Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of cancer by providing new options in addition to existing therapies. However, peptide vaccination therapies still represent an attractive approach, because of the antigen specificity. We identified survivin 2B peptide (SVN‐2B), a 9‐mer antigenic peptide encoded by survivin, and an SVN‐2B peptide vaccine‐based phase II randomized clinical trial targeting unresectable and refractory pancreatic carcinoma was undertaken. The SVN‐2B peptide vaccine did not have any statistically significant clinical benefits in that study. Therefore, we undertook an autopsy study to analyze the immune status of the pancreatic cancer lesions at the histological level. Autopsies were carried out in 13 patients who had died of pancreatic cancer, including 7 who had received SVN‐2B peptide vaccination and 6 who had not, as negative controls. The expression of immune‐related molecules was analyzed by immunohistochemical staining. Cytotoxic T lymphocytes were analyzed by tetramer staining and enzyme‐linked immunospot assay. Histological analysis revealed dense infiltration of CD8+ T cells in some lesions in patients who had received the SVN‐2B peptide vaccine. A high rate of programmed cell death ligand 1 expression in cancer cells was observed in these cases, indicating that CTLs were induced by SVN‐2B peptide vaccination and had infiltrated the lesions. The lack of a significant antitumor effect was most likely attributable to the expression of immune checkpoint molecules. These findings suggest that the combination of a tumor‐specific peptide vaccine and an ICI might be a promising approach to the treatment of pancreatic carcinoma in the future.
Keywords: autopsy, immunohistochemistry, pancreatic carcinoma, peptide vaccine, survivin
1. INTRODUCTION
After extensive research for more than a century, immunotherapy is now the fourth standard treatment after surgery, chemotherapy, and radiotherapy for various types of malignancy.1 Despite the great success of immune checkpoint inhibitors (ICIs) as monotherapy, more than half of patients are still not responsive to these agents, even those with tumors that are approved for these treatments, including melanoma, renal cell carcinoma, and non‐small‐cell lung carcinoma. Substantial numbers of patients are not eligible for this therapy because the type of malignancy is not sensitive to ICIs. Furthermore, ICIs are not specific for tumor cells, so serious immune‐related adverse events often result in discontinuation of treatment. Five years after the approval of ICIs, we are now at a crossroads in terms of further improvement in immunotherapy for cancer.
We have sought and identified many cancer‐specific and sarcoma‐specific antigens that are promising candidates for tumor‐specific immunotherapy.2 Immunization with HLA‐A24‐restricted survivin 2B 80‐88 (SVN‐2B) peptide, which is derived from a splice variant of survivin, a member of the inhibitor of apoptosis protein family, was observed to induce a strong immune reaction in in vitro studies.3, 4 Furthermore, encouraging results were obtained in phase I clinical studies of the use of this peptide vaccine in pancreatic, colorectal, and urothelial carcinoma.5, 6, 7 Importantly, no severe adverse effects occurred after administration of the SVN‐2B peptide vaccine. There is also a noteworthy report of a patient with advanced pancreatic carcinoma who received the SVN‐2B peptide vaccine as sole treatment more than 10 years ago and remains alive. Recently, we undertook an SVN‐2B vaccine‐based phase II randomized clinical trial targeting pancreatic carcinoma that was unresectable and refractory to existing therapies. The SVN‐2B peptide vaccine induces proliferation and activation of SVN‐2B peptide‐specific CTLs. However, the vaccine did not improve progression‐free survival, as reported by Shima et al. in this issue.8 Development of the next generation of tumor‐specific immunotherapy requires an understanding of the local effect in tumor tissue after administration of this vaccine.
In this report, we present the immunohistochemical analysis of tissue specimens obtained at autopsy from 13 patients who died of pancreatic carcinoma, including 7 who received the vaccine. Using formalin‐fixed paraffin‐embedded specimens, we evaluated expression of CD8, programmed cell death‐1 (PD‐1), and Forkhead box P3 (FOXP3) in infiltrated lymphocytes and PD ligand 1 (PD‐L1), survivin, and HLA class I expression in tumor cells. Interestingly, some lesions showed dense infiltration by CD8+ or PD‐1+ cells, which was significantly correlated with the proportion of PD‐L1+ tumor cells. These lesions were found in only inoculated cases, indicating that tumor‐specific peptide vaccination could be an upcoming partner of ICI treatment for malignancies.
2. MATERIALS AND METHODS
2.1. Patients and specimens
A total of 83 patients with unresectable and refractory pancreatic carcinoma were enrolled in the phase II clinical trial (clinical trial registration number: UMIN 000012146). All patients were found to have the HLA‐A24 allele and expression of HLA class I and survivin on examination of biopsy specimens. The protocol for this trial is described in detail in the parallel publication by Shima et al.8 Autopsies were carried out in 7 of these patients; the vaccination schedule for each of these patients is shown in Figure 1A. Five patients who had not participated in the clinical trial and 1 who had been treated with nivolumab were included as the control group. The clinicopathological factors analyzed in this study are summarized in Table 1. Written informed consent was obtained from each patient and the study was conducted according to the guidelines of the Declaration of Helsinki. There was no significant difference in the male to female patient ratio or in the mean age (with vaccination, 59.9 ± 17.0 years; without vaccination, 68.3 ± 5.6 years, P = .27).
Figure 1.
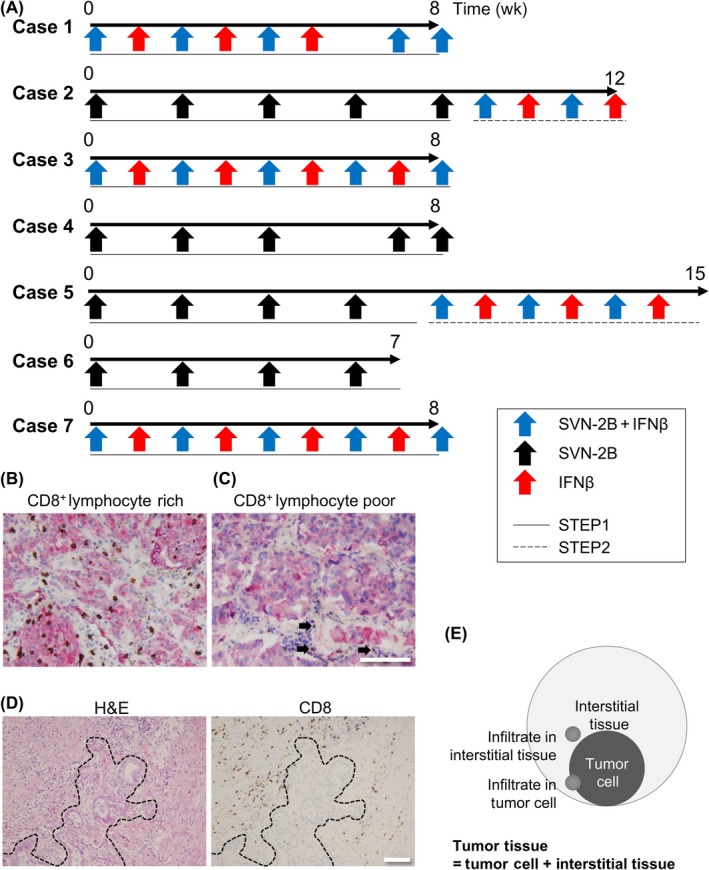
Vaccination schedule for each patient included in the clinical trial who was subsequently investigated at autopsy and the distinctive infiltration pattern of CD8+ cells in pancreatic carcinoma. A, In Step 1, the patients were treated until diagnosed as having progressive disease by RECIST or clinically apparent progressive disease. In Step 2, all patients who had given their consent were treated with survivin 2B peptide (SVN‐2B) and β‐interferon (IFNβ) until diagnosed as immune‐related response criteria (irRC) immune‐related progression of disease (irPD) or for a maximum 7 mo. B,C, Double staining pictures showing pan‐cytokeratin (red) expressed in tumor cells and CD8 (brown) in lymphocytes. CD8+ lymphocyte‐rich (B) and CD8+ lymphocyte‐poor (C) tumor tissue specimens are shown. There is a small number of infiltrated CD8+ lymphocytes in the CD8+ lymphocyte‐poor lesion (C) identified as the internal positive control (arrows). Bar = 50 μm. Original magnification, ×200. D, Heterogenous distribution of CD8+ lymphocytes in tumor tissue. Dotted line indicates the boundary between the tumor and interstitial tissue. Bar = 100 μm. Original magnification, ×100. E, Schematic diagram of lymphocyte infiltration into tumor tissue composed of tumor cells and interstitial tissue
Table 1.
Clinicopathological factors in this series of autopsy cases
| Patient | Age, years | Sex | MTS (%) | PFS | OS | Pretreatment |
|---|---|---|---|---|---|---|
| 1 | 78 | M | −60.2 | 56 | 84 | Surgery, GEM, S‐1 |
| 2 | 29 | F | −24.4 | 60 | 115 | Surgery, GEM + S‐1, FOLFIRINOX |
| 3 | 48 | M | −37.8 | 58 | 80 | GEM |
| 4 | 63 | M | −112.3 | 55 | 72 | Surgery, GEM, S‐1 |
| 5 | 60 | F | −35.8 | 55 | 145 | S‐1 |
| 6 | 65 | F | NA | 49 | 51 | GEM, GEM + S‐1 |
| 7 | 65 | F | −130.1 | 56 | 76 | Surgery, PBT, S‐1, GEM, FOLFIRINOX, GEM + nabPTX |
| 8 | 61 | F | — | — | — | Surgery, GEM, HIT, GEM + nabPTX, nivolumab |
| 9 | 65 | F | — | — | — | GEM, S‐1 |
| 10 | 71 | M | — | — | — | GEM + S‐1, HIT, S‐1, FOLFIRINOX |
| 11 | 65 | M | — | — | — | FOLFIRINOX, GEM + nabPTX, S‐1 |
| 12 | 76 | F | — | — | — | GEM, GEM + nabPTX |
| 13 | 72 | F | — | — | — | GEM, S‐1, FOLFIRINOX |
Abbreviations: F, female; FOLFIRINOX, leucovorin + fluorouracil + irinotecan + oxaliplatin; GEM, gemcitabine; HIT, heavy ion radiotherapy; M, male; MTS, maximum tumor shrinkage; nabPTX, nanoparticle albumin‐bound paclitaxel; OS, overall survival; PBT, proton beam therapy; PFS, progression‐free survival; S‐1, tegafur/gimeracil/oteracil potassium.
2.2. Immunohistochemical staining
The tumor tissues were fixed in 10% buffered formalin and embedded in paraffin. For immunohistochemistry, mouse anti‐CD8 mAb (clone C8/144B; Dako, Santa Barbara, CA, USA), rabbit anti‐PD‐L1 mAb (clone E1L3N; Cell Signaling Technology, Danvers, MA, USA), and mouse anti‐PD‐1 mAb (clone NAT105; Abcam, Cambridge, UK) were used according to the manufacturers’ instructions. Mouse anti‐HLA class I molecule (clone EMR8‐5; now commercially available from Hokudo, Sapporo, Japan) was generated previously in our laboratory and used at 0.5 μg/mL.9 Rabbit polyclonal Ab against survivin (2 μg/mL; Novus Biologicals, Littleton, CO, USA) was used. We compared 2 representative primary tumors and/or metastases to other organs with or without SVN‐2B peptide inoculation. For other analyses, all sampled tumor specimens were examined. Cell count was undertaken manually when dense infiltration of each type of marker‐positive cell (except for PD‐L1) was found. Three different ×400 fields were evaluated, and mean values are shown. For PD‐L1 expression, whole tumor cells in the specimen were investigated for PD‐L1 expression. In this report, infiltration of tumor cells includes attachment of lymphocytes to tumor cells and lymphocytes surrounded by tumor cells. We did not evaluate the normal tissue included in each specimen, and included lymph nodes and Glisson's sheath in the liver as interstitial tissue.
2.3. Cytotoxic T lymphocyte analysis
Peptide‐specific CTLs were detected using a tetramer binding assay. We used phycoerythrin‐labeled HLA‐A*2402/SVN‐2B peptide (80‐88; AYACNTSTL)‐specific tetramers purchased from Medical & Biological Laboratories (Nagoya, Japan). The number of γ‐interferon (IFNγ)‐producing CTLs was assessed using the enzyme‐linked immunospot assay. The anti‐IFNγ capture Ab was obtained from BD Biosciences (San Jose, CA USA).
2.4. Statistical analysis
Data are shown as the mean ± SD. Student's t test and Pearson's correlation coefficient were used for all analyses as appropriate. All statistical analyses were carried out using GraphPad Prism software (version 6.0 for Windows; GraphPad Software, La Jolla, CA, USA). P values <.05 were considered statistically significant.
3. RESULTS
3.1. Summary of clinical trial and autopsy study
A detailed analysis and discussion of the clinical trial are described in the parallel publication by Shima et al.8 Briefly, treatment with SVN‐2B peptide with or without IFNβ inoculation did not improve progression‐free survival, although some immunological effect was noted, namely, increased numbers of IFNγ‐producing and/or survivin 2B 80‐88‐specific CTLs in peripheral blood. All patients in whom an autopsy was carried out, including the controls, had died as a result of their cancer, and the primary or metastasized lesion was sampled postmortem. Histopathologically, we found the tumors varied from well to poorly differentiated adenocarcinoma and even included anaplastic carcinoma. Necrosis was occasionally observed, especially in the center of the lesions. However, the majority of tumor cells were viable in all cases.
3.2. Survivin and HLA class I expression in tumors
Before enrolling patients in the clinical trial, we confirmed that their tumor cells were positive for survivin and HLA class I molecules (Figure 2A, B). In the autopsy specimens, the tumors from all patients who had participated in the clinical trial expressed HLA class I molecules. However, only 2 (10.5%) of 19 lesions from these patients and 2 (16.7%) of 12 lesions from the control patients expressed survivin.
Figure 2.
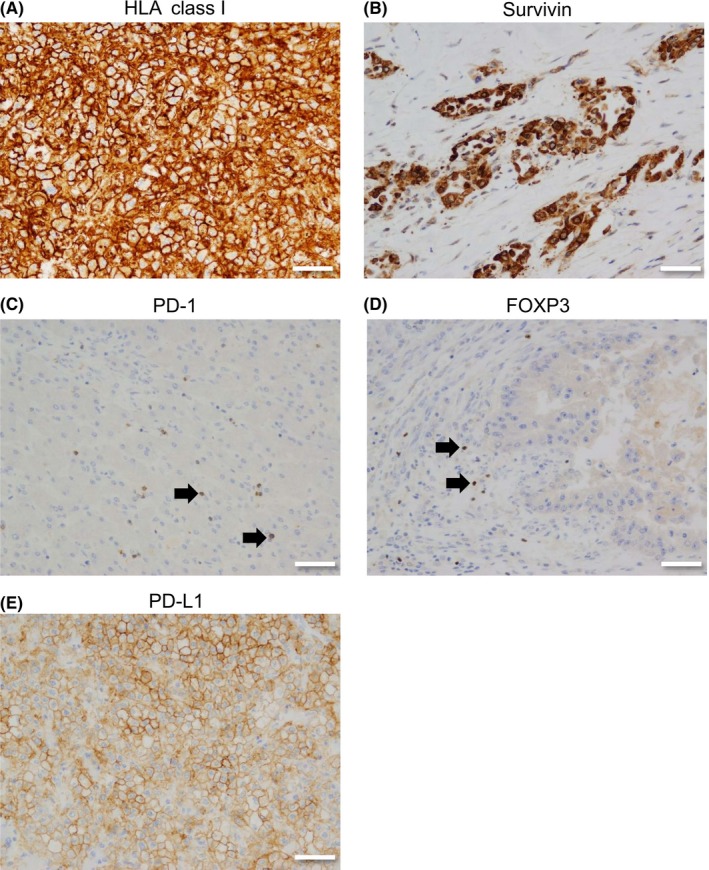
Immunohistochemistry for pancreatic carcinoma. A, HLA class I molecules in the surface of tumor cell. B, Survivin in the nucleus and/or cytoplasm of tumor cell. C, Programmed cell death‐1 (PD‐1) in the cell boundary of lymphocytes (arrows). D, Forkhead box P3 (FOXP3) in the nucleus of lymphocytes (arrows). E, PD ligand 1 (PD‐L1) in the surface of tumor cell. Bar = 50 μm. Original magnification, ×200
3.3. Infiltration of lymphocytes positive for CD8, PD‐1, or FOXP3
Although many clinical trials using peptide vaccination have been reported, there are few reports describing the tumor microenvironment in autopsy cases, and the immunological effects of peptide vaccination at the histological level are still unclear. Therefore, we investigated the tumor microenvironment of tumor specimens obtained at autopsy. We investigated the infiltration of CTLs as a surrogate marker of the antitumor reaction in the tumor tissue by counting the CD8+ cells. Interestingly, high numbers of these cells infiltrated some of the tumors (Figure 1B). However, there were also tumors that showed almost no CD8+ lymphocyte infiltration (Figure 1C). Importantly, we often found a substantial number of CD8+ cells in the interstitial tissue with infiltration of only a few of these cells in the tumor specimen (Figure 1D), indicating that the distribution of CD8+ cells was heterogenous. Therefore, we counted the CTLs that had infiltrated the tumor and interstitial tissue separately to distinguish these 2 patterns (Figure 1E) and found a correlation between CD8+ cells in tumor tissue and those in interstitial tissue (r = 0.45, P = .014; Figure S1A).
In the majority of the lesions, infiltration of CD8+ cells was greater in interstitial tissue than in the tumor (data not shown). A significantly higher number of CD8+ cells was observed to have infiltrated the tumors in patients who had received the SVN‐2B vaccine than the tumors in controls (P = .020; Figure 3A). No statistically significant difference in the extent of CD8+ lymphocyte infiltration into interstitial tissue was found between the 2 groups (Figure 3B). Of note, several lesions harbored abundant CD8 in the tumor cells, whereas others did not, even among the vaccinated population (Figure 3C). No statistically significant difference was found between patients who had received additional IFNβ inoculation and those who had not (data not shown), probably because of the small sample size. Interestingly, we found significant correlations between the systemic immune status, number of peptide‐responsive IFNγ‐producing or peptide‐specific CD8+ T cells, and infiltration of CD8+ cells into the tumor (r = 0.99, P < .001 and r = 0.95, P = .004, respectively; Figure S2A, B).
Figure 3.
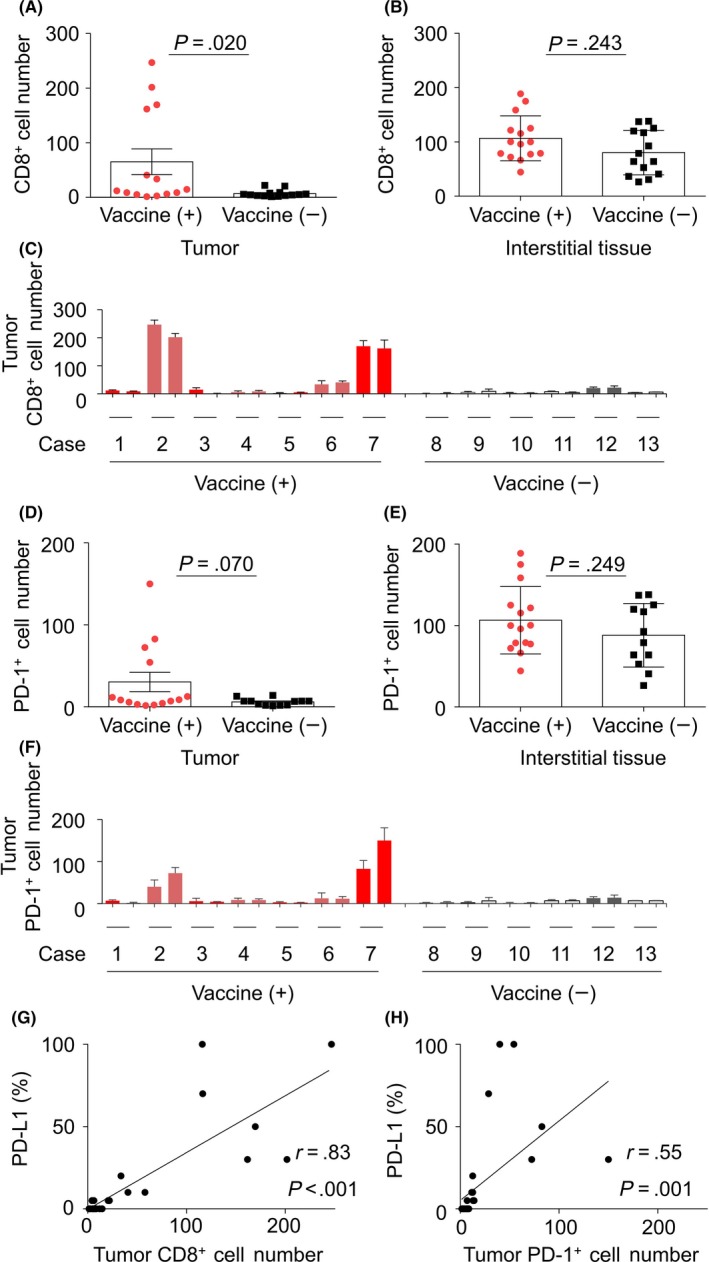
In situ investigation of infiltration of CD8+ and programmed cell death‐1 (PD‐1)+ lymphocytes into pancreatic carcinoma lesions. A,B, Comparison of infiltration of CD8+ cells in the tumor (A) and interstitial tissue (B) between patients who had and had not received the survivin 2B peptide (SVN‐2B) vaccine. C, Number of CD8+ lymphocytes that infiltrated each tumor lesion. D,E, Comparison of infiltration of PD‐1+ cells into the tumor (D) and interstitial tissue (E) between patients who had and had not received the SVN‐2B vaccine. F, Number of PD‐1+ lymphocytes that infiltrated each lesion. G,H, Correlation between proportions of the PD‐L1+ tumor and CD8+ (G) or PD‐1+ (H) lymphocyte infiltration in the lesions. PD‐L1 expression on the surface of tumor cells was deemed to be positive. Signal intensity was not evaluated
Recent advances in the treatment of cancer have provided the opportunity for another treatment strategy for some cancers. Given that interaction between PD‐1 and the PD‐L1 axis is a crucial immune checkpoint, and could be susceptible to molecules that act by inhibiting cancer immunity, we also investigated infiltration of PD‐1+ cells (Figure 2C). The results were similar to those for infiltration of CD8+ cells but did not reach statistical significance (Figure 3D–F; Figure S1B). As expected, there was a significant correlation between the number of CD8+ cells and the number of PD‐1+ cells that had infiltrated the tumors (r = 0.81, P < .001; Figure S3A). However, there was no such correlation between the numbers of CD8+ and PD‐1+ cells that had infiltrated the interstitial tissue (Figure S3B). This discrepancy probably reflects the fact that PD‐1 is not only expressed in activated CD8+ cells but also in CD4+ cells, especially those with the follicular helper T cell phenotype.
Regulatory T cells (Tregs) suppress antitumor immunity, so it is important that immunotherapy for cancer does not cause proliferation or activation of these cells.10 Therefore, we investigated Treg‐positive infiltration in the lesions using FOXP3 staining as a surrogate marker (Figure 2D). Most FOXP3+ cells were found only in the interstitial tissue. Furthermore, the numbers of lymphocytes that expressed FOXP3 were relatively small, as reported previously.11 In our study, there was no significant difference in infiltration of FOXP3+ cells between the patients who did and did not receive the vaccination (Figure S4A). The ratio of CD8+ cells and FOXP3+ cells in tumors was higher in the vaccination group than in the control group (P = .012; Figure S4B). However, the ratio of interstitial CD8+ cells to FOXP3+ cells was not significantly different between the groups (Figure S4C).
3.4. Correlation between PD‐L1 expression and CD8+ and PD‐1+ cell infiltration
Detecting PD‐L1 expression by immunohistochemistry using formalin‐fixed, paraffin‐embedded specimens is now recognized as a biomarker of the efficacy of treatment with PD‐1‐blocking ICIs in some types of malignancy.12, 13 To investigate the potential value of combination therapy consisting of tumor‐specific peptide vaccine and ICI, we examined PD‐L1 expression in the tumor specimens (Figure 2E). Although the lesions in patients who had received the SVN‐2B peptide vaccine tended to show higher PD‐L1 expression than those in patients who had not, the difference was not statistically significant (Figure S5). As reported for CD8, some lesions showed abundant expression of PD‐L1 and others did not (Figure S6). Interestingly, there was a significant correlation between the level of PD‐L1 expression and the numbers of CD8+ and PD‐1+ cells in each lesion (r = 0.83, P < .001 and r = 0.54, P = .001, respectively; Figure 3G,H).
3.5. Case presentations
Two cases are presented to demonstrate the significant immune reactions that occurred in peripheral blood and local tumor tissue.
The first case (Table 1, patient 2) was a 29‐year‐old woman with a pancreatic tumor that was initially diagnosed as acinar carcinoma. She was treated with gemcitabine (GEM) + tegafur/gimeracil/oteracil potassium (S‐1) and leucovorin + 5‐fluorouracil + irinotecan + oxaliplatin (FOLFIRINOX)‐based chemotherapy in addition to palliative surgery. The chemotherapy had no clinical effect by RECIST. She was entered into our clinical trial and initially assigned to the group that received SVN‐2B vaccination but not IFNβ (Figure 1A). She developed progressive disease, so was then given IFNβ in addition to the SVN‐2B peptide vaccine but died 115 days after the first vaccination.
When the immunological effect of the first vaccination was evaluated at 8 weeks (before addition of IFNβ to her treatment), the numbers of IFNγ‐producing and peptide‐specific CD8+ T cells were significantly increased in peripheral blood (Figure 4A, B). The gross appearance of the cut surface of a hepatic lesion obtained from this patient is shown in Figure 4C. Microscopically, the tumor showed undifferentiated morphology and high expression of PD‐L1 with abundant infiltration of CD8+ cells (Figure 4D).
Figure 4.
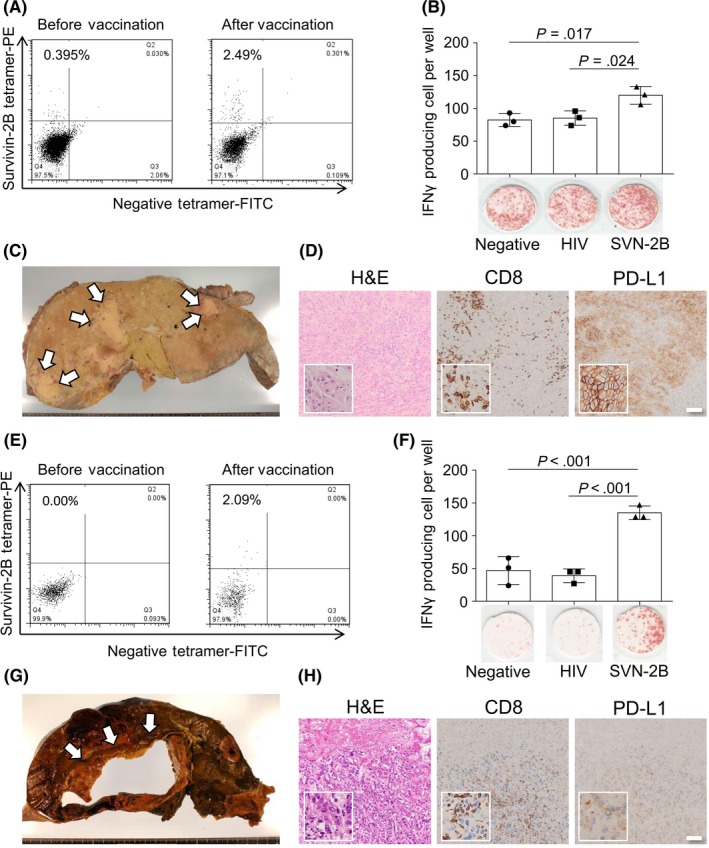
Immunological effects in peripheral blood and histopathological analysis of pancreatic carcinoma tumor specimens from cases 2 and 7. A,E, Flow cytometry analysis of peripheral blood for CD8+ T lymphocytes specific for survivin 2B peptide (SVN‐2B) peptide on the HLA‐A24 complex before (left panel) and 8 weeks after (right panel) the first vaccination in case 2 (A) and case 7 (E). B,F, γ‐Interferon (IFNγ) enzyme‐linked immunospot assay of PBMCs at 8 weeks after the first vaccination for no peptide (left), HIV (center), or SVN‐2B (right) specific peptide in case 2 (B) and case 7 (F). C,G, Gross appearance of the cut surface of a liver lesion after formalin fixation in case 2 and before fixation in case 7 (G). Arrows indicate metastatic lesions. D,H, Morphological image (H&E stain; left panel) and immunohistochemistry for CD8 (center panel) and programmed cell death ligand 1 (PD‐L1) (right panel) of a metastatic liver lesion in case 2 (D) and case 7 (H). Insets show high‐power magnification
The second case (Table 1, patient 7) was a 65‐year‐old woman who underwent surgery after S‐1‐based neoadjuvant chemotherapy. The patient developed local recurrence that was treated with proton beam therapy. She was subsequently treated with GEM + nanoparticle albumin‐bound paclitaxel and S‐1 + GEM. She then developed progressive disease, so she was entered into our clinical trial and assigned to receive SVN‐2B + IFNβ vaccination (Figure 1A). Unfortunately, the disease progressed and she died 76 days after the first vaccination.
The immunological findings in the peripheral blood of this patient at 8 weeks after the first vaccination were very similar to those in case 1, as were the immunopathological findings (Figure 4E,F,H). Figure 4G depicts the gross appearance of a lesion in the patient's liver that showed cystic change suggestive of central necrosis. Viable carcinoma with high PD‐L1 expression and harboring a larger number of CD8+ cells was observed at the margin, which was the area sampled for histological analysis.
4. DISCUSSION
Numerous antigen peptides derived from various proteins have been investigated as tumor‐specific immunotherapy in vitro and in vivo.14, 15 Unfortunately, few established targets have been identified for standard therapy so far, even though some immune effects have been observed. In these studies, although systemic immune status was monitored, it was not known how the immune cells were distributed in tumor tissue. As far as we know, this is the first investigation of local immune status using tissue derived from a series of autopsy cases. Although survivin‐based vaccination could not prolong survival, large infiltrations of CD8+ and PD‐1+ lymphocytes were identified in some lesions from patients who had received the vaccine. Lesions from patients with pancreatic carcinoma who had received the SVN‐2B peptide vaccine harbored a significantly greater number of CD8+ cells than those from patients who did not, but not in the interstitial tissue. This observation implies that tumor antigen‐specific CTLs were appropriately mobilized and recognized cancer cells. Notably, these lesions showed a large proportion of tumor cells that were positive for PD‐L1, which is a cardinal marker of PD‐1‐inhibiting immune therapy. Our results show that, although the tumor cells were attacked by IFNγ from CTLs, the tumor remained viable, probably because of immune suppression mediated by the PD‐1/PD‐L1 axis, and this is why we could not prevent disease progression. It is tempting to hypothesize that combination treatment with a tumor‐specific peptide vaccine and ICI would be a promising approach for these lesions.16 Few studies have investigated PD‐L1 expression in pancreatic carcinoma. In these reports, PD‐L1 expression in more than 5% of tumor cells was designated as positive. The results are varied; 12.5–49.4% pancreatic carcinoma is reported to be positive for PD‐L1.17, 18 With this criterion, 33.3% (2/6) of nonvaccinated cases, and 42.9% (3/7) of vaccinated tumors were PD‐L1+ in our cases. The results are compatible with our current results. However, we would like to point out that a higher proportion of tumor cells tended to be positive for PD‐L1 expression in vaccinated cases, although the difference did not reach statistical significance. Importantly, some types of malignancy with relatively few genetic alterations, including carcinoma of the pancreas, harbor small numbers of neoantigens, leading to low antigenicity.19, 20 Immune checkpoint inhibitors are considered to be less effective for these tumors. Enhancing the antigenicity of these malignancies by treatment with a tumor vaccine might allow adaptation of ICI therapies to improve their efficacy. Furthermore, a combination of these treatments could lead to a tumor cell‐specific immune reaction and reduce the risk of harmful side‐effects. It is worth noting that the numbers of CD8+ and PD‐1+ lymphocytes that infiltrated the tumors were as low as those in so‐called “immune deserts” in more than half of the cases.21 Vaccination using a tumor antigen peptide could increase the number and activity of tumor‐specific CTLs. However, more effective immunotherapy is needed to achieve appropriate sensitization, trafficking, and recognition of the antigen by tumor cells.21 Particularly, to facilitate CTL mobilization, it is important that the malignant cell itself or interstitial cells, including immune cells or fibroblasts, that are in direct interaction with tumor cells, release CTL‐attracting chemokines. Timely generation of costimulatory molecules and inflammatory cytokines is also required. Achieving this cancer‐immune circuitry will require complementing the effects of as many factors as necessary.
In addition, overcoming immune suppression and/or escape are crucially important issues to be addressed in the development of immunotherapy for cancer. Some animal models have shown that FOXP3+ Tregs play a prominent role in immunity to pancreatic cancer.22, 23 Indeed, in the biopsy specimens obtained from our patients before vaccination, although the number of FOXP3+ cells that had infiltrated was not correlated with progression‐free survival, the ratio of CD8+ to FOXP3+ cells affected progression‐free survival in the patients who received the survivin vaccine (2018). In these autopsy specimens of human pancreatic carcinoma, relatively small infiltrations of FOXP3+ cells were found, as previously reported.11 Nevertheless, the ratio of CD8+ cells to FOXP3+ cells in the tumors was significantly different between the vaccination and control groups. These results suggest that the effect of FOXP3+ Tregs on local immunity is not negligible in pancreatic cancer. However, the cellular origin of the immune‐suppressive mediators includes the tumor cells themselves, myeloid‐derived suppressor cells, and regulatory B cells,24, 25 consideration of which was beyond the scope of this study. Analysis of additional immune suppressor cells is required in the future.
In this investigation, almost all tumor cells expressed HLA class I antigen, which is a prerequisite for CTL‐based immunotherapy in cancer. However, we had a few cases in which we immunohistochemically analyzed survivin, which was a target in this trial. All the tumor cells that were positive for survivin were detected using the same immunohistochemistry protocol in biopsy specimens, suggesting immune escape through loss of the target. From the biological point of view, survivin is a member of the inhibitor of apoptosis protein family, which inhibits apoptosis‐related caspase activity.26 Therefore, given the amount of research demonstrating the functional importance of survivin in the survival of tumor cells, we consider that survivin expression might not have been appropriately detected by immunohistochemistry in our study, probably because of the altered fixation conditions brought about by use of formalin in autopsy specimens, which might have affected staining for survivin. Other researchers reported that only 16.7% of control tumors were positive for survivin whereas survivin was detected in more than 75% of pancreatic adenocarcinomas.27, 28 A sophisticated fixation procedure or antisurvivin mAb would be needed.
In conclusion, vaccination with survivin 2B 80‐88 peptide, a tumor‐specific antigen, can be a potent immunological manipulation for pancreatic carcinoma, which is a cancer with relatively low amounts of immunogenic neoantigen. Combined use of this cancer vaccine and ICI therapy to inhibit the PD‐1 axis might be worth attempting in expanded indications and could have less severe adverse effects. However, more than half of the vaccinated patients did not show infiltration of CTLs into the tumor at autopsy, indicating that some of the mechanisms that promote cancer immunity are still unknown. Comprehensive understanding and a combinational approach should lead us to the next generation of cancer immunotherapy, which hopefully will prove to be highly effective as standard therapy.
DISCLOSURE
K. Matsuo is an employee of Sapporo Clinical Laboratory Inc. The other authors have no conflicts of interest in regard to this study.
Supporting information
ACKNOWLEDGMENTS
This work was supported by a Grant‐in‐Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS; grant no. 17H01540) and the Project for Cancer Research and Therapeutic Evolution (P‐CREATE) from the Japan Agency for Medical Research and Development (AMED) to T. Torigoe.
Kubo T, Tsurita G, Hirohashi Y, et al. Immunohistological analysis of pancreatic carcinoma after vaccination with survivin 2B peptide: Analysis of an autopsy series. Cancer Sci. 2019;110:2386–2395. 10.1111/cas.14099
Kubo and Tsurita equally contributed to this work.
Clinical trial registration number: UMIN 000012146
Contributor Information
Yoshihiko Hirohashi, Email: hirohash@sapmed.ac.jp.
Toshihiko Torigoe, Email: torigoe@sapmed.ac.jp.
REFERENCES
- 1. Couzin‐Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432‐1433. [DOI] [PubMed] [Google Scholar]
- 2. Sato N, Hirohashi Y, Tsukahara T, et al. Molecular pathological approaches to human tumor immunology. Pathol Int. 2009;59:205‐217. [DOI] [PubMed] [Google Scholar]
- 3. Hirohashi Y, Torigoe T, Maeda A, et al. An HLA‐A24‐restricted cytotoxic T lymphocyte epitope of a tumor‐associated protein, survivin. Clin Cancer Res. 2002;8:1731‐1739. [PubMed] [Google Scholar]
- 4. Schmidt SM, Schag K, Muller MR, et al. Survivin is a shared tumor‐associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571‐576. [DOI] [PubMed] [Google Scholar]
- 5. Kameshima H, Tsuruma T, Kutomi G, et al. Immunotherapeutic benefit of alpha‐interferon (IFNalpha) in survivin2B‐derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2013;104:124‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kameshima H, Tsuruma T, Torigoe T, et al. Immunogenic enhancement and clinical effect by type‐I interferon of anti‐apoptotic protein, survivin‐derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci. 2011;102:1181‐1187. [DOI] [PubMed] [Google Scholar]
- 7. Honma I, Kitamura H, Torigoe T, et al. Phase I clinical study of anti‐apoptosis protein survivin‐derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58:1801‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shima H, Tsurita G, Wada S et al. Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA-A24-positive pancreatic adenocarcinoma. Cancer Sci 2019; 110:2378‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torigoe T, Asanuma H, Nakazawa E, et al. Establishment of a monoclonal anti‐pan HLA class I antibody suitable for immunostaining of formalin‐fixed tissue: unusually high frequency of down‐regulation in breast cancer tissues. Pathol Int. 2012;62:303‐308. [DOI] [PubMed] [Google Scholar]
- 10. Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang Y, Du Z, Yang F, et al. FOXP3 + lymphocyte density in pancreatic cancer correlates with lymph node metastasis. PLoS ONE. 2014;9:e106741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. New Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. New Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 14. Tsukahara T, Hirohashi Y, Kanaseki T, et al. Peptide vaccination therapy: towards the next generation. Pathol Int. 2016;66:547‐553. [DOI] [PubMed] [Google Scholar]
- 15. Hirayama M, Nishimura Y. The present status and future prospects of peptide‐based cancer vaccines. Int Immunol. 2016;28:319‐328. [DOI] [PubMed] [Google Scholar]
- 16. Ribas A, Hu‐Lieskovan S. What does PD‐L1 positive or negative mean? J Exp Med. 2016;213:2835‐2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7‐H1 and B7‐1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059‐1065. [DOI] [PubMed] [Google Scholar]
- 18. Soares KC, Rucki AA, Wu AA, et al. PD‐1/PD‐L1 blockade together with vaccine therapy facilitates effector T‐cell infiltration into pancreatic tumors. J Immunother. 2015;38:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajasagi M, Shukla SA, Fritsch EF, et al. Systematic identification of personal tumor‐specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JM, Chen DS. Immune escape to PD‐L1/PD‐1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27:1492‐1504. [DOI] [PubMed] [Google Scholar]
- 22. Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar‐Sagi D. Crosstalk between regulatory T cells and tumor‐associated dendritic cells negates anti‐tumor immunity in pancreatic cancer. Cell Rep. 2017;20:558‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keenan BP, Saenger Y, Kafrouni MI, et al. A Listeria vaccine and depletion of T‐regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146:1784‐1794. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veglia F, Perego M, Gabrilovich D. Myeloid‐derived suppressor cells coming of age. Nat Immunol. 2018;19:108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guzman‐Genuino RM, Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. 2017;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altieri DC. Survivin – the inconvenient IAP. Semin Cell Dev Biol. 2015;39:91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271‐278. [DOI] [PubMed] [Google Scholar]
- 28. Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


