Abstract
Ulcerative colitis is closely associated with colorectal cancer, the long-standing chronic inflammation being the key etiology of ulcerative colitis. The aim of the present study was to identify the anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis. MTT assay was used to obtain the optimal concentrations of lipopolysaccharide (LPS) and taraxasterol for cell treatments in vitro. A mouse model of colitis was established via dextran sodium sulphate (DSS) administration. Levels of IL-6 and TNF-α were detected through ELISA. Flow cytometry and western blotting were used to detect apoptosis and related protein expression levels, respectively. Hematoxylin and eosin staining was performed to detect the pathological damage. The results from the MTT assay identified the optimal concentration of LPS and taraxasterol, and ELISA results demonstrated that taraxasterol treatment decreased the expression levels of IL-6 and TNF-α in vitro and in vivo, in a dose-dependent manner. Taraxasterol treatment inhibited apoptosis, and reduced the protein levels of p53, Bcl-2 associated X (BAX) and caspase-3. Finally, pathological damages were reduced in colonic tissues of mice treated with taraxasterol. Taken together, taraxasterol treatment markedly inhibited inflammation and apoptosis in ulcerative colitis. Therefore, taraxasterol may be a promising agent for decreasing the inflammatory response in ulcerative colitis and other inflammation-related diseases.
Keywords: colitis, taraxasterol, dextran sodium sulphate, lipopolysaccharide
Introduction
Ulcerative colitis (UC), a chronic intestinal inflammation condition, is characterized by repeated flare-ups of inflammation that can result in oncogenic insults to the colonic epithelial. UC is a known risk factor for intestinal cancer, especially for colorectal cancer. The etiology of UC is unknown but it is generally considered to be the result of a combination of genetic susceptibility, changes in the commensal enteric flora, and an immune imbalance, in which the inflammatory response is considered to be one of the important pathogenic factors (1–3). Markers of inflammation, TNF-α (tumor necrosis factor-α) and IL-6 (interleukin-6), are useful in assessing disease activity in patients with UC, as they have been demonstrated to contribute to the development of colon mucosal damage and lead to a chronic inflammatory process (4).
LPS (lipopolysaccharide), the specialized structural component of bacteria cell walls, represents a major virulence factor of gram-negative bacteria, as it initiates the occurrence and development of a strong inflammatory response (5). Oral administration of dextran sodium sulphate (DSS) in mice triggers intestinal inflammation that is closely related to human ulcerative colitis (6).
Dandelion is a perennial herb which is used to treat acute conjunctivitis, malignant boils, skin ulcers and other inflammatory conditions (7). Taraxasterol is an active compound extracted from dandelion that has a molecular structure resembling a steroid hormone, and has been demonstrated to display anti-inflammatory effects in vivo and in vitro (8). However, there are no reports on the treatment of UC using taraxasterol. In the present study, taraxasterol was demonstrated to significantly decrease expression of inflammatory factors IL-6 and TNF-α, in vivo and vitro. In addition, taraxasterol significantly decreased the apoptosis rate, as well as the protein expression levels of caspase-3 and p53, which suggested that the inhibitory effects of taraxasterol on inflammatory factors may have an effect on apoptosis. Finally, taraxasterol decreased the pathological damage of colonic epithelial cells in a dose-dependent manner. The present study provided the foundation for further investigation into the anti-inflammatory mechanism of taraxasterol.
Materials and methods
Drugs and reagents
Taraxasterol (purity, 98% HPLC) was purchased from Chengdu Herbpurify Co., Ltd. (Chengdu, China). Dextran sodium sulfate (DSS) was purchased from MP Biomedicals, LLC (Santa Ana, CA, USA). The ELISA kits for TNF-α (cat. no. A0119), IL-6 (cat. no. A0107) were purchased from Nanjing Jiancheng Bioengineering Institute (China). All plastic materials were purchased from Falcon Labware (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). RPMI Medium-1640, fetal bovine serum (FBS), and phosphate buffer saline (PBS) were obtained from Gibco (Thermo Fisher Scientific, Inc. Waltham, MA, USA). Penicillin G/streptomycin, MTT, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Merck KGaA Darmstadt, Germany). Apoptosis Assay kit was purchased from Nanjing KeyGen Biological Co., Ltd. (Nanjing, China). Rabbit anti-human Caspase antibody (cat no. 9661; 1:1,000; Cell Signaling Technology, Inc.), rabbit anti-human BAX antibody (cat no. 2774; 1:1,000; Cell Signaling Technology, Inc.), rabbit anti-human p53 antibody (cat no. 48818; 1:1,000; Cell Signaling Technology, Inc.) and rabbit anti-human β-actin antibody (cat no. 622101; 1:2,000) were purchased from BioLegend Inc. (San Diego, CA, USA). Human colon cancer epithelial cells (HT-29) were purchased from the Chinese Academy of Sciences Shanghai Cell Bank (Shanghai, China).
Experimental animals
Adult male C57BL/6 mice (20–24 g) were purchased from the Laboratory Animal Services Center, The First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). All animals were housed in accordance with the National Institutes of Health Guide for Laboratory animals' use. The study was approved by the Animal Ethics Committee of The First Affiliated Hospital of Zhengzhou University. Animals were housed under standard environment condition of temperature at 20–25°C under a 12-h dark/light cycle and allowed free access to sterilized water and standard food.
Colitis mouse model and related treatments
A total of 60 male C57BL/6 mice were randomly divided into 5 groups (n=12); control group, model group (DSS group), low, medium and high dose (25, 50 and 100 mg/kg/day respectively) taraxasterol groups. Colonic inflammation was established by oral gavage of 5% DSS solution for 5 days. Establishment of a successful model was determined based on thin stools, fecal blood, weight loss and other symptoms and morphological changes. Intestinal inflammation was graded in a blinded fashion, according to Cooper et al (9): 0, no inflammation; 1, mild inflammation of the mucosal intrinsic layer and submucosal layer; 2, severe inflammation of the mucosal intrinsic layer and submucosal layer; 3, mild inflammation of the whole colon wall; and 4, severe inflammation of the whole colon wall.
For taraxasterol treatments, C57BL/6 mice were fed with low, medium or high dose (25, 50 and 100 mg/kg/day) taraxasterol. Mice in the control group were treated with normal saline.
Colon histomorphology
Changes in mucosa and serosal surfaces of colonic tissue were observed via microscope. Fresh colonic tissue was isolated (size ~1×1×0.2 cm). The embedding box was fixed in 4% paraformaldehyde for 24 h. The tissue was embedded in paraffin and stained continuously for 4 µm. Tissue lesions were observed via microscopic observation through hematoxylin and eosin (HE) staining. The criteria was as follows: 0, normal colonic mucosa; 1, crypt defect of 1/3; 2, crypt defect of 2/3; 3, the inherent layer covers the monolayer epithelium with mild inflammatory cell infiltration; and 4, erosion and ulcer in mucosa with plentiful inflammatory cell infiltration.
Immunohistochemistry (IHC)
Paraffin-embedded colonic tissues were continuously sliced into 4 µm thick sections, at 65°C for 2 h. The streptavidin-peroxidase (SP) method was used where sections were dewaxed, hydrated then 0.01 M sodium citrate buffer solution was used for antigen repair for 15 min (1:300) at 95°C. Sections were washed and blocked with goat serum (cat. no. ab7481; Abcam) at room temperature for 20 min. Samples were incubated with primary antibody anti-p53 (1:300; cat no. 48818; Cell Signaling Technology, Inc.) at 4°C overnight. Sections were then washed three times for 5 min using PBS with Tween (PBS-T) then incubated with horseradish peroxidase linked secondary antibody (1:2,000; cat no. 7076; Cell Signaling Technology, Inc.) at 37°C for 1 h. Following washes, SP was added at room temperature for 30 min. DAB staining upon the observation of the slices, and finally the slices were dehydrated, transparent and sealed. Images were taken using an image scanner (Zeiss 125 AxioScan.Z1; Zeiss AG).
A total of 10 non-repeatable fields were selected at ×100 magnification to analyze the % of positively stained cells and coloring intensity. The intensity was scored as: 0, negative; 1, weak; 2, medium; 3, strong. The % of positive-stained cancer cells was scored as: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The intensity number multiplied by extent of staining number produced the final staining score of p53 (range 0–12).
Cell culture
Human colon cancer epithelium HT-29 cells were removed from liquid nitrogen, thawed in a water bath at 37°C, then transferred into a centrifuge tube with RPMI 1640 medium containing 10% fetal bovine serum, and centrifuged at 200 × g for 10 min. The cell pellet was resuspended with complete medium in a 75 ml standard flask, then incubated under standard conditions (37°C, 5% CO2). For LPS and taraxasterol treatments, either LPS (10 µg/ml) or taraxasterol (2.5, 5 or 10 µg/ml respectively) were added into the medium.
MTT assay
In the logarithmic growth phase, 5,000 cells/well were seeded in 5 wells of a 96-well plate. Following 12 h, 1640 medium with 1% serum containing no taraxasterol was added to the control group and culture medium 1640 with 1% serum containing 2.5, 5 or 10 µg/ml taraxasterol was added to the model group. A total of 100 µl of medium containing MTT (0.5 g/l) was added to each well then samples were incubated for 4 h under standard conditions (37°C, 5% CO2). The purple formazan crystals were dissolved using dimethyl sulfoxide and viability was subsequently analyzed at a wavelength of 490 nm with a microplate reader (BIO-RAD680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Measurement of cytokines
HT-29 cells were treated with different concentrations of taraxasterol (2.5, 5 or 10 µg/ml) for 24 h. After digestion, cells were collected and centrifuged at 800 × g for 5 min then the supernatant was collected. For animal experiments, colon tissue was cut into 2–3 mm samples, homogenized with PBS, centrifuged at 12,000 × g for 15 min then the supernatant was collected. Levels of TNF-α and IL-6 in the supernatant were measured according to the instructions of the kit. The experiment was repeated at least three times.
Apoptosis analysis in vitro
HT-29 cells were treated with different concentrations of taraxasterol (2.5, 5 or 10 µg/ml) for 24 h. Cells were washed with PBS twice, digested and collected by centrifugation. Cells were resuspended with 500 µl binding-buffer, followed by 5 µl Annexin-V/fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (PI) for 5 min. Cells were detected by flow cytometry (BD FACSCanto A) and analyzed using FlowJo v10 (FlowJo LLC). FITC-/PI- were deemed viable cells, FITC-/PI+ were necrotic cells, FITC+/PI- were early apoptotic cells, and FITC+/PI+ were late apoptotic cells. The apoptosis rate was calculated as follows: Apoptosis rate % = (number of early apoptotic cells + number of late apoptotic cells)/total number of cells ×100%. The experiment was repeated at least three times.
Western blot analysis
Proteins were extracted from cell suspension and tissue homogenates with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology). The concentration of proteins were detected via Bicinchoninic acid Protein Assay Kit. Proteins (30 µg) were separated via for SDS-PAGE on a 12% gel for 2 h, then the proteins were transferred for 2 h to a polyvinylidene difluoride membrane. Blocking of the membrane with 5% skim milk was performed at 37°C for 2 h. The membranes were incubated with primary antibodies anti-p53 (cat no. 48818; 1:1,000; Cell Signaling Technology, Inc.), BAX (cat no. 2774; 1:1,000; Cell Signaling Technology, Inc.) and cleaved caspase-3 (cat no. 9661; 1:1,000; Cell Signaling Technology, Inc.) overnight at 4°C. Membranes were washed with PBS for 15 min then incubated with secondary antibody IgG (cat on. 7076; 1:2,000; Cell Signaling Technology, Inc.) for 1.5 h at room temperature. Electrochemiluminescence imaging with gel imaging analysis system (Bio-Rad Laboratories, Inc.) was used to measure protein band density value. The experiment was repeated at least three times with β-actin as the loading control.
Statistical analysis
Statistical analysis was performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). All results were presented as mean ± standard error of the mean (SEM). Comparisons were performed using one-way analysis of variance followed by Bonferroni's post hoc test. P<0.05 was considered to indicate significant difference.
Results
Effect of taraxasterol and LPS on HT-29 cell toxicity
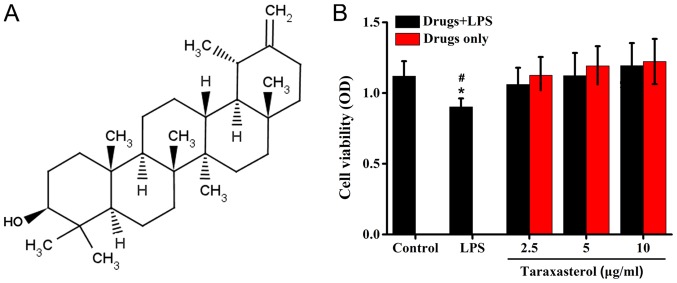
To obtain the optimal concentration of taraxasterol and LPS, HT-29 cells were treated with LPS or taraxasterol at different concentrations, and the cell viability was detected via MTT assay. The results demonstrated that 10 µg/ml LPS decreased the cell viability slightly, while the taraxasterol concentrations used in the experiments did not affect the survival of HT-29 cells compared with the control group (Fig. 1).
Figure 1.
Effect of taraxasterol on the survival of HT-29 cells. (A) Structure of taraxasterol. (B) HT-29 cells were treated with LPS, LPS+2.5 µg/ml taraxasterol, LPS+5 µg/ml taraxasterol or LPS+10 µg/ml taraxasterol. MTT assay was performed to assess cell viability. There was no differences between control and Drugs+LPS groups. *P<0.05 vs. control; #P<0.05 vs. Drugs+LPS groups. LPS, lipopolysaccharide.
Effect of taraxasterol on expression of inflammatory factors TNF-α and IL-6 in vitro
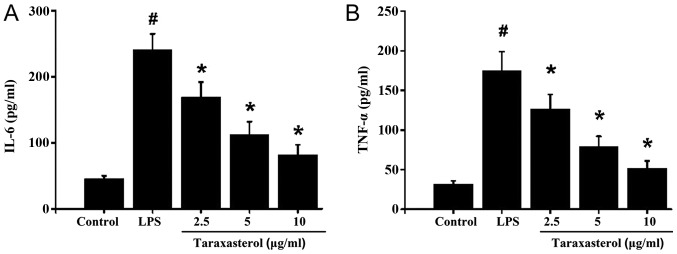
Next, the effect of taraxasterol on the expression of inflammation factors was investigated. To this effect, HT-29 cells were treated with LPS to induce an inflammatory response. LPS stimulation significantly increased TNF-α and IL-6 levels in the supernatant (Fig. 2A and B). Taraxasterol treatment decreased the LPS-induced TNF-α and IL-6 expression in a dose-dependent manner which suggested that taraxasterol exerted a significant anti-inflammatory effect.
Figure 2.
Effect of taraxasterol on secretion of IL-6 and TNF-α. HT-29 cells were treated with 10 µg/ml LPS, then with 2.5, 5 or 10 µg/ml taraxasterol. The supernatants were collected to detect the expression levels of (A) IL-6 and (B) TNF-α. #P<0.05 vs. control; *P<0.05 vs. LPS only group. TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6; LPS, lipopolysaccharide.
Effect of taraxasterol on LPS-induced apoptosis
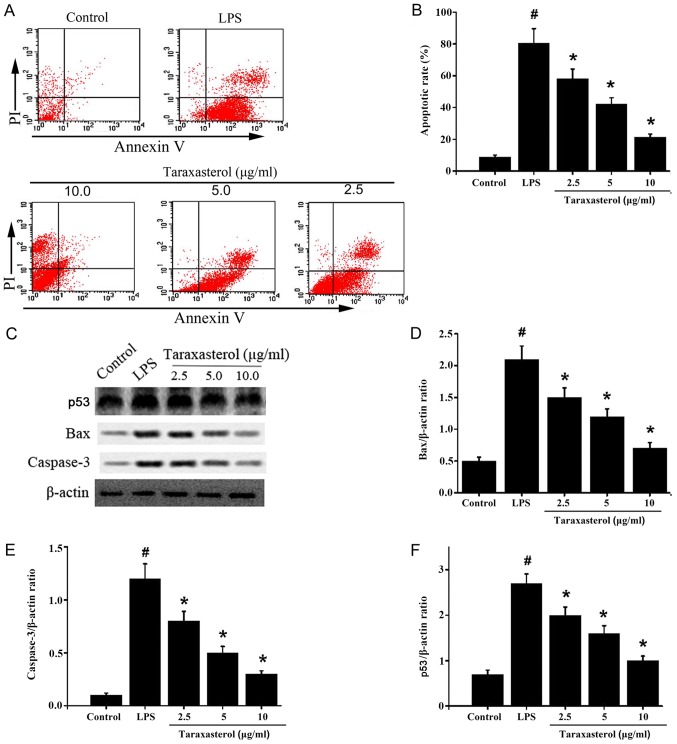
Since taraxasterol could reduce the expression of inflammatory factors, the effect of taraxasterol on apoptosis activity was investigated. HT-29 cells were treated with LPS or taraxasterol, flow cytometry was used to detect the apoptosis rates. The results demonstrated that LPS induced apoptosis in HT-29 cells. In comparison, taraxasterol significantly decreased apoptosis in HT-29 cells (Fig 3A and B). Next, the expression levels of apoptosis-related proteins were detected via western blotting. The results demonstrated that expression of p53, BAX and cleaved caspase-3 were significantly higher in the LPS-treated group compared with the control group (Fig. 3C-F). Taraxasterol significantly decreased p53, BAX and cleaved caspase-3 protein levels in a dose dependent manner (Fig. 3C-F), which suggests that taraxasterol could reduce LPS-induced apoptosis in vitro.
Figure 3.
Effect of taraxasterol on apoptosis rate and protein expression of BAX, cleaved caspase-3 and p53. HT-29 cells were treated with 10 µg/ml LPS, then treated with 2.5, 5 or 10 mg/ml taraxasterol. (A) Cells were analyzed by flow cytometry and (B) the apoptosis rate (%) was calculated. (C) Representative images from western blot analysis of apoptosis-related proteins and quantification of protein expression levels of (D) BAX, (E) cleaved caspase-3 and (F) p53, as a ratio to β-actin. #P<0.05 vs. control; *P<0.05 vs. LPS only group. LPS, lipopolysaccharide; BAX, bcl-2-associated X protein; p53, tumor protein p53.
Effect of taraxasterol on colon histopathological changes induced by DSS
Animal experiments were used to identify the effects of taraxasterol on UC in vivo, after DSS was used to establish a UC mouse model. The structure of the control group colonic tissue was distinct with smooth intact mucosa and no erosion of the tissue or inflammatory cell infiltration (Fig. 4A). In the UC model group, mucosal erosion, granulation tissue hyperplasia and glandular enlargement was observed in Fig. 4A (indicated by the red arrows). In the taraxasterol groups, there was no obvious erosion in the colonic mucosa of mice with minimal inflammatory cell infiltration (Fig. 4A). Colonic pathological changes in the 100 mg/kg taraxasterol dosage group were significantly reduced compared with the 50 mg/kg or 25 mg/kg taraxasterol treatment groups (Fig. 4A). The histopathological scores of the model group and taraxasterol treated groups were significantly higher compared with the control group (Table I; P<0.05). The histopathological score of the 100 mg/kg group (1.5±0.2) was significantly lower compared with the 25 mg/kg group (2.7±0.3; P<0.05).
Figure 4.
Colonic histopathological micrographs and p53 expression levels. Mice were randomly divided into 5 groups (n=12); control group, model group (DSS group) and low, medium and high dose (25, 50 and 100 mg/kg/day respectively) taraxasterol groups. (A) Colonic tissues from each group were stained with H&E (magnification, ×100). The red arrows indicate granulation tissue hyperplasia and glandular enlargement. (B) p53 expression levels (brown staining) were measured by immunohistochemistry assay (magnification, ×100). p53, tumor protein p53; DSS, dextran sodium sulphate; H&E, hematoxylin and eosin.
Table I.
Effect of taraxasterol on histopathological score, p53 IHC score and expression of TNF-α and IL-6 in vivo.
| Group | n | Histopathological score | p53 IHC score | IL-6 (pg/ml) | TNF (pg/ml) |
|---|---|---|---|---|---|
| Model | 12 | 9.12±1.12 | 16.27±2.12 | 271.36±24.28 | 342.182±24.283 |
| 25 mg/kg | 12 | 7.43±1.02 | 13.28±1.87 | 215.37±24.28c | 254.281±24.283c |
| 50 mg/kg | 12 | 5.38±0.93 | 10.27±1.68 | 162.27±24.28b,c | 182.472±24.283b,c |
| 100 mg/kg | 12 | 4.58±0.98 | 9.12±1.57 | 135.21±24.28a–c | 152.811±16.482a–c |
| Control | 12 | 1.95±0.47 | 5.27±1.20 | 69.26±11.45a | 83.127±9.281a |
P<0.05 vs. model group
P<0.05 vs. the 25 mg/kg taraxasterol group
P<0.05 vs. control group. p53, tumor protein p53; IHC, immunohistochemistry; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6.
Effect of taraxasterol on p53 protein expression in colon tissues
Immunohistochemical assay was used to determine p53 protein levels in colon tissues. The results demonstrated that positive p53 staining (brown) was mainly located within nuclei (Fig. 4B). Expression of p53 in colonic mucosa was higher in the UC model groups and the taraxasterol treated groups compared to the control groups (Table I; P<0.01). Treatment with 100 mg/kg taraxasterol significantly decreased p53 levels compared with the UC model group (Fig. 4B). There was no significant difference in IHC score between 100 mg/kg and 50 mg/kg taraxasterol treatment groups, however both had lower IHC scores than the 25 mg/kg treatment groups (Table I; P<0.01).
Effect of taraxasterol on the expression of TNF-a and IL-6 in vivo
The expression levels of TNF-α and IL-6 in the supernatant of colonic mucosa tissues was determined. TNF-α and IL-6 expression levels were lower in taraxasterol-treated groups compared with UC model groups (Table I; P<0.01). The levels of TNF-α and IL-6 in the taraxasterol-treated groups were significantly higher compared with the control group (Table I; P<0.05). In taraxasterol-treated groups, the levels of TNF-α and IL-6 in the 100 mg/kg treatment group were significantly lower than those in the 25 mg/kg treatment group (Table I; P<0.05). These results suggested that taraxasterol significantly decreased the expression of TNF-α and IL-6 in vivo.
Discussion
Cytokines are immunomodulators that are released in response to injury or inflammation with inflammatory cytokines serving an important role in the development of UC. The present study investigated the anti-inflammatory and anti-apoptosis activity of taraxasterol in relation to UC. Taraxasterol is an active compound extracted from dandelion with a molecular structure resembling a steroid hormone and has been demonstrated to have anti-inflammatory effects in vivo and in vitro (8). LPS is the specialized structural component of bacteria cell walls, a major virulence factor of gram-negative bacteria (10) and can induce production of a variety of proinflammatory cytokines, including TNF-α, IL-1 and IL-6 (11). TNF-α is a prototypical proinflammatory cytokine that has been implicated in carcinogenesis due to its participation in chronic inflammatory diseases (12). IL-6 is another proinflammatory cytokine with a typical protumorigenic effect, with elevated IL-6 serum levels detected in patients with systemic cancers (13). Previous studies have reported that TNF-α and IL-6 are closely associated with UC (14).
In the present study, first, the optimal concentrations of LPS and taraxasterol on HT-29 cells were determined, and the results demonstrated that the different concentrations of taraxasterol and LPS examined had no obvious cytotoxic effects on HT-29 cells. The levels of TNF-α and IL-6 were increased both in the in vitro UC model induced by LPS and in the in vivo UC model induced by DSS. Of note, taraxasterol significantly decreased the inflammatory cytokines TNF-α and IL-6 secretion in vivo and in vitro. These results revealed that taraxasterol could reduce inflammation through inhibiting TNF-α and IL-6 secretion.
Apoptosis is programmed cell death and maintains the balance between healthy cell survival and death. Numerous studies have identified that apoptosis in intestinal epithelial cells is driven by increased cytokine activity such as TNF, IL, and interferon family members (15). Increased apoptosis of intestinal epithelial cells has been observed at acute UC inflammatory sites which can disrupt intestinal mucosal integrity and barrier function eventually leading to inflammation (16). Identifying how best to decrease colonic epithelial cell apoptosis and how to repair mucosal tissues has become the focus of therapy of UC (17). Studies have identified p53, BAX, caspase-3 as essential proteins involved in apoptosis (18). In the present study, results demonstrated that LPS significantly increased apoptosis as well as the protein expression levels of p53, BAX and caspase-3. Taraxasterol treatment decreased apoptosis rate, and decreased the protein levels of these apoptosis-related proteins. In addition, taraxasterol treatment effectively reduced pathological injury of colonic epithelial tissues in UC mouse models. Furthermore, p53 expression in colonic tissue was decreased and TNF-α and IL-6 levels were reduced following taraxasterol treatment.
In summary, the present study identified that taraxasterol significantly inhibited secretion of TNF-α and IL-6 in vivo and in vitro, and decreased apoptosis and associated protein levels. Taken together, these findings suggest that taraxasterol could decrease inflammation and apoptosis associated with UC.
Acknowledgements
The authors would like to thank the members of The First Affiliated Hospital of Zhengzhou University, for providing helpful discussions and technical support concerning the present study.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HJL analyzed and interpreted the main data regarding the cell function study and immunofluorescence. XYL was responsible for design and drafting of the manuscript. DBJ was responsible for statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institute Research Medical Ethics Committee of The First Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Scarpa M, Castagliuolo I, Castoro C, Pozza A, Scarpa M, Kotsafti A, Angriman I. Inflammatory colonic carcinogenesis: A review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J Gastroenterol. 2014;20:6774–6785. doi: 10.3748/wjg.v20.i22.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisamatsu T, Kanai T, Mikami Y, Yoneno K, Matsuoka K, Hibi T. Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacol Ther. 2013;137:283–297. doi: 10.1016/j.pharmthera.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg L, Nanda KS, Zenlea T, Gifford A, Lawlor GO, Falchuk KR, Wolf JL, Cheifetz AS, Goldsmith JD, Moss AC. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol. 2013;11:991–996. doi: 10.1016/j.cgh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mankowska-Wierzbicka D, Swora-Cwynar E, Poniedzialek B, Adamski Z, Dobrowolska A, Karczewski J. Usefulness of selected laboratory markers in ulcerative colitis. Eur Cytokine Netw. 2015;26:26–37. doi: 10.1684/ecn.2015.0363. [DOI] [PubMed] [Google Scholar]
- 5.Wei C, Tan CK, Xiaoping H, Junqiang J. Acanthoic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts. Inflammation. 2015;38:896–901. doi: 10.1007/s10753-014-0051-7. [DOI] [PubMed] [Google Scholar]
- 6.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 7.Herz W, Mirrington RN. Identification of pyrethrol with taraxasterol. J Pharm Sci. 1966;55:104. doi: 10.1002/jps.2600550123. [DOI] [PubMed] [Google Scholar]
- 8.Piao T, Ma Z, Li X, Liu J. Taraxasterol inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Eur J Pharmacol. 2015;756:38–42. doi: 10.1016/j.ejphar.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 10.Kinoshita T, Imamura R, Kushiyama H, Suda T. NLRP3 mediates NF-κB activation and cytokine induction in microbially induced and sterile inflammation. PLoS One. 2015;10:e0119179. doi: 10.1371/journal.pone.0119179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 12.Liverani E, Rico MC, Yaratha L, Tsygankov AY, Kilpatrick LE, Kunapuli SP. LPS-induced systemic inflammation is more severe in P2Y12 null mice. J Leukoc Biol. 2014;95:313–323. doi: 10.1189/jlb.1012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RA, Motiwala MN, Mahajan UN, Sabre SG. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol. 2018;219:222–232. doi: 10.1016/j.jep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki D, Adams A, Warner KA, Zhang Z, Pearson AT, Misawa K, McLean SA, Wolf GT, Nör JE. Anti-tumor effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma. Oncotarget. 2015;6:22822–22835. doi: 10.18632/oncotarget.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 17.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn's disease. Dis Colon Rectum. 2003;46:1498–1507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 18.Al-Assaf AH, Alqahtani AM, Alshatwi AA, Syed NA, Shafi G, Hasan TN. Mechanism of cadmium induced apoptosis in human peripheral blood lymphocytes: The role of p53, Fas and Caspase-3. Environ Toxicol Pharmacol. 2013;36:1033–1039. doi: 10.1016/j.etap.2013.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.