Abstract
Context
Patients with very severe hypertriglyceridemia (triglyceride levels ≥2000 mg/dL; 22.6 mmol/L) require aggressive treatment. However, little research exists on the underlying etiologies and management of very severe hypertriglyceridemia.
Objective
We hypothesized (i) very severe hypertriglyceridemia in adults is mostly associated with secondary causes and (ii) most patients with very severe hypertriglyceridemia lack appropriate follow-up and treatment.
Design
We queried electronic medical records at Parkland Health and Hospital Systems for lipid measurements in the year 2016 and identified patients with serum triglyceride levels ≥2000 mg/dL (22.6 mmol/L). We extracted data on demographics, underlying causes, lipid-lowering therapy, and follow-up.
Results
One hundred sixty-four serum triglyceride measurements were ≥2000 mg/dL (22.6 mmol/L) in 103 unique patients. Of these, 60 patients were admitted to the hospital (39 for acute pancreatitis). Most were Hispanic (79%). The major conditions associated with very severe hypertriglyceridemia included uncontrolled diabetes mellitus (74%), heavy alcohol use (10%), medication use (7%), and hypothyroidism (2%). Two patients were known to have monogenic causes of hypertriglyceridemia. After the index measurement of triglycerides ≥2000 mg/dL (22.6 mmol/L), the use of triglyceride-lowering drugs increased, most prominently the use of fish oil supplements, which increased by 80%. However, in follow-up visits, hypertriglyceridemia was addressed in only 50% of encounters, and serum triglycerides were remeasured in only 18%.
Conclusion
In summary, very severe hypertriglyceridemia was quite prevalent (∼0.1% of all lipid measurements) in our large county health care system, especially in Hispanic men. Most cases were related to uncontrolled diabetes mellitus, and follow-up monitoring was inadequate.
Keywords: triglycerides, pancreatitis, hypertriglyceridemia
Approximately one-third of the US population has elevations in circulating triglyceride levels [1, 2]. Most of these elevations are mild to moderate in nature, and the pathophysiology involves hepatic overproduction of very low‒density lipoproteins (VLDLs), the principal triglyceride-carrying lipoprotein circulating in the fasting state [3, 4]. A subset of patients have “very severe hypertriglyceridemia,” defined by the Endocrine Society as serum triglyceride concentration ≥2000 mg/dL (22.6 mmol/L) [5]. In addition to VLDL overproduction, the pathophysiology of very severe hypertriglyceridemia involves defective triglyceride clearance due to either saturation or dysfunction of the enzyme lipoprotein lipase (LPL), resulting in accumulation of chylomicrons and VLDLs [3, 6, 7].
Very severe hypertriglyceridemia is associated with either primary (genetic) causes or secondary conditions. Primary causes of very severe hypertriglyceridemia involve biallelic mutations in LPL, apolipoprotein C2 (APOC2), lipase maturation factor 1 (LMF1), apolipoprotein 5 (APOA5), and glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1); patients with pathogenic mutations in these genes are referred to as having familial chylomicronemia syndrome or type 1 hyperlipoproteinemia [8–18]. Secondary associated conditions include a long list of possibilities, such as uncontrolled diabetes mellitus, nephrotic syndrome, excessive alcohol use, polygenic causes, and medication use (e.g., protease inhibitors, vitamin A derivatives, l-asparaginase, and estrogen) [4, 19–22].
Very severe hypertriglyceridemia can manifest with eruptive xanthomas, lipemia retinalis, hepatosplenomegaly, and lipemic plasma. The lipemia can interfere with measurements of some serum analysts. Most concerning, however, very severe hypertriglyceridemia predisposes patients to attacks of acute pancreatitis [23–25], a serious condition that can be complicated by multiorgan failure, pancreatic necrosis, and mortality rates as high as 20% [26, 27]. Fortunately, acute pancreatitis episodes can be prevented by reducing serum triglyceride levels below 1000 mg/dL (11.3 mmol/L) by addressing associated secondary causes along with a combination of low-fat diet, fibrates, and very long‒chain omega-3 fatty acids from fish oils [20, 23].
In multiethnic populations, as in the United States, few prior investigations have reported clinical characteristics or management of very severe hypertriglyceridemia [21, 28]. To address these issues, we sought to study patient characteristics, such as conditions associated with secondary hypertriglyceridemia and the burden of acute pancreatitis, as well as clinical management patterns in patients with very severe hypertriglyceridemia in a large community-based health care system.
1. Methods
The study was approved by the institutional review board of University of Texas Southwestern Medical Center. We performed a retrospective observational study by querying electronic medical records (EMRs) at Parkland Health and Hospital Systems, a county hospital providing both inpatient and outpatient care for indigent populations of Dallas, Texas, for all serum lipid measurements in the year 2016. Parkland Hospital is part of the public community health system in Dallas and has an average of >1 million patient visits per year. It has 870 patient beds, and in 2016, it had 74,394 total hospital discharges; 1,080,668 outpatient visits; and 258,047 emergency visits (www.parklandhospital.com/phhs/parklands-statistics.aspx). In 2016, Hispanic patients were the largest ethnic/racial group seen in the system, compromising 51% of the total system population.
The results of the EMR query were filtered for patients with at least one single measurement of triglyceride levels ≥2000 mg/dL (22.6 mmol/L). In the case of patients with multiple triglyceride measurements ≥2000 mg/dL, the first instance was used as an index date for further data collection. For patients with very severe hypertriglyceridemia, we performed a retrospective review of EMRs to extract data on demographics, underlying causes, lipid-lowering therapy, and follow-up. Heavy alcohol use was defined as binge drinking on 5 or more days in the past month per the Substance Abuse and Mental Health Services Administration of the US Department of Health & Human Services (www.samhsa.gov).
A. Statistical Methods
Categorical variables were reported as percentages and continuous variables as means ± SD or median (25th to 75th percentile) depending on their distributions. Triglycerides were right censored at 4425 mg/dL, the upper limit at which the hospital laboratory reports triglyceride results. The median for triglyceride levels was based on patients with triglyceride levels ≤4425 mg/dL. Comparisons between those with and without acute pancreatitis were made using the Fisher exact test for categorical variables and the t test for Gaussian-distributed continuous variables or the Wilcoxon rank sum test for non‒Gaussian-distributed continuous variables. A logistic regression model was constructed to predict a patient’s acute pancreatic risk using independent variables of categorized age (<30, 30 to 39, 40 to 49, 50 to 59, and ≥60 years in five groups), race (white vs other races), alcohol status, hypertension, categorized body mass index (BMI; <25, 25 to 29, and ≥30 kg/m2 in three groups), and categorized serum triglyceride level in three groups (<3000, 3000 to 4425, and >4425 mg/dL). A two-sided P value <0.05 was considered statistically significant. SAS 9.4 (SAS Institute, Cary, NC) was used in the data analysis.
2. Results
A. Patient Characteristics
There were 95,836 lipid measurements (71,495 unique patients) in 2016 in the Parkland Health and Hospital System. Of these, 164 serum triglyceride measurements were ≥2000 mg/dL (22.6 mmol/L) from 103 unique patients (0.14% of total unique patients; 32 women, 71 men) (Table 1). These patients were predominantly Hispanic (n = 78; 79%), with a high prevalence of diabetes mellitus (76%), hypertension (49%), and obesity (51%, defined as BMI ≥30 kg/m2).
Table 1.
Descriptive Characteristics of All Patients With Very Severe Hypertriglyceridemia (vs HTG) and Patients With Pancreatitis vs Those Without
| All Patients (n = 103) | Patients With Pancreatitis (n = 39) | Patients Without Pancreatitis (n = 64) | P Valuea | |
|---|---|---|---|---|
| Age, yb | 42 ± 11 | 40 ± 11 | 43 ± 11 | 0.23 |
| Male, n (%) | 71 (69%) | 25 (64%) | 46 (67%) | 0.51 |
| BMI, kg/m2b | 30 ± 5 | 30 ± 5 | 30 ± 5 | 0.98 |
| Ethnicity and race, n (%) | 0.31 | |||
| Hispanic white | 81 (79%) | 34 (87%) | 47 (73%) | |
| Non-Hispanic white | 9 (9%) | 2 (5%) | 7 (11%) | |
| Black | 10 (10%) | 3 (8%) | 7 (11%) | |
| Asian, n (%) | 3 (2%) | 0 | 3 (5%) | |
| Triglyceride levels in all patients, mg/dLc | 3043 (2439, >4425) | 4131 (2953, 4426) | 2703.5 (2382.5, 3268.5) | 0.0002 |
| Triglyceride levels among patients with <4425 mg/dL (n = 77) | 3,042 (2444, 4409) | 3003 (2602, 3675) | 2548 (2326, 3113) | 0.03 |
| n of patients with triglyceride levels ≥4425 mg/dL | 26 (25%) | 17 (44%) | 9 (14%) | 0.0008 |
| Total cholesterol level, mg/dLc | 444 (373, 598) | 525 (424, 680) | 425 (345, 503) | 0.008 |
| HDL-C level, mg/dLc | 31 (25, 38) | N/A | 32 (26,42) | N/A |
| HbA1c, % (n = 86) | 10.4 (8.2, 12.6) | 9.7 (8.0, 12.4) | 10.8 (8.2, 12.9) | 0.50 |
| Any alcohol use, n (%) | 45 (48%) | 17 (44%) | 28 (44%) | 1.0 |
| Any smoking history, n (%) | 27 (26%) | 9 (23%) | 18 (28%) | 0.65 |
| DM, n (%) | 78 (76%) | 29 (74%) | 49 (77%) | 0.82 |
| HTN, n (%) | 50 (49%) | 15 (38%) | 35 (55%) | 0.15 |
| CAD, n (%) | 9 (9%) | 4 (10%) | 5 (8%) | 0.73 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; HbA1c: hemoglobin A1c; HDL-C, high density lipoprotein-cholesterol; HTN, hypertension; N/A, not applicable; IQR, interquartile range.
P values are for patients with pancreatitis vs patients without pancreatitis.
Values are mean ± SD.
Values are median (IQR).
B. Lipid Levels
Median serum triglyceride level was 3043 mg/dL (range, 2027 to >4425 mg/dL), and total cholesterol level was 444 mg/dL (range, 373 to 598 mg/dL). High-density lipoprotein cholesterol (HDL-C) level was not reported in 93% of patients (per the Parkland laboratory, the HDL-C assay is a homogeneous enzymatic colorimetric test and is not accurate when triglyceride levels are >1200 mg/dL because of false-positive results and the contribution of non‒HDL-C in the measurement; in the patients with available HDL-C levels, the median interquartile range (IQR) level was 31 (25, 38) mg/dL.
Among all patients with very severe hypertriglyceridemia, 26 had serum triglyceride levels above the limit reported by the hospital laboratory (>4425 mg/dL); their total cholesterol level [median (IQR)] was 642 (619, 824) mg/dL, and their average age was 39 years; 21 patients (80%) were Hispanic, and 17 (65%) were male. Of the patients with an index serum triglyceride level >4425 mg/dL, 20 (76%) were admitted to the hospital; of these, 18 (69%) had acute pancreatitis, eight of whom required admission to the intensive care unit (ICU).
C. Presentation and Characteristics of Inpatients
Of patients with an index serum triglyceride measurement ≥2000 mg/dL (22.6 mmol/L), 42% of the measurements were obtained during outpatient visits, whereas 58% were obtained during hospitalization. Of the hospitalized patients, acute pancreatitis was diagnosed in 39 (38%); 22 (21%) were admitted for diabetic ketoacidosis (DKA), 19 of whom had type 2 diabetes mellitus and three of whom had type 1 diabetes mellitus; 16 (16%) required ICU admission; and seven received plasmapheresis as part of their treatment. An inpatient endocrinology consult was requested in 32 cases (31%), mostly to address whether plasmapheresis should be initiated. Three patients’ medical records documented eruptive xanthomas, and none documented lipemia retinalis.
Of the seven patients who received plasmapheresis, the mean age was 47 years, six were Hispanic, the mean BMI was 33 kg/m2, all had type 2 diabetes mellitus, three presented with DKA, one had newly diagnosed type 2 diabetes mellitus, six were admitted to the ICU, three had serum triglyceride levels above the detectable limit, and the remainder had serum triglyceride levels of 2800 to 4124 mg/dL upon admission. At the time of discharge, their serum triglyceride levels ranged from 366 to 2629 mg/dL.
D. Patients With Acute Pancreatitis
For the 39 patients with acute pancreatitis as diagnosed by inpatient primary teams, 34 (87%) were Hispanic and the average age was 40 years (range: 20 to 64 years). The median (IQR) serum triglyceride level was 3003 (2602, 3675) mg/dL. In this subset of patients, 14 (35%) presented with DKA, most 29 (74%) had type 2 diabetes mellitus, and the average BMI was 30 kg/m2 (range: 21 to 39.8 kg/m2). All had evidence of acute pancreatitis on imaging studies; four patients (15%) had evidence of pancreatic necrosis, 23 (88%) had inflammation, and two (8%) had calcifications. Twenty-three patients (59%) had more than one serum triglyceride measurement >1000 mg/dL in the EMR, either before or after hospital admission. Most had more than one episode of pancreatitis (Table 2).
Table 2.
Clinical Characteristics of Patients With Very Severe Hypertriglyceridemia (vs HTG) According to Number of Lifetime Episodes of Acute Pancreatitis
| No Pancreatitis | 1 Episode | 2–4 Episodes | 5–9 Episodes | 10 or More Episodes | |
|---|---|---|---|---|---|
| No. of patients | 64 | 15 | 17 | 4 | 3 |
| Average age, y | 43 | 41 | 37 | 40 | 44 |
| Ethnicity | |||||
| Hispanic | 47 (73%) | 12 (85%) | 16 (94%) | 4 (100%) | 2 (67%) |
| Non-Hispanic white | 7 (11%) | 2 (14%) | 1 (6%) | 1 (33%) | |
| Black | 7 (11%) | ||||
| Asian | 3 (5%) | ||||
| BMI mean, kg/m2; | 30 | 31 | 30 | 31 | 29 |
| Sex, M:F | 46:18 | 9:6 | 11:6 | 2:2 | 3:0 |
| TG level, median (IQR), mg/dL | 2548 (2350, 3103) | 3641 (3040, 4124) | 2959 (2575, 3278) | 2869 | 2843 (2222, 3225) |
| 9 >4425 | 5 >4425 | 9 >4425 | 3 >4425 | ||
| TC level, median (IQR), mg/dL | 393 (342, 456) | 633 (505, 753) | 418 (340, 460) | 773 (620, 849) | 444 (420, 457) |
| Encounters with TG >1000 mg/dL; mean | 3 | 1.8 | 3.5 | 7.3 | 7.7 |
| Presentation | |||||
| Outpatient | 40 (62%) | 1 (7%) | 1 (6%) | 0 | 1 (33%) |
| Inpatient | 24 (38%) | 13 (93%) | 16 (94%) | 4 (100%) | 2 (67%) |
| ICU stay | 4 (6%) | 6 (43%) | 5 (29%) | 1 (25%) | 0 |
| DKA | 8 (12%) | 7 (50%) | 6 (35%) | 0 | 0 |
| Plasmapheresis | 0 | 3 (21%) | 3 (17%) | 1 (25%) | 0 |
| Diabetes, % | 49 (76%) | 8 (57%) | 13 (76%) | 4 (100%) | 3 (100%) |
| Alcohol abuse, % | 28 (44%) | 7 (50%) | 7 (41%) | 2 (50%) | 1 (33%) |
| Obesity, % | 32 (50%) | 8 (57%) | 9 (52%) | 3 (75%) | 1 (33%) |
| Medication use at initial presentation with vsHTG | |||||
| Fibrate | 31 (48%) | 11 (78%) | 9 (52%) | 4 (100%) | 1 (33%) |
| Fish oil | 18 (28%) | 6 (43%) | 3 (17%) | 3 (75%) | 1 (33%) |
| Statin | 26 (40%) | 2 (14%) | 4 (24%) | 1 (25%) | 1 (33%) |
| Follow-up | |||||
| Referred to lipid clinic | 27 (42%) | 7 (50%) | 8 (47%) | 2 (50%) | 2 (67%) |
| Seen in lipid clinic | 23 (35%) | 2 (14%) | 5 (29%) | 2 (50%) | 0 |
| Had FU visit | 45 (70%) | 11 (78%) | 12 (71%) | 2 (50%) | 0 |
| TG checked before FU visit | 9 (14%) | 2 (14%) | 1 (6%) | 1 (25%) | N/A |
| HTG addressed at FU visit | 32 (50%) | 10 (71%) | 5 (29%) | 2 (50%) | N/A |
Data presented as N (%).
Abbreviations: BMI, body mass index; DKA, diabetic ketoacidosis; FU, follow-up; HTG, hypertriglyceridemia; ICU, intensive care unit; TC, total cholesterol; TG, triglycerides.
One patient died during admission for hypertriglyceridemia-induced acute pancreatitis, a 64-year-old woman with a medical history of type 2 diabetes mellitus, hypothyroidism, hypertriglyceridemia, hypertension, and chronic obstructive pulmonary disease who was admitted to the ICU for acute pancreatitis due to hypertriglyceridemia (serum triglycerides 2800 mg/dL or 31.6 nmol/L) as well as DKA (hemoglobin A1c value of 17.4%) and non‒ST elevation myocardial infarction. Her hospital course was complicated by respiratory failure (acute respiratory distress syndrome) requiring intubation, hypotension requiring pressor support, and metabolic acidosis requiring continuous renal replacement therapy. She was treated with an insulin-glucose infusion as well as plasmapheresis, which reduced her serum triglyceride level to 1037 mg/dL. After a prolonged hospital course with minimal improvement, she transitioned to comfort care measures and eventually died of hypoxia, bradycardia, and cardiac arrest.
E. Pancreatitis vs No Pancreatitis
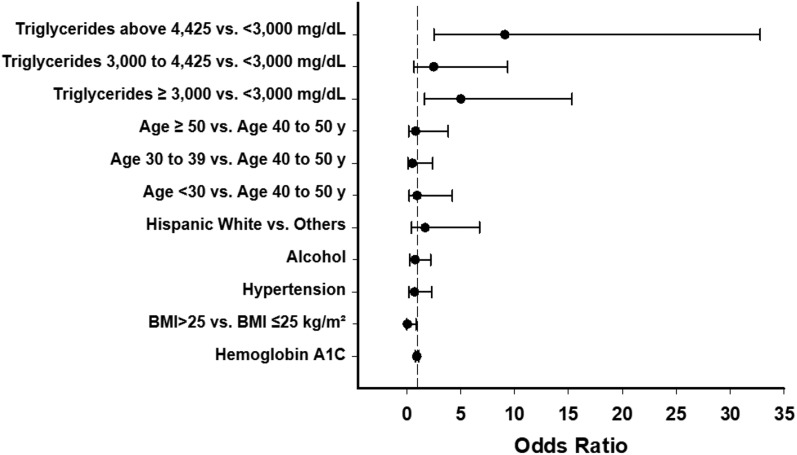
Table 1 describes the baseline characteristics for patients with acute pancreatitis and for those without pancreatitis. The subgroup with pancreatitis was 3 years younger, had higher serum triglyceride and total cholesterol levels, and a higher prevalence of serum triglycerides above the detectable level (>4425 mg/dL; n = 17; 65%). No significant differences were seen in ethnic/racial demographics and BMI. Regarding signs and symptoms, patients with pancreatitis experienced nausea, vomiting, and abdominal pain more commonly than patients without pancreatitis (Table 3). In logistic regression models, only triglyceride level reached statistical significance as a risk factor for pancreatitis (Fig. 1).
Table 3.
Characteristics of Patient Presentation With First Measurement of Triglyceride Level ≥2000 mg/dL
| All Patients (n = 103) | Patients With Pancreatitis (n = 39) | Patients Without Pancreatitis (n = 64) | P Valuea | |
|---|---|---|---|---|
| Outpatient | 43 (42%) | 1 (3%) | 40 (63%) | |
| Inpatient | 60 (58%) | 38 (97%) | 24 (38%) | <0.0001 |
| Intensive care unit admission | 16 (16%) | 12 (31%) | 4 (6%) | 0.23 |
| Diabetic ketoacidosis | 22 (21%) | 14 (35%) | 8 (13%) | 0.01 |
| Plasmapheresis | 7 (7%) | 7 (18%) | 0 | 0.03 |
| Presenting symptoms | ||||
| Abdominal pain | 38 (37%) | 33 (84%) | 5 (8%) | <0.0001 |
| Nausea | 35 (34%) | 31 (79%) | 4 (6%) | <0.0001 |
| Vomiting | 29 (28%) | 27 (69%) | 2 (3%) | <0.0001 |
| Chest pain | 8 (8%) | 3 (8%) | 5 (8%) | 1.0 |
| Asymptomatic | 35 (34%) | 2 (5%) | 33 (52%) | <0.0001 |
| Other symptomsb | 23 (22%) | 3 (8%) | 20 (31%) | 0.007 |
Data are presented as n (%).
P values are for patients with pancreatitis vs patients without pancreatitis.
Other symptoms include polydipsia and polyuria (n = 8), altered mental status (n = 3), dizziness (n = 3), fatigue (n = 2), palpitations (n = 2), fever (n = 1), diarrhea (n = 1), myalgias (n = 1), headache (n = 1), and rash (n = 1).
Figure 1.
Risk factors for acute pancreatitis among patients with very severe hypertriglyceridemia. Error bars represent 95% CI.
F. Conditions Associated With Very Severe Hypertriglyceridemia
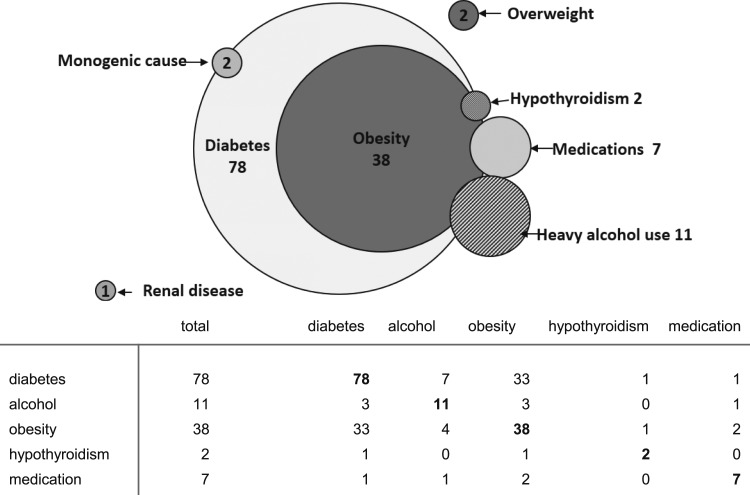
Major conditions associated with very severe hypertriglyceridemia included uncontrolled diabetes mellitus (n = 78), heavy alcohol use (n = 11), medication use (n = 7; 5 taking HIV-1 protease inhibitors and 2 taking estrogen), uncontrolled hypothyroidism (n = 2), and kidney disease (focal segmental glomerulosclerosis, n = 1). Many patients had overlapping conditions associated with very severe hypertriglyceridemia (Fig. 2).
Figure 2.
Schematic representation (Venn diagram) of associated conditions for serum triglyceride levels ≥2000 mg/dL (very severe hypertriglyceridemia). Shared conditions are represented by the areas of overlap among the circles (see tabular data for n in each category). Numbers represent totals for each category. Note: Genetic screening was not done in all patients.
For the 78 patients with diabetes (31% female, 69% male), the mean hemoglobin A1c value around the time of the index measurement was 11.2%. Three patients (4%) had type 1 diabetes mellitus, and 75 patients (96%) had type 2 diabetes mellitus. Eighteen patients had information about duration of diabetes in the EMR: average age at diagnosis was 39 years (range: 7 to 52 years), and average duration was 4.6 years (range: 0 to 16 years).
Two patients were known to have monogenic causes of hypertriglyceridemia [29] (no other patients had genetic testing information available). As previously reported [29], one patient harbored biallelic mutations in GCKR. The other patient had novel homozygous LPL p.(Ile22IAsn) (c.662T>A) mutation.
G. Medications
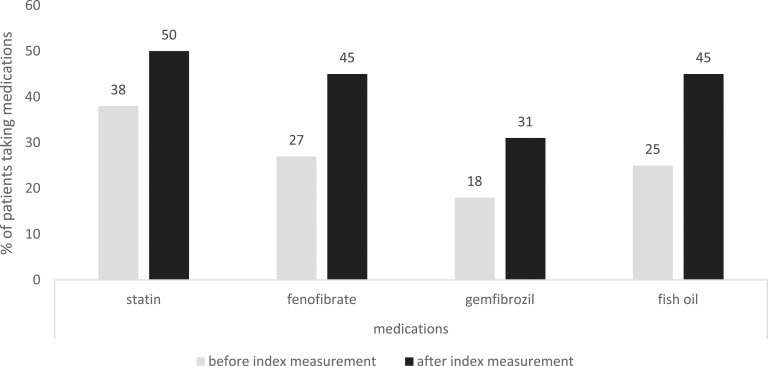
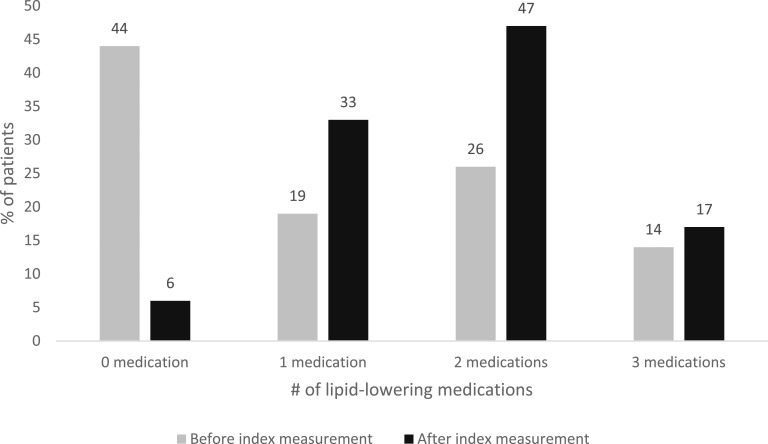
At the time of the index measurement of triglycerides ≥2000 mg/dL (22.6 mmol/L), 43% of patients were not taking any triglyceride-lowering medication, 38% were taking a statin, 45% were taking a fibrate, and 25% were taking fish oil supplements. After an index measurement of triglycerides ≥2000 mg/dL, the use of statins increased by 31%, use of fibrate by 68%, and use of fish oil by 80% (Fig. 3). Before detection of elevated triglyceride levels, 43% of patients were taking one or two lipid-lowering medications, and 14% were taking three different lipid-lowering medications. After the index measurement, the proportion of patients taking one or two medications increased by 44%, and the proportion taking three medications increased by 18% (Fig. 4).
Figure 3.
Changes in the use of triglyceride-lowering medications after index measurement of triglyceride levels ≥2000 mg/dL.
Figure 4.
Number of lipid-lowering medications before and after index measurement of triglyceride levels ≥2000 mg/dL.
H. Follow-Up
Seventy percent of the patients attended a clinic visit after the index measurement of serum triglyceride levels ≥2000 mg/dL (22.6 mmol/L). Health care providers addressed hypertriglyceridemia in only 50% of these follow-up visits, and serum triglyceride levels were remeasured in only 18%. Although 45% of patients (n = 46) were referred to a specialty lipid clinic, only one-third of referred patients (n = 33 of 103) were eventually seen there.
3. Discussion
We found very severe hypertriglyceridemia to be quite prevalent (∼0.1% of all lipid measurements) in our large county health care system, especially in Hispanic men. Most cases were associated with uncontrolled diabetes, and almost 40% of patients presented with acute pancreatitis. On follow-up, several gaps in care emerged. Hypertriglyceridemia was addressed in only 50% of follow-up visits, triglyceride concentration was rechecked in only 18% of follow-up visits, and few patients were eventually seen in the lipid specialty clinic.
Few prior studies characterized multiethnic cohorts of adult patients with severe elevations in triglyceride levels. Previous publications had few patients with triglyceride levels ≥2000 mg/dL (22.6 mmol/L) [2] or lacked data on ethnicity/race and underlying causes of hypertriglyceridemia. In the US National Health and Nutrition Examination Survey (NHANES) multiethnic population, 0.4% of the population-based cohort had serum triglyceride levels >1000 mg/dL [2]. However, only three patients with triglyceride levels ≥2000 mg/dL were identified, and no description of underlying causes was available [30]. Toth et al. [25] identified a US cohort of 1964 patients with serum triglyceride levels >1500 mg/dL. Unfortunately, because of the nature of the data (secondary data from administrative claims data repositories), no information was available on key patient characteristics such as race/ethnicity, BMI, and—perhaps most importantly—associated causes of hypertriglyceridemia. Outside the United States, a study from Norway identified 862 patients with serum triglyceride levels >885 mg/dL among 681,990 participants across three population-based studies [31]. Unfortunately, limited data on underlying causes of hypertriglyceridemia were available. A study from Denmark that included 108,711 individuals from the Copenhagen General Population Study reported findings resembling ours, in that type 2 diabetes mellitus and obesity were the top risk factors identified [32].
Concerning underlying causes of very severe hypertriglyceridemia, our data resemble that of prior publications [19, 24, 33–35]. Most recently, Patni et al. [36] found that in children with very severe hypertriglyceridemia, the most common associated factor was uncontrolled diabetes (31%) followed by l-asparaginase and high-dose steroid use for children with acute lymphoblastic leukemia (28%). Fourteen percent were known to have monogenic causes (type 1 hyperlipoproteinemia). Another US study found that among patients with hypertriglyceridemia-induced pancreatitis (mean triglyceride levels, 4587 mg/dL), 72% had diabetes and 23% had heavy alcohol use; no monogenic cases were mentioned [24]. In a study from Japan (n = 251 with triglyceride levels >1000 mg/dL), most patients (59%) had hypertriglyceridemia due to a combination of diabetes and heavy alcohol intake. Two percent had a monogenic cause [35].
Common small-effect variants in GCKR are associated with small elevations in triglyceride levels in genome-wide association studies and contribute to polygenic susceptibility to severe hypertriglyceridemia [37–40]. Certain rare large-effect variants in GCKR are seen in some families with severe hypertriglyceridemia [38, 39], but these patients with type 1 hypolipoproteinemia due to balletic mutations do not behave as typical in LPL, APOC2, APOA5, LMF1, and GPIHBP1.
Four patients with triglyceride levels ≥2000 mg/dL (22.6 mmol/L) in our cohort had no clear associated conditions to cause very severe hypertriglyceridemia besides excess weight. Of note, none of these patients were genotyped, so they may have a monogenic cause. Because some of these patients were not evaluated in a lipid clinic, they could have conditions such as partial lipodystrophy, a clinical diagnosis dependent on physical examination findings from an experienced health care provider. Alternatively, these patients could possibly be taking undisclosed medications associated with hypertriglyceridemia or may have had an incomplete medical history.
In our cohort of patients with very severe hypertriglyceridemia, Hispanics were the most represented ethnicity/race (79%). Hispanic individuals are known to have more hypertriglyceridemia than people of other races/ethnicities [2, 21, 41]. In NHANES, for example, 40% of Hispanic men and 35% of Hispanic women had hypertriglyceridemia, the highest prevalence among all racial/ethnic groups. In addition, in children with very severe hypertriglyceridemia [36], the most common race/ethnicity was Hispanic (28%) followed by white (17%).
It is worth noting that only three patients were reported to have an eruptive xanthoma (or xanthomata), which is commonly reported in patients with very severe hypertriglyceridemia. This may be due to a lack of awareness of this clinical finding, or perhaps the actual prevalence is lower than previously described.
Predicting which patients with very severe hypertriglyceridemia will develop acute pancreatitis remains difficult. Our logistic regression model identified serum triglyceride level as the only important risk factor, similar to prior reports [25, 42]. For example, Murphy et al. [43] reported that risk of acute pancreatitis increased by 4% for every 100-mg/dL increase in serum triglyceride concentration. They also reported a multivariate hazard ratio of 3.20 (1.99 to 5.16) for first pancreatitis hospitalization among individuals with triglyceride levels >500 mg/dL.
One prior report found that among patients with serum triglyceride levels >1500 mg/dL, fewer than 10% saw a lipid specialist [25]. Their findings, together with our observation that only 15% of similar patients were seen in specialty lipid clinics, identifies a gap in the care of patients with very severe hypertriglyceridemia: few get access to experienced health care providers. Possible barriers to follow-up or to obtaining specialist care include cost of care, lack of insurance coverage, long clinic wait times, difficulty scheduling appointments, effects of other medical or physical comorbidities, difficulties related to medical interpretation, being part of a minority ethnic population, and lack of knowledge within a patient population about the existence of lipid specialists [44–49].
Underutilization of triglyceride-lowering agents is also a major issue, with prior publications reporting few treated patients. In the NHANES, fewer than 2% of patients with triglyceride levels >1000 mg/dL were taking any triglyceride-lowering drugs [2]. In US claims databases, fibrates were taken by 35% and fish oils by 8% of patients with triglyceride levels >1500 mg/dL [25]. At least one report found a benefit of being seen in a lipid specialty clinic: fibrate use increased from 24% to 84% [33].
Future efforts should focus on identifying and treating very severe hypertriglyceridemia before patients develop acute pancreatitis. Awareness of associated secondary conditions, referrals to specialty lipid clinics, and increased use of triglyceride-lowering medications may all improve outcomes. Patients with serum triglyceride levels >1500 mg/dL have an associated 300% increase in all-cause costs [25, 50–52]; therefore, managing very severe hypertriglyceridemia more aggressively and preventing acute pancreatitis may generate cost savings [25].
Our study had a few limitations. It was performed in a single health care system, although that health care system serves a large multiethnic community. Our sample was retrospective, based on medical record review, and dependent on appropriate documentation by providers. It included a relatively small sample over a 1-year period. We may be missing follow-up data, as patients may have had follow-up visits in different institutions, clinics, or hospitals. Because our laboratory did not measure the actual triglyceride levels in subjects with serum triglyceride values >4425mg/dL (n = 26), the relationship between triglyceride levels >4425 mg/dL (the limit of detection for the laboratory) and clinical findings could not be fully evaluated. Our data did not allow determination of whether the indigent nature of our patient population was relevant to our findings; such patients may have limited access to health care professionals and thus present to providers only when their condition(s) reach an extreme level. Finally, because the ethnic/racial distribution varies across the United States, our findings may not be completely generalizable.
In conclusion, uncontrolled diabetes mellitus was the major condition associated with very severe hypertriglyceridemia. Unfortunately, few of these patients had appropriate treatment and follow-up, so it remains unclear whether very severe hypertriglyceridemia resolved after achievement of glycemic control. Future efforts are warranted to improve care for this group of patients, who are at the highest risk for potentially life-threatening acute pancreatitis.
Acknowledgments
Disclosure Summary: A.G. has received consulting fees from Aegerion Pharmaceuticals, Venture Point, Candace Consulting, Axon Consulting, and Deerfield Consulting and has served as research investigator for Pfizer, Inc., Ionis Pharmaceuticals Inc., Aegerion Pharmaceuticals, Quintiles, and InterCept Regeneron Pharmaceuticals. Z.A. has received consulting and speaking fees from Amgen and Akcea and has served as research investigator for Regeneron. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- DKA
diabetic ketoacidosis
- EMR
electronic medical record
- HDL-C
high-density lipoprotein cholesterol
- ICU
intensive care unit
- IQR
interquartile range
- LPL
low-density lipoprotein
- NHANES
US National Health and Nutrition Examination Survey
- VLDL
very low‒;density lipoprotein
References and Notes
- 1. Carroll M, Kit B, Lacher D. Trends in elevated triglyceride in adults: United States, 2001-2012. NCHS Data Brief. 2015;(198):198. [PubMed] [Google Scholar]
- 2. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169(6):572–578. [DOI] [PubMed] [Google Scholar]
- 3. Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, Kastelein JJ, Péterfy M, Nieuwdorp M. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hassing HC, Surendran RP, Mooij HL, Stroes ES, Nieuwdorp M, Dallinga-Thie GM. Pathophysiology of hypertriglyceridemia. Biochim Biophys Acta. 2012;1821(5):826–832. [DOI] [PubMed] [Google Scholar]
- 5. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome--part 1. Endocr Pract. 2015;21(11):1291–1300. [DOI] [PubMed] [Google Scholar]
- 6. Poapst M, Reardon M, Steiner G. Relative contribution of triglyceride-rich lipoprotein particle size and number to plasma triglyceride concentration. Arteriosclerosis. 1985;5(4):381–390. [DOI] [PubMed] [Google Scholar]
- 7. Lewis GF, Xiao C, Hegele RA. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev. 2015;36(1):131–147. [DOI] [PubMed] [Google Scholar]
- 8. Benlian P, De Gennes JL, Foubert L, Zhang H, Gagné SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med. 1996;335(12):848–854. [DOI] [PubMed] [Google Scholar]
- 9. Nordestgaard BG, Abildgaard S, Wittrup HH, Steffensen R, Jensen G, Tybjaerg-Hansen A. Heterozygous lipoprotein lipase deficiency: frequency in the general population, effect on plasma lipid levels, and risk of ischemic heart disease. Circulation. 1997;96(6):1737–1744. [DOI] [PubMed] [Google Scholar]
- 10. Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am. 1998;27(3):551–567, viii. [DOI] [PubMed] [Google Scholar]
- 11. Péterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE, Pullinger CR, Frost PH, Kane JP, Malloy MJ, Reue K, Pajukanta P, Doolittle MH. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet. 2007;39(12):1483–1487. [DOI] [PubMed] [Google Scholar]
- 12. Deep SS, Brunzell JD. Familial lipoprotein lipase deficiency, Apo C-II deficiency, and hepatic lipase deficiency. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G, eds. Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; 2006. [Google Scholar]
- 13. Wang J, Hegele RA. Homozygous missense mutation (G56R) in glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPI-HBP1) in two siblings with fasting chylomicronemia (MIM 144650). Lipids Health Dis. 2007;6(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29(6):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Priore Oliva C, Carubbi F, Schaap FG, Bertolini S, Calandra S. Hypertriglyceridaemia and low plasma HDL in a patient with apolipoprotein A-V deficiency due to a novel mutation in the APOA5 gene. J Intern Med. 2008;263(4):450–458. [DOI] [PubMed] [Google Scholar]
- 16. Marçais C, Verges B, Charrière S, Pruneta V, Merlin M, Billon S, Perrot L, Drai J, Sassolas A, Pennacchio LA, Fruchart-Najib J, Fruchart JC, Durlach V, Moulin P. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest. 2005;115(10):2862–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Priore Oliva C, Pisciotta L, Li Volti G, Sambataro MP, Cantafora A, Bellocchio A, Catapano A, Tarugi P, Bertolini S, Calandra S. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2005;25(2):411–417. [DOI] [PubMed] [Google Scholar]
- 18. Priore Oliva C, Tarugi P, Calandra S, Pisciotta L, Bellocchio A, Bertolini S, Guardamagna O, Schaap FG. A novel sequence variant in APOA5 gene found in patients with severe hypertriglyceridemia. Atherosclerosis. 2006;188(1):215–217. [DOI] [PubMed] [Google Scholar]
- 19. Bessembinders K, Wielders J, van de Wiel A. Severe hypertriglyceridemia influenced by alcohol (SHIBA). Alcohol Alcohol. 2011;46(2):113–116. [DOI] [PubMed] [Google Scholar]
- 20. Karalis DG. A review of clinical practice guidelines for the management of hypertriglyceridemia: a focus on high dose omega-3 fatty acids. Adv Ther. 2017;34(2):300–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin SX, Carnethon M, Szklo M, Bertoni A. Racial/ethnic differences in the association of triglycerides with other metabolic syndrome components: the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2011;9(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steiner G, Poapst M, Davidson JK. Production of chylomicron-like lipoproteins from endogenous lipid by the intestine and liver of diabetic dogs. Diabetes. 1975;24(3):263–271. [DOI] [PubMed] [Google Scholar]
- 23. Brunzell JD. Hypertriglyceridemia. N Engl J Med. 2007;357(10):1009–1017. [DOI] [PubMed] [Google Scholar]
- 24. Fortson MR, Freedman SN, Webster PD III. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90(12):2134–2139. [PubMed] [Google Scholar]
- 25. Toth PP, Grabner M, Ramey N, Higuchi K. Clinical and economic outcomes in a real-world population of patients with elevated triglyceride levels. Atherosclerosis. 2014;237(2):790–797. [DOI] [PubMed] [Google Scholar]
- 26. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400. [DOI] [PubMed] [Google Scholar]
- 27. Gloor B, Müller CA, Worni M, Martignoni ME, Uhl W, Büchler MW. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001;88(7):975–979. [DOI] [PubMed] [Google Scholar]
- 28. Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999-2006. Am J Cardiol. 2010;106(7):969–975. [DOI] [PubMed] [Google Scholar]
- 29. Shetty S, Xing C, Garg A. Type 1 hyperlipoproteinemia due to compound heterozygous rare variants in GCKR. J Clin Endocrinol Metab. 2016;101(11):3884–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christian JB, Bourgeois N, Snipes R, Lowe KA. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol. 2011;107(6):891–897. [DOI] [PubMed] [Google Scholar]
- 31. Retterstøl K, Narverud I, Selmer R, Berge KE, Osnes IV, Ulven SM, Halvorsen B, Aukrust P, Holven KB, Iversen PO. Severe hypertriglyceridemia in Norway: prevalence, clinical and genetic characteristics. Lipids Health Dis. 2017;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pedersen SB, Varbo A, Langsted A, Nordestgaard BG. Chylomicronemia risk factors ranked by importance for the individual and community in 108 711 women and men. J Intern Med. 2018;283(4):392–404. [DOI] [PubMed] [Google Scholar]
- 33. Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36(1):54–62. [DOI] [PubMed] [Google Scholar]
- 35. Tada H, Kawashiri MA, Nakahashi T, Yagi K, Chujo D, Ohbatake A, Mori Y, Mori S, Kometani M, Fujii H, Nohara A, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J Clin Lipidol. 2015;9(4):519–524. [DOI] [PubMed] [Google Scholar]
- 36. Patni N, Li X, Adams-Huet B, Garg A. The prevalence and etiology of extreme hypertriglyceridemia in children: data from a tertiary children’s hospital. J Clin Lipidol. 2018;12(2):305–310. [DOI] [PubMed] [Google Scholar]
- 37. Johansen CT, Hegele RA. Genetic bases of hypertriglyceridemic phenotypes. Curr Opin Lipidol. 2011;22(4):247–253. [DOI] [PubMed] [Google Scholar]
- 38. Johansen CT, Wang J, Lanktree MB, Cao H, McIntyre AD, Ban MR, Martins RA, Kennedy BA, Hassell RG, Visser ME, Schwartz SM, Voight BF, Elosua R, Salomaa V, O’Donnell CJ, Dallinga-Thie GM, Anand SS, Yusuf S, Huff MW, Kathiresan S, Hegele RA. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010;42(8):684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rees MG, Raimondo A, Wang J, Ban MR, Davis MI, Barrett A, Ranft J, Jagdhuhn D, Waterstradt R, Baltrusch S, Simeonov A, Collins FS, Hegele RA, Gloyn AL. Inheritance of rare functional GCKR variants and their contribution to triglyceride levels in families. Hum Mol Genet. 2014;23(20):5570–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tam CH, Wang Y, Lee HM, Luk AO, Tong PC, Chan MH, Ozaki R, Kong AP, So WY, Chan JC, Ma RC. Early gene-diet interaction between glucokinase regulatory protein (GCKR) polymorphism, vegetable and fish intakes in modulating triglyceride levels in healthy adolescents. Nutr Metab Cardiovasc Dis. 2015;25(10):951–958. [DOI] [PubMed] [Google Scholar]
- 41. Garcia-Garcia G, Aviles-Gomez R, Luquin-Arellano VH, Padilla-Ochoa R, Lepe-Murillo L, Ibarra-Hernandez M, Briseño-Renteria G. Cardiovascular risk factors in the Mexican population. Ren Fail. 2006;28(8):677–687. [DOI] [PubMed] [Google Scholar]
- 42. Lloret Linares C, Pelletier AL, Czernichow S, Vergnaud AC, Bonnefont-Rousselot D, Levy P, Ruszniewski P, Bruckert E. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas. 2008;37(1):13–2. [DOI] [PubMed] [Google Scholar]
- 43. Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164. [DOI] [PubMed] [Google Scholar]
- 44. Howard AF, Kazanjian A, Pritchard S, Olson R, Hasan H, Newton K, Goddard K. Healthcare system barriers to long-term follow-up for adult survivors of childhood cancer in British Columbia, Canada: a qualitative study. J Cancer Surviv. 2018;12(3):277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee BW, Murakami Y, Duncan MT, Kao AA, Huang JY, Lin S, Singh K. Patient-related and system-related barriers to glaucoma follow-up in a county hospital population. Invest Ophthalmol Vis Sci. 2013;54(10):6542–6548. [DOI] [PubMed] [Google Scholar]
- 46. Palmer NR, Weaver KE, Hauser SP, Lawrence JA, Talton J, Case LD, Geiger AM. Disparities in barriers to follow-up care between African American and white breast cancer survivors. Support Care Cancer. 2015;23(11):3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Story M, Reynolds B, Bowser M, Xu H, Lyon M. Barriers to outpatient stress testing follow-up for low-risk chest pain patients presenting to an ED chest pain unit. Am J Emerg Med. 2016;34(5):790–793. [DOI] [PubMed] [Google Scholar]
- 48. Unzueta M, Globe D, Wu J, Paz S, Azen S, Varma R; Los Angeles Latino Eye Study Group. Compliance with recommendations for follow-up care in Latinos: the Los Angeles Latino Eye Study. Ethn Dis. 2004;14(2):285–291. [PubMed] [Google Scholar]
- 49. Wilcox A, Levi EE, Garrett JM. Predictors of non-attendance to the postpartum follow-up visit. Matern Child Health J. 2016;20(Suppl 1)22–27. [DOI] [PubMed] [Google Scholar]
- 50. Wadhwa V, Patwardhan S, Garg SK, Jobanputra Y, Lopez R, Sanaka MR. Health care utilization and costs associated with acute pancreatitis. Pancreas. 2017;46(3):410–415. [DOI] [PubMed] [Google Scholar]
- 51. Nichols GA, Arondekar B, Jacobson TA. Hospital use and medical care costs up to 5 years after triglyceride lowering among patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6(5):443–449. [DOI] [PubMed] [Google Scholar]
- 52. Nichols GA, Arondekar B, Garrison LP Jr. Patient characteristics and medical care costs associated with hypertriglyceridemia. Am J Cardiol. 2011;107(2):225–229. [DOI] [PubMed] [Google Scholar]