Abstract
Context
No consensus has been reached regarding the glucocorticoid (GC) to use for congenital adrenal hyperplasia (CAH) during adulthood. Dexamethasone (DEX), because of its longer half-life, could improve compliance; however, no data are available regarding the long-term effects of DEX therapy.
Objective
To analyze the metabolic effect of DEX therapy for adults with CAH.
Design
Retrospective analysis of a CAH cohort receiving DEX therapy.
Setting
Medical School Hospital, São Paulo University, Brazil.
Participants
Sixty patients with well-controlled classic CAH (41 women; 30 with salt-wasting) receiving DEX after achievement of final height.
Interventions
None.
Main Outcome Measures
Clinical, laboratory, and metabolic data were compared immediately before DEX and at the last evaluation.
Results
The mean age at the last evaluation was 31.9 ± 9.6 years, and the duration of DEX therapy was 11.5 ± 4.9 years. The mean DEX dose was 0.18 ± 0.07 mg/m2/d. The body mass index SD score (1.6 ± 1.6 vs 1.5 ± 1.5 mg/m2; P = 0.65) and obesity prevalence (27% vs 27%) did not differ between evaluations. However, the waist/height ratio (WtHR) had increased from 0.54 ± 0.08 to 0.56 ± 0.1 (P = 0.001). An increase in the homeostatic model assessment for insulin resistance index (2.5 ± 1.3 vs 2.8 ± 1.7; P = 0.03) was observed and positively correlated with the WtHR (r = 0.54). The prevalence of metabolic syndrome (7% vs 10%; P = 0.7) and hypertension (15% vs 13.3%; P = 0.8) did not differ significantly between the two evaluations.
Conclusions
Long-term and low-dose DEX therapy did not lead to increases in obesity or metabolic syndrome, although it was associated with an increased WtHR and greater homeostatic model assessment for insulin resistance observed with chronic use of GCs. DEX appears to be an acceptable option to treat adult CAH.
Keywords: congenital adrenal hyperplasia, waist circumference, dexamethasone, obesity, metabolic syndrome
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is a common autosomal recessive disorder caused by mutations in the 21-hydroxylase gene (CYP21A2). These mutations result in a complex imbalance of adrenal steroids with decreased glucocorticoid (GC) secretion, accompanied by mineralocorticoid deficiency and androgen excess [1, 2]. Different mutations will cause variable degrees of residual enzymatic activity, leading to a spectrum of clinical manifestations. These manifestations have been classified into the classic form, including the salt-wasting (SW) and simple virilizing (SV) forms, and the nonclassic form (NC). The classic forms have an incidence of 1:10,000 to 1:20,000 live births [3, 4], and the NC has an estimated prevalence of 1:1,000 of the general population [5].
The aims of CAH therapy are to replace deficient hormones, prevent an adrenal crisis, and control androgen excess. A substantial interindividual variability in GC dose requirements make the achievement of these therapeutic goals a challenging task, and patients are frequently exposed to under- or overtreatment. No consensus has yet been reached regarding which type of GC and daily regimen should be used for adult patients with CAH [6–8].
Short-acting GCs such as hydrocortisone are indicated for the treatment of children with CAH because these will have less effect on the children’s growth [9]. These can also be used in adults. However, they require at least two to three daily doses and, for a life-long chronic disorder such as CAH, adherence can be an issue. Long-acting GCs such as dexamethasone (DEX) are another option for treating adults according to the Endocrine Society guidelines [8]. However, some investigators have reported that its use has been related to a greater frequency of adverse metabolic outcomes [6, 7, 10]. Therefore, hydrocortisone has been suggested as a first-choice therapy by many investigators, because it will result in fewer metabolic side effects. However, no reported studies have compared both regimens.
Relatively few studies have been performed of adults with CAH receiving long-term treatment, and many of these have reported a greater prevalence of obesity, hypercholesterolemia, insulin resistance, osteopenia, and/or hypertension than in the normal population [6, 11–14]. However, these were usually cross-sectional studies and involved patients using distinct types and regimens of GCs, which impaired the correlation of these findings with a specific type of GC. At present, no longitudinal studies with a homogeneous cohort of patients with classic CAH using DEX to evaluate its long-term metabolic effects have been performed. The objective of the present study was to analyze the metabolic effects of DEX therapy in adult patients with well-controlled CAH followed up in a single tertiary center after the diagnosis.
1. Subjects and Methods
A. Subjects
We selected 60 adult patients with classic CAH (41 women). Of the 41 women, 21 had the SV form and 20 had the SW form. All the patients had basal 17 hydroxy-progesterone (17OHP) levels >50 ng/mL at diagnosis and molecular genetic test results predicting for classic form phenotypes [15]. The inclusion criteria were the exclusive use of a short-action GC during childhood and puberty (cortisone acetate, 18 to 20 mg/m2/d); introduction of DEX therapy in elixir (0.1 mg/mL) or tablet (0.5 and 0.75 mg) formulations only after the achievement of final height; good compliance with treatment during adulthood; and adequate hormonal control [normal serum electrolytes, normal androgen levels according to sex and age, and no suppression of serum 17OHP levels (<5 ng/mL) in the previous 5 years]. The SW patients also received fludrocortisone (55.7 ± 10.6 µg/d). Mineralocorticoid replacement was monitored by the blood pressure and plasma renin activity. We excluded patients who had developed SW crises during the study period and those receiving cytochrome P450 inductors.
The local ethics committee approved the present study, and all individuals gave their written informed consent.
B. Methods
We compared the clinical and laboratory data immediately before the beginning of DEX therapy and at the last evaluation. The clinical data included the body mass index (BMI), waist/height ratio (WtHR), and blood pressure. The BMI data of all the patients were converted to the SD score (SDS) according to the World Health Organization growth reference graphs [16], because 28 of the 60 patients had initiated DEX therapy before they were 19 years old. The clinical data were compared with data extracted from a paired population matched by age and sex available from a Brazilian national health research study conducted in 2013 [17].
The laboratory data included measurements of glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) and calculation of the homeostatic model assessment for insulin resistance (HOMA-IR) index. The serum 17OHP and androstenedione levels were measured at baseline using a radioimmunoassay and at the end of the study using liquid chromatography-mass spectrometry. The intra-assay and interassay coefficients of variation ranged from 5% to 10%.
The presence of metabolic syndrome (MetS) in adults was defined in accordance with the National Cholesterol Education Program Adult Treatment Panel III as the presence of three or more of the following abnormalities: waist circumference (WC) ≥102 cm in men and ≥88 cm in women, blood pressure ≥130/85 mm Hg, fasting glucose >110 mg/dL, fasting serum TG ≥150 mg/dL, and low HDL-C levels (men, <40 mg/dL; women, <50 mg/dL) [18]. For patients aged <19 years, the modified Adult Treatment Panel III criteria [19] were used, defined as the presence of three or more of the following abnormalities: WC ≥90th percentile for age and sex, blood pressure ≥90th percentile for age and sex, fasting glucose ≥100 mg/dL, fasting serum TG ≥90th percentile for age and sex, and HDL-C levels <40 mg/dL.
C. Statistical Analysis
Data are presented as the mean ± SD, unless otherwise stated. Analyses of the metabolic profile before and after DEX therapy were undertaken using the paired Student t test or Wilcoxon signed-rank test for numerical continuous variables, as appropriate. The categorical variables were compared between groups using the χ2 test or Fisher exact test, as appropriate. Linear and multiple regression analysis were performed to evaluate the correlation between the metabolic data and DEX doses and/or the duration of treatment. Statistical analysis was performed using Minitab, version 17, software, and a P value < 0.05 was considered to indicate statistical significance.
2. Results
The mean patient age at the transition from the short-acting GC to DEX therapy was 20.5 ± 9.8 years. The mean patient age at the last evaluation was 31.9 ± 9.6 years. The mean duration of GC therapy since the diagnosis, including the short-acting GC, was 27.1 ± 7.7 years, and the mean duration of DEX therapy was 11.5 ± 4.9 years. DEX was administered once daily at bedtime, and the mean daily dose in the previous 5 years was 0.18 ± 0.07 mg/m2/d. The patients with SW also received fludrocortisone (55.7 ± 10.6 µg/d). The differences in the baseline and last hormonal 17OHP levels were not statistically significant (Table 1). However, the levels of androstenedione were lower at the last evaluation, which could suggest better compliance.
Table 1.
Metabolic and Hormonal Parameters at Baseline and Last Evaluation of Dexamethasone Therapy
| Data | Baseline | Last Evaluation | P Value |
|---|---|---|---|
| BMI-SDS | 1.6 ± 1.6 | 1.5 ± 1.5 | NS |
| Obesity, % | 27 | 27 | NS |
| WC, cm | |||
| Women | 78.6 ± 10.3 | 80.8 ± 11.5 | NS |
| Men | 92.6 ± 15.3 | 97.4 ± 17.9 | NS |
| WtHR | 0.54 ± 0.08 | 0.56 ± 0.1 | <0.01 |
| Hypertension, % | 15 | 13.3 | NS |
| Glucose, mg/dL | 83.6 ± 7.7 | 82.6 ± 10.8 | NS |
| HOMA-IR | 2.5 ± 1.3 | 2.8 ± 1.7 | 0.03 |
| TG, mg/dL | 84.2 ± 40.5 | 94.2 ± 51.1 | 0.051 |
| HDL-C, mg/dL | |||
| Women | 59.8 ± 13.1 | 58 ± 10.9 | NS |
| Men | 51.9 ± 12.8 | 49.9 ± 20 | NS |
| Low HDL-C, % | 22 | 25 | NS |
| LDL-C, mg/dL | 108.3 ± 28 | 109.9 ± 31.6 | NS |
| MetS, % | 7 | 10 | NS |
| 17-OHP, ng/mL | 53.2 (31.8–79) | 45.3 (34.9–59.1) | NS |
| Androstenedione, ng/mL | 5.9 ± 6.3 | 2.8 ± 1.2 | 0.001 |
Data presented as percentage, mean ± SD, or median (interquartile range), as appropriate.
Abbreviations: LDL-C, low-density lipoprotein cholesterol; NS, not significant.
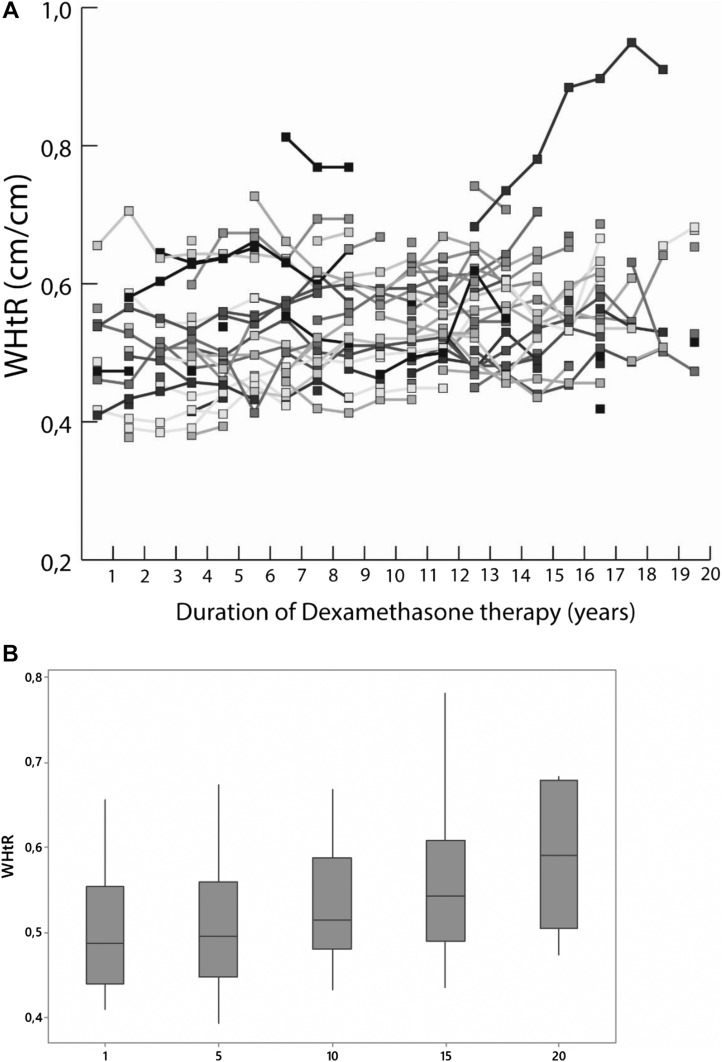
When we compared the metabolic variables immediately before DEX therapy and at the last evaluation (Table 1), we observed a small but statistically significant increase in the WtHR, from 0.54 ± 0.08 to 0.56 ± 0.1 (P = 0.001). The individual values and increase in the WtHR during DEX therapy are shown in Fig. 1. Furthermore, a statistically significant increase occurred in the HOMA-IR index (from 2.5 ± 1.3 to 2.8 ± 1.7; P = 0.03), which correlated positively with the WtHR (r = 0.54). The difference in the BMI-SDS was not statistically significant during the DEX period (1.6 ± 1.6 kg/m2 at the transition to DEX and 1.5 ± 1.5 mg/m2 at the last clinical visit; P = 0.65). Moreover, the prevalence of obesity according to the BMI was 27% in both evaluations, and 33.3% of patients were overweight immediately before the introduction of DEX and 35% at the last visit (P = 0.842). The mean DEX dose did not differ between those of normal weight and those who were overweight or obese (0.17 ± 0.07 vs 0.18 ± 0.08 mg/m2/d; P = 0.7).
Figure 1.
(A) Individual values of WHtR in our cohort during 20 y of DEX therapy. (B) Boxplots showing the evolution of WtHR for every 5 y of DEX treatment.
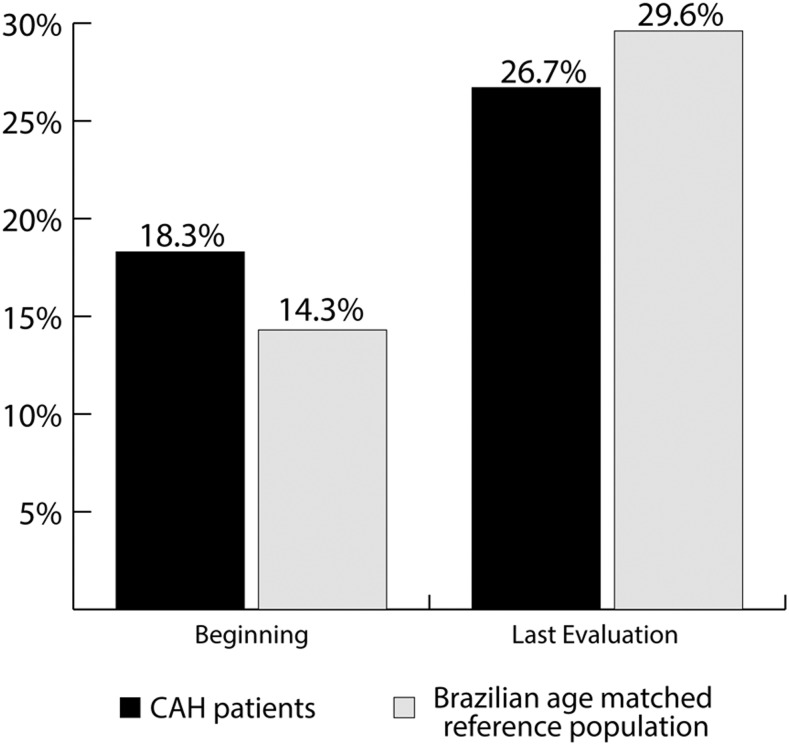
The comparison of the WHtR between the CAH cohort and the reference Brazilian population paired by age [17] showed a similar prevalence of an increased WC at the beginning of the DEX therapy in the first group (18.3% vs 14.3%, respectively) and at the last evaluation (26.7% vs 29.6%, respectively; Fig. 2).
Figure 2.
Prevalence of increased WC in our CAH cohort and the Brazilian reference population.
No other metabolic parameter was significantly different during DEX therapy, including HDL-C according to sex, low-density lipoprotein cholesterol, fasting glucose, TG, or blood pressure. MetS was observed in four patients (7%) at the transition to DEX and in six (10%) at the last evaluation (P = 0.743). The results of the linear and multiple regression analysis showed no correlation between the metabolic variables and DEX therapy (duration and doses), sex, or clinical form of CAH.
3. Discussion
CAH is the most common genetic endocrine disorder and, with the availability of treatment with GCs, most of these patients have been reaching adulthood. However, no standard regimen has been determined for treating adults [6, 13].
DEX offers the advantage of a once-daily dose, which could improve treatment compliance. However, its use has been feared because it has been suggested that its use could be related to a greater risk of the development of an adverse metabolic profile [7, 20]. In addition to the lack of longitudinal studies, one drawback in the available data on metabolic disturbances in these patients has been the scarcity of homogeneous cohorts to evaluate the specific effects of the disease and each GC regimen. To focus on the metabolic effects of long-term DEX therapy, we selected only patients with classic CAH forms and adequate compliance and who had used exclusively DEX since the attainment of their adult height.
According to the World Health Organization, the prevalence of obesity in the Brazilian population was 17% in men and 24% in women and the prevalence of overweight was 38% in men and 29% in women. In our group, 27% of patients were obese and 33.3% were overweight immediately before DEX introduction. These rates did not increase during the use of DEX and were similar to, or even lower than, that in other CAH cohorts receiving diverse types of GC. For instance, in a cross-sectional study conducted in the United Kingdom [CaHASE (United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive)] [6], which evaluated 165 adult patients with CAH (103 women), 41% had a BMI in the obese range and 37% in the overweight range. Moreover, in a French cross-sectional study [21], which had included 70 adult patients with CAH (45 women; 53 with SW), 44% had a BMI >25 kg/m2. Finally, in an American cross-sectional study [13], which had include 44 adults with CAH (25 women; 26 with SW), one third of the patients were obese.
Although the BMI did not change with DEX, we observed a small, but substantial, increase in the WtHR, compatible with an undesirable body fat distribution. The WtHR is an effective anthropometric indicator and correlates better than the WC alone with cardiovascular health [22]. The WHtR cutoff value of 0.5 was proposed after an analysis of data from 14 different countries and was a stronger predictor of the development of diabetes and cardiovascular disease than the BMI [23]. Furthermore, the final WC in our female patients was lower than that from the CaHASE cohort (80.8 cm and 99.5 cm, respectively), in which 26% of the patients had received hydrocortisone, 43% prednisolone, 19% DEX, and 10% a combination of GCs [6]. Only one study evaluated visceral adipose tissue (VAT) [24]. They evaluated 28 subjects with CAH (aged 15.6 ± 3.2 years), including 8 treated with DEX, and increased VAT was found compared with paired healthy controls [24].
A substantial increase in the HOMA-IR index was also observed during the use of DEX, with 38% of patients having a HOMA-IR >2.5 (compatible with insulin resistance) at our last evaluation. Moreover, in our sample, the HOMA-IR presented a moderate positive correlation with WtHR (r = 0.54), consistent with the correlation seen between WtHR and VAT in the American cohort (r = 0.84) [24].
The prevalence of MetS and hypertension remained unchanged during DEX therapy in our series. These results have varied among studies. For example, in a Norwegian study of 64 adult patients with CAH [25], a higher diastolic blood pressure was observed compared with that in the normal population. In the CaHASE study [6], an increase in diastolic blood pressure in female patients with CAH was also observed. Regarding the prevalence of MetS, in the American cohort [13], it was identified in 18% of adults.
It is possible that we saw few substantial metabolic effects with DEX treatment in our patients because they were still young (mean age, 31.9 ± 9.6 years). This cohort of patients was organized within the previous 20 years and did not have enough older patients from which to draw further conclusions about elderly patients with CAH. Continued follow-up of the cohort will hopefully result in more information as the population ages. However, they have received DEX treatment for a considerable time (11.5 ± 4.9 years). In addition, our results are similar to those from other young CAH cohorts receiving mixed GC regimens. We could not rule out that the undesirable effects observed for the WHtR could be related to aging and long-term GC exposure and not exclusively related to the use of DEX.
One limitation of the present study was the absence of a control group using other GC regimens. This was because our study was a real-life setting study in a tertiary center in which we had opted to improve compliance by the use of a once-daily dose of DEX, which is more accessible in Brazil. The lack of an age-matched control group was partially resolved through a comparison with data from a large Brazilian population and with data from patients with CAH described in other studies. Second, some concerns exist regarding the long-term DEX effects on bone health. However, a recent study reported the 10-year follow-up data for patients with CAH who had been receiving mixed GC regimens (89% of the patients receiving DEX therapy) [26]. They found no correlation between bone mineral density and the cumulative dose of GC. Therefore, the investigators suggested that the risk of osteoporosis in this cohort could be similar to that of the normal population [26]. Finally, the use of DEX should not be used during pregnancy, because it crosses the placenta and reaches the fetal circulation.
4. Conclusions
These preliminary data have indicated that DEX therapy appears to be an acceptable option to treat adult patients with CAH, because it was not associated with an increase in the prevalence of obesity, MetS, or hypertension. Furthermore, DEX could be an interesting option to improve patient compliance. Prospective studies are still necessary to compare the long-term effects of each GC formulation.
Acknowledgments
Financial Support: T.A.S.S.B. and B.B.M. were partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development; grant 303088/2015-0 and 303002/2016-6, respectively). T.A.S.S.B. was partially supported by a grant from FAPESP (São Paulo Research Foundation) 09/54294-4.
Glossary
Abbreviations:
- 17OHP
17 hydroxy-progesterone
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- DEX
dexamethasone
- GC
glucocorticoid
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment for insulin resistance
- NC
nonclassic form
- SDS
SD score
- SV
simple virilizing
- SW
salt-wasting
- TG
triglycerides
- VAT
visceral adipose tissue
- WC
waist circumference
- WtHR
waist/height ratio
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Krone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf). 2007;66(2):162–172. [DOI] [PubMed] [Google Scholar]
- 2. White PC, Bachega TA. Congenital adrenal hyperplasia due to 21 hydroxylase deficiency: from birth to adulthood. Semin Reprod Med. 2012;30(5):400–409. [DOI] [PubMed] [Google Scholar]
- 3. Pang SY, Wallace MA, Hofman L, Thuline HC, Dorche C, Lyon IC, Dobbins RH, Kling S, Fujieda K, Suwa S. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81(6):866–874. [PubMed] [Google Scholar]
- 4. Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101(4 Pt 1):583–590. [DOI] [PubMed] [Google Scholar]
- 5. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- 6. Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE). Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(7):2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joint LWPES/ESPE CAH Working Group. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab. 2002;87(9):4048–4053. [DOI] [PubMed] [Google Scholar]
- 10. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10(2):115–124. [DOI] [PubMed] [Google Scholar]
- 11. Falhammar H, Filipsson Nyström H, Wedell A, Thorén M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164(2):285–293. [DOI] [PubMed] [Google Scholar]
- 12. Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, Nihoul-Fékété C, Kuttenn F, Polak M, Touraine P. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2007;67(6):268–276. [DOI] [PubMed] [Google Scholar]
- 13. Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim MS, Merke DP. Cardiovascular disease risk in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Semin Reprod Med. 2009;27(4):316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bachega TA, Billerbeck AE, Madureira G, Marcondes JA, Longui CA, Leite MV, Arnhold IJ, Mendonca BB. Molecular genotyping in Brazilian patients with the classical and nonclassical forms of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1998;83(12):4416–4419. [DOI] [PubMed] [Google Scholar]
- 16. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Health Survey, 2013. Available at: https://ww2.ibge.gov.br/home/estatistica/populacao/pns/2013_vol3/default_microdados.shtm. Accessed 1 November 2018.
- 18. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 19. Dhuper S, Cohen HW, Daniel J, Gumidyala P, Agarwalla V, St Victor R, Dhuper S. Utility of the modified ATP III defined metabolic syndrome and severe obesity as predictors of insulin resistance in overweight children and adolescents: a cross-sectional study. Cardiovasc Diabetol. 2007;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han TS, Stimson RH, Rees DA, Krone N, Willis DS, Conway GS, Arlt W, Walker BR, Ross RJ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2013;78(2):197–203. [DOI] [PubMed] [Google Scholar]
- 21. Bachelot A, Golmard JL, Dulon J, Dahmoune N, Leban M, Bouvattier C, Cabrol S, Leger J, Polak M, Touraine P. Determining clinical and biological indicators for health outcomes in adult patients with childhood onset of congenital adrenal hyperplasia. Eur J Endocrinol. 2015;173(2):175–184. [DOI] [PubMed] [Google Scholar]
- 22. Shen S, Lu Y, Qi H, Li F, Shen Z, Wu L, Yang C, Wang L, Shui K, Yao W, Qiang D, Yun J, Zhou L. Waist-to-height ratio is an effective indicator for comprehensive cardiovascular health. Sci Rep. 2017;7(1):43046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23(2):247–269. [DOI] [PubMed] [Google Scholar]
- 24. Kim MS, Ryabets-Lienhard A, Dao-Tran A, Mittelman SD, Gilsanz V, Schrager SM, Geffner ME. Increased abdominal adiposity in adolescents and young adults with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(8):E1153–E1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nermoen I, Brønstad I, Fougner KJ, Svartberg J, Øksnes M, Husebye ES, Løvås K. Genetic, anthropometric and metabolic features of adult Norwegian patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(4):507–516. [DOI] [PubMed] [Google Scholar]
- 26. Ceccato F, Barbot M, Albiger N, Zilio M, De Toni P, Luisetto G, Zaninotto M, Greggio NA, Boscaro M, Scaroni C, Camozzi V. Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2016;175(2):101–106. [DOI] [PubMed] [Google Scholar]