA (n = 6) | Placebo (n = 5) | CE/BZA (n=6) | P a | |
| IVGTT b | |||||||
| AIRg, µU/l−1. min−1 | 331 (190, 374) | 179 (135, 272) | 181 (68, 382) | 634 (104, 716) | −25 (−47, −9) | 189 (−31, 444) | 0.273 |
| Gb, mg/dL | 87.3 (81.7, 88.8) | 90.9 (84.5, 100.8) | 92 (78.5, 93.8) | 85.0 (81.3, 90.3) | 2.7 (0.9, 4.9) | −5.2 (−9.2, −1.7) | 0.029 |
| DI | 658 (497, 946) | 651 (286, 1051) | 1519 (395, 1733) | 892 (595, 1892) | 267 (28, 572) | 500 (−19, 841) | 0.855 |
| SI, µU/L−1.min−1 | 3.42 (1.76, 3.94) | 3.59 (1.25, 6.53) | 4.54 (2.85, 5.77) | 3.26 (1.60, 5.79) | 1.35 (1.12, 1.82) | −0.24 (−1.50, 0.19) | 0.029 |
| SG, min−1 | 0.01 (0.007, 0.016) | 0.016 (0.006, 0.018) | 0.016 (0.014, 0.024) | 0.013 (0.010, 0.018) | 0.008 (0.008, 0.009) | 0.002 (−0.009, 0.005) | 0.068 |
| Ib , μU/mL | 8.1 (7.3, 16.3) | 6.7 (2.3, 18.8) | 8.9 (6.5, 14.9) | 14.0 (2.6, 37.0) | 0 (−0.8, 0.8) | 1.2 (−0.7, 2.9) | 0.465 |
| P2, min−1 | 0.033 (0.025, 0.053) | 0.026 (0.020, 0.031) | 0.032 (0.015, 0.04) | 0.018 (0.005, 0.045) | −0.010 (−0.010, −0.000) | −0.001 (−0.019, 0.025) | 0.855 |
| P3, µU/L.min−2 | 9.97e-6 (5.72e-6, 1.83e-5) | 8.53e-6 (2.58e-6, 1.99e-5) | 9.14e-6 (8.84e-6, 1.81e-5) | 4.59e-6 (1.96e-6, 7.81e-6) | −1.38e-7 (−8.73e-7, 3.43e-6) | −1.99e-7 (−8.75e-6, 8.53e-7) | 0.584 |
| G0, mg/dL | 200 (198, 219) | 249 (228, 254) | 217 (215, 254) | 251 (234, 268) | 14.6 (1.5, 46.2) | 4.2 (−12.7, 30.0) | 0.273 |
| GEZI, min−1 | 5.40e-3 (3.88e-3, 1.32e-2) | 9.93e-3 (5.31e-3, 1.57e-2) | 1.15e-2 (9.74e-3, 1.96e-2) | 8.85e-3 (6.94e-3, 9.26e-3) | 6.84e-3 (6.45e-3, 7.61e-3) | −5.80e-4 (−7.29e-3, 3.23e-3) | 0.100 |
| HOMA | |||||||
| β cell, µU/mM | 110.7 (101.3, 432.4) | 61.8 (32.9, 315.6) | 110.8 (75.8, 392.6) | 299.1 (52.0, 636.2) | −25.5 (−39.9, 0.1) | 18.5 (−0.9, 320.6) | 0.045 |
| IR, mM.µU/L−2 | 1.78 (1.59, 2.98) | 1.67 (0.51, 3.93) | 2.03 (1.50, 2.75) | 2.81 (0.53, 8.01) | −0.01 (−0.10, 0.24) | 0.15 (−0.18, 0.34) | 0.855 |
| HOMA2 | |||||||
| % β cell | 126 (125, 178) | 104 (83, 125) | 128 (101, 171) | 134 (77, 154) | −9.9 (−22.8, 1.7) | 12.1 (−0.4, 42.9) | 0.068 |
| IR | 1.91 (1.75, 2.02) | 1.50 (1.4, 2.14) | 1.75 (1.66, 1.95) | 1.76 (1.61, 1.88) | −0.25 (−0.27, 0.20) | 0.09 (−0.11, 0.31) | 0.584 |
| Insulin clearance | |||||||
| FICc | 13.9 (8.2, 16.6) | 13.6 (4.9, 26.8) | 13.9 (8.0, 15.9) | 10.5 (3.9, 20.7) | −0.25 (−0.70, −0.00) | −3.50 (−11.56, 0.78) | 0.715 |
| GSICd | 127 (93, 219) | 154 (79, 355) | 153 (134, 217) | 122 (57, 236) | −1.85 (−20.5, 25.8) | −32.8 (−123.6, 6.9) | 0.201 |
Data are expressed as median (IQR). Note that for technical reasons, the IVGTT serum was processed in only 11 of 12 subjects (CE/BZA n = 6).
Abbreviations: G0, glucose at time zero; GEZI, glucose effectiveness at zero insulin; Ib, basal insulin concentration; P2, removal rate of insulin from the interstitial space; P3, movement of circulating insulin to the interstitial space.
From Wilcoxon rank sum test.
IVGTT data derived by MINMOD Millennium software.
FIC derived from fasting C-peptide to insulin ratio.
GSIC derived from molar ratio of C-peptide to insulin AUC over first 20 min of IVGTT.
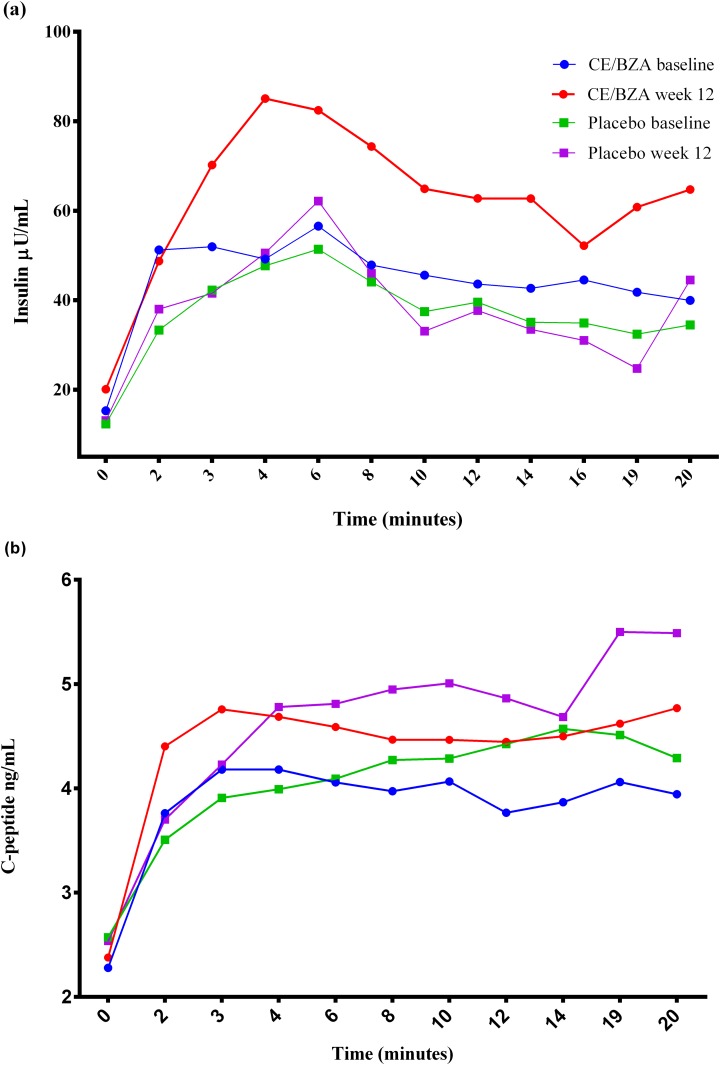
Figure 2.
Insulin and C-peptide concentrations. (a) Mean serum insulin concentrations first 20 min of the IVGTT. (b) Mean serum C-peptide concentrations first 20 min of the IVGTT.
Table 4.
Effect of CE/BZA on Insulin Resistance and Inflammation Markers
| Baseline | Week 12 | Change from Baseline to Week 12 | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 5) | CE/BZA (n = 7) | Placebo (n = 5) | CE/BZA (n = 7) | Placebo (n = 5) | CE/BZA (n = 7) | P a | |
| Serum biomarkers | |||||||
| Leptin ng/mL | 54.4 (53.1, 62.1) | 54.3 (38.7, 60.1) | 64.5 (46.8, 72.6) | 56.6 (41.4, 68.5) | 0.7 (−0.1, 10.5) | −1.6 (−7.2, 9.5) | 0.465 |
| Adiponectin µg/mL | 12.9 (7.7, 13.1) | 7.5 (4.4, 14.6) | 10.3 (5.9, 11.9) | 7.4 (3, 10.2) | −2.5 (−2.6, −0.4) | −1.4 (−2.8, −0.5) | 0.808 |
| LAR | 4.12 (3.87, 8.06) | 7.99 (5.06, 8.80) | 7.93 (5.42, 8.15) | 8.92 (5.59, 21.90) | 1.55 (0.02, 4.03) | 2.71 (−0.04, 4.96) | 0.808 |
| PAI-1 ng/mL | 10.1 (10.1, 10.6) | 14 (9.4, 16.7) | 11.4 (9.4, 13.4) | 8.9 (8.8, 14.9) | 1.3 (−0.4, 3.3) | −1.8 (−5.2, −1.4) | 0.088 |
| FGF-21 pg/mL | 361 (316, 556) | 261 (189, 415) | 241 (227, 330) | 171 (149, 315) | −89 (−226, −48) | −134 (−208, 24) | 0.685 |
| LCN2 ng/mL | 17.9 (16.7, 22.7) | 14.8 (13.5, 15.7) | 17.8 (12.9, 20.9) | 13.8 (11.5, 24.1) | −3.8 (−4.9, −2.2) | −1.7 (−3, 3.5) | 0.223 |
| CRP mg/mL | 4.58 (1.83, 6.78) | 3.49 (2.34, 4.59) | 4.67 (1.44, 5.96) | 2.82 (1.93, 3.49) | −0.39 (−0.82, −0.06) | −0.41 (−2.66, 0.45) | 0.935 |
| RBP4 µg/mL | 40.2 (32.7, 46.9) | 32.9 (15.9, 35.3) | 28.7 (28.6, 45.8) | 28.1 (19.7, 44.6) | −4.0 (−24.1, 4.6) | −0.4 (−5.6, 14.5) | 0.465 |
| TBARS nmol/mL | 6.1 (5.5, 8.4) | 6.5 (4.0, 8.2) | 5.5 (4.9, 5.9) | 3.8 (2.2, 7.1) | −1.2 (−1.2, 0.0) | −1.7 (−3.3, −0.4) | 0.372 |
| Intact OCN ng/mL | 10.1 (7.4, 11.3) | 5.2 (1.5, 9.5) | 10.4 (6.3, 10.6) | 4.6 (4.3, 10.3) | −0.9 (−1.1, 2.7) | 0.8 (−0.7, 3.1) | 0.465 |
| Glu-OC ng/mL | 4.6 (4.6, 8.6) | 4.4 (2.5, 5.9) | 3.8 (3.4, 24.4) | 4.8 (1.8, 11.4) | −1.2 (−1.9, 2.1) | 0.0 (−0.7, 4.7) | 0.372 |
| Gla-OC ng/mL | 4.0 (3.4, 4.4) | 4.2 (2.9, 4.3) | 5.9 (3.6, 7.0) | 4.2 (3.6, 4.9) | 1.5 (0.5, 3.0) | 0.6 (−0.6, 0.8) | 0.168 |
| Glu/Gla ratio | 1.05 (0.66, 1.36) | 0.98 (0.80, 1.30) | 0.9 3 (0.44, 1.28) | 0.98 (0.58, 2.27) | −0.10 (−0.42, 0.62) | 0.04 (−0.14, 1.29) | 0.685 |
Data are expressed as median (IQR).
Abbreviations: Gla-OC, carboxylated osteocalcin; Glu-OC, undercarboxylated osteocalcin; LAR, leptin:adeponectin ratio; OCN, osteocalcin.
From Wilcoxon rank sum test.
3. Discussion
The main finding of this study is treatment of obese menopausal women with the combination of CE with BZA improves β cell function [calculated by HOMA-B (P = 0.045) and to a lesser extent by HOMA2-B (P = 0.068)] and lowers Gb. Large randomized controlled trials suggest estrogen therapy with or without a progestin reduces the incidence of T2D in postmenopausal women [1–3, 6, 34–36]. The exact mechanism of this antidiabetic effect is not understood clearly but could involve the beneficial effect of estrogen therapy on pancreatic β cell function and insulin secretion in postmenopausal women [6]. Indeed, our findings are consistent with preclinical studies showing that estradiol provides pancreatic islet protection from metabolic injuries in culture and in vivo [17, 37–43]. Estrogens also promote human islet protection in an in vivo diabetic environment [37, 44]. Additionally, BZA, a SERM, displays estrogen agonistic activity in female β cells, thus providing the beneficial effects of estrogens on β cell survival and insulin secretion [17]. Accordingly, treatment with CE/BZA prevents T2D induced by estradiol deficiency and Western diet in female mice [16], and mitigates β cell apoptosis, thus maintaining insulin secretion and delaying diabetes onset in female diabetic mouse models of β cell failure [17, 45]. CE and BZA provide similar protection in cultured islets from female human donors [17]. Therefore, the observed improvement in HOMA-B in menopausal women assigned to CE/BZA may reflect protection of β cell function. Another randomized controlled trial found no effect of CE/BZA on blood glucose in postmenopausal women [11]. However, the study participants were not obese and measures of β cell function were not reported. Surprisingly, we observe no effect of CE/BZA on the AIRg. However, the AIRg explores the IV (exogenous) glucose stimulation of the β cell. The HOMA-B reflects a different aspect of β cell function, i.e., the fasting insulin secretion in response to endogenous glucose production. Therefore, it is possible that CE/BZA improves fasting insulin secretion, which is not observed during IV glucose infusion.
Because insulin and C-peptide are secreted in equimolar quantities but only insulin, not C-peptide, is extracted by the liver, one can quantify prehepatic insulin secretion and hepatic insulin clearance from the ratio of plasma insulin and C-peptide concentrations. In menopausal women, studies are consistent with the combination of estrogens and progestins enhancing hepatic insulin clearance [46–48]. Here, women assigned to CE/BZA do not show any difference in insulin clearance compared with women assigned to placebo, both in fasting and glucose-stimulated states (Fig. 2). This may explain why the HOMA2-B, which uses C-peptide, is not increased substantially (Table 3). Although preclinical studies have shown that treatment with the CE/BZA improves markers of inflammation and ameliorates IR [16], our study did not show improvement in IR and inflammation. Insulin sensitivity assessed by mathematical modeling using the MINMOD increased in the placebo arm, which likely occurred by chance as is often the case in small and short-term studies. There are limitations to our pilot study. First, the small sample size (n = 12) associated with high dropout rate (33%) limits the power to detect findings and some findings may have occurred by chance. Second, the finding of significant P values may have been influenced by the multiple comparisons. Finally, treatment with CE and BZA lasted for only 12 weeks and longer duration of treatment may be needed to observe changes.
In conclusion, a 12-week treatment with CE/BZA in obese menopausal women improves β cell function (HOMA-B) and Gb without change in AIRg, HOMA-IR, DI, insulin clearance, body composition, or markers of systemic inflammation. A larger study is warranted.
Acknowledments
Financial Support: The study was supported by an investigator-initiated award from Pfizer, Inc to F.M.J. F.M.J. was also supported by National Institutes of Health (NIH) awards DK074970 and DK107444 and a Department of Veterans Affairs Merit Review Award (#BX003725). D.L. was supported by NIH Award K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Scholar and U54 GM104940 from the National Institute of General Medical Sciences, which funds the Louisiana Clinical and Translational Science Center. K.M.U. was supported by the Department of Veteran Affairs.
Clinical Trial Information: ClinicalTrials.gov no. NCT02237079 (registered 11 September 2014).
Disclosure Summary: Dr. Mauvais-Jarvis was a scientific advisory board member of Pfizer, Inc. The other authors declare no conflict of interests.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References and Notes.
Glossary
Abbreviations:
- AIRg
acute insulin response to glucose
- AUC
area under the curve
- BZA
bazedoxifene
- BMI
body mass index
- CE
conjugated estrogens
- CRP
C-reactive protein
- DI
disposition index
- DXA
dual-energy X-ray absorptiometry
- FDA
Food and Drug Administration
- FGF-21
fibroblast growth factor 21
- FIC
fasting insulin clearance
- G0
glucose at time zero
- Gb
basal glucose concentration
- GEZI
glucose effectiveness at zero insulin
- GSIC
glucose stimulated insulin clearance
- HDL
high-density lipoprotein
- HOMA
homeostatic model assessment
- Ib
basal insulin concentration
- IQR
interquartile range
- IR
insulin resistance
- IVGTT
IV glucose tolerance test
- LCN2
lipocalin 2
- LDL
low-density lipoprotein
- LMP
last menstrual period
- MHT
menopause hormone therapy
- P2
removal rate of insulin from the interstitial space
- P3
the movement of circulating insulin to the interstitial space
- PAI-1
plasminogen activator inhibitor-1
- RBP4
retinol binding protein 4
- SG
glucose effectiveness
- SI
insulin sensitivity
- SERM
selective estrogen receptor modulator
- T2D
type 2 diabetes
- TBARS
thiobarbituric acid reactive substances
- TC
total cholesterol
- WC
waist circumference
- WHR
waist-to-hip ratio
Contributor Information
Dragana Lovre, Email: dlovre@tulane.edu.
Franck Mauvais-Jarvis, Email: fmauvais@tulane.edu.
References and Notes
- 1. Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E; Heart and Estrogen/progestin Replacement Study. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. [DOI] [PubMed] [Google Scholar]
- 2. Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV; Women’s Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. [DOI] [PubMed] [Google Scholar]
- 3. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. [DOI] [PubMed] [Google Scholar]
- 4. Manson JE, Rimm EB, Colditz GA, Willett WC, Nathan DM, Arky RA, Rosner B, Hennekens CH, Speizer FE, Stampfer MJ. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2(5):665–673. [DOI] [PubMed] [Google Scholar]
- 5. de Lauzon-Guillain B, Fournier A, Fabre A, Simon N, Mesrine S, Boutron-Ruault MC, Balkau B, Clavel-Chapelon F. Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort. Diabetologia. 2009;52(10):2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santen RJ, Kagan R, Altomare CJ, Komm B, Mirkin S, Taylor HS. Current and evolving approaches to individualizing estrogen receptor-based therapy for menopausal women. J Clin Endocrinol Metab. 2014;99(3):733–747. [DOI] [PubMed] [Google Scholar]
- 8. Komm BS, Mirkin S. Evolution of the tissue selective estrogen complex (TSEC). J Cell Physiol. 2013;228(7):1423–1427. [DOI] [PubMed] [Google Scholar]
- 9. Komm BS. A new approach to menopausal therapy: the tissue selective estrogen complex. Reprod Sci. 2008;15(10):984–992. [DOI] [PubMed] [Google Scholar]
- 10. Komm BS, Mirkin S. Incorporating bazedoxifene/conjugated estrogens into the current paradigm of menopausal therapy. Int J Womens Health. 2012;4:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, Constantine G. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038. [DOI] [PubMed] [Google Scholar]
- 12. Gruber C, Gruber D. Bazedoxifene (Wyeth). Curr Opin Investig Drugs. 2004;5(10):1086–1093. [PubMed] [Google Scholar]
- 13. Komm BS, Vlasseros F, Samadfam R, Chouinard L, Smith SY. Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone. 2011;49(3):376–386. [DOI] [PubMed] [Google Scholar]
- 14. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052. [DOI] [PubMed] [Google Scholar]
- 15. Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149(12):6084–6091. [DOI] [PubMed] [Google Scholar]
- 16. Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab. 2014;3(2):177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu B, Allard C, Alvarez-Mercado AI, Fuselier T, Kim JH, Coons LA, Hewitt SC, Urano F, Korach KS, Levin ER, Arvan P, Floyd ZE, Mauvais-Jarvis F. Estrogens promote misfolded proinsulin degradation to protect insulin production and delay diabetes. Cell Reports. 2018;24(1):181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RRID:AB_2800327. https://scicrunch.org/resolver/RRID:AB_2800327.
- 19. RRID:AB_2800332. https://scicrunch.org/resolver/RRID:AB_2800332.
- 20. RRID:AB_2783729. https://scicrunch.org/resolver/RRID:AB_2783729.
- 21. RRID:AB_2800328. https://scicrunch.org/resolver/RRID:AB_2800328
- 22. RRID:AB_2800329. https://scicrunch.org/resolver/RRID:AB_2800329.
- 23. RRID:AB_2800330. https://scicrunch.org/resolver/RRID:AB_2800330.
- 24. RRID:AB_2800331. https://scicrunch.org/resolver/RRID:AB_2800331.
- 25. RRID:AB_2800326. https://scicrunch.org/resolver/RRID:AB_2800326.
- 26. RRID:AB_2575140. https://scicrunch.org/resolver/RRID:AB_2575140.
- 27. RRID:AB_2800333. https://scicrunch.org/resolver/RRID:AB_2800333.
- 28. RRID:AB_2800334. https://scicrunch.org/resolver/RRID:AB_2800334.
- 29. RRID:AB_2800325. https://scicrunch.org/resolver/RRID:AB_2800325.
- 30. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003–1015. [DOI] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 32. Lovre D. Supplemental Table 1- Effect of CE/BZA on BMD and Lean Indices. https://figshare.com/articles/Supplemental_Table_1_docx/7719206/1. Accessed 29 March 2019.
- 33. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418. [DOI] [PubMed] [Google Scholar]
- 34. Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, Bush TL. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21(10):1589–1595. [DOI] [PubMed] [Google Scholar]
- 35. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806. [DOI] [PubMed] [Google Scholar]
- 37. Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74(9):1252–1259. [DOI] [PubMed] [Google Scholar]
- 38. Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103(24):9232–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58(10):2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121(8):3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou Z, Ribas V, Rajbhandari P, Drew BG, Moore TM, Fluitt AH, Reddish BR, Whitney KA, Georgia S, Vergnes L, Reue K, Liesa M, Shirihai O, van der Bliek AM, Chi NW, Mahata SK, Tiano JP, Hewitt SC, Tontonoz P, Korach KS, Mauvais-Jarvis F, Hevener AL. Estrogen receptor α protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J Biol Chem. 2018;293(13):4735–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–351. [DOI] [PubMed] [Google Scholar]
- 43. Kilic G, Alvarez-Mercado AI, Zarrouki B, Opland D, Liew CW, Alonso LC, Myers MG Jr, Jonas JC, Poitout V, Kulkarni RN, Mauvais-Jarvis F. The islet estrogen receptor-α is induced by hyperglycemia and protects against oxidative stress-induced insulin-deficient diabetes. PLoS One. 2014;9(2):e87941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu S, Kilic G, Meyers MS, Navarro G, Wang Y, Oberholzer J, Mauvais-Jarvis F. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia. 2013;56(2):370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim J, Mauvais-Jarvis F. The combination of conjugated equine estrogens with bazedoxifene prevents streptozotocin-induced diabetes in female mice. Matters. 2016;2(6):1–6. [Google Scholar]
- 46. Godsland IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, Stevenson JC. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism. 1993;42(7):846–853. [DOI] [PubMed] [Google Scholar]
- 47. Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC. Effects of oral and transdermal 17beta-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism. 2000;49(6):742–747. [DOI] [PubMed] [Google Scholar]
- 48. Godsland IF, Manassiev NA, Felton CV, Proudler AJ, Crook D, Whitehead MI, Stevenson JC. Effects of low and high dose oestradiol and dydrogesterone therapy on insulin and lipoprotein metabolism in healthy postmenopausal women. Clin Endocrinol (Oxf). 2004;60(5):541–549. [DOI] [PubMed] [Google Scholar]