Abstract
Cervical cancer (CC) is the second most common malignant cancer among women. Gefitinib was one of the first-generation epidermal growth factor receptor-tyrosine kinase inhibitors in clinical trials. However, the underlying mechanism of gefitinib in regulating CC progression remains unknown. In the current study, two CC cell lines, HeLa and Siha, were used to investigate the effects of gefitinib. Cell counting kit-8 assays demonstrated that treatment with gefitinib exerted strong cytotoxicity in HeLa and Siha cells. Flow cytometry was used to examine cell cycle progression and apoptosis. Treatment with gefitinib enhanced the number of cells in the G0/G1 phase and increased apoptosis in HeLa and Siha cells. Furthermore, treatment with gefitinib decreased the protein expression level of Bcl-2 and increased the protein expression level of Bax. Taken together, these results suggest that gefitinib may suppress CC cell proliferation and induce cell cycle arrest and apoptosis. The current study also demonstrated that treatment with gefitinib suppressed epithelial-mesenchymal transition (EMT) as the expression level of the epithelial marker, E-cadherin was increased, while the expression level of the mesenchymal marker, vimentin was decreased. The current study demonstrated that treatment with gefitinib decreased the protein expression levels of phosphorylated-GSK3β and β-catenin, which suggests that gefitinib may be a potential novel therapeutic strategy in CC by suppressing the Wnt/β-catenin signaling pathway and EMT to inhibit tumor metastasis in CC cells. In conclusion, gefitinib may suppress the EMT process during cell invasion and induce cell apoptosis and cell cycle arrest via inhibition of the Wnt/β-catenin signaling pathway.
Keywords: cervical cancer, gefitinib, Wnt/β-catenin signaling, epithelial-mesenchymal transition
Introduction
Cervical cancer (CC) is the second most common malignant cancer among women (1). CC is characterized by high incidence and recurrence rates, as well as high resistance to systemic therapies (2). Therefore, prognosis for patients with CC remains relatively poor. Increasing evidence has demonstrated that the human papillomavirus (HPV) is correlated with the development of a high-grade precursor lesions and invasion in CC (3). HPV can infect epithelial cells, which remain active in cell-cycle progression and no longer undergo apoptosis (4).
Studies have demonstrated that the Wnt signaling pathway serves a key role in cell differentiation, proliferation, migration and polarity (5,6). In addition, the Wnt signaling pathway serves a key role in maintaining protein stability, subcellular localization and transcriptional activity (7). In the progression of tumors, the Wnt/β-catenin signaling pathway is an evolutionarily conserved and versatile pathway (8). Aberrant activation of the Wnt/β-catenin pathway can lead to abnormal accumulation of β-catenin in the nucleus, which accelerates the epithelial-mesenchymal transition (EMT) process (9,10). It is therefore important to maintain appropriate Wnt signaling.
Gefitinib was one of the first-generation epidermal growth factor receptor-tyrosine kinase inhibitors in clinical trials, and is now widely used for the treatment of several types of cancer (11,12). An increasing number of studies have been designed to identify the efficacy and toxicity of gefitinib (13,14). However, the underlying mechanism of gefitinib in regulating CC progression remains unknown. Therefore, the aim of the current study was to investigate the specific role and underlying mechanism of gefitinib in CC using human CC cell lines.
Materials and methods
Cell culture
Human cervical cancer cell lines HeLa and Siha were obtained from the Institute of Life Sciences Cell Resource Center (Shanghai, China). HeLa and Siha cells were cultured in minimal essential medium (MEM, HyClone; GE Healthcare Life Sciences) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences), penicillin (100 U/ml)-streptomycin (100 U/ml) liquid (Thermo Fisher Scientific, Inc.) and 0.25 µg/ml amphotericin B (Ameresco, Inc.). Cells were cultured at 37°C in a humidified incubator containing 5% CO2.
Cell proliferation assay
HeLa and Siha cell proliferation was examined using the cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.), according to the manufacturer's protocol. Exponentially growing cells were seeded into 96-well plates at a density of 5×103 cells/well in a final volume of 100 µl MEM and cultured under normal conditions for 24 h at 37°C in a 5% CO2-humidified incubator. Subsequently, different concentrations (0.3125, 0.625, 1.25, 2.5, 5, 10, 20, 40 and 80 µmol/l) of gefitinib (cat. no. SML1657; Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) were added to each well, with DMSO used as the vehicle control. After 48 h, 10 µl CCK-8 reagent was added to each well and incubated for 1 h at 37°C. Cell proliferation was calculated by measuring the absorbance at a wavelength of 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). Growth inhibition was calculated as a percentage of the untreated controls. All experiments were performed in triplicate and the data were expressed as the mean value ± standard deviation of five wells per treatment. For each cell line, the half maximal inhibitory concentration (IC50) was determined using the four-parameter logistic model.
Cell cycle analysis
HeLa and Siha cells were seeded into six-well plates at the density of 106 cells/well. Following treatment with 10 µmol/l of gefitinib for 48 h, cells were harvested using trypsin without EDTA, washed three times with ice-cold PBS and fixed with 70% ethanol overnight at 4°C. Cells were subsequently stained 25 µl propidium iodide with 10 µl RNase A at 37°C for 30 min in dark using the Cell cycle and apoptosis analysis kit (cat. no. C1052; Beyotime Institute of Biotechnology, Haimen, China). Cell cycle analysis was performed using a BD FACSCalibur system and CellQuest pro software (version 2.0; BD Biosciences, Franklin Lakes, NJ, USA). All experiments were performed in triplicate.
Flow cytometry evaluation of apoptosis
Cell apoptosis was examined using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit (BD Biosciences). Following treatment with 10 µmol/l of gefitinib for 48 h, cells were harvested using trypsin without EDTA and washed three times with ice-cold PBS. Cells were subsequently suspended at 1×104 cells/ml in Annexin V-Binding buffer and incubated with 5 µl Annexin V-FITC for 15 min at 37°C in dark, followed by staining with 5 µl PI. Apoptotic cells were immediately analyzed using a BD FACSCalibur system and CellQuest pro software. All experiments were performed in triplicate.
Dual-luciferase reporter assay
HeLa and Siha cells were seeded into 24-well plates at a density of 1×105 cells/well and incubated overnight. After 24 h of culture, cells were co-transfected with 200 ng pTOP-Flash (Promega Corporation, Madison, WI, USA) or pFOP-Flash reporter plasmids (Promega Corporation) and 200 ng β-galactosidase (β-gal) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) to monitor for transfection efficiency. After transfection for 48 h, the activity was measured. The TCF-responsive TOP-Flash reporter contains three TCF binding sites, and the corresponding FOP-Flash contains three mutated TCF sites (15). Cells were treated with 10 µmol/l gefitinib for 4 h at 37°C and luciferase activity was analyzed using a Dual-Luciferase Reporter assay system (Promega Corporation), according to the manufacturer's protocol. Luciferase activity was normalized for β-gal activity. The experiment was performed in triplicate.
Western blot analysis
Total cellular protein was extracted from cells on ice for 15 min using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) supplemented with fresh proteinase inhibitor cocktail and phosphatase inhibitor (Sigma-Aldrich; Merck KGaA). Samples were centrifuged at 11,000 × g for 20 min at 4°C. Total protein was quantified using a bicinchoninic acid assay (Sigma-Aldrich; Merck KGaA) and 20 µg protein/well was separated via SDS-PAGE on a 10% gel. The fractionated proteins were transferred onto polyvinylidene difluoride membranes (EMD Millipore) and blocked for 1 h at room temperature with 5% non-fat skimmed milk. The membranes were incubated with primary antibodies, including E-cadherin (1:1,000; cat. no. ab1416), Vimentin (1:1,000; cat. no. ab8978), GSK3β (1:1,000; cat. no. ab93926), p-GSK3β (1:1,000; cat. no. ab131097), β-catenin (1:1,000; cat. no. ab32572) and GAPDH (1:5,000; cat. no. ab181602) overnight at 4°C (all from Abcam, Cambridge, UK). Following primary antibody incubation, membranes were incubated with horseradish peroxidase-conjugated secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000; cat. no. ZB-2306; OriGne Technologies, Inc.), for 1 h at room temperature. Protein bands were visualized using a Western Lightning® Chemiluminescence Reagent Plus according to the manufacturer's protocol (cat. no. NEL105001EA; PerkinElmer, Inc., Waltham, MA, USA). Protein expression was quantified using ImageJ (version 1.8.0; National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
HeLa and Siha cells were cultured in a six-well plate with glass coverslips and following treatment with 10 µM gefitinib at 37°C for 48 h, cells were fixed with 4% paraformaldehyde for 30 min at room temperature. Cells were washed three times with PBS for 5 min and blocked for 2 h at room temperature with 8% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA). Subsequently, cells were incubated with primary antibodies against E-cadherin (1:50; cat. no. ab1416) and vimentin (1:50; cat. no. ab92547; both Abcam) in a humidified chamber overnight at 4°C. Cells were washed three times with PBS. Following primary incubation, cells were incubated with tetramethylrhodamine-conjugated anti-rabbit IgG (1:500; cat. no. ZDR5209; OriGene Technologies, Inc.) at room temperature for 30 min. Cell nuclei were counterstained with DAPI (1:1,000; cat. no. C0060; Beijing Solarbio Science & Technology Co., Ltd.) for 20 min at room temperature. Cells were subsequently washed three times with PBS in the dark and the coverslips were mounted with mounting medium at room temperature for ~1 h in the dark. Fluorescence intensity was observed under a fluorescence microscope (magnification, ×40; XDS-500D; Shanghai Caikon Optical Instrument Co., Ltd.).
Statistical analysis
Data were presented as the mean ± standard error of the mean of at least three experiments. All statistical analyses were performed using GraphPad Prism software (version 5.0; GraphPad Software, Inc.). All experimental data were analyzed using the unpaired Student's t-test or one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Gefitinib reduces proliferation and induces cell cycle arrest in CC cells
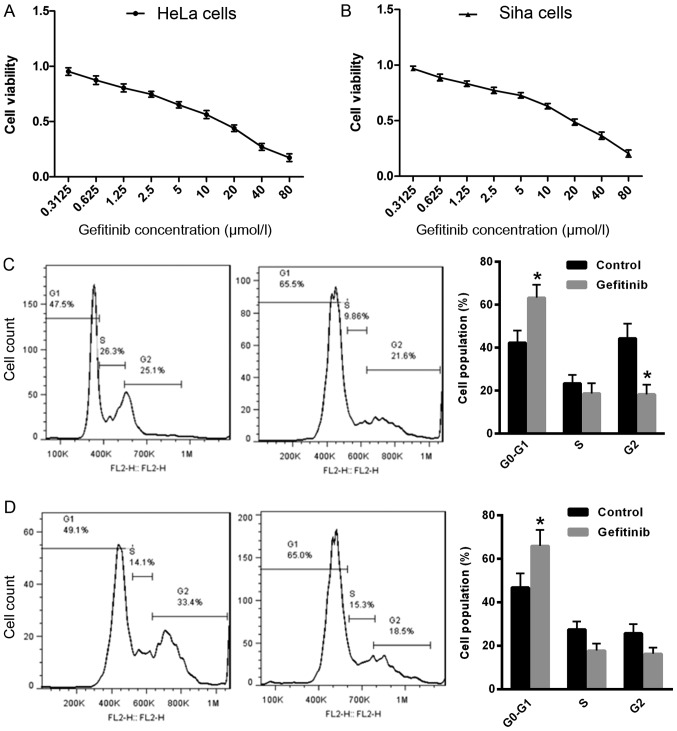
To determine the effect of gefitinib on CC cell growth, cell proliferation was examined in HeLa and Siha cells following treatment with gefitinib. Treatment with gefitinib exhibited strong cytotoxicity in HeLa cells [IC50, 16.19±0.26 µmol/l; 95% confidence interval (CI): 1.077–1.341; Fig. 1A] and Siha cells (IC50, 11.87±0.21 µmol/l, 95% CI: 1.003–1.146; Fig. 1B) compared with the control. Furthermore, the effect of gefitinib on cell cycle distribution was examined in HeLa and Siha cells. The number of cells in the G0/G1 phase was significantly increased in Hela and Siha cells following treatment with gefitinib compared with the control (Fig. 1C and D), which suggests that gefitinib may induce CC cell cycle arrest in the G0/G1 phase.
Figure 1.
Gefitinib reduces cell proliferation and induces cell cycle arrest in HeLa and Siha cells. Cell proliferation was examined by CCK-8 assay in (A) HeLa and (B) Siha cells following treatment with gefitinib. Cell cycle analysis was determined by flow cytometry in (C) HeLa and (D) Siha cells following treatment with gefitinib. *P<0.05 vs. control. CC, cervical cancer; CCK-8, cell counting kit-8.
Gefitinib induces apoptosis in CC cells
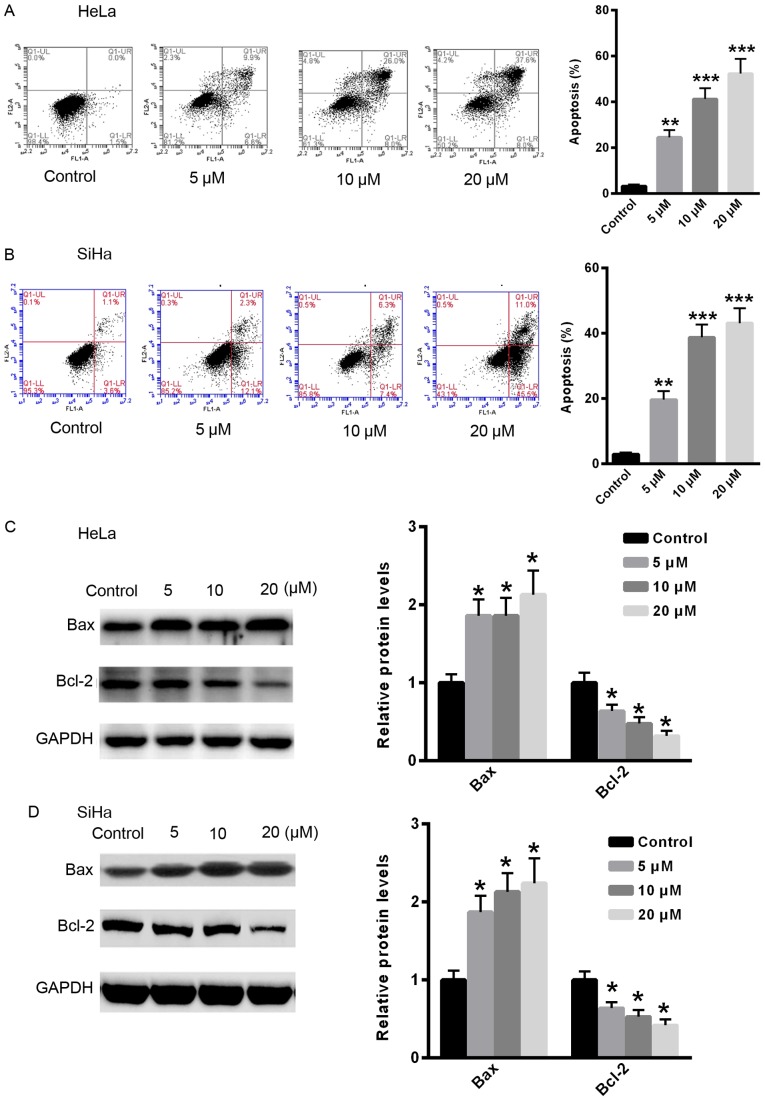
To examine the effect of gefitinib on CC cell apoptosis, apoptosis was examined in HeLa and Siha cells following treatment with gefitinib. Treatment with gefitinib significantly induced apoptosis in CC cells in a dose-dependent manner compared with the control (Fig. 2A and B). Furthermore, the relative protein expression levels of apoptosis-related proteins, Bax and Bcl-2, were examined. Following treatment with gefitinib, the relative protein expression level of Bcl-2 was significantly reduced, whereas the protein expression level of Bax was significantly increased in HeLa and Siha cells compared with the control (Fig. 2C and D).
Figure 2.
Gefitinib induces apoptosis in HeLa and Siha cells. Cell apoptosis was examined by flow cytometry in (A) HeLa and (B) Siha cells following treatment with gefitinib. The relative protein expression levels of Bcl-2 and Bax were determined by western blot analysis in (C) HeLa and (D) Siha cells following treatment with gefitinib. *P<0.05, **P<0.01 and ***P<0.001 vs. control. Con, control.
Gefitinib suppresses EMT in CC cells
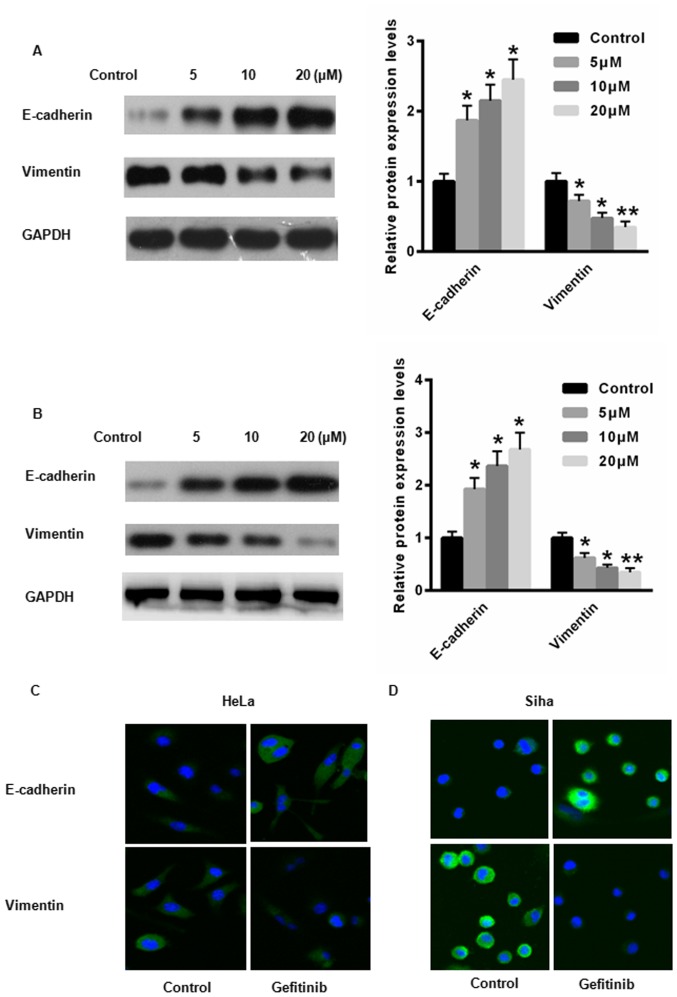
EMT is a key regulator of CC progression (16). Therefore, the expression levels of E-cadherin, an epithelial cell marker, and vimentin, a mesenchymal cell marker were examined in HeLa and Siha cells following treatment with gefitinib. Following treatment with gefitinib, the protein expression level of E-cadherin was significantly increased, whereas the protein expression level of vimentin was significantly reduced in HeLa and Siha cells compared with the control (Fig. 3A and B). Immunofluorescence demonstrated increased E-cadherin expression and reduced vimentin expression in HeLa and Siha cells following treatment with gefitinib compared with the control (Fig. 3C and D. These results suggest that gefitinib may suppress the EMT process in CC cells.
Figure 3.
Gefitinib suppresses EMT in HeLa and Siha cells. The relative protein expression levels of E-cadherin and vimentin were determined by western blot analysis in (A) HeLa and (B) Siha cells following treatment with gefitinib. E-cadherin and vimentin immunostaining in (C) HeLa and (D) Siha cells following treatment with 20 µM gefitinib (magnification, ×40). *P<0.05 and **P<0.01 vs. control. EMT, epithelial-mesenchymal transition.
Gefitinib suppresses EMT via the Wnt/β-catenin signaling pathway in CC cells
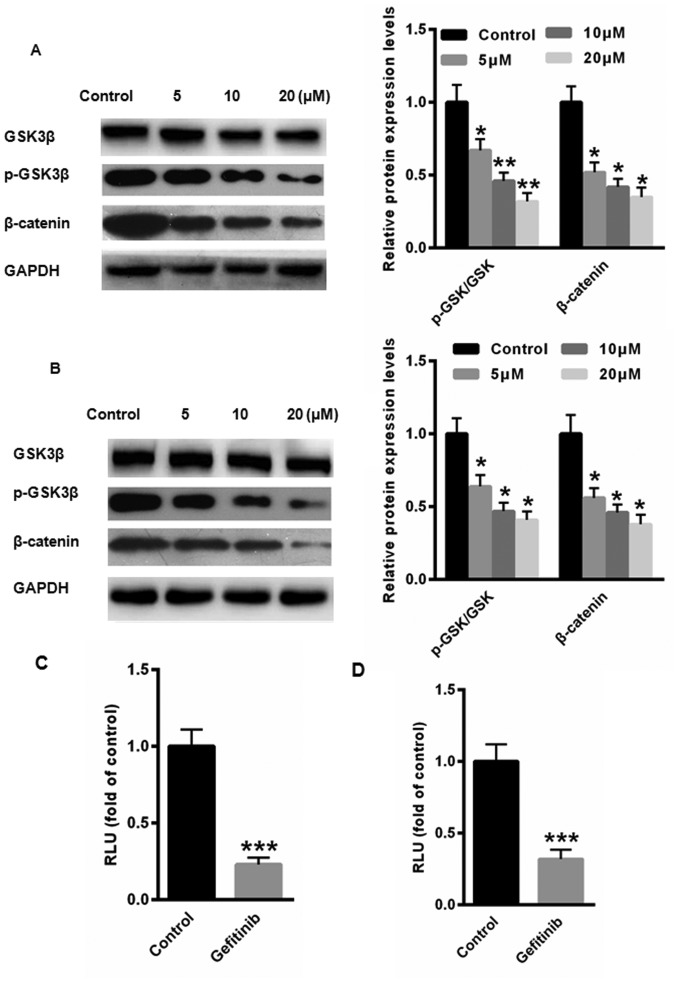
The Wnt/β-catenin signaling pathway is a major contributor to CC tumorigenesis (17). GSK3β is a known negative regulator of β-catenin. Therefore, the phosphorylation of GSK3β at Ser9, an indicator of the activation state of GSK3β, was examined by western blot analysis in HeLa and Siha cells following treatment with gefitinib. The protein expression levels of p-GSK3β and β-catenin and were significantly reduced in HeLa and Siha cells following treatment with gefitinib compared with the control (Fig. 4A and B). Furthermore, luciferase reporter assays demonstrated that activation of the Wnt/β-catenin pathway was significantly suppressed following treatment with gefitinib in HeLa and Siha cells compared with the control (Fig. 4C and D). Taken together, these results suggest that gefitinib may inactivate the Wnt/β-catenin signaling pathway to inhibit EMT in CC cells.
Figure 4.
Gefitinib suppresses EMT via the Wnt/β-catenin signaling pathway in HeLa and Siha cells. The relative protein expression levels of GSK3β, p-GSK3β and β-catenin were determined by western blot analysis in (A) HeLa and (B) Siha cells following treatment with gefitinib. The activation of the Wnt/β-catenin pathway was determined by the luciferase reporter assay in (C) HeLa and (D) Siha cells following treatment with gefitinib. *P<0.05, **P<0.01 and ***P<0.001 vs. control. EMT, epithelial-mesenchymal transition; p-, phosphorylated.
Discussion
In recent years, significant advances have been made in the diagnosis and treatment of CC, however, the overall 5-year survival rate remains poor (18). Currently, the most common treatment strategy for CC includes surgery with platinum-based chemotherapy (19). Unfortunately, patients with advanced CC relapse after primary treatment, and the majority of patients succumb to recurrence and metastasis (20). It is therefore necessary to identify novel therapeutic strategies for CC.
The in vitro and in vivo antitumor activity of gefitinib has been reported in several types of human cancer, including head and neck, colorectal, breast and lung cancer (21–23). However, the effect of gefitinib in CC remains unknown. In the current study, two CC cell lines, HeLa and Siha, were used to investigate the effects of gefitinib. CCK-8 assays demonstrated that gefitinib exerted strong cytotoxicity in HeLa and Siha cells. Flow cytometry was performed to examine cell cycle progression and apoptosis in CC following treatment with gefitinib. The current study demonstrated that treatment with gefitinib enhanced the number of cells in the G0/G1 phase and increased apoptosis in HeLa and Siha cells. Furthermore, treatment with gefitinib reduced the protein expression level of Bcl-2, and enhanced the protein expression level of Bax. Taken together, these results suggest that gefitinib may suppress CC cell proliferation and induce cell cycle arrest and apoptosis.
To further investigate the underlying mechanism of gefitinib in regulating CC progression, the EMT process was examined in CC cells following treatment with gefitinib. In the progression of CC, EMT is a key regulator that promotes cancer cell proliferation and invasion (4). The current study demonstrated that treatment with gefitinib suppressed the EMT process by increasing the expression level of the epithelial marker, E-cadherin, and decreasing the expression level of the mesenchymal marker, vimentin. These results suggest that gefitinib may suppress the EMT process in CC.
The canonical Wnt/β-catenin signaling pathway serves an important role in EMT (25,26). Abnormal activation of Wnt/β-catenin signaling is reported to increase cancer cell proliferation, survival, differentiation and EMT (27,28). The current study examined the potential association between gefitinib and the Wnt/β-catenin signaling pathway in CC cells. The current study demonstrated that treatment with gefitinib decreased the protein expression levels of p-GSK3β and β-catenin, which suggests that gefitinib may be a potential novel therapeutic strategy in CC by suppressing the Wnt/β-catenin signaling pathway and EMT to inhibit tumor metastasis in CC cells.
In conclusion, the current study demonstrated that gefitinib may suppress EMT during cell invasion and induce apoptosis and cell cycle arrest by inhibiting the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from Puyang Oil Field General Hospital (grant no. 20160783).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
JZ performed the experiments and revised the manuscript for important intellectual content. JY performed the cell proliferation experiments and was involved in drafting the manuscript. MY and LT designed the experiments, analyzed the data and gave final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang R, Lu H, Lyu YY, Yang XM, Zhu LY, Yang GD, Jiang PC, Re Y, Song WW, Wang JH, et al. E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep. 2017;7:44744. doi: 10.1038/srep44744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrami A, Hasanzadeh M, ShahidSales S, Yousefi Z, Kadkhodayan S, Farazestanian M, Joudi Mashhad M, Gharib M, Mahdi Hassanian S, Avan A. Clinical significance and prognosis value of Wnt signaling pathway in cervical cancer. J Cell Biochem. 2017;118:3028–3033. doi: 10.1002/jcb.25992. [DOI] [PubMed] [Google Scholar]
- 3.Chung MT, Lai HC, Sytwu HK, Yan MD, Shih YL, Chang CC, Yu MH, Liu HS, Chu DW, Lin YW. SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol Oncol. 2009;112:646–653. doi: 10.1016/j.ygyno.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Cui N, Yang WT, Zheng PS. Slug inhibits the proliferation and tumor formation of human cervical cancer cells by up- regulating the p21/p27 proteins and down-regulating the activity of the Wnt/β-catenin signaling pathway via the trans-suppression Akt1/p-Akt1 expression. Oncotarget. 2016;7:26152–26167. doi: 10.18632/oncotarget.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hua F, Liu S, Zhu L, Ma N, Jiang S, Yang J. Highly expressed long non-coding RNA NNT-AS1 promotes cell proliferation and invasion through Wnt/beta-catenin signaling pathway in cervical cancer. Biomed Pharmacother. 2017;92:1128–1134. doi: 10.1016/j.biopha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Kloth JN, Fleuren GJ, Oosting J, de Menezes RX, Eilers PH, Kenter GG, Gorter A. Substantial changes in gene expression of Wnt, MAPK and TNFalpha pathways induced by TGF-beta1 in cervical cancer cell lines. Carcinogenesis. 2005;26:1493–1502. doi: 10.1093/carcin/bgi110. [DOI] [PubMed] [Google Scholar]
- 7.Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG, Cheung AN, Ngan HY. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One. 2013;8:e53597. doi: 10.1371/journal.pone.0053597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan K, Zhao Y, Fan Y, Ma B, Yang S, Liu Q, Linghu H, Wang H. Sulfiredoxin may promote cervical cancer metastasis via Wnt/β-catenin signaling pathway. Int J Mol Sci. 2017;18:E917. doi: 10.3390/ijms18050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Yoon YS, Chung JH. Epigenetic silencing of the WNT antagonist DICKKOPF-1 in cervical cancer cell lines. Gynecol Oncol. 2008;109:270–274. doi: 10.1016/j.ygyno.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Wang T, Tang S. SOX14 promotes proliferation and invasion of cervical cancer cells through Wnt/β-catenin pathway. Int J Clin Exp Pathol. 2015;8:1698–1704. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu CR, Zhong WZ, Zhou Q, Zhang XC, Yang JJ, Wu YL. Heterogeneity of the resistance to gefitinib treatment in a non-small cell lung cancer patient with active epidermal growth factor receptor mutation. Thorac Cancer. 2017;8:51–53. doi: 10.1111/1759-7714.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang RF, Yu B, Zhang RQ, Wang XH, Li C, Wang P, Zhang Y, Han B, Gao XX, Zhang L, Jiang ZM. Bevacizumab and gefitinib enhanced whole-brain radiation therapy for brain metastases due to non-small-cell lung cancer. Braz J Med Biol Res. 2017;51:e6073. doi: 10.1590/1414-431x20176073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XB, Chai XS, Wu WY, Long SQ, Deng H, Pan ZQ, He WF, Zhou YS, Liao GY, Xiao SJ. Gefitinib plus Fuzheng Kang'ai formula () in patients with advanced non-small cell lung cancer with epidermal growth factor receptor mutation: A randomized controlled trial. Chin J Integr Med. 2018;24:734–740. doi: 10.1007/s11655-017-2819-8. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y, Mao C, Tang J. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: A meta-analysis. Int J Cancer. 2017;140:2805–2819. doi: 10.1002/ijc.30691. [DOI] [PubMed] [Google Scholar]
- 15.Xu S, Gotlieb AI. Wnt3a/β-catenin increases proliferation in heart valve interstitial cells. Cardiovasc Pathol. 2013;22:156–166. doi: 10.1016/j.carpath.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2015;356:321–331. doi: 10.1016/j.canlet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Fan Y, Li D, Liu Y, Chen X. Glycoprotein nonmetastatic melanoma protein B accelerates tumorigenesis of cervical cancer in vitro by regulating the Wnt/β-catenin pathway. Braz J Med Biol Res. 2018;52:e7567. doi: 10.1590/1414-431x20187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Yang F, Wang M, Cao W, Yang Z. miR-378 functions as an onco-miRNA by targeting the ST7L/Wnt/β-catenin pathway in cervical cancer. Int J Mol Med. 2017;40:1047–1056. doi: 10.3892/ijmm.2017.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Ma S, Liu H, Han H, Wang S. HCFU inhibits cervical cancer cells growth and metastasis by inactivating Wnt/β-catenin pathway. J Cell Biochem. 2017 Dec 12; doi: 10.1002/jcb.26570. (Epub ahead of print) doi: 10.1002/jcb.26570. [DOI] [PubMed] [Google Scholar]
- 20.Liu XF, Li XY, Zheng PS, Yang WT. DAX1 promotes cervical cancer cell growth and tumorigenicity through activation of Wnt/β-catenin pathway via GSK3beta. Cell Death Dis. 2018;9:339. doi: 10.1038/s41419-018-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann S, Neckel N, Seher A, Mutzbauer G, Brands RC, Linz C, Kübler AC, Müller-Richter UD. Erlotinib and gefitinib responsiveness in head and neck cancer cell lines-a comparing analysis with cetuximab. Clin Oral Investig. 2016;20:759–769. doi: 10.1007/s00784-015-1566-5. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Zhang D, Chen X, He L, Li T, Xu X, Li M. Nuclear PKM2 contributes to gefitinib resistance via upregulation of STAT3 activation in colorectal cancer. Sci Rep. 2015;5:16082. doi: 10.1038/srep16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng D, Sun D, Zhang L, Zhang W. The therapy of gefitinib towards breast cancer partially through reversing breast cancer biomarker arginine. Afr Health Sci. 2015;15:594–597. doi: 10.4314/ahs.v15i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Xu R, Li XX, Wang YY, Liang WQ, Zeng JD, Zhang SS, Xu XY, Yang Y, Zhang MY, et al. p53R2 overexpression in cervical cancer promotes AKT signaling and EMT, and is correlated with tumor progression, metastasis and poor prognosis. Cell Cycle. 2017;16:1673–1682. doi: 10.1080/15384101.2017.1320629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernando G, Paul F, Laura J, Alejandra AM, Gabriela M, Alberto PL. Is the Wnt/β catenin signalling pathway activated in Seminoma? An immunohistochemical study. J Cancer Res Ther. 2016;12:1075–1079. doi: 10.4103/0973-1482.147392. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Du W, Yang D. Inhibition of green tea polyphenol EGCG((−)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical wnt/β-catenin signalling pathway. Int J Food Sci Nutr. 2016;67:818–827. doi: 10.1080/09637486.2016.1198892. [DOI] [PubMed] [Google Scholar]
- 27.Mittag S, Valenta T, Weiske J, Bloch L, Klingel S, Gradl D, Wetzel F, Chen Y, Petersen I, Basler K, Huber O. A novel role for the tumour suppressor Nitrilase1 modulating the Wnt/β-catenin signalling pathway. Cell Discov. 2016;2:15039. doi: 10.1038/celldisc.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zuylen WJ, Rawlinson WD, Ford CE. The Wnt pathway: A key network in cell signalling dysregulated by viruses. Rev Med Virol. 2016;26:340–355. doi: 10.1002/rmv.1892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.