Abstract
The aim of the present study was to explore the amplitude of low-frequency fluctuations (ALFF; a measurement of spontaneous brain activity) in different brain regions of patients with retinal vein occlusion (RVO) and its association with vision changes measurements. A total of 24 RVO patients (12 males and 12 females) and 24 healthy controls (HCs, 12 males and 12 females) were recruited, and they were closely matched regarding age, gender and education level (classified according to nine-year compulsory education in China and higher education, all including primary school, junior school, high school and university). ALFF values of different brain regions were gathered and analyzed, and statistical analysis software was used to explore the correlations between the average ALFF signals and clinical features. The ability of ALFF values to distinguish between subjects with RVO and HCs was analyzed by receiver operating characteristic (ROC) curves. The results indicated that the subjects from the RVO group had higher ALFF values than the HCs in the posterior lobe of the left cerebellum, inferior temporal gyrus, cerebellar anterior lobe, right cerebellum posterior/anterior lobe, and lower ALFF values in the medial frontal gyrus, right precuneus, left middle frontal gyrus, right angular gyrus and right superior frontal gyrus. The ROC curve analysis of each brain region indicated that the accuracy of the area under the ROC curves regarding the prediction of RVO was excellent. The best-corrected visual acuity (VA) in the left eye was positively correlated with the ALFF value of the right precuneus (r=0.767, P=0.004) and the best-corrected VA in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.935, P<0.001). The central subfield retinal thickness in the left eye was negatively correlated with the ALFF value of the right precuneus (r=−0.895; P<0.001). The duration of RVO in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.868; P<0.001). In conclusion, the present results indicate that RVO is associated with dysfunction of diverse brain regions, including language- and movement-associated areas, which may reflect the underlying pathogenic mechanisms of RVO (trial registry no. CDYFY-LL-2017025).
Keywords: retinal vein occlusion, amplitude of low-frequency fluctuation, functional magnetic resonance imaging, resting state, spontaneous activity
Introduction
Retinal vein occlusion (RVO) is a type of ophthalmic vascular lesion characterized by tortuous expansion and hemorrhage along a retinal vein that may be visualized on funduscopic exams. The prevalence of these lesions determined by epidemiological studies varies between 0.1 to 0.5% in middle-aged and elderly individuals. It is the second most common retinal vascular disease after diabetic retinopathy (1,2). The major pathogenesis of RVO is venous thrombosis (3). According to different occlusion sites, Hayreh (4) proposed that RVO may be divided into three categories: Central retinal vein occlusion (CRVO), top or bottom hemi-(H)CRVO and branch retinal vein occlusion. Depending on the presence of ocular fundus ischemia, CRVO and HCRVO may be sub-divided into ischemic and non-ischemic groups. If left untreated, RVO may lead to blindness. RVO is affected by systemic diseases and is more likely to occur in individuals with hypertension, hyperlipidemia (5), diabetes (6), coagulation disorders (7), anti-phospholipid antibody syndrome (8) and migraine (9). The control of systemic risk factors may effectively reduce the occurrence of RVO. At our center, fundus fluorescein angiography (FFA) is commonly used to diagnose RVO. The degree of retinal edema is observed by optical coherence tomography (OCT), but elucidation of the underlying pathophysiology is rarely achieved through neuroimaging. A previous study reported significantly decreased functional connectivity in the occipital visual cortex of early blind patients (10). If untreated, patients with RVO may eventually become blind. Therefore, the investigation of RVO-associated brain processes using modern imaging techniques may lead to a better understanding of the underlying visual mechanisms.
Recently, increasing attention has been paid to resting-state functional magnetic resonance imaging (RS-fMRI) for the study of ongoing neuronal processes during rest (11). As a reliable method to measure the correlation amplitude, ALFF has proven to be a valuable technique for investigating the intensity of spontaneous neural activity (12), and it has been applied in studies of neurophysiological activity and diseases occurring in different brain regions. In 2007, Zang et al (13) first proposed the ALFF index. By calculating the low-frequency amplitude of each individual element, this method directly represents the intensity of blood oxygen level dependent (BOLD), and reflects the level of spontaneous activity of each brain area in the resting state from the perspective of energy. The reduction of ALFF represents the decrease of the BOLD signal in this brain region. To date, ALFF has been applied in ophthalmology research on conditions including glaucoma (14,15), amblyopia (16), strabismus (17), high myopia (18), optic neuritis (19), eye trauma (20), blindness (21), retinal detachment (22), diabetic retinal diseases (23) and acute eye pain (24). To the best of our knowledge, the present study is the first to explore ALFF in different brain regions of patients with RVO.
Materials and methods
Participants
A total of 24 patients with RVO (12 males and 12 females; age range, 27–85 years; average age, 54 years) were recruited from the First Affiliated Hospital of Nanchang University (Nanchang, China) with the following inclusion criteria: i) Signs of RVO on ophthalmoscopy; ii) indication of macular edema on OCT; iii) FFA indicating occlusion of a retinal vein (Fig. 1); iv) no history of nervous system diseases (e.g. cerebral hemorrhage, cerebral infarction or brain atrophy); v) no history of psychiatric illness, myocardial infarction and/or cerebral infarction disease; and vi) ability to undergo MRI examination. The exclusion criteria for the RVO group were as follows: i) History of ophthalmic surgery (intraocular or extraocular surgery) within three months; ii) history of other eye diseases (glaucoma, cataracts, infections, inflammation, congenital pathology and hereditary eye diseases); iii) systemic diseases that affect the appearance of the eye; iv) presence of a cardiac pacemaker or other implanted metal implants that may represent a counterindication for MRI scans; v) medical history of risk factors, including hypertension, hyperlipidemia, diabetes, coagulation disorders, anti-phospholipid antibody syndrome and migraine headaches. Anti-phospholipid antibodies may have a role in the development of atherosclerosis. Induction of a prothrombotic vascular endothelial microenvironment may be involved in the pathogenesis of RVO (25). The aforementioned additional risk factors were excluded according to data obtained from previous studies (8,9).
Figure 1.
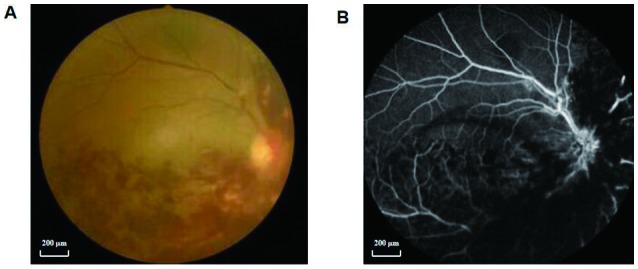
Example of RVO seen on fundus colorized photography and FFA. (A) Colorized image of the fundus of an eye with RVO. (B) FFA of the same eye with RVO. RVO, retinal vein occlusion; FFA, fundus fluorescein angiography.
Furthermore, 24 healthy controls (HCs; 12 males and 12 females) of comparable age and educational status to those of the RVO subjects were enrolled. The inclusion criteria were as follows: i) No ocular disease history; ii) no history of nervous system diseases (e.g. cerebral hemorrhage, cerebral infarction or brain atrophy); iii) no history of psychiatric illness, myocardial infarction and/or cerebral infarction disease; and iv) ability to undergo MRI examination.
MRI parameters
The MRI scanning was performed using a Trio 3-Tesla MRI scanner (Siemens AG). For these MRI examinations, each of the subjects was instructed to relax, keep their eyes closed and continue to breathe steadily until the end of the scan. The functional data were obtained using a 3D metamorphic gradient recalled-echo pulse sequence. First, 176 structural images with the parameters set as follows: Acquisition matrix, 256×256; field of view, 250×250 mm; echo time, 2.26 msec; repetition time, 1,900 msec; thickness, 1.0 mm; gap, 0.5 mm; flip angle, 9°. Subsequently, 240 functional images were obtained with the following settings: Acquisition matrix, 64×64; field of view, 220×220 mm; thickness, 4.0 mm; gap, 1.2 mm; repetition time, 2,000 msec; echo time, 30 msec; flip angle, 90°, 29 axial.
RS-fMRI data analysis
Functional data from different brain regions were differentiated by using MRIcro software (REST; http://www.restfmri.net), and unqualified data were eliminated. Qualified data were processed using software from rs-fMRI (DPARSFA 2.3, http://rfmri.org/DPARSF), including digital image form conversion, slice time, head action adjustment, spatial standardization, and with a smooth Gaussian core 6×6×6 mm3 widescreen at half-peak. If during the scan, the patient's head moved by >1.5 mm along the x-, y- or z-axis and the angle range was >1.5 mm, the data were deemed ineligible. A previous study indicated that the higher-order model is more effective in eliminating head movement errors (26). Using linear regression to help eliminate uncontrollable variables, signals from the central white matter of the brain were excluded (27). The longer the scan, the more agitated the patients became, accompanied by an increase in body movements, including head movements, and therefore, brain function images were extracted after corrections were performed to compensate for any movement artifacts (13).
Brain-visual acuity and other eye parameters correlation analysis
These other eye parameters include onset time of RVO and central subfield retinal thickness. First, Brain areas with different ALFF findings between groups were classified as regions of interest using the resting-state fMRI data analysis toolkit software. Second, the average ALFF values for the different brain regions were calculated. Finally, a linear correlation analysis was performed to define the correlation between the behavior of the RVO group and the average ALFF value in the different brain regions (P<0.05).
Statistical analysis
The clinical data, including the duration of the onset of RVO, best-corrected VA and central subfield retinal thickness were recorded and analyzed in the study with independent sample t-test (SPSS 24.0; IBM Corp.). The differences in ALFF values of the RVO and HC groups were collected and receiver operatin characteristic (ROC) curves were plotted and analyzed. The ALFF values in RVO patients were also compared with clinical features, and the correlation was analyzed through the Pearson's correlation analysis software and the scatter diagram was generated. P<0.05 was considered to indicate statistical significance.
Results
Demographics and clinical behavioral results
As demonstrated in Table I, there were no significant differences in body weight (P=0.916), gender (P>0.999) or age (P=0.753) between the subjects with RVO and the HCs. In addition, there were obvious differences in best-corrected VA-right (P=0.001), best-corrected VA-left (P=0.001), central subfield retinal thickness (P=0.012) and cube average thickness (P=0.009) between the RVOs and the HCs (Table I).
Table I.
Demographics and clinical measurements in the two groups.
| Characteristics | RVO (n=24) | HC (n=24) | T | P-value |
|---|---|---|---|---|
| Males/females | 12/12 | 12/12 | N/A | >0.99 |
| Age (years) | 54.04±4.93 | 56.76±5.87 | 0.097 | 0.753 |
| Body weight (kg) | 66.46±6.12 | 68.11±5.98 | 0.154 | 0.916 |
| Right-handedness | 24 | 24 | N/A | >0.99 |
| Duration of RVO (days) | 66.67±24.28 | N/A | N/A | N/A |
| Best-corrected VA | ||||
| Right eye | 0.16±0.07 | 0.95±0.12 | −0.432 | 0.001 |
| Left eye | 0.22±0.10 | 0.98±0.22 | −0.396 | 0.001 |
| IOP (mmHg) | ||||
| Right eye | 14.69±1.11 | 16.54±1.23 | 0.078 | 0.673 |
| Left eye | 14.62±0.99 | 15.18±2.32 | 0.085 | 0.731 |
| Central subfield retinal thickness (µm) | 701.13±81.61 | 301.58±46.18 | 0.683 | 0.012 |
| Cube average thickness (µm) | 746.83±86.67 | 312.34±44.52 | 0.531 | 0.009 |
VA, visual acuity; RVO, retinal vein occlusion; HC, healthy controls; N/A, not applicable; IOP, intraocular pressure.
Differences in ALFF
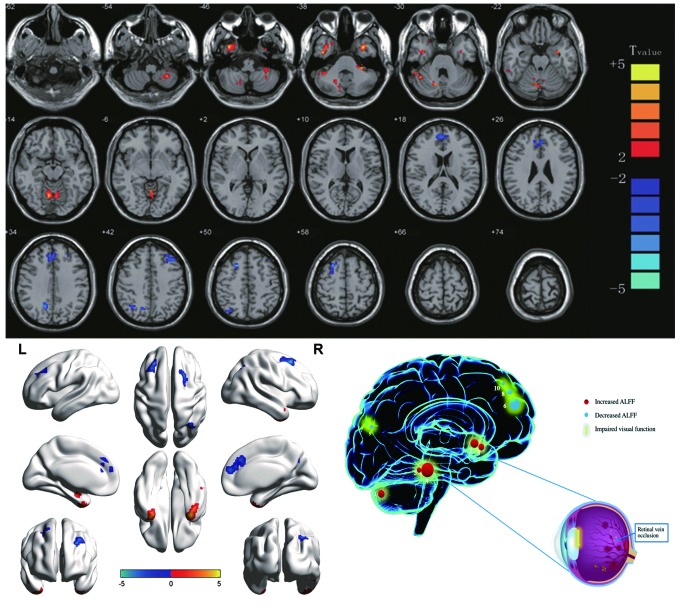
Compared with the HCs, subjects with RVOs had significantly higher ALFF values areas in the posterior lobe of the left cerebellum, inferior temporal gyrus, anterior lobe of the cerebellum, right cerebellar posterior/anterior lobe, lower area of the medial frontal gyrus, the right precuneus, left middle frontal gyrus, right angular gyrus and right superior frontal gyrus (Figs. 2 and 3; Table II).
Figure 2.
Significant differences in spontaneous brain activity between the retinal vein occlusion group and healthy controls. The sizes of the spots denote the degree of quantitative changes. The different brain regions were observed in the left cerebellar posterior lobe, right inferior temporal gyrus, left inferior temporal gyrus, bilateral cerebellar anterior lobe, right cerebellar posterior/anterior lobe, bilateral medial frontal gyrus, right precuneus, left middle frontal gyrus, right angular gyrus and right superior frontal gyrus. The red areas denote that patients with RVO exhibit higher ALFF in brain areas than HCs and the blue areas denote brain regions with a lower ALFF [P<0.001 for multiple comparisons using Gaussian random field theory (z.2.3, P<0.001, cluster >13 voxels, Alphasim corrected)]. ALFF, amplitude of low-frequency fluctuation; L, left; R, right. RVO, retinal vein occlusion; HCs, healthy controls; 1, left cerebellar posterior lobe; 2, right inferior temporal gyrus; 3, right cerebellar posterior/anterior lobe; 4, left inferior temporal gyrus; 5, bilateral cerebellar anterior lobe; 6, right angular gyrus; 7, bilateral medial frontal gyrus; 8, right superior frontal gyrus; 9, left middle frontal gyrus; 10, right precuneus.
Figure 3.
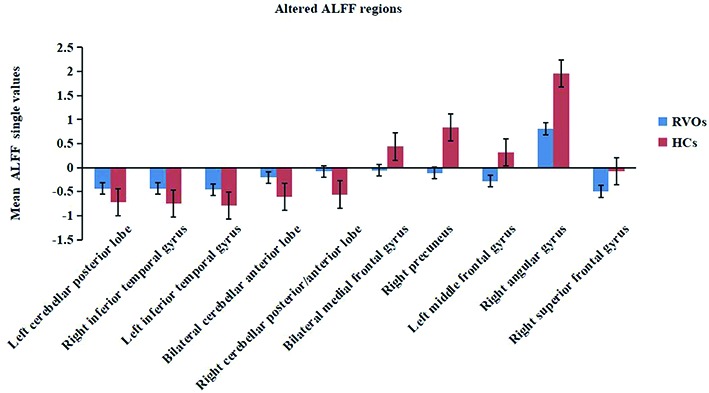
Means of altered spontaneous brain activity between the retinal vein occlusion group and HCs. All differences were statistically significant (P<0.05). ALFF, amplitude of low-frequency fluctuation; HCs, healthy controls.
Table II.
Brain areas with significantly different ALFF values between groups.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Brain area | BA | X | Y | Z | Peak voxels | T-value | P-values |
| RVO>HC | |||||||
| Left cerebellar posterior lobe | 0 | −30 | −36 | −39 | 81 | 4.4823 | P<0.001 |
| Right inferior temporal gyrus | 20 | 42 | −6 | −39 | 97 | 5.1104 | P<0.001 |
| Left inferior temporal gyrus | 20 | −33 | 3 | −39 | 66 | 4.9095 | P<0.001 |
| Bilateral cerebellar anterior lobe | 0 | 9 | −63 | −12 | 175 | 4.8534 | P<0.001 |
| Right cerebellar posterior/anterior lobe | 0 | 42 | −54 | −30 | 60 | 4.3742 | P<0.001 |
| RVO<HC | |||||||
| Bilateral medial frontal gyrus | 9 | 0 | 36 | 30 | 150 | −4.2564 | P<0.001 |
| Right precuneus | 0 | 15 | −63 | 36 | 41 | −4.4224 | P<0.001 |
| Left middle frontal gyrus | 9 | −30 | 39 | 39 | 69 | −4.8306 | P<0.001 |
| Right angular gyrus | 7 | 33 | −66 | 45 | 48 | −4.0212 | P<0.001 |
| Right superior frontal gyrus | 0 | 24 | 18 | 54 | 41 | −5.2273 | P<0.001 |
The significance level was set at voxel level with P<0.001 for multiple comparisons using Gaussian random field theory (z.2.3, P<0.001, cluster >13 voxels, Alphasim corrected). ALFF, amplitude of low-frequency fluctuation; BA, Brodmann's area; RVO, retinal vein occlusion; HC, healthy control; MNI, Montreal Neurological Institute.
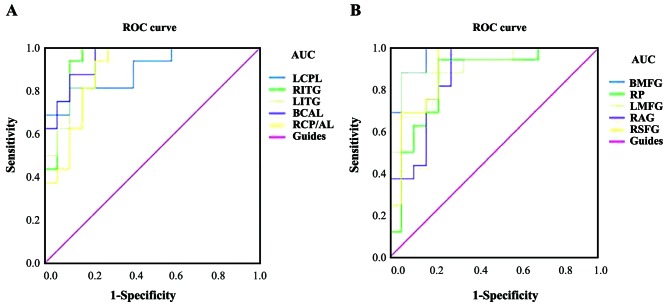
ROC curve analysis
The mean ALFF values of the two groups were analyzed using ROC curves, with a larger the area under the curve (AUC) indicating a higher diagnostic rate. The following AUCs were determined for ALFF values (RVOs>HCs) in the different brain regions: Left cerebellar posterior lobe, 0.897 (P<0.001); right inferior temporal gyrus, 0.949 (P<0.001); left inferior temporal gyrus, 0.926 (P<0.001); bilateral anterior lobes of the cerebellum, 0.949 (P<0.001); right posterior/anterior lobe of the cerebellum, 0.893 (P<0.001; Fig. 4A). For ALFF values (RVOs<HCs), the following AUCs were determined: Bilateral medial frontal gyrus, 0.967 (P<0.001); right precuneus, 0.849 (P=0.001); left middle frontal gyrus, 0.919 (P<0.001); right angular gyrus, 0.868 (P<0.001); and right superior frontal gyrus, 0.904 (P<0.001; Fig. 4B).
Figure 4.
ROC curve analysis of the mean ALFF values for altered brain regions. (A) The AUCs of different brain regions were as follows: LCPL, 0.897 (P<0.001; 95% CI: 0.790–1.000); RITG, 0.949 (P<0.001; 95% CI: 0.874–1.000); LITG, 0.926 (P<0.001; 95% CI: 0.841–1.000); BCAL, 0.949 (P<0.001; 95% CI: 0.881–1.000); RCP/AL, 0.893 (P<0.001; 95% CI: 0.783–1.000). (B) The AUCs of different brain regions were as follows: BMFG, 0.967 (P<0.001; 95% CI: 0.916–1.000); RP, 0.849 (P=0.001; 95% CI: 0. 711–0.988); LMFG, 0.919 (P<0.001; 95% CI: 0.822–1.000); RAG, 0.868 (P<0.001; 95% CI: 0.742–0.993), RSFG, 0.904 (P<0.001; 95% CI: 0.798–1.000). ALFF, amplitude of low-frequency fluctuation; ROC, receiver operating characteristic; AUC, area under the ROC curve; LCPL, left cerebellar posterior lobe; RITG, right inferior temporal gyrus; LITG, left inferior temporal gyrus; BCAL, bilateral cerebellar anterior lobe; RCP/AL, right cerebellar posterior/anterior lobe; BMFG, bilateral medial frontal gyrus; RP, right precuneus; LMFG, left middle frontal gyrus; RAG, right angular gyrus; RSFG, right superior frontal gyrus.
Correlation analysis of eye parameters and ALFF value
The best-corrected VA in the left eye was positively correlated with the ALFF value of the right precuneus (r=0.767; P=0.004) and the best-corrected VA in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.935; P<0.001). Furthermore, the central subfield retinal thickness of the left eye was negatively correlated with the ALFF value of the right precuneus (r=−0.895; P<0.001). The duration of RVO in the right eye was also positively correlated with the ALFF value of the left middle frontal gyrus (r=0.868; P<0.001; Fig. 5). It can be inferred that with the extension of the onset time of RVO and the improvement of the best corrected VA in the right eye, the ALFF value in the left middle frontal gyrus increases gradually. Furthermore, with the improvement of the best corrected VA in the left eye, the ALFF value of the right precuneus increased gradually. However, the central subfield retinal thickness in the left eye was negatively correlated with the ALFF value of right precuneus. That is, with the increase of the central subfield retinal thickness in the left eye, the ALFF value of right precuneus decreases gradually.
Figure 5.
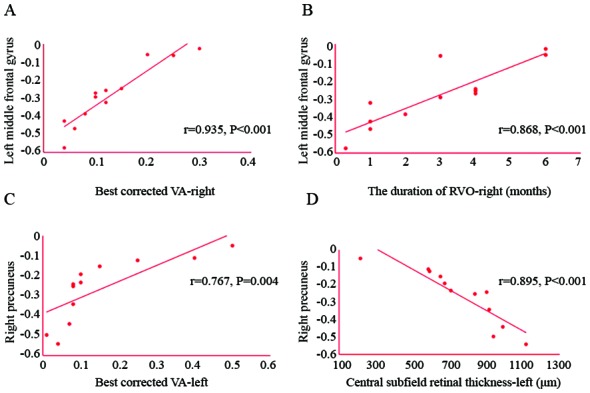
Correlation between the mean ALFF values of different brain areas and clinical behavior. (A) The best-corrected VA in the left eye was positively correlated with the ALFF value of the right precuneus (r=0.767, P=0.004). (B) The duration of RVO in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.868, P<0.001). (C) The best-corrected VA in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.935, P<0.001). (D) The central subfield retinal thickness in the left eye was negatively correlated with the ALFF value of the right precuneus (r=−0.895, P<0.001). RVO, retinal vein occlusion; VA, visual acuity. ALFF, amplitude of low-frequency fluctuation.
Discussion
The current study assessed the association between clinical behavior and brain changes in patients with RVO on resting-state brain activity using the ALFF technique. The clinical behaviors assessed include the duration of the onset of RVO, best-corrected VA and central subfield retinal thickness. Compared to the HCs, the subjects with RVO of the present study exhibited distinctly increased ALFF values in the left posterior lobe of the cerebellum, bilaterally in the inferior temporal gyri, bilaterally in the anterior lobes of the cerebellum, as well as the right posterior/anterior lobes of the cerebellum. By contrast, the ALFF values of the medial frontal gyri, right precuneus, left middle frontal gyrus, right angular gyrus and right superior frontal gyrus were significantly decreased.
Reduced ALFF values in the subjects with RVO vs. HCs were first analyzed. The middle frontal gyrus is thought to be associated with mental and physical balance. Carter et al (28) used fMRI to synchronously track and delay the acquisition of explicit knowledge in a conditioned reflex paradigm. Their experiments demonstrated that activity in the frontal gyrus was associated with the accuracy of clear emergency awareness in each trial. Leung et al (29) used fMRI to study activity in the MFG, revealing that, during more demanding tasks, it may provide up to 24 sec of memory, and the signal changes in this area are greater than those in other pre-frontal areas. Therefore, the middle frontal gyrus constitutes an important part of the brain's memory storage. Japee et al (30) investigated the function of the middle frontal gyrus and concluded that it has an important role in attention control. Talati and Hirsch (31) reported that the middle frontal gyrus participates in advanced execution and decision making. Furthermore, previous studies have indicated that patients with depression have dysfunctional middle frontal gyri (32,33). In adjustment disorder, stress is not proportional to physical and mental stimulus. In other words, a more obvious the clinical manifestation may not necessarily be proportional to the pressure (34). The best-corrected VA in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.935, P<0.001). Therefore, it may be hypothesized that, with the decrease of the ALFF value, the best-corrected VA may also decrease, which means that the dysfunction of the left middle frontal gyrus would increase. The duration of RVO in the right eye was positively correlated with the ALFF value of the left middle frontal gyrus (r=0.868, P<0.001). With the extension of the course and progression of the disease, the left middle frontal gyrus impairment of RVO patients became aggravated. The angular gyrus has the function of integrating and transmitting multi-organ sensory information in different ways. Therefore, the role of the angular gyrus in isolation cannot be determined. Yazar et al (35) performed a study on 61 healthy individuals and 61 patients with disease of the angular gyrus, indicating that the ability of patients with angular gyrus disease to acquire context features in diverse modes was reduced, and that the angular gyrus was required for memory experiences. Yazar et al (36) then indicated that reduced stimulation of the angular gyrus was associated with lower levels of subjective recall.
The superior frontal gyrus is part of the frontal lobe, dividing it into three sub-regions. A positive correlation has been identified between the default network and the cognitive control network in the anterior medial inferior region of the frontal gyrus (37). There was a positive correlation between the superior dorsolateral sub-area default network and the cognitive execution network (38). Studies using fMRI experiments have indicated that the superior frontal gyrus is involved in self-awareness (39) and laughter (40). The present study suggested that ALFF values of RVO patients were lower in the right superior frontal gyrus, which suggested that there was brain dysfunction in this area.
The precise function of the precuneus remains elusive. Three different functional modes may exist in the precuneus: The anterior precuneus exhibits functional connectivity with sensorimotor regions, the central precuneus appears to be a cognitive/associative region, and the posterior precuneus displays functional connectivity with adjacent visual cortical regions (41). Uchimura et al (42) reported that the right precuneus is involved in visuospatial cognitive tasks. This is similar to the result of another study, according to which the right precuneus has a causal role in visual short-term memory capacity, particularly in bilateral visual displays (43). Therefore, the precuneus is considered ‘an active region that can continuously collect information about the surrounding world’ (44). Chen et al (45) indicated that responses in the right anterior cingulate gyrus were enhanced in individuals with higher language creativity. Therefore, it may be concluded that the size of the anterior right wedge is positively correlated with the individual's linguistic creativity. Functional fMRI studies have indicated that this region of the brain participates in the integration of behavior, visual images and consciousness (46). The present study determined that the best-corrected VA in the left eye was positively correlated with the ALFF value of the right precuneus (r=0.767, P=0.004). Furthermore, the central subfield retinal thickness in the left eye was negatively correlated with the ALFF value of the right precuneus (r=−0.895, P<0.001). The average thickness of the macular fovea was increased in RVO patients vs. HCs. The increase in thickness of the central subfield retinal reflects the severity of RVO, so the ALFF value reduction of the right precuneus may be associated with the severity of RVO.
Increased ALFF values in the subjects with RVO vs. HCs were then analyzed. The inferior temporal gyrus is located on the outer and lower surfaces of the temporal neocortex. The temporal cortex is composed of three parts, of which the inferior temporal gyrus (20, 21 and 37 region) is associated to visual information processing, and is particularly important for promoting cognitive processing and emotional regulation (47). Stoeter et al (48) suggested that somatic pain disorder was associated with increased temporal lobe activation, which was consistent with the present result that ALFF values were significantly increased in the sub-temporal region. This may be associated with the activation of multiple cortical regions when patients are anxious that a serious eye disease may lead to blindness (49).
Anatomically, the cerebellar is located in the posterior fossa. Cerebellar function includes cognition, coordination and balance, and fine regulation of the eye (50). Ataxia occurs when the cerebellar is damaged. According to studies, the functions of the cerebellar include cognition and memory (51), and dysfunction of the cerebellar is associated with Alzheimer's disease (52), bipolar disorder (53), depression (54) and schizophrenia (55). This is consistent with the present study, as RVO patients were generally anxious over their condition while they underwent fMRI scanning. Therefore, it is likely that the high ALFF value of the inferior lobe of the cerebellar was the result of anxiety in these patients. The ALFF method has been successfully applied to patients with ophthalmological diseases (Table III) and is expected to have huge prospects for development. In the present study, the mean ALFF values of specific ROIs were collected and subjected to ROC curve analyses. The accuracy was considered excellent if the AUC was >0.8. In the present ROC curve analysis, excellent AUC values were obtained for all ROIs, including the right precuneus and the left middle frontal gyrus, indicating that ALFF methodology may provide promising biological indicators for distinguishing patients with RVO from HCs.
Table III.
Amplitude of low-frequency fluctuation method applied in ophthalmological diseases.
| First author (year) | Disease | (Refs.) |
|---|---|---|
| Liu (2014) | Glaucoma | (14) |
| Huang (2015) | Glaucoma | (15) |
| Shao (2015) | Optic neuritis | (19) |
| Huang (2016) | Strabismus | (17) |
| Tan (2017) | Amblyopia | (16) |
| Huang (2016) | High myopia | (18) |
| Tan (2016) | Open-globe injury | (20) |
| Li (2016) | Monocular blindness | (21) |
| Wang (2017) | Diabetic retinopathy | (23) |
| Xin (2017) | Retinal detachment | (22) |
| Pan(2018) | Acute eye pain | (24) |
Of note, the present study had several limitations. First, the small sample size may have affected the experimental results. Furthermore, the best corrected visual acuity is easily influenced by subjective factors. In addition, the spontaneous activity in different brain regions was affected by different scanning times. At present, it remains elusive whether lowering blood pressure, blood lipid and/or blood sugar levels may improve vision or complications of RVO, and this may be worthy of further study. In the future, more objective indicators may be used to record and analyze data, and the determination of spontaneous activity changes in different brain regions in RVO patients may also be improved. This may provide a theoretical basis for further study of the pathophysiological changes and treatment of RVO.
In conclusion, the present study was the first, to the best of our knowledge, to report that brain activity disorders occur in RVO patients. In the future, ALFF may provide guidance in the early detection of the neuropathological mechanisms of RVO and provide a basis for clinical diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81660158), the Natural Science Key Project of Jiangxi Province (grant no. 20161ACB21017), the Youth Science Foundation of Jiangxi Province (grant nos. 20151BAB215016 and 20161BAB215198), the Key Research Foundation of Jiangxi Province (grant no. 20181BBG70004), the Teaching Reform of Degree and Graduate Education Research Project of Jiangxi Province (grant no. JXYJG-2018-013) and the Health Development Planning Commission Science TCM Foundation of Jiangxi Province (grant no. 2018060).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YS and QZ designed the current study. QY and BL recruited healthy controls. YQS performed MRI scanning. YM, PZ and WS collected and analyzed the data. YW wrote the manuscript. All the authors read and approved the final manuscript.
Ethical approval and consent to participate
The study methods and protocols were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (Nanchang, China) and followed the principles of the Declaration of Helsinki. All subjects were notified of the objectives and content of the study and latent risks, and then provided written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
This study did not receive any industrial support. The authors have no competing interests to declare regarding this study.
References
- 1.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: The beaver dam eye study. Trans Am Ophthalmol Soc. 2000;98:133–143. [PMC free article] [PubMed] [Google Scholar]
- 2.Ho M, Liu DT, Lam DS, Jonas JB. Retinal vein occlusions, from basics to the latest treatment. Retina. 2016;36:432–448. doi: 10.1097/IAE.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 3.Sivaprasad S, Amoaku WM, Hykin P. The royal college of ophthalmologists guidelines on retinal vein occlusions: Executive summary. Eye (Lond) 2015;29:1633–1638. doi: 10.1038/eye.2015.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol. 1994;42:109–132. [PubMed] [Google Scholar]
- 5.Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, Park KH, Jo YJ, Korean RVO Study Baseline characteristics and risk factors of retinal vein occlusion: A study by the Korean RVO study group. J Korean Med Sci. 2013;28:136–144. doi: 10.3346/jkms.2013.28.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International Eye Disease Consortium The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319.e1. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhli-Hattenbach C, Inge S, Lüchtenberg M, Hattenbach LO. Coagulation disorders and the risk of retinal vein occlusion. Thromb Haemost. 2010;103:299–305. doi: 10.1160/TH09-05-0331. [DOI] [PubMed] [Google Scholar]
- 8.Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilleul J, Glacet-Bernard A, Coscas G, Soubrane G, Souied EH. Underlying conditions associated with the occurrence of retinal vein occlusion. J Fr Ophtalmol. 2011;34:318–324. doi: 10.1016/j.jfo.2010.12.002. (In French) [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130:2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- 11.Biswal BB. Resting state fMRI: A personal history. Neuroimage. 2012;62:938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 12.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 13.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Liu Z, Li J, Liu Z, Tang Z, Xie X, Yang D, Wang N, Tian J, Xian J. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: A resting-state fMRI study. Invest Ophthalmol Vis Sci. 2014;56:322–329. doi: 10.1167/iovs.14-14974. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Zhong YL, Zeng XJ, Zhou F, Liu XH, Hu PH, Pei CG, Shao Y, Dai XJ. Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: A fMRI study. Neuropsychiatr Dis Treat. 2015;11:1877–1883. doi: 10.2147/NDT.S87596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang A, Chen T, Zhang J, Gong Q, Liu L. Abnormal spontaneous brain activity in patients with anisometropic amblyopia using resting-state functional magnetic resonance imaging. J Pediatr Ophthalmol Strabismus. 2017;54:303–310. doi: 10.3928/01913913-20170320-05. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Li SH, Zhou FQ, Zhang Y, Zhong YL, Cai FQ, Shao Y, Zeng XJ. Altered intrinsic regional brain spontaneous activity in patients with comitant strabismus: A resting-state functional MRI study. Neuropsychiatr Dis Treat. 2016;12:1303–1308. doi: 10.2147/NDT.S118326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Zhou FQ, Hu YX, Xu XX, Zhou X, Zhong YL, Wang J, Wu XR. Altered spontaneous brain activity pattern in patients with high myopia using amplitude of low-frequency fluctuation: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2016;12:2949–2956. doi: 10.2147/NDT.S118326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao Y, Cai FQ, Zhong YL, Huang X, Zhang Y, Hu PH, Pei CG, Zhou FQ, Zeng XJ. Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: A resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2015;11:3065–3073. doi: 10.2147/NDT.S92968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:2015–2020. doi: 10.2147/NDT.S110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong YL, Jiang N, Shao Y. Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: A resting-state functional MRI study. Clin Interv Aging. 2016;11:1773–1780. doi: 10.2147/CIA.S117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Li D, Li HJ, Zhong YL, Freeberg S, Bao J, Zeng XJ, Shao Y. Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: A resting-state functional MRI study. Neuropsychiatr Dis Treat. 2017;13:2849–2854. doi: 10.2147/NDT.S147645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZL, Zou L, Lu ZW, Xie XQ, Jia ZZ, Pan CJ, Zhang GX, Ge XM. Abnormal spontaneous brain activity in type 2 diabetic retinopathy revealed by amplitude of low-frequency fluctuations: A resting-state fMRI study. Clin Radiol. 2017;72:340.e1–e7. doi: 10.1016/j.crad.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L, Ma MY, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–257. doi: 10.2147/NDT.S150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: A form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021–1026. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 26.Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015;11:207–214. doi: 10.2147/NDT.S73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2005;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line strage of spatial memoranda. J Cognit Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- 30.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: A case study. Front Syst Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on ‘what,’ ‘when,’ and ‘where’ related information: An fMRI study. J Cogn Neurosci. 2005;17:981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- 32.Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, Boyd BD, Krishnan KR, MacFall JR, Steffens DC, Payne ME. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011;193:1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JD, Craig JP, Akpek EK, Azar DT, Belmonte C, Bron AJ, Clayton JA, Dogru M, Dua HS, Foulks GN, et al. TFOS DEWS II introduction. Ocul Surf. 2017;15:269–275. doi: 10.1016/j.jtos.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Gnanavel S, Robert RS. Diagnostic and statistical manual of mental disorders, fifth edition, and the impact of events scale-revised. Chest. 2013;144:1974–1975. doi: 10.1378/chest.13-1691. [DOI] [PubMed] [Google Scholar]
- 35.Yazar Y, Bergström ZM, Simons JS. Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One. 2014;9:e110414. doi: 10.1371/journal.pone.0110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazar Y, Bergström ZM, Simons JS. Reduced multimodal integration of memory features following continuous theta burst stimulation of angular gyrus. Brain Stimul. 2017;10:624–629. doi: 10.1016/j.brs.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino J, Gabarrós A, Deus J, Juncadella M, Acebes JJ, Torres A, Pujol J. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience. 2011;179:131–142. doi: 10.1016/j.neuroscience.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 38.Owen AM. The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp Brain Res. 2000;133:33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg I, Harel M, Malach R. When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Fried I, Wilson CL, MacDonald KA, Behnke EJ. Electric current stimulates laughter. Nature. 1998;391:650. doi: 10.1038/35536. [DOI] [PubMed] [Google Scholar]
- 41.Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchimura M, Nakano T, Morito Y, Ando H, Kitazawa S. Automatic representation of a visual stimulus relative to a background in the right precuneus. Eur J Neurosci. 2015;42:1651–1659. doi: 10.1111/ejn.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraft A, Dyrholm M, Kehrer S, Kaufmann C, Bruening J, Kathmann N, Bundesen C, Irlbacher K, Brandt SA. TMS over the right precuneus reduces the bilateral field advantage in visual short term memory capacity. Brain Stimul. 2015;8:216–223. doi: 10.1016/j.brs.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen QL, Xu T, Yang WJ, Li YD, Sun JZ, Wang KC, Beaty RE, Zhang QL, Zuo XN, Qiu J. Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia. 2015;75:441–449. doi: 10.1016/j.neuropsychologia.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 47.Noppeney U, Price CJ. Retrieval of visual, auditory, and abstract semantics. Neuroimage. 2002;15:917–926. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- 48.Stoeter P, Bauermann T, Nickel R, Corluka L, Gawehn J, Vucurevic G, Vossel G, Egle UT. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: An fMRI study. Neuroimage. 2007;36:418–430. doi: 10.1016/j.neuroimage.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 49.Schunck T, Mathis A, Erb G, Namer IJ, Demazières A, Luthringer R. Effects of lorazepam on brain activity pattern during an anxiety symptom provocation challenge. J Psychopharmacol. 2010;24:701–708. doi: 10.1177/0269881109104864. [DOI] [PubMed] [Google Scholar]
- 50.Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 51.Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: Language, learning and memory. Trends Cogn Sci. 1998;2:355–362. doi: 10.1016/S1364-6613(98)01211-X. [DOI] [PubMed] [Google Scholar]
- 52.Thomann PA, Schläfer C, Seidl U, Santos VD, Essig M, Schröder J. The cerebellum in mild cognitive impairment and Alzheimer's disease-a structural MRI study. J Psychiatr Res. 2008;42:1198–1202. doi: 10.1016/j.jpsychires.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Baldaçara L, Nery-Fernandes F, Rocha M, Quarantini LC, Rocha GG, Guimarães JL, Araújo C, Oliveira I, Miranda-Scippa A, Jackowski A. Is cerebellar volume related to bipolar disorder? J Affect Disord. 2011;135:305–309. doi: 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 54.Bledsoe JC, Semrud-Clikeman M, Pliszka SR. Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder-combined type. J Am Acad Child Adolesc Psychiatry. 2011;50:593–601. doi: 10.1016/j.jaac.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreasen NC, Paradiso S, O'Leary DS. ‘Cognitive dysmetria’ as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.