Abstract
Context
Patients with lipodystrophy have dyslipidemia and insulin resistance. Leptin treatment with metreleptin in lipodystrophy decreases insulin resistance and lowers triglycerides without changing high-density lipoprotein. Detailed measurement of lipoprotein particles with nuclear magnetic resonance (NMR) spectroscopy can offer insights into cardiovascular disease (CVD) risk and lipid metabolism beyond a standard lipid panel. We hypothesized that patients with lipodystrophy would have a more atherogenic lipid profile than controls at baseline, which would be ameliorated with metreleptin treatment.
Objective
To characterize the lipoprotein profile in patients with lipodystrophy compared with controls and to evaluate effects of metreleptin treatment.
Design, Setting, Patients, and Intervention
Patients with lipodystrophy (N = 17) were studied before and after metreleptin for 2 weeks and 6 months and compared with 51 insulin-sensitive sex-matched controls.
Main Outcome Measures
Lipoprotein profiles were measured by NMR with the LP4 deconvolution algorithm, which reports triglyceride-rich lipoprotein particles (TRLPs), high-density lipoprotein particles (HDLPs), and low-density lipoprotein particles (LDLPs).
Results
Patients with lipodystrophy had elevated large TRLPs and smaller HDLPs and LDLPs compared with controls. Five patients with lipodystrophy had chylomicrons, compared with zero controls. Metreleptin decreased the size and concentration of TRLPs, eliminated chylomicrons in all but one patient, decreased LDLPs, and increased LDLP size. Metreleptin treatment did not have major effects on HDLPs.
Conclusions
Patients with lipodystrophy had an atherogenic lipoprotein profile at baseline consistent with elevated CVD risk, which improved after metreleptin treatment. The presence of fasting chylomicrons in a subset of patients with lipodystrophy suggests saturation of chylomicron clearance by lipoprotein lipase.
Keywords: lipoproteins, triglycerides, LDL-C, HDL-C, lipodystrophy
Lipodystrophy syndromes are a heterogeneous group of disorders characterized by selective deficiency of adipose tissue. Generalized lipodystrophy involves a nearly complete absence of adipose tissue, whereas partial lipodystrophy involves a deficiency confined to select areas, usually the limbs and buttocks, with other fat depots preserved or even hypertrophied [1]. Deficiency of adipose tissue in lipodystrophy results in low levels of the adipokine leptin. Low leptin and deficient adipose storage capacity lead to ectopic lipid in the muscle and liver, insulin resistance [2], and dyslipidemia [high triglycerides (TGs) and low high-density lipoprotein cholesterol (HDL-C)] [3].
Treatment with recombinant human methionyl leptin (metreleptin) improves metabolic disease in patients with lipodystrophy [4]. Specifically, hypertriglyceridemia and ectopic lipid storage improve in patients with both generalized and partial lipodystrophy [5]. Despite the large reductions in TGs with metreleptin therapy, HDL-C does not change with this treatment [6].
Plasma TGs are present mainly in triglyceride-rich lipoprotein particles (TRLPs), including very low-density lipoproteins (VLDLs) and chylomicrons. Certain TRLPs s may be better indicators of cardiovascular risk than conventional measures of TG concentration [7]. These lipoprotein parameters can now be assessed via nuclear magnetic resonance (NMR). NMR measures the resonance signal of terminal methyl groups of lipids in lipoproteins, and subsequent deconvolution of the resulting spectra provides exquisite phenotyping of lipoprotein subclasses. Elevations in large very low-density lipoprotein particles (VLDLPs), small high-density lipoprotein particles (HDLPs), and small low-density lipoprotein particles (LDLPs) have been associated with increased risk of coronary artery disease [8, 9]. This atherogenic lipoprotein profile has been described in patients with insulin resistance due to type 2 diabetes [10]. Lipoprotein profiles in patients with lipodystrophy may provide additional information about the atherogenic profile in these patients beyond that of a typical lipid panel.
In addition to measuring major categories of small, medium, and large HDLPs, LDLPs, and TRLPs, the LipoProfile-4 (LP4) spectral deconvolution algorithm provides better resolution of TRLP and HDLP subclasses. The LP4 further subdivides TRLPs and HDLPs into 24 and 7 particle size subcategories, respectively. A novel feature of this analysis is the ability to identify and separate chylomicrons (very large TRLPs). Because lipoprotein particles change as they accumulate and clear lipids, characterizing the distribution of different-sized particles may provide hypothesis-generating data on where the dysfunction in lipid clearance and accumulation occurs in patients with lipodystrophy.
The goal of the study was to better characterize the lipid abnormalities in patients with lipodystrophy by using high-resolution separation of lipoprotein particles. The first aim was to compare lipoprotein profiles in patients with lipodystrophy to those of a healthy sex-matched control population. The second aim was to examine the short-term effects of metreleptin on the lipoprotein profile in patients with lipodystrophy during hospitalization with constant food intake and the long-term effects of metreleptin in these patients in a free-living environment. We hypothesized that patients with lipodystrophy would have a more atherogenic lipoprotein profile compared with controls and that the lipoprotein profile would become less atherogenic after metreleptin treatment, indicating improvements in lipid metabolism.
1. Methods
All studies were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board. Subjects or their legal guardians provided written informed consent; minors provided written assent.
A. Subjects
Seventeen patients with lipodystrophy who had not previously received metreleptin treatment were studied to evaluate the short- and long-term effects of metreleptin on metabolic disease (NCT01778556). This was a part of a larger study, the main results of which have been previously published [5]. Two additional patients were added to the data set since the previous publication.
Inclusion criteria included a clinical diagnosis of lipodystrophy based on generalized or regional absence of subcutaneous adipose tissue, fasting leptin <12.0 ng/mL (women) or <8.0 (men), and one or more metabolic complications of lipodystrophy, including diabetes (defined by 2007 American Diabetes Association criteria), fasting insulin >30 µU/mL, or fasting TG >200 mg/dL. Exclusion criteria included HIV-associated lipodystrophy, active inflammatory disease or glucocorticoid use, and changes in diabetes or lipid-lowering medications within the past 6 weeks.
Healthy control subjects were selected from a metabolic phenotyping protocol (NCT00428987). Selected subjects had lipid NMR performed; were insulin sensitive based on insulin sensitivity index (SI) >3.0 as measured by frequently sampled intravenous glucose tolerance test; did not have a diagnosis of diabetes, hyperlipidemia, cancer, cirrhosis, or heart disease; and did not take any lipid- or glucose-modifying medications (e.g., statins, metformin, fibrates). All control subjects were >18 years old. Among eligible controls, three matched controls were selected for each subject with lipodystrophy based on sex (n = 51).
B. Study Design
Details of the study design have been published [5]. Briefly, metreleptin-naive patients with lipodystrophy were hospitalized on a metabolic unit for 19 days. Patients were admitted on their home diet (day −5). A weight maintenance diet with controlled macronutrient content (20% ± 5% protein, 25% ± 5% fat, 55% ± 5% carbohydrate) was then initiated and maintained for the next 19 days. Research dietitians used the Mifflin–St. Jeor equations for men with an activity factor of 1.5 to estimate total caloric requirements (for both male and female subjects). The subjects were instructed on the importance of eating 100% of the food given and not consuming any additional food. Metreleptin treatment (5 mg metreleptin twice daily) was initiated after 5 days of the diet (day 0) and was continued until the end of the inpatient stay (day 14). After discharge, patients continued metreleptin treatment (the dosage was lowered for patients with generalized lipodystrophy to prevent excessive weight loss), and food intake was not controlled by investigators. Blood samples were obtained after a supervised 10- to 12-hour fast on day −5, day 0, day 14, and the 6-month follow-up visit, and plasma was frozen at −80°C until the time of analysis. Standard clinical lipid panel, fasting glucose, and serum insulin levels were measured. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as glucose (mg/dL) × insulin (μU/mL)/405. Total cholesterol, HDL-C, and TGs were run on the Roche Cobas 6000 Analyzer (Basel, Switzerland). Low-density lipoprotein cholesterol (LDL-C) was calculated via the Friedewald equation, LDL-C = Total cholesterol – (HDL-C + TG/5) and was evaluated only if the specimen had TGs ≤400 mg/dL. At day 0, day 14, and the 6-month follow-up visit, insulin sensitivity was evaluated via hyperinsulinemic euglycemic clamp. A detailed description of the hyperinsulinemic euglycemic clamp has been previously described [11]. Briefly, patients received a primed, continuous infusion of insulin (120 mU/m2/min) for 3 hours with infusion of 20% dextrose to maintain blood glucose at 100 ± 5 mg/dL. Insulin sensitivity (M) was defined as the glucose infusion rate during the final 30 minutes of the clamp, normalized for fat-free mass (in mg/kgFFM/min).
Except for insulin and sulfonylureas, patients continued their preadmission medications throughout the study, including oral hypoglycemic agents, lipid-lowering medications, and other medications either related or unrelated to lipodystrophy and its complications. Because of metreleptin’s effects on insulin sensitivity, subjects on insulin and sulfonylureas were at risk for hypoglycemia, so the dosage was reduced as needed throughout treatment.
C. Lipid NMR
The lipoprotein profile was characterized via NMR spectroscopy on samples obtained on day −5, day 0, day 14 and the 6-month follow-up visit. Of the 17 patients, three subjects’ day −5 lipid NMR data were not included because of poor sample correlation in the LP4 algorithm, one subject had missing data at day 14, and two subjects’ 6-month data were not used (one because of noncompliance with metreleptin and the second because of initiation of an appetite-stimulating medication). This resulted in a total of 14 patients for comparison with controls (day −5), 16 patients for the evaluation of short-term metreleptin treatment (day 0 vs day 14), and 13 patients for the evaluation of long-term metreleptin treatment (day −5 vs 6 months). Plasma samples were thawed and analyzed with the 400-MHz proton Vantera Clinical Analyzer (LipoScience, Inc, Morrisville, NC). The NMR analysis uses an advanced algorithm (LP4) to quantify lipoprotein subspecies. Particle size, concentration, and concentration of subclasses by size (e.g., large, medium, small) were measured for all lipoprotein classes (LDLPs, HDLPs, and TRLPs). Additionally, concentrations of very small and very large particles were quantified for TRLPs. HDLPs and TRLPs were further subdivided into seven and 24 additional particle size subcategories, respectively. The additional 24 TRLP parameters were not available for control subjects. The diameters of the various parameters are in Table 1. This technique also provides derived measures for apolipoprotein A1 (apoA1) and apolipoprotein B (apoB).
Table 1.
Description of Lipoprotein Parameters
| Parameter | Description | Diameter Range (nm) |
|---|---|---|
| LDLP concentrations, nmol/L | ||
| Total LDLP | Sum of LDLP subclass concentrations | 19–23 |
| Small LDLP | Small LDLP concentration | 19–20.4 |
| Medium LDLP | Medium LDLP concentration | 20.5–21.4 |
| Large LDLP | Large LDLP concentration | 21.5–23 |
| HDLP concentrations, μmol/L | ||
| Total HDLP | Sum of HDLP concentrations | 7.5–13 |
| Small HDLP | Small HDL concentration | 7.4–7.8 |
| Medium HDLP | Medium HDL concentration | 8.7–9.5 |
| Large HDLP | Large HDL concentration | 10.3–12 |
| TRLP concentrations, nmol/L | ||
| Total TRLP | Sum of TRLP subclass concentrations | 24–240 |
| Very small TRLP | Very small TRLP concentration | 24–29 |
| Small TRLP | Small TRLP concentration | 30–36 |
| Medium TRLP | Medium TRLP concentration | 37–49 |
| Large TRLP | Large TRLP concentration | 50–89 |
| Very large TRLP | Very large TRLP concentration | 90–240 |
| Mean particle sizes, nm | ||
| LDLP size | Mean LDLP size | 19–22.5 |
| HDLP size | Mean HDLP size | 7.4–13 |
| TRLP size | Mean TRLP size | 30–100 |
D. Statistics
Comparisons between patients with lipodystrophy and controls were evaluated via unpaired t tests for normally distributed data, Mann-Whitney tests for nonparametric data (continuous variables), or Fisher exact test (categorical variables). Changes in lipoprotein parameters and metabolic and phenotypic characteristics between time points (day −5 vs 6 months and day 0 vs day 14) were evaluated by one-sample t tests for normally distributed data or Wilcoxon signed rank tests for nonparametric data. Correlations between changes in M and lipoprotein parameters were evaluated via Pearson correlation for parametric data and Spearman correlation for nonparametric data. Because M values were not available on day −5, both short-term and long-term changes with metreleptin were calculated as changes from day 0.
Values shown in tables are means ± SD for normally distributed data and median (25th percentile, 75th percentile) for nonnormally distributed variables. Analyses were performed in GraphPad Prism version 7 (GraphPad Software, La Jolla, CA). A P < 0.05 was considered statistically significant.
2. Results
A. Baseline Characteristics
Baseline characteristics in patients with lipodystrophy and controls are shown in Table 2. Seventeen patients with lipodystrophy (3 male, 14 female) and 51 sex-matched control patients (9 male, 42 female) were studied. Five patients with lipodystrophy had congenital generalized lipodystrophy (three AGPAT2, two BSCL2) and 12 had familial partial lipodystrophy (four LMNA, one PPARG, and seven unknown). Two patients with partial lipodystrophy were second-degree relatives. Among patients with lipodystrophy, 11 (64.7%) used insulin, 12 (70.6%) used metformin, and 5 (29.4%) used other diabetes medications (thiazolidinediones, glucagon-like peptide-1 analogs, dipeptidyl peptidase IV inhibitors). Fourteen patients (82%) used fibrates, 6 (35.3%) used fish oil, and 2 (11.8%) used niacin. Nine patients (52.9%) used angiotensin system antagonists, and 8 (47.1%) used other antihypertensive drugs.
Table 2.
Baseline Characteristics of Subjects
| Lipodystrophy | Controls | P | |
|---|---|---|---|
| Age | 29 ± 16 | 35 ± 8 | 0.06 |
| Sex | 3 M, 14 F | 9 M, 42 F | >0.99 |
| Weight, kg | 70.5 ± 17.9 | 73.5 ± 17.1 | 0.54 |
| BMI, kg/m2 | 24.7 ± 4.9 | 27.5 ± 6.1 | 0.18 |
| Body fat, % | 22.5 ± 11.4 | 36.0 ± 8.5 | <0.0001 |
| Total cholesterol, mg/dL | 237 (174, 377)a | 164 (145, 182) | 0.0001 |
| HDL-C, mg/dL | 25 (22, 32)b | 55 (46, 65) | <0.0001 |
| LDL-C, mg/dL, by Friedewald | 76 (60, 118)c | 90 (68, 112) | 0.43 |
| LDL-C, mg/dL, by NMR | 102.7 ± 30.3 | 93.5 ± 23.4 | 0.22 |
| TG, mg/dL | 553 (291, 3248)a | 83 (58, 107) | <0.0001 |
| HOMA-IR | 11.6 (4.2, 25.5) | 1.3 (0.72, 1.9) | <0.0001 |
| Fasting glucose, mg/dL | 159 (108, 308) | 88.7 (83.7, 93.7) | <0.0001 |
Values are means ± SD for normally distributed data and median (25th percentile, 75th percentile) for variables without normal distribution. If either the patients with lipodystrophy cohort or control cohorts did not have normal distribution, the medians (25th percentile, 75th percentile) are reported for both. HOMA-IR was calculated as glucose (mg/dL) × insulin (μU/mL)/405.
Abbreviations: F, female; M, male.
n = 16.
n = 12.
n = 6.
Age, weight, and body mass index (BMI) were not significantly different between patients with lipodystrophy and controls. As expected, patients with lipodystrophy had lower body fat. When compared with controls, patients with lipodystrophy had significantly lower HDL-C [25 (22, 32) vs 55 (46, 65) mg/dL] and significantly higher levels of TG [553 (291, 3248) vs 83 (58, 107) mg/dL] and total cholesterol [237 (174, 377) in lipodystrophy vs 164 (145, 182) in controls]. LDL-C was not different in patients vs controls either when calculated via the Friedewald equation (available in only six patients with lipodystrophy due to TG >400 mg/dL in others) or derived via NMR spectroscopy. Patients with lipodystrophy also had higher fasting glucose and insulin resistance as assessed by HOMA-IR than controls.
B. Comparison of Lipoprotein Parameters in Patients With Lipodystrophy vs Controls
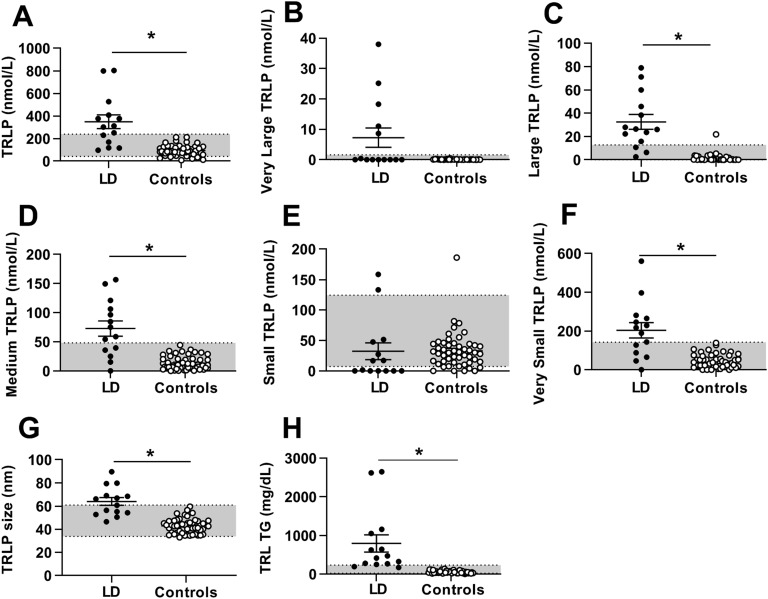
At baseline, most TRLP parameters were higher in patients with lipodystrophy than in controls (Fig. 1). Specifically, concentrations of total TRLPs, large TRLPs (equivalent to large VLDLs), medium TRLPs (equivalent to medium VLDLs), very small TRLPs [equivalent to intermediate-density lipoproteins (IDLs)], TG content within TRLPs, and average size of TRLPs were all significantly higher in patients with lipodystrophy than in controls. Concentration of very large TRLPs (equivalent to chylomicrons) was not significantly elevated in patients with lipodystrophy compared with controls. However, five patients with lipodystrophy had very large TRLPs >1 nmol/L in the fasting state, compared with 0 controls (P = 0.0002).
Figure 1.
TRLP parameters in patients with lipodystrophy before metreleptin treatment (black circles) vs healthy controls (open circles). Bars indicate the mean and SEM. (A) Total TRLPs, (B) very large TRLPs, (C) large TRLPs, (D) medium TRLPs, (E) small TRLPs, (F) very small TRLPs, (G) TRLP size, and (H) TG content within TRLPs. *P < 0.05 for comparison of controls vs patients with lipodystrophy. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)].
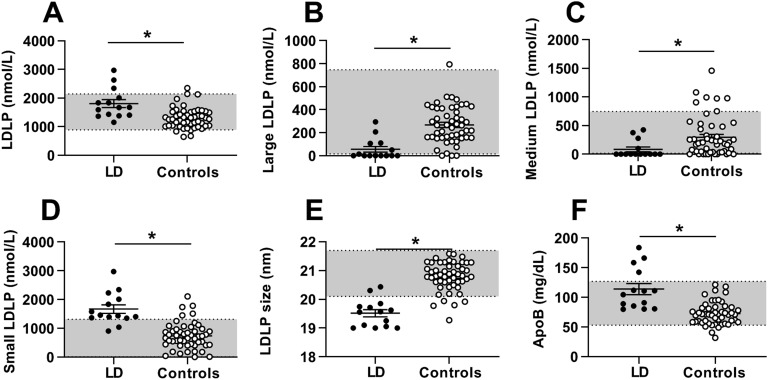
LDL-C was not different in patients with lipodystrophy vs controls, but all LDL NMR parameters were significantly different (Fig. 2). Total LDLPs was markedly higher in patients with lipodystrophy, primarily because of increased small LDLPs, whereas large LDLPs and medium LDLPs were lower in patients with lipodystrophy than in controls. Average LDL size was therefore smaller and apoB higher in patients with lipodystrophy compared with controls.
Figure 2.
LDLP parameters in patients with lipodystrophy before metreleptin treatment (black circles) vs healthy controls (open circles). Bars indicate the mean and SEM. (A) Total LDLPs, (B) large LDLPs, (C) medium LDLPs, (D) small LDLPs, (E) LDLP size, and (F) apoB. *P < 0.05 for comparison of controls vs patients with lipodystrophy. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)].
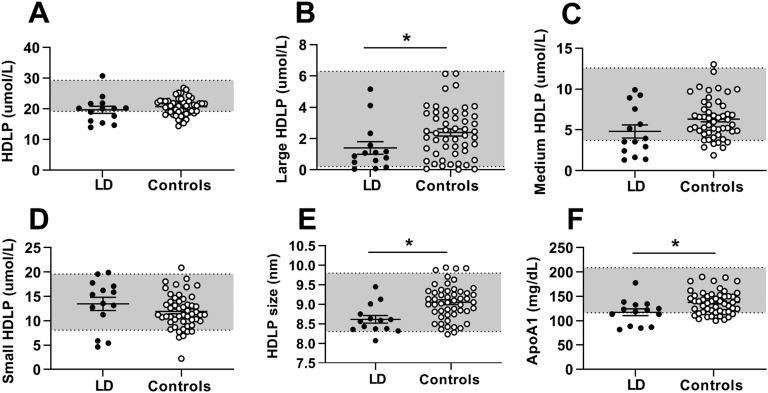
HDL-C and apoA1 were markedly lower in patients with lipodystrophy than in controls. These differences were attributable mainly to smaller HDLPs and lower concentrations of large HDLPs (Fig. 3). Total HDLP, small HDLP, and medium HDLP concentrations were not significantly different between the two groups. Seven HDLP subclasses in patients with lipodystrophy compared with controls are shown in Table 3.
Figure 3.
HDLP parameters in patients with lipodystrophy before metreleptin treatment (black circles) vs healthy controls (open circles). Bars indicate the mean and SEM. (A) Total HDLPs, (B) large HDLPs, (C) medium HDLPs, (D) small HDLPs, (E) HDLP size, and (F) apoA1. *P < 0.05 for comparison of controls vs patients with lipodystrophy. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)].
Table 3.
Additional TRLP and HDLP Parameters
| Parameter | Day −5 | Day 0 | Day 14 | 6 Mo | Controls |
|---|---|---|---|---|---|
| TRLPs | |||||
| Very large TRLPs (chylomicrons) | |||||
| VVL-255 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| VVL-240 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-225 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.01) | 0 (0, 0) | |
| VVL-215 | 0 (0, 0.25) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-190a | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-190b | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-180 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-170 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-160 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| VVL-150 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.02) | 0 (0, 0) | |
| VVL-130 | 0 (0, 4.9) | 0 (0, 0) | 0 (0, 0.1) | 0 (0, 0.02) | |
| VVL-110 | 0 (0, 0) | 0 (0, 3.3) | 0 (0, 0.1) | 0 (0, 0.05) | |
| VVL-95 | 0 (0, 2.2) | 0.04 (0, 8.2) | 0.1 (0, 0.8) | 0 (0, 0) | |
| Large TRLPs | |||||
| VL-80 | 3.2 (0, 29.5) | 5.4 (0, 27.5) | 0.1 (0, 15.1) | 0.3 (0, 3.2)a | |
| VL-65 | 9.8 (2.9, 22.2) | 7.4 (1.5, 16.7) | 6.4 (1.3, 13.9) | 3.2 (1.2, 13.1) | |
| VL-55 | 0 (0, 0) | 0 (0, 0.2) | 0 (0, 0) | 0 (0, 8.8) | |
| Medium TRLPs | |||||
| VM-48 | 16.5 (0, 26.0) | 25.2 (4.6, 52.6) | 31.2 (15.6, 36.7) | 15.7 (6.6, 23.2) | |
| VM-42 | 26.1 (0.5, 65.3) | 11.8 (0, 54.3) | 0 (0, 15.4) | 0 (0, 23.6)a | |
| VM-38 | 0 (0, 31.3) | 0 (0, 0) | 0.3 (0, 5.9) | 0 (0, 3.5) | |
| Small TRLPs | |||||
| VS-35 | 0 (0, 21.4) | 0 (0, 9.4) | 0 (0, 3.8) | 7.9 (0, 30.0) | |
| VS-33 | 0 (0, 7.3) | 0 (0, 5.7) | 0.6 (0, 17.9) | 7 (0, 16.6)a | |
| VS-30 | 0 (0, 0.4) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Very small TRLPs (IDLs) | |||||
| VVS-28 | 0 (0, 17.0) | 0 (0, 23.4) | 0 (0, 8.5) | 0 (0, 28.2) | |
| VVS-27 | 201.3 (56.0, 269.4) | 104.2 (19.1, 239.0) | 42.8 (11.4, 110.3)b | 57.3 (18.1, 100.7)a | |
| HDLPs | |||||
| Large HDLPs | |||||
| H7P (12.0) | 0 (0, 0.01) | 0 (0, 0.01) | 0.01 (0, 0.1)1 | 0 (0, 0.1) | 0.1 (0.02, 0.4)c |
| H6P (10.8) | 0 (0, 0.8) | 0.1 (0, 0.2) | 0.1 (0, 0.8) | 0.05 (0, 0.4) | 0.5 (0, 1.4) |
| H5P (10.3) | 0.5 (0.1, 1.2) | 0.3 (0.05, 1) | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.6) | 1.04 (0.4, 1.9) |
| Medium HDLPs | |||||
| H4P (9.5) | 0.6 (0.2, 1.2) | 0.5 (0.1, 1.8) | 1.1 (0.3, 1.4) | 1 (0.8, 1.4)a | 2.03 (1.42, 2.54)c |
| H3P (8.7) | 2.8 (1.6, 7.5) | 1.8 (0.2, 4.4) | 1 (0.6, 3.2) | 2.8 (0.7, 4.6)a | 3.84 (2.71, 5.33) |
| Small HDLPs | |||||
| H2P (7.8) | 12.04 (4.2, 14.9) | 12.5 (8.1, 14.9) | 10.9 (8.0, 13.8) | 11.0 (8.7, 13.2) | 9.8 (7.9, 11.1) |
| H1P (7.4) | 2.3 (1.1, 3.7) | 2.0 (1.1, 3.0) | 3.0 (1.7, 3.3) | 1.8 (1.1, 2.7) | 1.6 (1.0, 2.4) |
The additional TRLP and HDLP parameters are distinguished by size. The number in each TRLP parameter indicates the approximate diameter in nm. For HDLP parameters, the number in parentheses indicates the approximate diameter in nm. Values shown are means ± SD for normally distributed data and median (25th percentile, 75th percentile) for variables without normal distribution. Units are nmol/L for TRLPs and μmol/L for HDLPs.
Indicates significant long-term (day −5 vs 6 mo) change (P < 0.05).
Indicates significant short-term (day 0 vs day 14) change (P < 0.05).
Indicates a significant difference between patients with lipodystrophy and controls (for HDLP parameters) (P < 0.05).
C. Effects of Metreleptin
Metreleptin treatment resulted in expected changes in the baseline metabolic parameters in patients with lipodystrophy. These changes are shown in Table 4. Over the short term (day 0 to day 14) inpatient treatment period, weight, BMI, total cholesterol, TGs, and fasting glucose significantly decreased. Over the 6-month period, weight, BMI, body fat percentage, total cholesterol, TGs, fasting glucose, and LDL-C by NMR significantly decreased.
Table 4.
Changes in Anthropometric and Metabolic Parameters With Metreleptin
| Change After 2 Wka | Change After 6 Mob | |
|---|---|---|
| Weight, kg | −0.88 ± 1.2c | −2.9 ± 2.7c |
| BMI, kg/m2 | −0.29 ± 0.43c | −1.1 ± 0.94c |
| Body fat, % | −0.35 ± 0.76 | −1.1 ± 1c,d |
| Total cholesterol, mg/dL | −33 (−105.8, −14.6)c | −101.6 ± 95.6c |
| HDL-C, mg/dL | −0.88 ± 4.4 | 2.5 ± 7.6 |
| LDL-C, mg/dL | −10.3 (−28.9, 18.4)e | −16 (−40, −15.5)f |
| LDL-C, mg/dL, by NMR | −5.9 ± 23.0 | −22.3 ± 12.8c |
| TG, mg/dL | −138.3 (−692.5, −60.5)c | −225.5 (−2358, −49)c |
| HOMA-IR | −1.6 (−9.4, 0.57) | −0.64 (−13.7, 0.30) |
| Fasting glucose, mg/dL | −18.8 ± 26.2c | −63.5 ± 85.3c |
Values are means ± SD for normally distributed data and median (25th percentile, 75th percentile) for variables without normal distribution.
Day 0 to day 14.
Day −5 to 6 mo.
P < 0.05.
Body fat percentage was not done at day −5, so the 6-mo time point was compared with day 0.
n = 6.
n = 5.
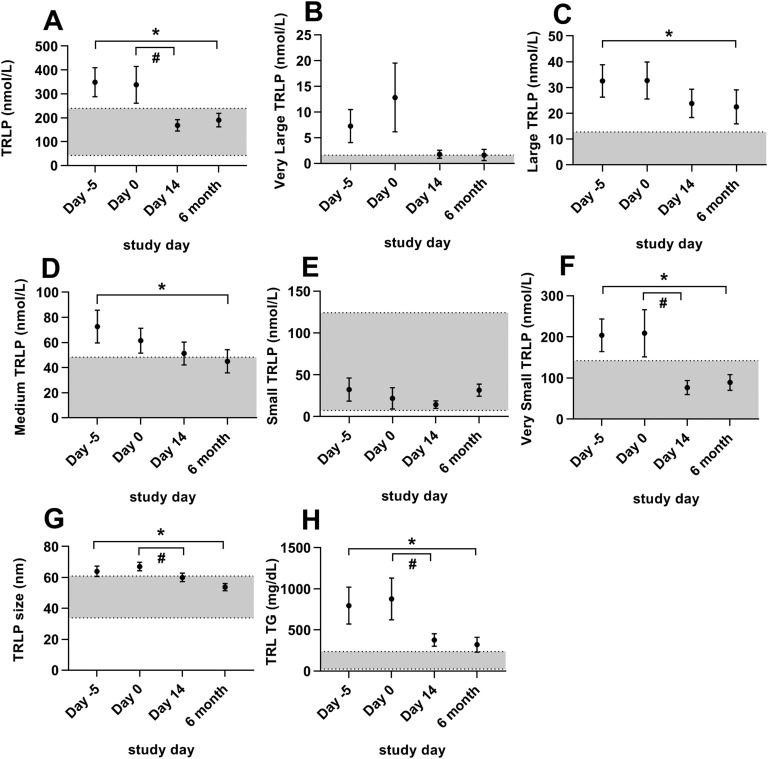
Metreleptin treatment improved many of the abnormal TRLP parameters in patients with lipodystrophy (Fig. 4). After short-term (14 days) metreleptin treatment on constant food intake, total TRLPs, very small TRLPs, average TRLP size, and TGs within TRLPs significantly decreased. After long-term (6 months) metreleptin on home diet, total TRLPs markedly decreased, primarily because of reductions in very small TRLPs with associated reductions in medium and large TRLPs. This decrease led to an overall reduction in TRLP size and TGs within TRLPs. Although long-term changes in very large TRLPs were not significant, only one patient with lipodystrophy had chylomicrons in the fasting state after 6 months of leptin treatment, compared with five in the metreleptin- naive state. Changes in all 24 TRLP subclasses are listed in Table 3.
Figure 4.
Effects of metreleptin on TRLPs in patients with lipodystrophy. Bars indicate the mean and SEM. Patients were studied before metreleptin on hospital admission with home diet (day −5), after 5 d of controlled inpatient diet (day 0), after 2 wk of metreleptin on controlled inpatient diet (day 14), and after 6 mo of metreleptin on home diet (6 mo). Statistical comparisons were performed off vs on short-term metreleptin on controlled diet (day 0 vs day 14) and off vs on long-term metreleptin on home diet (day −5 vs 6 mo). *P < 0.05 for day −5 vs 6 mo. #P < 0.05 for day 0 vs day 14. (A) Total TRLPs, (B) very large TRLPs, (C) large TRLPs, (D) medium TRLPs, (E) small TRLPs, (F) very small TRLPs, (G) TRLP size, and (H) TG content within TRLPs. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)].
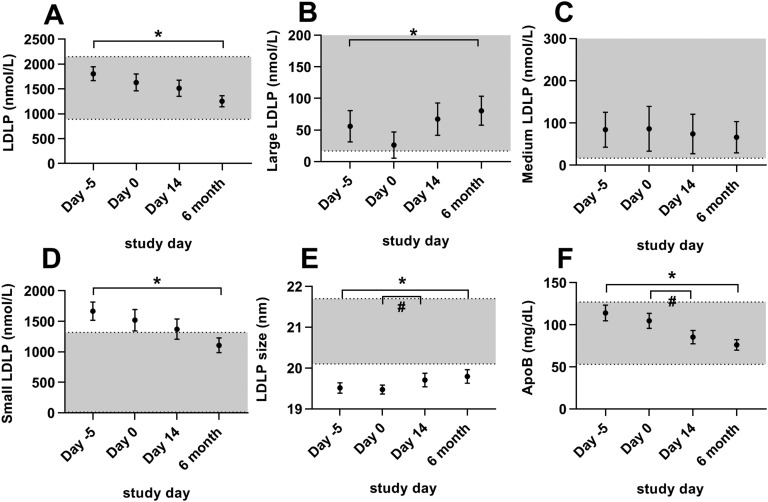
Metreleptin treatment resulted in a less atherogenic LDLP distribution in patients with lipodystrophy (Fig. 5). Average LDL size significantly increased during both short-term metreleptin treatment with constant food intake (14 days), and during long-term metreleptin treatment (6 months). ApoB significantly decreased during both periods. After long-term (but not short-term) metreleptin treatment, total LDLPs decreased significantly because of reductions in small LDLPs, whereas there was a mild increase in large LDLPs.
Figure 5.
Effects of metreleptin on LDLPs in patients with lipodystrophy. Bars indicate the mean and SEM. Patients were studied before metreleptin on hospital admission with home diet (day −5), after 5 d of controlled inpatient diet (day 0), after 2 wk of metreleptin on controlled inpatient diet (day 14), and after 6 mo of metreleptin on home diet (6 mo). Statistical comparisons were performed off vs on short-term metreleptin on controlled diet (day 0 vs day 14) and off vs on long-term metreleptin on home diet (day −5 vs 6 mo). *P < 0.05 for day −5 vs 6 mo. #P < 0.05 for day 0 vs day 14. (A) Total LDLPs, (B) large LDLPs, (C) medium LDLPs, (D) small LDLPs, (E) LDLP size, and (F) apoB. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)]. The upper limit of the normal range for large LDLPs (B) is 748 nmol/L and for medium LDLPs (C) is 1377 nmol/L (not shown on graph).
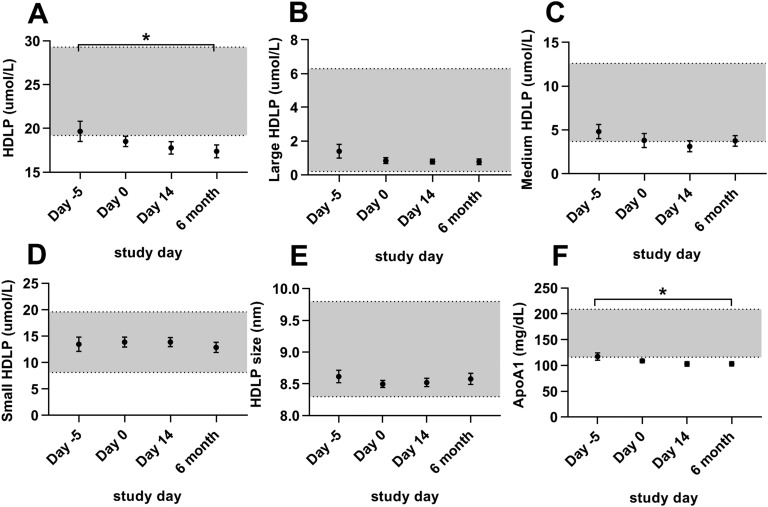
Total HDLPs and apoA1 decreased minimally with long-term metreleptin treatment. No other HDLP parameter changed with metreleptin treatment (Fig. 6). Changes in the additional seven HDLP subclasses are listed in Table 3.
Figure 6.
Effects of metreleptin on HDLPs in patients with lipodystrophy. Bars indicate the mean and SEM. Patients were studied before metreleptin on hospital admission with home diet (day −5), after 5 d of controlled inpatient diet (day 0), after 2 wk of metreleptin on controlled inpatient diet (day 14), and after 6 mo of metreleptin on home diet (6 mo). Statistical comparisons were performed off vs on short-term metreleptin on controlled diet (day 0 vs day 14) and off vs on long-term metreleptin home diet (day −5 vs 6 mo). *P < 0.05 for day −5 vs 6 mo. #P < 0.05 for day 0 vs day 14. (A) Total HDLPs, (B) large HDLPs, (C) medium HDLPs, (D) small HDLPs, (E) HDLP size, and (F) apoA1. Gray shading indicates manufacturer-provided normal range [5th to 95th percentile of a sample of the population (n = 698) of healthy men (n = 284) and women (n = 414) aged 18 to 94 (mean 39 y)].
D. Insulin Sensitivity
On day 0, insulin sensitivity in patients with lipodystrophy [M (mg/kgFFM/min)] was negatively correlated with very large TRLPs (r = −0.76, P = 0.0007), TRLP size (r = −0.52, P = 0.04), and TRLP TGs (r = −0.58, P = 0.02). Short-term changes (day 0 to day 14) and long-term changes (day 0 to 6 months) in insulin sensitivity were not significantly correlated with changes in any lipoprotein parameters.
3. Discussion
This study provides an in-depth characterization of lipoprotein particles in patients with lipodystrophy syndromes compared with controls, as well as a comparison of lipoprotein particles in patients with lipodystrophy before and after metreleptin treatment. We found that before metreleptin treatment, patients with lipodystrophy have a more atherogenic lipoprotein profile than controls, consistent with their numerous metabolic risk factors for cardiovascular disease (CVD). The hypertriglyceridemic lipoprotein profile was characterized by presence of chylomicrons in the fasting state in almost one-third of patients, suggesting impaired TRLP clearance, as well as increases in atherogenic small HDLPs and LDLPs. Metreleptin ameliorated many of the abnormal lipoprotein parameters, resulting in a less atherogenic profile and indicating a partial normalization of lipoprotein accumulation and clearance.
A. Patients With Lipodystrophy vs Controls
Patients with lipodystrophy are well known to have elevations in TGs measured via standard lipid panels [5]. In small studies, patients with lipodystrophy had an increase in TRLP TG production rate, a decrease in fractional catabolic rate of TRLPs, and lower lipoprotein lipase (LPL) activity [12, 13]. In the current study, we have added to this knowledge by demonstrating increases in the most atherogenic TRLPs in patients with lipodystrophy (Fig. 1), as well as demonstrating the existence of chylomicrons in the fasting state in a subset of patients with lipodystrophy. The five patients who had chylomicrons in the fasting state included both patients with partial lipodystrophy (n = 2) and patients with generalized lipodystrophy (n = 3) due to multiple genetic causes, suggesting that fasting chylomicronemia is not restricted to a specific patient subgroup. Chylomicrons (90 to 240 nm) are separated from endogenously synthesized VLDLs (30 to 80 nm) based on size [14]. TRLPs >80 nm (very large TRLPs) cannot pass through the hepatic fenestrae and therefore can only be chylomicrons due to their large size. Separating very large chylomicrons from other TRLPs such as VLDLs by size via NMR and other techniques is well established and has been validated by methods including density ultracentrifugation [15].
Similar elevations in TRLPs have been observed in other insulin-resistant states [10]. These lipid abnormalities are cause for concern clinically, because large VLDLs are associated with endothelial dysfunction [16], probably because of their preferential uptake by macrophages, leading to formation of foam cells in vessel walls [17]. This could be a mechanism behind the increased CVD that has been observed in patients with lipodystrophy [18, 19]. The ability of LPL to clear lipids from TRLPs is impaired in lipodystrophy by the limited adipose tissue LPL and the limited adipose tissue available for fatty acid uptake [20]. Because chylomicrons and VLDLs compete for LPL, saturation of this pathway by VLDLs could be a reason for the persistence of chylomicrons and increased large and medium TRLPs in the fasting state in some patients with lipodystrophy.
LDL-C calculated via the Friedewald equation was unmeasurable in 11 patients with lipodystrophy because their TG levels were >400 mg/dL. The NMR lipoprotein profile overcame this limitation and demonstrated more atherogenic, smaller LDLPs [9, 21] in patients with lipodystrophy compared with controls, increasing cardiovascular risk prediction.
Similarly, the NMR lipoprotein profile demonstrated lower levels of atheroprotective HDL parameters (large HDLPs and apoA1) and smaller HDL in patients with lipodystrophy than controls. This indicates inefficient reverse cholesterol transport, increasing cardiovascular risk [22].
B. Effects of Metreleptin Treatment
Proposed effects of metreleptin are shown in Fig. 7. Treatment with metreleptin significantly altered the size distribution of TRLPs in patients with lipodystrophy. In the current study, we demonstrated that metreleptin decreased the concentration of multiple TRLP parameters while food intake was held constant, supporting mechanisms of metreleptin to alter TRLP metabolism independent of food intake. There was marked reduction in very small TRLPs (IDLs) over both the short-term and long-term metreleptin treatment periods, which is important because of the key pathogenic role of IDLs in CVD [27, 28]. In addition, four out of five patients with elevated very large TRLPs before metreleptin had <1 nmol/L of very large TRLPs after treatment. This provides evidence that metreleptin improves chylomicron clearance, possibly through decreased competition of chylomicrons with VLDLs for LPL. We cannot determine whether food intake–independent reductions in TRLPs are due to decreased VLDL synthesis, increased clearance of TRLPs via LPL, or both. Metreleptin has been shown to decrease angiopoietin-like 3, an inhibitor of LPL [24], which could contribute to improved TRLP clearance with metreleptin. Possible mechanisms for decreased VLDL synthesis include reductions in fatty acid substrate for TG synthesis. Metreleptin treatment decreases lipolysis [11, 29], and experiments in rodents have shown that leptin decreased expression of enzymes and transcription factors regulating fatty acid synthesis [25] and increased those involved in fatty acid oxidation [30].
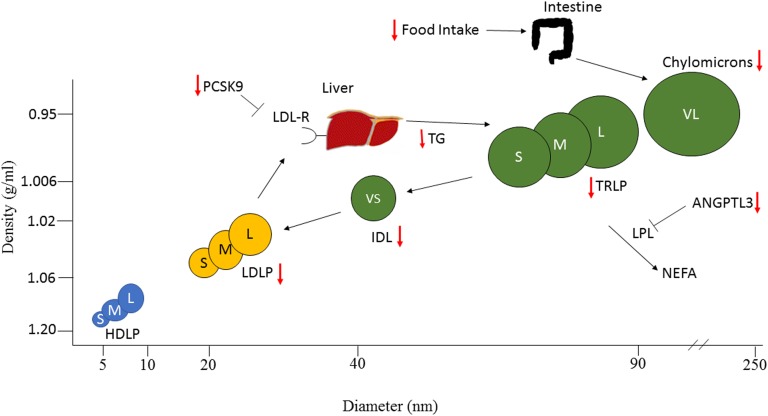
Figure 7.
Possible effects of metreleptin therapy on lipid metabolism in patients with lipodystrophy. Schematic representation of lipoprotein particles identified by NMR, arranged by diameter and density. Small, medium, and large (S, M, L) HDLPs are shown in blue; small, medium, and large (S, M, L) LDLPs in yellow; and very small, small, medium, large, and very large (VS, S, M, L, VL) TRLPs in green. Known effects of metreleptin are indicated with red arrows. Metreleptin lowers TRLPs through multiple mechanisms. Metreleptin decreases food intake [23], which probably decreases chylomicrons. It decreases angiopoietin-like protein 3 (ANGPTL3) [24], which should LPL activity, resulting in increased catabolism of TRLPs and chylomicrons. It also decreases transcription factors involved in de novo lipogenesis [25], which should decrease VLDL production. Metreleptin decreases LDLs via decreased proprotein convertase subtilisin/kexin type 9 (PCSK9) [26]. NEFA, nonesterified fatty acids.
Metreleptin treatment increased the average size of LDLPs, resulting in a less atherogenic LDLP profile. The reductions in small and total LDLPs with metreleptin may be mediated by reductions in proprotein convertase subtilisin/kexin type 9 (PCSK9), which was previously shown to decrease with metreleptin in women with lipodystrophy [26]. Metreleptin-induced reductions in PCSK9 probably lead to increased LDL clearance. Importantly, most LDL parameters (except LDL size) did not change during short-term metreleptin treatment with controlled diet, suggesting that LDL changes may be mediated primarily by changes in diet or food intake or that it takes a longer time for metreleptin to exert its effects on LDL.
Previous studies have shown that despite reductions in TGs, HDL-C does not change with long-term metreleptin treatment in patients with lipodystrophy [6]. Consistent with this finding, we did not observe improvements in most HDL parameters measured by NMR after either short-term or long-term metreleptin treatment.
Because lipoprotein abnormalities have been associated with insulin resistance, we evaluated correlations in lipoprotein parameters with insulin sensitivity measured by the hyperinsulinemic euglycemic clamp. Although insulin sensitivity was negatively correlated with very large TRLPs, TRLP size, and TRLP TG content before metreleptin treatment, there was no association between changes in insulin sensitivity and changes in most lipoprotein parameters with metreleptin treatment. This finding suggests that although some changes in lipoprotein parameters may be mediated by changes in insulin sensitivity, it is likely that metreleptin also has direct effects on lipoprotein metabolism.
In conclusion, patients with lipodystrophy had a more atherogenic lipid profile than healthy controls, which improved after metreleptin therapy. The NMR lipoprotein profile provided information about cardiovascular risk beyond that available from a standard lipid panel, including the observation that LDL parameters were more atherogenic in patients than in controls, despite no difference in LDL-C. We further observed that chylomicrons, not previously separated from other TG-rich lipoproteins, were present in the fasting state in a subset of patients with lipodystrophy and improved after metreleptin treatment. This study was limited by small sample size and by the heterogeneous genotypes and phenotypes of the patients studied. In addition, although the NMR lipoprotein particle analysis can help in generating hypotheses about the mechanisms leading to dyslipidemia in patients with lipodystrophy, additional mechanistic studies are needed to elucidate the changes in lipid accumulation and clearance that are observed with metreleptin therapy.
Acknowledgments
Financial Support: This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (to R.J.B. and R.M.), the National Heart, Lung, and Blood Institute (to R.D.S.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to M.L.).
Clinical Trial Information: ClinicalTrials.gov nos. NCT01778556 (registered 29 January 2013) and NCT00428987 (registered 30 January 2007).
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- apoA1
apolipoprotein A1
- apoB
apolipoprotein B
- BMI
body mass index
- C
cholesterol
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HDLP
high-density lipoprotein particle
- HOMA-IR
homeostatic model assessment of insulin resistance
- IDL
intermediate-density lipoprotein
- LDL
low-density lipoprotein
- LDLP
low-density lipoprotein particle
- LP4
LipoProfile-4
- LPL
lipoprotein lipase
- M
insulin sensitivity
- NMR
nuclear magnetic resonance
- PCSK9
proprotein convertase subtilisin/kexin type 9
- TG
triglyceride
- TRLP
triglyceride-rich lipoprotein particle
- VLDL
very low-density lipoprotein
- VLDLP
very low-density lipoprotein particle
References and Notes
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):2237–2238. [DOI] [PubMed] [Google Scholar]
- 3. Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O’Rahilly S, Savage DB. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119(2):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. [DOI] [PubMed] [Google Scholar]
- 5. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joseph J, Shamburek RD, Cochran EK, Gorden P, Brown RJ. Lipid regulation in lipodystrophy versus the obesity-associated metabolic syndrome: the dissociation of HDL-C and triglycerides. J Clin Endocrinol Metab. 2014;99(9):E1676–E1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64(23):2525–2540. [DOI] [PubMed] [Google Scholar]
- 8. Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. [DOI] [PubMed] [Google Scholar]
- 9. Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881. [PubMed] [Google Scholar]
- 10. Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–462. [DOI] [PubMed] [Google Scholar]
- 11. Brown RJ, Valencia A, Startzell M, Cochran E, Walter PJ, Garraffo HM, Cai H, Gharib AM, Ouwerkerk R, Courville AB, Bernstein S, Brychta RJ, Chen KY, Walter M, Auh S, Gorden P. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wajchenberg BL, Amâncio RF, Santomauro AT, Maranhão RC. Metabolism of chylomicrons in patients with congenital lipoatrophic diabetes: a study with emulsion models of chylomicrons. Clin Endocrinol (Oxf). 2004;61(3):347–352. [DOI] [PubMed] [Google Scholar]
- 13. Stacpoole PW, Alig J, Kilgore LL, Ayala CM, Herbert PN, Zech LA, Fisher WR. Lipodystrophic diabetes mellitus. Investigations of lipoprotein metabolism and the effects of omega-3 fatty acid administration in two patients. Metabolism. 1988;37(10):944–951. [DOI] [PubMed] [Google Scholar]
- 14. German JB, Smilowitz JT, Zivkovic AM. Lipoproteins: when size really matters. Curr Opin Colloid Interface Sci. 2006;11(2-3):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savorani F, Kristensen M, Larsen FH, Astrup A, Engelsen SB. High throughput prediction of chylomicron triglycerides in human plasma by nuclear magnetic resonance and chemometrics. Nutr Metab (Lond). 2010;7(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson RA, Evans ML, Ellis GR, Graham J, Morris K, Jackson SK, Lewis MJ, Rees A, Frenneaux MP. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154(2):475–483. [DOI] [PubMed] [Google Scholar]
- 17. Palmer AM, Nova E, Anil E, Jackson K, Bateman P, Wolstencroft E, Williams CM, Yaqoob P. Differential uptake of subfractions of triglyceride-rich lipoproteins by THP-1 macrophages. Atherosclerosis. 2005;180(2):233–244. [DOI] [PubMed] [Google Scholar]
- 18. Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103(18):2225–2229. [DOI] [PubMed] [Google Scholar]
- 19. Bidault G, Garcia M, Vantyghem MC, Ducluzeau PH, Morichon R, Thiyagarajah K, Moritz S, Capeau J, Vigouroux C, Béréziat V. Lipodystrophy-linked LMNA p.R482W mutation induces clinical early atherosclerosis and in vitro endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2013;33(9):2162–2171. [DOI] [PubMed] [Google Scholar]
- 20. Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–1210. [DOI] [PubMed] [Google Scholar]
- 21. Tani M, Kawakami A, Mizuno Y, Imase R, Ito Y, Kondo K, Ishii H, Yoshida M. Small dense LDL enhances THP-1 macrophage foam cell formation. J Atheroscler Thromb. 2011;18(8):698–704. [DOI] [PubMed] [Google Scholar]
- 22. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13–14):1331–1346. [DOI] [PubMed] [Google Scholar]
- 23. McDuffie JR, Riggs PA, Calis KA, Freedman RJ, Oral EA, DePaoli AM, Yanovski JA. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab. 2004;89(9):4258–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muniyappa R, Abel BS, Asthana A, Walter MF, Cochran EK, Remaley AT, Skarulis MC, Gorden P, Brown RJ. Metreleptin therapy lowers plasma angiopoietin-like protein 3 in patients with generalized lipodystrophy. J Clin Lipidol. 2017;11(2):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297(5579):240–243. [DOI] [PubMed] [Google Scholar]
- 26. Levenson AE, Haas ME, Miao J, Brown RJ, de Ferranti SD, Muniyappa R, Biddinger SB. Effect of leptin replacement on PCSK9 in ob/ob mice and female lipodystrophic patients. Endocrinology. 2016;157(4):1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoji T, Nishizawa Y, Kawagishi T, Kawasaki K, Taniwaki H, Tabata T, Inoue T, Morii H. Intermediate-density lipoprotein as an independent risk factor for aortic atherosclerosis in hemodialysis patients. J Am Soc Nephrol. 1998;9(7):1277–1284. [DOI] [PubMed] [Google Scholar]
- 28. Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation. 1993;88(6):2762–2770. [DOI] [PubMed] [Google Scholar]
- 29. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prieur X, Tung YC, Griffin JL, Farooqi IS, O’Rahilly S, Coll AP. Leptin regulates peripheral lipid metabolism primarily through central effects on food intake. Endocrinology. 2008;149(11):5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]