Abstract
Glioma, the most common human primary brain tumor, is characterized by invasive capabilities and angiogenesis. Vasorin (VASN), a transmembrane protein, is reported to be associated with vascular injury repair and is overexpressed in some human tumors. However, its role in tumor progression and angiogenesis in glioma is unknown. In this study, VASN was shown to be overexpressed in high‐grade gliomas, and the expression level correlated with tumor grade and microvessel density in glioma specimens. Glioma patients with high VASN expression had a shorter overall survival time. Knockdown of VASN in glioma cells by shRNA significantly inhibited the malignancy of glioma, including cell proliferation, colony formation, invasion, and sphere formation. Ectopic expression of VASN increased glioma progression in vitro. The expression of VASN correlated with the mesenchymal type of glioblastoma multiforme (GBM) subtyped by gene set enrichment analysis (GSEA). Our results showed that the concentration of VASN was increased in the conditioned medium (CM) from glioma cells with VASN overexpression, and the CM from glioma cells with knockdown or overexpressed VASN inhibited or promoted HUVEC migration and tubulogenesis in vitro, respectively. Glioma growth and angiogenesis were stimulated upon ectopic expression of VASN in vivo. The STAT3 and NOTCH pathways were found to be activated and inhibited by VASN overexpression. Our findings suggest that VASN stimulates tumor progression and angiogenesis in glioma, and, as such, represents a novel therapeutic target for glioma.
Keywords: VASN, glioma, tumor progression, angiogenesis, STAT3
1. INTRODUCTION
Gliomas, the most common primary brain tumors, have been classified by the World Health Organization (WHO) into 4 grades of ascending malignancy according to histological criteria. Glioblastoma multiforme (GBM, WHO grade IV) is the most aggressive form of malignant glioma and can arise de novo or from pre–existing lower grade tumors.1 GBM prognosis remains poor, with the median survival time <15 months.2 Currently, GBM biology, in general, is not fully understood, and the clinical treatment failure could be ascribed to multiple factors.
Vascularization is an important pathological feature of GBM and has a critical role in tumor progression and metastatic dissemination by displaying a high degree of vascular proliferation and endothelial cell hyperplasia.3 GBM use multiple mechanisms to ensure adequate access to the vasculature.4, 5 Hypoxia is one of the principal vascularization stimuli leading to the synthesis of angiogenic growth factors and the induction of the formation of new blood vessels.6, 7, 8 Vasorin (VASN) was reported to be regulated by hypoxia‐inducible factor‐1 (HIF‐1) and induced by hypoxia. VASN, also known as SLIT‐like 2, is a type I transmembrane glycoprotein.9 It was originally found to play a role in vascular injury repair,9, 10, 11 and its expression was restricted to the aorta, the kidney, and the placenta.9 VASN expression was found to increase gradually during embryonic development in mice.11 Recent reports showed that VASN was also highly expressed in some cancer cells, including hepatocellular carcinoma, breast cancer and GBM, and played an important role in facilitating the progression of tumors by inhibiting hypoxia‐induced apoptosis.12, 13, 14 It was also shown that VASN promoted migration of recipient HUVEC in hepatocarcinoma and angiogenesis in breast cancer.15, 16 Recently, VASN was identified as a key regulator of NOTCH signaling in glioma stem cells (GSC) in the hypoxic niche.14 However, the influence of VASN on tumor progression and angiogenesis in glioma remains unclear.
In this study, we reported that VASN expression was increased in human gliomas, especially in GBM. Of note, the increased expression of VASN induced an infiltrative behavior within glioma cells, with increased migration and tube formation of vascular endothelial cells in vitro and stimulated glioma growth and angiogenesis in vivo. Thus, our results identified a functional protein that is expressed in tumor cells and affects tumor proliferation and angiogenesis in glioma.
2. MATERIALS AND METHODS
2.1. Patients and tissue specimens
Paraffin‐embedded samples of glioma were collected from 100 patients in Shenzhen People's Hospital, China. None of the patients received radiotherapy or chemotherapy before surgery, and none of them had multiple cancers in other organs. A total of 100 glioma specimens spanning WHO grade I (n = 8), grade II (n = 46), grade III (n = 30) and grade IV (n = 16) were divided into low grade (grade I and II, n = 54) glioma and high grade (grade III and IV, n = 46) glioma. The characteristics of the patients are detailed in Table 1.
Table 1.
Relationships between vasorin (VASN) expression and clinical pathological parameters in glioma patients
| Variables | N | Vasorin expression | P | ||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ++++ | |||
| Sex | |||||||
| Male | 57 | 8 | 14 | 11 | 18 | 6 | 0.855 |
| Female | 43 | 4 | 10 | 11 | 15 | 3 | |
| Agea | |||||||
| <20 | 13 | 2 | 3 | 1 | 4 | 3 | 0.774 |
| 20~ | 51 | 7 | 13 | 14 | 15 | 2 | |
| 40~ | 27 | 3 | 5 | 4 | 13 | 2 | |
| ≥60 | 8 | 0 | 3 | 3 | 1 | 1 | |
| Histological grade | |||||||
| I | 8 | 6 | 2 | 0 | 0 | 0 | 0.000b |
| II | 46 | 6 | 22 | 13 | 5 | 0 | |
| III | 30 | 0 | 0 | 8 | 19 | 3 | |
| IV | 16 | 0 | 0 | 1 | 9 | 6 | |
Abbreviations: N, number of cases; P, P value.
1 case without age.
Statistically significant.
Table 2.
Relationships between glioma MVD and clinical pathological parameters in glioma patients
| Variables | N | MVD | P |
|---|---|---|---|
| Mean ± SD | |||
| Sex | |||
| Male | 57 | 26.393 ± 15.796 | 0.718 |
| Female | 43 | 28.891 ± 18.989 | |
| Agea | |||
| <20 | 13 | 32.424 ± 23.100 | 0.800 |
| 20~ | 51 | 25.843 ± 15.078 | |
| 40~ | 27 | 30.247 ± 19.438 | |
| ≥60 | 8 | 22.167 ± 11.946 | |
| Histological grade | |||
| I | 8 | 8.750 ± 4.356 | 0.000b |
| II | 46 | 18.457 ± 7.684 | |
| III | 30 | 37.044 ± 18.238 | |
| IV | 16 | 46.000 ± 12.372 | |
| Vasorin expression | |||
| ‐ | 12 | 8.833 ± 3.106 | 0.000b |
| + | 24 | 17.139 ± 6.405 | |
| ++ | 22 | 22.788 ± 9.321 | |
| +++ | 33 | 38.657 ± 15.783 | |
| ++++ | 9 | 53.250 ± 14.719 | |
Abbreviations: MVD, microvessel density; N, number of cases; P, P value.
1 case without age.
Statistically significant.
2.2. Immunohistochemical staining and scoring
Tissue sections were incubated with antibodies against VASN (1:100; R&D Systems) and CD34 (1:100; ZSGB‐BIO) for 3 hours followed by incubation with secondary antibody (Dako REAL EnVision). Immunoreacted cells were visualized using diaminobenzidine, and nuclei were counterstained with hematoxylin. PBS was substituted for the primary antibody as a negative control. Two independent pathologists evaluated the sections. VASN‐positive glioma cells were assessed. The frequency was determined with a semi‐quantitative score ranging from − to ++++ (− = 0%‐10%, + = 10%‐25%, ++ = 25%‐50%, +++ = 50%‐75%, ++++ ≥ 75% of all cells showing positive staining). Both +++ and ++++ were scored as high expression and the others were scored as low expression. CD34‐positive individual microvessel counts were performed on ×400 fields, and 3 area microvessel counts were averaged to obtain the microvessel density (MVD). Evaluation and photographic documentation were performed using a Leica Microsystems DM6000B light microscope (Leica).
2.3. Cell lines and cell culture
Human glioma cell lines U87‐MG, U251 and U118‐MG (U118) were obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai) and cultured in DMEM with high glucose, 10% FBS. HUVEC were purchased from ScienCell (San Diego) and cultured in Endothelial Cell Growth Medium‐2 (EGM‐2) (Lonza). GSC grew in a stem cell‐permissive medium comprised of 1:1 of DMEM/F12 and neurobasal medium supplemented with 2% B27 (Invitrogen), 20 ng/mL bFGF and EGF (PeproTech). All cell types were maintained at 5% CO2.
2.4. Plasmids production
Expression and purification of lentiviruses were performed as previously described with minor modifications.17 For the generation of lentiviral vectors encoding shRNA (short hairpin RNA) targeting VASN, corresponding shRNA oligos (sequences including shRNA#1, TRCN0000244686; shRNA#2, TRCN0000244687; shRNA#3, TRCN0000257443 referenced from https://www.sigmainformatics.com) were cloned into the PLKO.1‐TRC vector (Sigma‐Aldrich). For lentiviral vectors expressing VASN or luciferase (control), corresponding cDNAs were cloned into the pSIN‐Puro‐FLAG vector (gift from Dr Guangjin Pan).
2.5. Quantitative real‐time PCR
Total RNA was extracted with TRIzol and reverse transcribed with oligo dT (Takara Bio). Quantitative real‐time PCR (qPCR) was performed following the manufacturer's recommendations. GAPDH was used for the normalization of human samples. All the data were measured in triplicate. All primer sequences are listed in Table S1.
2.6. Proliferation assays with Cell Counting Kit‐8
Living cells metabolize the Cell Counting Kit‐8 (CCK‐8) solution (KeyGen BioTech) to produce water‐soluble crystals, and the absorbance (OD value) can be directly detected by a microplate reader (Winooski). The specific method is as follows: glioma cells were seeded into 96‐well plates at a density of 1 × 104 cells. One plate was tested every day where 10 μL/well of CCK‐8 was added, and the absorbance was read at a wavelength of 450 nm after 1 hour of incubation. The average value was calculated from 5 replicates, and a growth curve was calculated from the average values. The above experiment was repeated 3 times. The OD value, determined by the relative absorbance of CCK‐8, was assessed by probit regression analysis in SPSS 13.0 statistical software.
2.7. Single‐cell clonal expansion assay
To determine the clonal expansion efficiency of transfected glioma cells, 2000 cells were seeded in a Matrigel‐coated 6‐well plate and cultured until clear cell colonies formed (approximately 2 weeks). The relative colony number was then determined by crystal violet staining.
2.8. Cell migration assay
For transwell migration assays, the migration of U118 and U251 cells in vitro was performed as previously described.18 The top chamber of the transwell was seeded with 5 × 104 cells in 100 μL basal medium (without serum). Complete culture medium (600 μL) was aliquoted into the bottom chamber. After 24 hours, the cells were fixed with methanol and stained with crystal violet. The number of cells that invaded through the membrane was counted under a light microscope.
The wound‐healing assay was performed as described previously.19 HUVEC were planted in a 12‐well plate. Before the wound was created, HUVEC were exposed to serum‐free endothelial cell medium for 8 hours. Conditioned media was harvested 24 hours after the cell density reached 80%. HUVEC were incubated with 200 μL EGM‐2 with 2% serum and 600 μL of conditioned medium containing 20 ng/mL bFGF after the wound induction. Results were recorded 8 hours after the treatment.
2.9. HUVEC tube formation
Matrigel (Corning) was mixed (1:1) with serum‐free DMEM and added to a 96‐well plate with 50 μL per well. The Matrigel was allowed to solidify at 37°C for 30 minutes. After the Matrigel solidified, HUVEC (2 × 104 cells) were plated into the wells with 100 μL of the same mixed media. Results were recorded 8 hours after the treatment.
2.10. Western Blot
Total cell protein lysate was made in RIPA buffer with proteinase and phosphatase inhibitor cocktails, resolved by SDS‐PAGE, and transferred onto an Immobilon‐PSQ Transfer Membrane, as previously described.20 Blots were incubated with antibodies against GAPDH (1:5000, Proteintech), VASN (2 μg/mL, R&D Systems), STAT3 (1:2000, Proteintech), p‐STAT3 (1:1000, Cell Signaling Technology), Notch1 (1:1000, 20687‐1‐AP, Proteintech), NICD (1:500, Cell Signaling Technology) and VEGF (1:1000, PeproTech) overnight at 4°C. All immunoblots were performed at least 3 times.
2.11. ELISA
The concentration of vasorin and VEGF in the conditioned medium were determined by using the Human Vasorin ELISA Kit (EK3851; Signalway Antibody LLC) and the Human VEGF ELISA Kit (KE00085; Proteintech), according to the manufacturer's instructions. In brief, 2 × 105 U251 and U118 cells were seeded on 6‐well plates. After 8 hours, cells were attached and the cell culture mediums were replaced with fresh DMEM with FBS. Supernatants were collected 24 hours later and centrifuged at 400 g to delete the cell debris. Supernatants were assayed and the concentration of human Vasorin and VEGF were calculated according to the standard curve.
2.12. Bioinformatics analysis
TCGA diffuse glioma data, including RNAseq and patient survival, were downloaded from the TCGA Research Network ( https://cancergenome.nih.gov). Gene expression of VASN and other genes in the TCGA diffuse glioma database were assessed using publicly available platforms, including the biostatistics program R Studio v3.5.0 and GraphPad Prism 7 software. Gene set enrichment analysis (GSEA) was performed by generating the Gene MatriX file (.gmx) by using published signatures for Angiogmatrix,21 the TCGA GBM subtypes22 and the Phillips GBM subtypes.23 The gene cluster text file (.gct) was generated from the TCGA diffuse glioma database. The number of permutations for GSEA was set to 1000, and we used the TCGA gene list as the chip platform.
2.13. Experimental glioma assay in chicken chorioallantoic membrane xenografts
On embryonic day 7, a plastic ring was placed on the chicken chorioallantoic membrane xenografts (CAM), and 4 × 106 glioma cells were deposited on each CAM. Photos were taken on day 4 after transplantation by stereomicroscope (Olympus SZX2). Tumor volume was measured using a vernier caliper. The experimental gliomas were fixed with 4% paraformaldehyde and caveolin 1 (1:200; Thermo Fisher Scientific) was used to detect chicken vessels in tumors by immunohistochemical staining.
2.14. Statistical analysis
The results were presented as the mean ± SEM or SD. Student's t test, Spearman's correlation coefficient for ranked data, and one‐way ANOVA were performed using SPSS/PC+ statistics 11.0 software (SPSS). P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Vasorin is highly expressed and correlates with poor overall survival in glioma
Vasorin was originally found to play a role in vascular injury repair. Recent reports showed that VASN was also highly expressed in some cancer cells.14 To further assess the role of VASN in glioma, we measured the expression levels of VASN in 100 glioma specimens spanning grade I, grade II, grade III, and grade IV (GBM). The expression of VASN increased with the histological grading in glioma tissues and was significantly upregulated in high‐grade gliomas (Table 1, Figure 1A,B). VASN was reported to localize to both the cell plasma membrane and mitochondria. In the present study, we found that VASN localized not only to the cell plasma membrane but also to the nucleus. Our results were partly inconsistent with the previous report, so we further confirmed the protein localization by western blot, which showed that VASN protein was expressed in the cell plasma membrane and the nucleus of glioma cells (Figure 2C). Furthermore, we queried TCGA diffuse glioma data and analyzed VASN levels in 660 glioma patients, which were separated into low‐grade glioma and GBM. Increased expression of VASN was also observed in GBM (Figure 1C), and high VASN expression was identified as a predictor for poor progression‐free survival (P = 0.0036; Figure 1D). These results indicate that the expression of VASN protein is elevated in more aggressive gliomas and predicts a poor prognosis of glioma.
Figure 1.
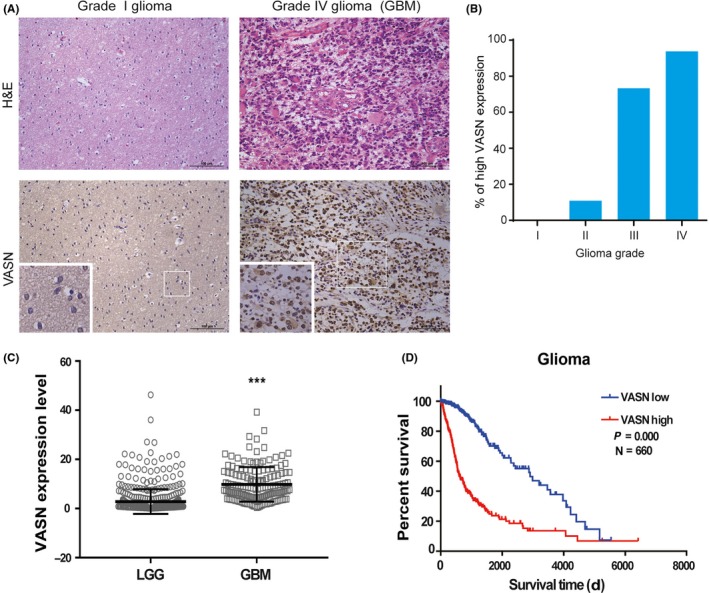
Vasorin (VASN) expression was elevated and correlates with poor prognosis in glioma. A, Representative H&E staining and immunohistochemical (IHC) staining images of VASN expression in glioma patients. B, The histogram illustrates the percentage of high VASN expression in gliomas across the 4 grades. C, VASN expression in low‐grade gliomas (LGG) and glioblastoma (GBM) from the TCGA diffuse glioma database. ***P < 0.001. (D) Kaplan‐Meier survival analysis of glioma patients stratified by VASN expression. Median VASN expression was used for stratification into VASN‐high and VASN‐low tumors. Scale bar, 100 μm
Figure 2.
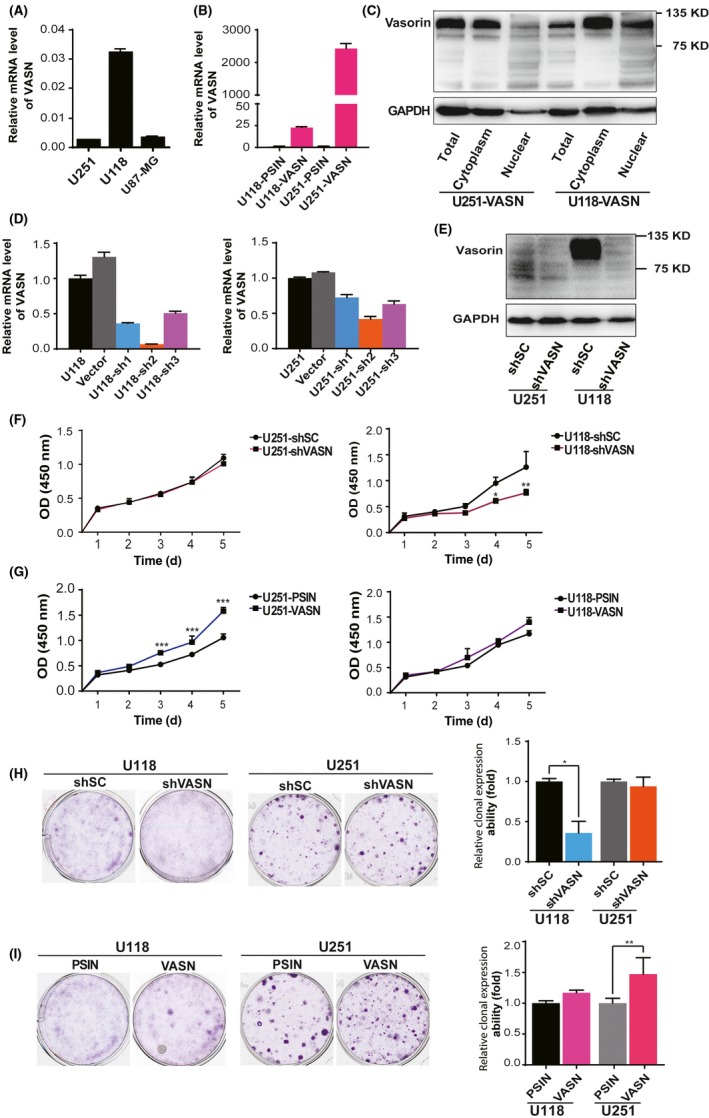
Vasorin (VASN) expression promoted cell growth in glioma cells. A, Expression levels of VASN in glioma cell lines. B, The ectopic expression of the VASN gene was confirmed by quantitative real‐time PCR (qPCR). C, The ectopic expression and protein localization of the VASN was confirmed by western blot. The knockdown efficiency of VASN shRNA was confirmed by qPCR (D) and western blot (E). CCK‐8 assays were performed at different time points to detect the effect of knockdown (F) ectopic expression (G) of VASN on the growth of glioma cells. (H, I) Colony‐forming assays were performed on the same cell lines with either VASN knockdown or ectopic expression. The histograms illustrate the number of colonies counted. Data are presented as mean ± SD of 3 independent experiments. *P < 0.05, **P < 0.01. PSIN, negative control of ectopic expression of VASN; shSC, negative control of shRNA of VASN
3.2. Vasorin regulates the proliferation and colony formation in glioblastoma multiforme cells
To decipher the biological function of VASN in glioma, we analyzed the mRNA level of VASN in glioma cell lines, and then selected the U118 and U251 cell lines, which had different expression levels of VASN, to be used for further study (Figure 2A). We generated stable ectopic expression and knockdown of VASN cell lines by transfecting U118 and U251 cells with lentivectors expressing VASN and shRNA of VASN (Figure 2B‐E). VASN protein was mainly expressed in the cell plasma membrane in glioma cells after transfection with a VASN plasmid. We then investigated the effect of VASN in the proliferation of glioma cells using CCK‐8 assays. The results showed that the growth of U118 and U251 cells was inhibited after knockdown of VASN and promoted after overexpression of VASN (Figure 2F,G). Colony formation assays demonstrated that knockdown of VASN efficiently decreased the ability of glioma cells to proliferate in semi‐solid matrices, whereas overexpression of VASN elevated this ability of glioma cells (Figure 2H,I). These results suggest that VASN promotes the proliferative ability of glioma cells.
3.3. Vasorin promotes cell invasion of glioma cells
Transwell invasion assays showed that overexpression of VASN in U118 and U251 cells increased their invasive ability (Figure 3A). Knockdown of VASN in glioma cells inhibited cell invasion, although it did not reach statistical significance (Figure 3B). GBM is divided into 4 subtypes, with different biological signatures in the TCGA database, including mesenchymal, proneural, neural and classical. To examine whether VASN activation differs among GBM subtypes, we used GSEA to determine the degree of VASN expression for the 4 TCGA GBM subtypes. As shown in Figure 3C, GBM tumors with high VASN expression were enriched for TCGA mesenchymal gene signature, but not for the signatures of the other 3 subtypes. Furthermore, we also analyzed the same GBM cohort for the enrichment of another set of GBM subtype‐specific gene signatures: Phillips mesenchymal, proneural and proliferation signatures. GBM with high VASN expression were enriched for the Phillips mesenchymal gene signature, but not the Phillips proneural and proliferation signatures (Figure 3D). These findings indicate that high VASN expression may be associated with the highly infiltrative and aggressive characteristics of mesenchymal GBM cells.
Figure 3.
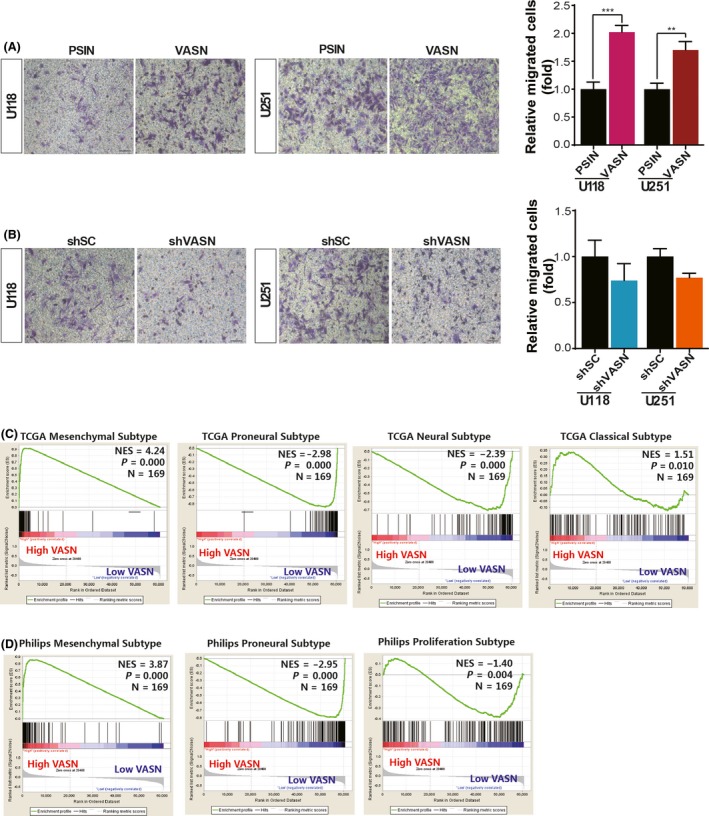
Vasorin (VASN) promoted cell invasion and correlated with mesenchymal glioblastoma (GBM) gene signatures. Representative transwell invasion images and statistics for the number of counted glioma cells with VASN knockdown (A) and overexpression (B) that were able to migrate. GSEA was performed using the signatures for the TCGA GBM subtypes (C) or Phillips GBM subtypes (D). Patients were separated by high or low VASN mRNA expression. Scale bar, 50 μm
3.4. Vasorin promotes the stemness property of glioma cells
Cancer stem cells are thought to contribute to tumor recurrence and metastasis. The ability to form spheroids is considered a stemness characteristic of cells. A spheroid formation assay showed that shVASN‐transfected U118 cells formed a reduced number of smaller‐sized spheroids, whereas VASN overexpressed U251 cells formed an increased number of spheroids (Figure 4A,B). VASN overexpressed glioma spheres displayed increased expression of stem cell genes CD133, CD15 and CD90 (Figure 4C).
Figure 4.
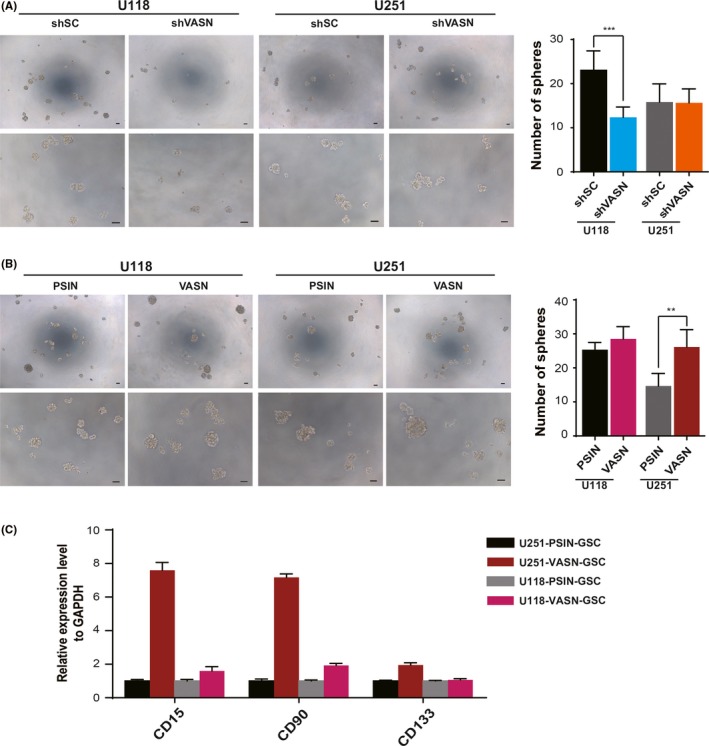
Vasorin (VASN) promoted tumor sphere formation in glioma cells. Representative images of sphere formation and statistics for the number of tumor spheres counted in glioma cells knockdown (A) and overexpression of VASN (B). C, The expression of cancer stem cell‐related genes (CD15, CD90 and CD133) was confirmed by quantitative real‐time PCR (qPCR). The data represent mean ± SD from 3 biological repeats. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar, 50 μm
3.5. Increased vasorin expression correlates with increased angiogenesis in glioma tissues
To elucidate whether VASN regulates angiogenesis in glioma, tumor capillaries were examined by anti–CD34 immunostaining. As shown in Figure 5A, tumor sections from GBM showed a significant increase in CD34 staining, compared with a grade II glioma sample. By analyzing the quantification of MVD (CD34‐positive microvessel number) in gliomas, we found that MVD was elevated in high‐grade gliomas relative to low‐grade gliomas (Table 2), and the elevated expression levels of VASN were positively correlated with the MVD (Figure 5B). GSEA with an AngioMatrix signature has been shown to be associated with tumor vascularity in GBM. Our results show that GBM with high VASN expression had significant enrichment with the AngioMatrix signature (Figure 5C,D). To further confirm this observation, we correlated VASN expression with vascularity markers and found that VASN was significantly associated with CD34, CD31 and CDH5 (Figure 5E). Taken together, these data suggest that VASN expression is correlated with increased angiogenesis in gliomas.
Figure 5.
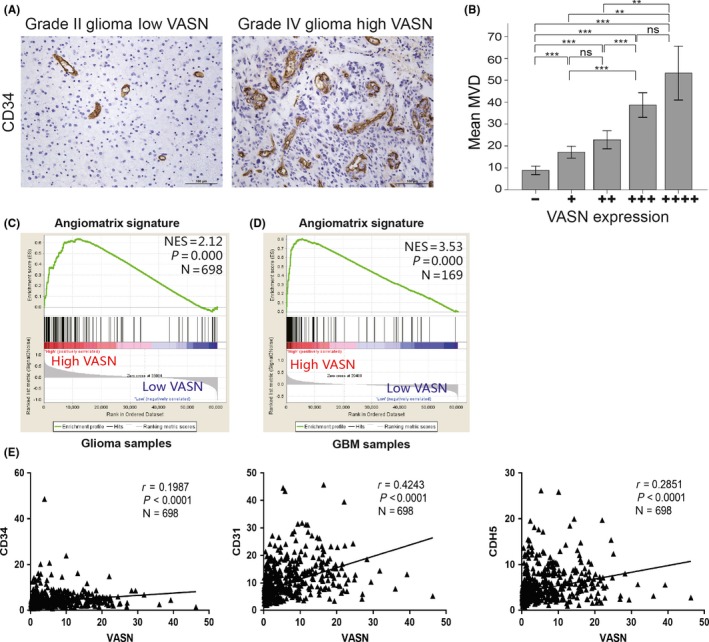
Increased levels of vasorin (VASN) were associated with increased angiogenesis of glioma samples. A, CD34 staining for endothelial cells revealed increased tumor vascularization in high VASN expressing tissues; representative images are shown. B, The histogram illustrates microvessel density (MVD) among the 5 groups. Data represent the mean ± SD of 3 replicates. **P < 0.01, ***P < 0.001. Gene set enrichment analysis (GSEA) was performed using the AngioMatrix signature that is representative of glioblastoma (GBM) angiogenesis (C, D). Patients were separated by high or low VASN expression using TCGA glioma and GBM datasets. (E) VASN expression was correlated with markers of tumor vascularity using CD34, CD31 and CDH5. Scale bar, 100 μm
3.6. Increased vasorin expression in glioblastoma multiforme cells promotes angiogenesis in vitro
To examine whether overexpression of VASN stimulates glioma cells to influence angiogenesis in a tumor microenvironment, we collected the conditioned medium (CM) from U118 and U251 cells, treated HUVEC with the CM, and then analyzed the migration of the HUVEC in a wound‐healing assay. Treatment with the CM obtained from U118 or U251 cells with VASN knockdown significantly inhibited the cell migration of HUVEC (Figure 6A). However, the CM obtained from glioma cells transfected with VASN overexpression significantly accelerated the cell migration of HUVEC (Figure 6B). The ability of cultured HUVEC to form vessel‐like structures (tubulogenesis) on Matrigel was also investigated. Knockdown of VASN inhibited endothelial cell tubulogenesis in terms of the number of branch points compared to the CM from control glioma cells (Figure 6C).
Figure 6.
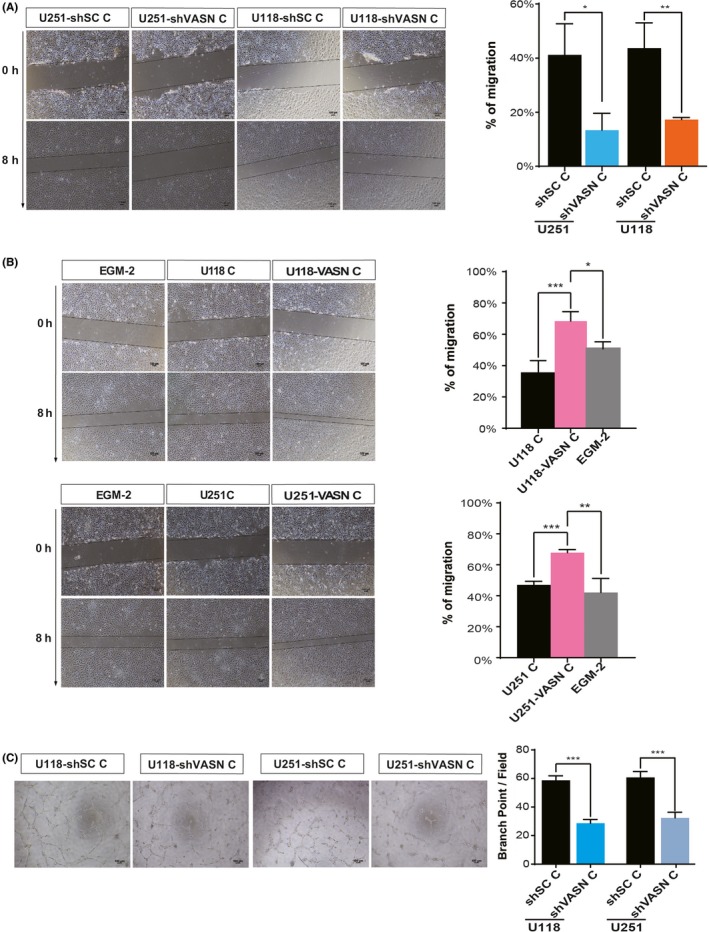
Vasorin (VASN)‐expressing tumor cells strongly promoted HUVEC migration and tube formation. Micrographs and histograms of the scratch wound‐healing assay of HUVEC grown in conditioned medium (CM) from glioma cell knockdown (A) and overexpression (B) of VASN. EGM‐2 served as a control. C, Micrographs of tube formation by HUVEC grown in CM from shVASN‐transfected glioma cells as indicated. Quantification of the number of branch points formed during the tube formation assay. Mean number of branching points per ×100 field ±SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar, 100 μm
3.7. Vasorin promotes glioma progression and angiogenesis by activating STAT3 and inhibiting NOTCH pathways
To investigate the mechanism of VASN‐induced angiogenesis, we detected the concentration of vasorin in CM by ELISA. Results showed that vasorin in the CM obtained from U118 or U251 cells with VASN knockdown decreased and that with VASN overexpression increased significantly (Figure 7A). We also detected the concentration of VEGF, an important angiogenic factor, and found that VEGF was decreased in the CM obtained from U251 cells with VASN knockdown, but there were no significant changes in the CM obtained from U118 cells (Figure 7B). qPCR and western blot were performed on the glioma cells with VASN knockdown or overexpression. Results showed that angiogenic factors, such as ANGPT2, CCR2 and VEGF‐A, were overexpressed in glioma cells with high VASN (Figure 7C,D). Furthermore, we investigated the important signaling pathways involved in tumor growth and angiogenesis by western blot; the results showed that VASN promoted STAT3 pathway activation and inhibited NOTCH pathway (Figure 7E).
Figure 7.
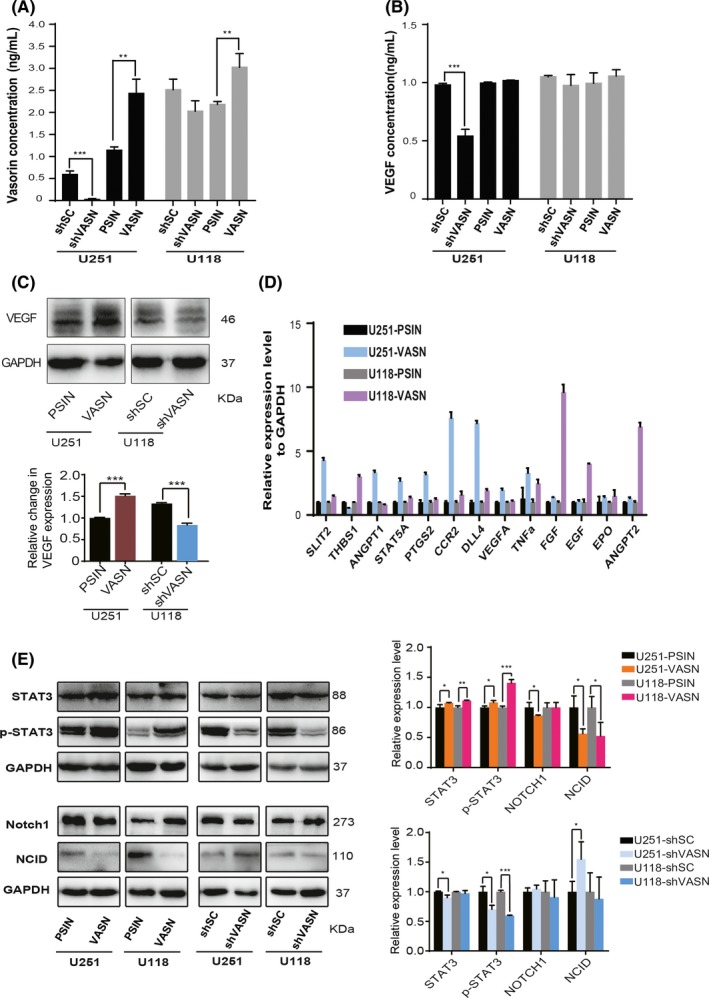
Vasorin (VASN) promoted glioma progression and angiogenesis by activating STAT3 and inhibiting NOTCH signaling. The concentrations of vasorin (A) and VEGF (B) in the conditioned medium (CM) from glioma cells with VASN knockdown or overexpression were detected by ELISA assay. C, Western blot analysis of VEGF in U118 cells knockdown and U251 cells overexpression of VASN. (D) qPCR analysis of angiogenic factors in glioma cells transfected with VASN. (E) Western blot analysis of STAT3, p‐STAT3, NOTCH1 and NICD in glioma cell knockdown or overexpression of VASN. *P < 0.05, **P < 0.01, ***P < 0.001
3.8. Vasorin promotes angiogenesis and tumor growth in vivo
To study the effect of VASN on angiogenesis and tumor growth in vivo, we used the experimental glioma assay developed on the chicken CAM, as previously described.24 This assay allows the first steps of tumor angiogenesis to be observed. Ectopic‐expressing VASN U118 and U251 cells were deposited on the CAM of fertilized eggs, and the tumors were analyzed 4 days later. VASN overexpression resulted in increased tumor size (Figure 8A,B). We observed that an area of the tumor in the control was necrotic. The necrotic area was significantly reduced in VASN‐overexpressing glioma cells. We also found increased capillaries in the margin of the tumor with high VASN expression (Figure 8C). We assessed angiogenesis by staining for caveolin 1 and found it to be increased in VASN‐overexpressing glioma. This confirms our in vitro data and indicates that angiogenesis can be regulated by VASN.
Figure 8.
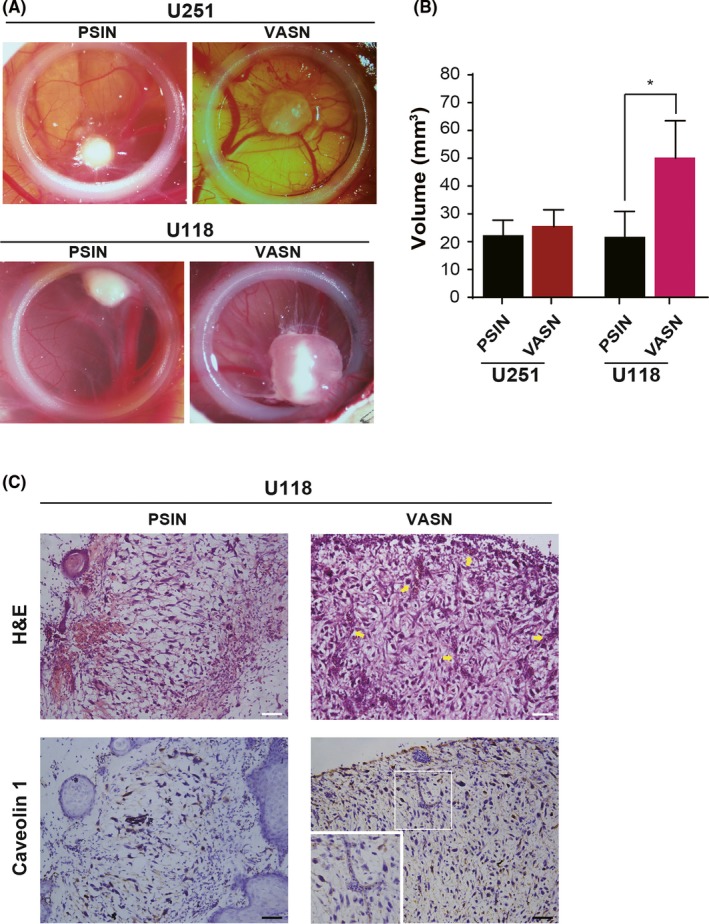
Transfection of vasorin (VASN) promoted tumor growth in vivo on chorioallantoic membranes (CAM). Glioma cells transfected with VASN were deposited on the CAM of fertilized eggs, and tumors were analyzed 4 days later. A, Tumor growth was assessed by biomicroscopy (×3 magnification). B, Tumor volume was quantified from the tumors obtained in CAM (8‐10 tumors/condition). *P < 0.05. C, H&E staining of the corresponding tumor sections is shown, and blood vessels are indicated by an arrow. Tumor sections were stained for caveolin 1 to mark endothelial cells. Numerous irregular and dilated blood vessels are visible in the tumor sections of gliomas overexpressing VASN. Scale bar, 50 μm
4. DISCUSSION
Vasorin was initially reported to play a role in blood vessel injury repair.9 Recent studies have reported that VASN is frequently upregulated in some types of human tumors, and the elevated expression of VASN is correlated with poor prognosis of tumor patients.14 However, the critical role of VASN in tumor progression and angiogenesis of glioma has not been studied. In this study, we identified that increased expression of VASN in glioma is important for the acquisition of an aggressive and poor prognostic phenotype. We also demonstrated that augmented expression of VASN promoted angiogenesis in glioma cells both in vitro and in vivo. Thus, VASN expression could be used to identify glioma patients at increased risk of tumor progression and angiogenesis.
Our results show that VASN expression significantly correlated with tumor grade in glioma and was elevated in high‐grade gliomas, which is consistent with the previous study.14 However, the role of VASN in human glioma remains poorly studied. It was reported that high levels of VASN in GBM cells might protect them from hypoxia‐induced apoptosis.25 VASN was shown to be a critical link between hypoxia and notch signaling in GSC and maintained the GSC in the hypoxic niche.14 Tumor hypoxia is consistently associated with poor survival across multiple cancer types, especially for GBM, which is characterized by areas of hypoxia.26, 27, 28 VASN was demonstrated to be a HIF‐1 target protein and induced by hypoxia; this may predict its important role in glioma progression. In the current study, a series of assays were used to investigate the role of VASN in regulating the characteristic aggressive phenotype of glioma. We established knockdown and ectopic expression of VASN glioma cells in vitro and investigated the cell growth, colony formation and invasion of these cells. Results demonstrated that VASN could substantially promote the growth and aggressiveness of glioma cells.
To further confirm our findings, we queried VASN expression in TCGA diffuse glioma data and found elevated VASN expression in high‐grade gliomas. GBM is classified into different subtypes on the basis of gene expression profiles and distinct biological characteristics, including Phillips subtypes23 and TCGA subtypes.22 Both studies identified a common subtype, named the mesenchymal subtype, according to the expression of genes associated with a mesenchymal cell phenotype. A mesenchymal cell phenotype is the hallmark of tumor aggressiveness in human malignant glioma.29 Earlier studies identified markers for the mesenchymal subtype of GBM, such as tGLI1, which was found to be expressed at higher levels in mesenchymal GBM and associated with tumor recurrence and poor clinical outcome.30, 31 We used 2 independent mesenchymal gene signatures and found both signatures to be highly enriched in GBM with high expression of VASN. Our bioinformatic analyses revealed VASN activation as a hallmark of mesenchymal GBM. We also found that VASN promoted glioma spheroid formation and was positively associated with the expression of stem cell‐related genes, such as CD133, CD90 and CD15. Thus, these results demonstrate that VASN may regulate the stem cell characteristics of glioma.
Angiogenesis has been recognized as a key event in the progression to malignancy.32, 33 It is regulated by a series of stimulatory and inhibitory angiogenic factors acting on endothelial cells. Cell communication between tumor cells and endothelial cells is crucial to angiogenesis and tumor progression.34 The rapid proliferation of GBM cells may result in necrosis in part of the tumor, and, moreover, may select cells that are able to survive in the ensuing hypoxic environment.35 Under reduced oxygen tensions, the remaining GBM cell cohort initiates the HIF‐1 pathway, endowing cells with the ability to adapt to the hypoxic environment via the induction of angiogenesis.8 VASN, a HIF‐1α target gene, has been shown to promote the migration of recipient HUVEC through HepG2 cells‐derived exosomes in hepatocarcinoma.15 In human glioma tissues, we found elevated VASN expression in GBM, and upregulation of VASN correlated significantly with increased MVD. To further study the role of VASN in angiogenesis, we incubated the conditioned medium from glioma cells ectopically expressing VASN with HUVEC. Our results showed that VASN stimulated tumor angiogenesis in vitro and in vivo. VASN was reported to have 2 forms, the intracellular‐associated form and the soluble form.13 The soluble VASN contribute to the various functions including angiogenensis.36 Our results showed that the concentration of VASN in the CM obtained from glioma cells with VASN overexpression increased significantly, based on ELISA analysis. Previous studies have demonstrated that angiogenic factors, such as VEGF, PDGF‐β and TGF‐β, are related to glioma angiogenesis.32, 37 VASN was also shown to increase the expression levels of angiogenic factors, such as ANGPT2, CCR2 and VEGF‐A, in glioma cells in our study; however, there were no significant changes in the CM obtained from glioma cells, except the decreased VEGF in the CM obtained from U251 cells with VASN knockdown by ELISA. We speculated that the angiogenesis promoted by VASN was mainly attributed to the direct action of VASN, and accompanied with the upregulated angiogenic factors, such as VEGF by VASN. The underlying mechanism of VASN regulating angiogenesis remains to be addressed in depth.
At present, the mechanism of VASN contributing to accelerate human tumor progression and angiogenesis is unclear. VASN has been reported to inhibit the TGFβ pathway; however, our results showed no significant effect of VASN on the expression of genes downstream of TGFβ signaling, including SMAD2, SMAD3, SNAI1 and SNAI2 (data not shown). Previous studies showed that expression of STAT3 correlated with mesenchymal differentiation and predicted poor clinical outcome,38 the HIF‐1α/STAT3 complex conferred selective activation of VASN,14 VASN may regulated NOTCH pathway,14 and AKT pathway was also an important angiogenic pathway in gliomas.39 We investigated these novel signaling pathways involved in glioma progression, including STAT3, HIF‐1, AKT and NOTCH pathways. Results showed that VASN promoted the expression of STAT3 and p‐STAT3, and inhibited NOTCH1 and NICD expression significantly, but had no significant influence on AKT pathway and HIF‐1α expression (data not shown). Therefore, STAT3 and NOTCH1, which were shown to have important roles in tumor progression and angiogenesis, may be the target molecules of VASN. Detailed investigation of the mechanisms could help elucidate a novel pathway in the tumor progression and angiogenesis.
In summary, our study demonstrates that VASN promotes tumor progression and angiogenesis in glioma. We found that VASN regulated the STAT3 and NOTCH pathways. Although anti–angiogenesis drugs targeting VEGF have been increasingly used in the treatment of GBM, the effects were only transient and GBM recurrence was not avoided.40 This reflects multiple mechanisms of tumor adaptation to anti–angiogenic therapies, and then we may have a choice of inhibiting VASN for glioma therapy. Our data provide new insights into the relevance of anti–angiogenic therapeutics for cancer treatment.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGMENT
We thank Dr Guangjin Pan of the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences for providing pSIN‐Puro‐FLAG vector for this project.This work was supported by the Natural Science Foundation of Guangdong Province (grant no. 2017A030313117 and 2018A030313301), the Guangzhou Science Technology and Innovation Commission (grant no. 201804010075), the Fundamental Research Fund for the Central Universities (grant no. 21616116), and the Key Laboratory of Regenerative Biology, Guangzhou Institutes of Biomedicine andHealth, Chinese Academy of Sciences (grant no. KLRB201602 and KLRB201711).
Liang W, Guo B, Ye J, et al. Vasorin stimulates malignant progression and angiogenesis in glioma. Cancer Sci. 2019;110:2558–2572. 10.1111/cas.14103
Liang and Guo contributed equally to this work.
REFERENCES
- 1. Cavenee WK. High‐grade gliomas with chromosome 1p loss. J Neurosurg. 2000;92:1080‐1081. [PubMed] [Google Scholar]
- 2. Fine HA. How to get from here to there: tracking down invasive glioma cells. J Natl Cancer Inst. 2014;106:dju120. [DOI] [PubMed] [Google Scholar]
- 3. Wong ML, Prawira A, Kaye AH, Hovens CM. Tumour angiogenesis: its mechanism and therapeutic implications in malignant gliomas. J Clin Neurosci. 2009;16:1119‐1130. [DOI] [PubMed] [Google Scholar]
- 4. Boer JC, Walenkamp AM, den Dunnen WF. Recruitment of bone marrow derived cells during anti‐angiogenic therapy in GBM: the potential of combination strategies. Crit Rev Oncol Hematol. 2014;92:38‐48. [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417‐427. [DOI] [PubMed] [Google Scholar]
- 6. Skuli N, Monferran S, Delmas C, et al. Alphavbeta3/alphavbeta5 integrins‐FAK‐RhoB: a novel pathway for hypoxia regulation in glioblastoma. Can Res. 2009;69:3308‐3316. [DOI] [PubMed] [Google Scholar]
- 7. Xie Q, Bao X, Chen ZH, et al. Role of protease‐activated receptor‐1 in glioma growth. Acta Neurochir Suppl. 2016;121:355‐360. [DOI] [PubMed] [Google Scholar]
- 8. Wang M, Zhao X, Zhu D, et al. HIF‐1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up‐regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeda Y, Imai Y, Kumagai H, et al. Vasorin, a transforming growth factor beta‐binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc Natl Acad Sci USA. 2004;101:10732‐10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Yao JH, Zhang SH, Wang L, Song HD, Xue JL. Slit‐like 2, a novel zebrafish slit homologue that might involve in zebrafish central neural and vascular morphogenesis. Biochem Biophys Res Commun. 2005;336:364‐371. [DOI] [PubMed] [Google Scholar]
- 11. Krautzberger AM, Kosiol B, Scholze M, Schrewe H. Expression of vasorin (Vasn) during embryonic development of the mouse. Gene Expr Patterns. 2012;12:167‐171. [DOI] [PubMed] [Google Scholar]
- 12. Li S, Li H, Yang X, et al. Vasorin is a potential serum biomarker and drug target of hepatocarcinoma screened by subtractive‐EMSA‐SELEX to clinic patient serum. Oncotarget. 2015;6:10045‐10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malapeira J, Esselens C, Bech‐Serra JJ, Canals F, Arribas J. ADAM17 (TACE) regulates TGFbeta signaling through the cleavage of vasorin. Oncogene. 2011;30:1912‐1922. [DOI] [PubMed] [Google Scholar]
- 14. Man J, Yu X, Huang H, et al. Hypoxic induction of vasorin regulates Notch1 turnover to maintain glioma stem‐like cells. Cell Stem Cell. 2018;22:104‐118 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang A, Dong J, Li S, et al. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 2015;11:961‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeo HL, Fan TC, Lin RJ, et al. Sialylation of vasorin by ST3Gal1 facilitates TGF‐beta1 mediated tumor angiogenesis and progression. Int J Cancer. 2018;144:1996‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang W, Li J, Suzuki K, et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015;348:1160‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiu YW, Lin TH, Huang WS, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol. 2011;255:316‐326. [DOI] [PubMed] [Google Scholar]
- 19. Bossard C, Van den Berghe L, Laurell H, et al. Antiangiogenic properties of fibstatin, an extracellular FGF‐2‐binding polypeptide. Cancer Res. 2004;64:7507‐7512. [DOI] [PubMed] [Google Scholar]
- 20. Man J, Shoemake J, Zhou W, et al. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014;9:1812‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langlois B, Saupe F, Rupp T, et al. AngioMatrix, a signature of the tumor angiogenic switch‐specific matrisome, correlates with poor prognosis for glioma and colorectal cancer patients. Oncotarget. 2014;5:10529‐10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high‐grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157‐173. [DOI] [PubMed] [Google Scholar]
- 24. Hagedorn M, Javerzat S, Gilges D, et al. Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc Natl Acad Sci USA. 2005;102:1643‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choksi S, Lin Y, Pobezinskaya Y, et al. A HIF‐1 target, ATIA, protects cells from apoptosis by modulating the mitochondrial thioredoxin, TRX2. Mol Cell. 2011;42:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keith B, Simon MC. Hypoxia‐inducible factors, stem cells, and cancer. Cell. 2007;129:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF‐1. Cancer Cell. 2004;5:405‐406. [DOI] [PubMed] [Google Scholar]
- 28. Soeda A, Park M, Lee D, et al. Hypoxia promotes expansion of the CD133‐positive glioma stem cells through activation of HIF‐1alpha. Oncogene. 2009;28:3949‐3959. [DOI] [PubMed] [Google Scholar]
- 29. Carro MS, Lim WK, Alvarez MJ, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giladi ND, Ziv‐Av A, Lee HK, et al. RTVP‐1 promotes mesenchymal transformation of glioma via a STAT‐3/IL‐6‐dependent positive feedback loop. Oncotarget. 2015;6:22680‐22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rimkus TK, Carpenter RL, Sirkisoon S, et al. Truncated glioma‐associated oncogene homolog 1 (tGLI1) mediates mesenchymal glioblastoma via transcriptional activation of CD44. Cancer Res. 2018;78:2589‐2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaudhry IH, O'Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409‐415. [DOI] [PubMed] [Google Scholar]
- 33. Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc). 2008;73:751‐762. [DOI] [PubMed] [Google Scholar]
- 34. Heindryckx F, Gerwins P. Targeting the tumor stroma in hepatocellular carcinoma. World J Hepatol. 2015;7:165‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li D, Zhang T, Yang X, et al. Identification of functional mimotopes of human vasorin ectodomain by biopanning. Int J Biol Sci. 2018;14:461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta VK, Jaskowiak NT, Beckett MA, et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8:47‐54. [DOI] [PubMed] [Google Scholar]
- 38. Liu J, Xu X, Feng X, Zhang B, Wang J. Adenovirus‐mediated delivery of bFGF small interfering RNA reduces STAT3 phosphorylation and induces the depolarization of mitochondria and apoptosis in glioma cells U251. J Exp Clin Cancer Res. 2011;30:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu HB, Yang S, Weng HY, et al. Autophagy‐induced KDR/VEGFR‐2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy. 2017;13:1528‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709‐722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


