Abstract
Multi‐walled carbon nanotube‐7 (MWCNT‐7) fibers are biopersistent and have a structure similar to asbestos. MWCNT‐7 has been shown to induce malignant mesothelioma when administered by intrascrotal or intraperitoneal injection in rats and mice, and an inhalation study demonstrated that rats exposed to respirable MWCNT‐7 developed lung tumors. MWCNT‐N, which is similar to MWCNT‐7, was shown to induce both lung tumors and malignant mesothelioma in rats when administered by trans‐tracheal intrapulmonary spraying (TIPS). The present study was performed to investigate the carcinogenicity of MWCNT‐7 when administered by the TIPS method. Ten‐week‐old male F344/Crj rats were divided into 3 groups and administered 0.5 mL vehicle, 0.250 μg/mL MWCNT‐7 or 0.250 μg/mL crocidolite once a week for 12 weeks (total doses of 1.5 mg/rat) and then observed for up to 104 weeks. Rats in the MWCNT‐7 group began to die from pathologies associated with the development of malignant mesothelioma 35 weeks after the final TIPS administration. Overall, the incidence of malignant mesothelioma in the MWCNT‐7 group was significantly higher than in the vehicle or crocidolite groups.
Keywords: inhalation toxicity, intra‐tracheal intra‐pulmonary spraying, malignant mesothelioma, MWCNT‐7, rat
1. INTRODUCTION
Multi‐walled carbon nanotube‐7 (MWCNT‐7) fibers are biopersistent, have aerodynamic diameters that allow deposition of inhaled fibers beyond the ciliated airways, and have rigidity and lengths sufficient to impede clearance of the fibers by alveolar macrophages. Consequently, the fiber pathogenicity paradigm identifies MWCNT‐7 as a possible carcinogen.1 The carcinogenic potential of MWCNT‐7 was initially demonstrated in intra‐scrotal and intra‐peritoneal injection studies.2, 3, 4 A later nose‐only inhalation study demonstrated that inhalation exposure to MWCNT‐7 promoted the development of lung tumors.5 Finally, a whole‐body inhalation study established that inhaled MWCNT‐7 was a complete carcinogen in the rat lung.6
MWCNT‐N, which is similar to MWCNT‐7, was shown to induce both malignant lung tumors and malignant pleural mesothelioma when administered via the respiratory tract using intra‐tracheal intra‐pulmonary spraying (TIPS).7 TIPS administration is a sensitive and readily applied method for identifying potential human carcinogens in studies using test animals: TIPS uses commonly available equipment and techniques, and, consequently, can be widely used to test the carcinogenicity of respirable materials in test animals. TIPS administration bypasses the nasal cavity, and, therefore, the test material is not filtered in the nasal passages of the test animal. In addition, when administered by TIPS, the test material is present in the lungs of the test animal from the beginning of the experiment, and if the material is biopersistent (a crucial property of respired carcinogenic particles and fibers) carcinogenic processes promoted by the material can proceed for the entire study period.
The present study was performed to investigate the carcinogenicity of MWCNT‐7 when administered by the TIPS method. We found that administration of MWCNT‐7 via the airway using TIPS resulted in induction of malignant pleural mesothelioma in rats. This is the first study to demonstrate that administration of MWCNT‐7 via the airway can result in the development of mesothelioma in rats.
2. MATERIALS AND METHODS
2.1. Preparation of materials
MWCNT‐7 (Mitsui Chemicals, Tokyo, Japan) and crocidolite (Union for International Cancer Control grade) suspensions were supplied by Dr A. Hirose (one of the authors). The suspensions were adjusted to a concentration of 250 μg/mL with physiological saline containing 0.5% Pluronic F68 (PF68) (Sigma‐Aldrich, St. Luis, Mo, USA). To minimize aggregation of the administered materials, the MWCNT‐7 suspension was sonicated using a Sonifier 250 (Emerson Japan, Shinagawa, Tokyo) for 5 minutes before administration, and the crocidolite suspension was vortexed for 5 minutes before administration.
2.2. Animals and husbandry
Nine‐week‐old male F344/DuCrlCrj rats were purchased from Charles River Laboratories Japan (Yokohama, Japan). The animals were housed in animal rooms maintained under standard conditions (22 ± 3°C, humidity 55 ± 15% and a 12‐hour light‐dark cycle) and received oriental MF powdered diet (Oriental Yeast, Tokyo, Japan) and water ad libitum. After a 7‐day quarantine and acclimation period, the rats were randomly divided into 3 groups. The experimental protocol was approved by the Animal Care and Use Committee of Nagoya City University Medical School, and the research was conducted according to the Guidelines for the Care and Use of Laboratory Animals of Nagoya City University Medical School.
2.3. Intra‐tracheal intrapulmonary spraying method
At the beginning of the study, animals were placed under 3% isoflurane anesthesia and administered 0.5 mL of vehicle, MWCNT‐7 (250 μg/mL) or crocidolite suspension (250 μg/mL) using a microsprayer (DIMS Institute of Medical Science, Nagoya, Japan) connected to a 1‐mL disposable syringe, once a week for 12 weeks (total doses of 1.5 mg/rat). Animals under anesthesia were kept in a vertical position and the nozzle of a microsprayer was inserted into the trachea. The instillation was carried out by pressing the plunger at a constant speed during inhalation. Before being replaced in its cage, the animal was maintained in a vertical position until it recovered from the instillation procedure.
2.4. Body weight
Animals were weighed once a week from week 1 to 14, once every 2 weeks from week 14 to 26, and once every 4 weeks from week 26 to 104.
2.5. Autopsy and histology
Rats that died before the end of the study period were autopsied immediately upon discovery. Moribund animals and animals that survived to the end of the study period underwent terminal necropsy: these animals were placed under deep anesthesia and killed by exsanguination via the abdominal aorta. All organs were preserved using 10% buffered formalin solution. The major organs and the lung, pleural wall, heart, and mediastinal, submandibular and mesenteric lymph nodes were processed for histopathological examination. Confirmation of test materials in the tissues was performed using polarized light microscopy (Olympus, Tokyo, Japan).
2.6. Immunohistochemistry
Briefly, paraffin‐embedded slides were deparaffinized in xylene, hydrated through a graded series of ethanol, incubated in heat processor solution pH6 (715281: Nichirei Biosciences, Tokyo, Japan) at 100°C for 40 minutes, incubated in 3% H2O2 (715242: Nichirei Biosciences, Tokyo, Japan) at room temperature for 15 minute, and blocked with 5% BSA 5% goat serum in PBS at room temperature for 1 hour. For thyroid transcription factor‐1 (TTF‐1) staining, slides were incubated in anti–TTF‐1 (sc‐13040: Santa Cruz Biotechnology [this product has been discontinued]) diluted 1:500 in PBS containing 1% BSA 1% goat serum at 4°C overnight. Slides were then incubated with MAX‐PO MULTI 2˚Ab (714191: Nichirei Biosciences, Tokyo Japan) for 1 hour. at room temperature, visualized with DAB (725191: Nichirei Biosciences), and counterstained with hematoxylin.
2.7. Scanning electron microscopy
For high magnification viewing, H&E stained slides were immersed in xylene to remove the cover slip, and then dried and processed for scanning electron microscopy. Slides were observed using a scanning electron microscope (SEM) (Model S‐4700 Field Emission SEM; Hitachi High Technologies Corporation, Tokyo, Japan) at 5 to 15 kv. For the measurement of the MWCNT‐7 fibers in the lungs and lymph nodes, digestion of fixed tissues was performed as previously described,8 and the fibers were imaged by SEM.
2.8. Statistical and analysis
Survival rates were analyzed using the Kaplan‐Meier method. Tumor and epithelial type II cell hyperplasia incidences were analyzed using Fisher's exact probability test (2‐sided). Homogeneity of variance of MWCNT‐7 fiber length in the lungs and mediastinal lymph nodes was analyzed using the F test. The variances were heterogeneous; therefore, the difference in mean MWCNT‐7 fiber length in the lungs and mediastinal lymph nodes was assessed by Welch's t‐test (1‐sided). P‐values <0.05 were considered significant.
3. RESULTS
3.1. Survival and body weights
During intratracheal instillation, 1 animal in the crocidolite group and 1 animal in the MWCNT‐7 group died and were, therefore, excluded from the study. Figure 1 shows a Kaplan‐Meier survival plot. A significant decrease in the average survival time was observed in the MWCNT‐7 group compared to the vehicle group. There was no significant difference in average survival time between the crocidolite and vehicle groups (Table 1). The first rat that died from pathologies associated with the development of malignant mesothelioma died at week 47. The average survival period was 78.6 weeks in the MWCNT‐7 group, 102.4 weeks in the crocidolite group, and 101.5 weeks in the vehicle group. The final survival rate at week 104 was 5% in the MWCNT‐7 group, 79% in the crocidolite group, and 80% in the vehicle group.
Figure 1.
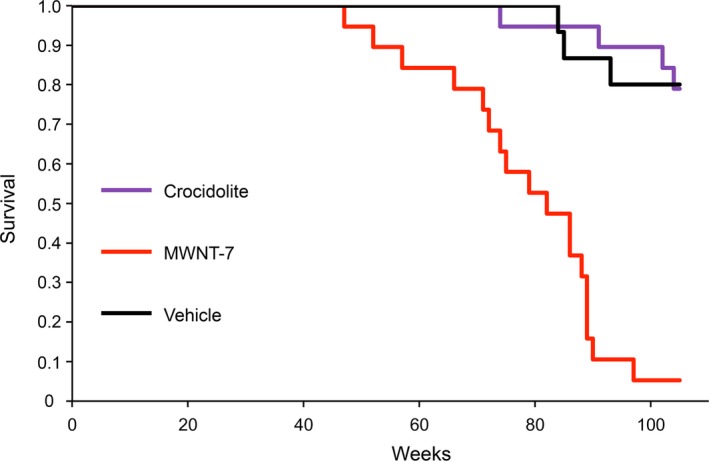
Kaplan‐Meier survival plot of the rats in the vehicle, MWCNT‐7, and crocidolite groups
Table 1.
Survival rate and average survival period
| Group | Number of rats examined | Survival at 104 wk (%) | Average survival (wk) |
|---|---|---|---|
| Vehicle | 15 | 80 | 101.5 |
| MWNT‐7 | 19 | 5 | 78.6 |
| Crocidolite | 19 | 79 | 102.4 |
The body weight curves are shown in Figure S1. A slight, but statistically significant, decrease in body weight was observed in the MWCNT‐7 group from week 6 to 80 and at weeks 88 and 96.
3.2. Histopathological observation
MWCNT‐7 fibers were distributed throughout all lobes of the lungs of the MWCNT‐7 treated rats, mostly encased in granulation tissue in the alveolar region (Figure 2A). The presence of MWCNT‐7 fibers was confirmed by polarized light microscopy (Figure 2B). No MWCNT‐7 fibers were found in the submandibular or mesenteric lymph nodes (data not shown). MWCNT‐7 fibers were also found in the mesotheliomas that developed in these rats, primarily associated with macrophages.
Figure 2.
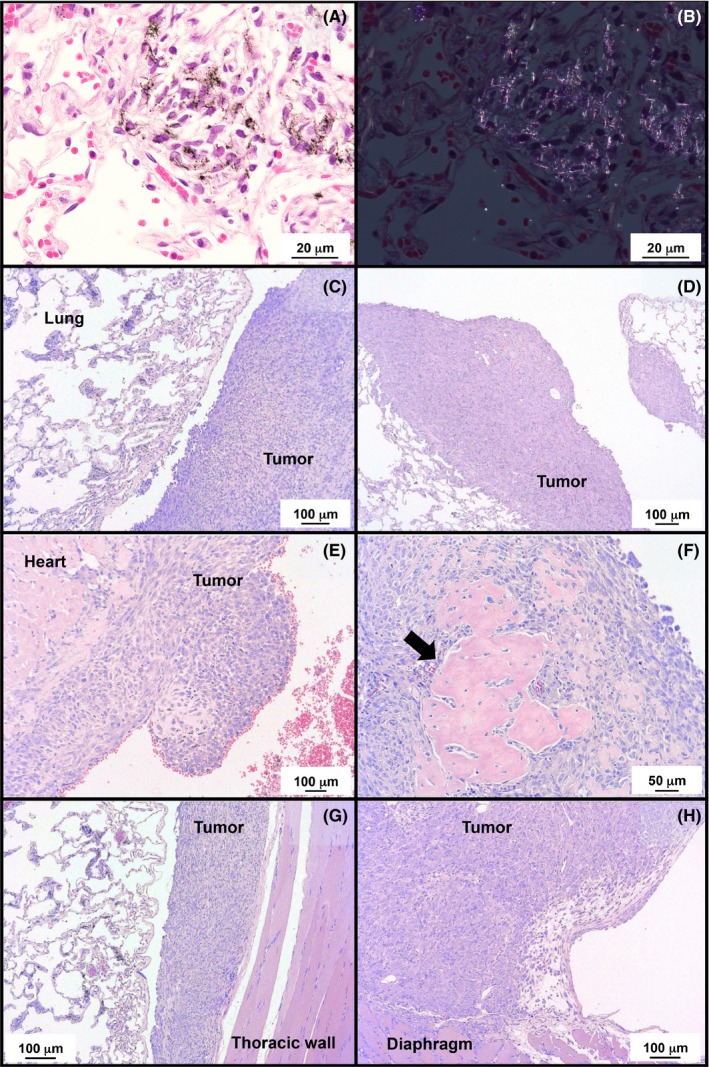
Deposition of MWCNT‐7 in the lung (A) and a polarized light microscope image (B). Representative histological imagines of malignant mesotheliomas located near the lung (C, D) and heart (E) in MWCNT‐7 administered rats. Several mesotheliomas had sites of ossification (arrow in F). Most mesotheliomas had adherence to the thoracic wall (G) or diaphragm (H)
The incidences of malignant mesothelioma and lung tumors are summarized in Table 2. In the MWCNT‐7 treated group, 18 of 19 rats developed malignant mesotheliomas: these mesotheliomas were all late‐stage mesotheliomas and readily identified as tumors, as opposed to reactive mesothelial hyperplasia. Because these tumors were all late‐stage mesotheliomas, the primary site of development could not be determined. One rat died from a spontaneous pituitary tumor in week 93: this rat had fibrous thickening of the parietal pleura but had not developed malignant mesothelioma at the time of death. No rats in the vehicle control or crocidolite groups developed malignant mesothelioma. Figure 2C, D and E show representative histopathological images of malignant mesotheliomas that developed in rats administered MWCNT‐7. Most tumors had partially ossified sites (Figure 2F) and adhesions to either the thoracic wall or diaphragm (Figure 2G and H). Diagnosis of mesothelioma was confirmed by staining for thyroid transcription factor‐1 (TTF‐1): lung adenocarcinomas will be positive for TTF‐1 while mesotheliomas will be negative for TTF‐1. Mesotheliomas from all 18 rats were stained for TTF‐1. All these tumors were TTF‐1 negative. Figure 3 shows representative results of TTF‐1 staining of the lung adenocarcinoma that developed in one of the vehicle control rats and the mesotheliomas that developed in the MWCNT‐7‐treated rats.
Table 2.
Incidence of obvious epithelial type II cell hyperplasia, lung tumors, and malignant pleural mesothelioma
| Group | Hyperplasia | Lung tumors | Malignant mesothelioma | Total tumor burden (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of rats | Number of rats | Incidence (%) | Adenoma | Adenocarcinoma | Combined | Incidence (%) | Number of rats | Incidence (%) | ||
| Vehicle | 15 | 1 | 7 | 0 | 1 | 1 | 7 | 0 | 0 | 7 |
| MWCNT‐7 | 19 | 17** | 89** | 1 | 0 | 1 | 5 | 18** | 95** | 95** |
| Crocidolite | 19 | 8* | 42* | 4 | 1 | 5 | 26 | 0 | 0 | 26 |
*,**Significantly different from the vehicle group at P < 0.05, P < 0.001.
Figure 3.
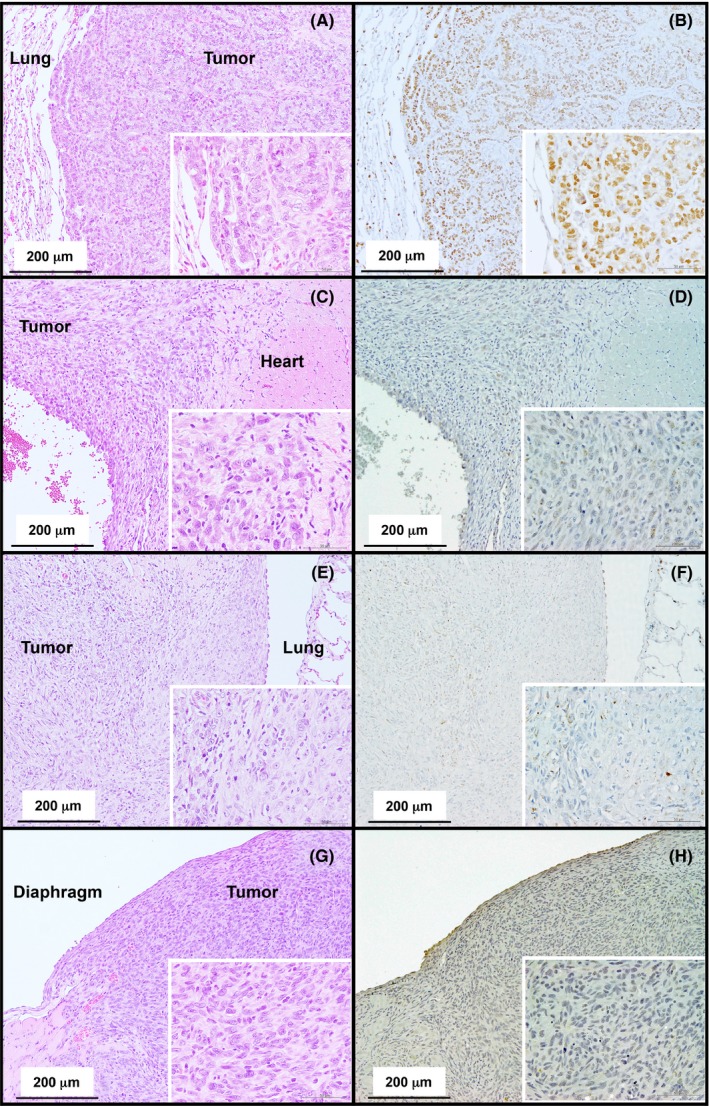
Representative histopathological images of the lung adenocarcinoma in the vehicle‐treated rat (A) and mesotheliomas that developed in the MWCNT‐7 treated rats (C, E, and G). Immunohistochemical staining of TTF‐1 (B, D, F and H). Nuclei in the adenocarcinoma are positive for TTF‐1 (B). The malignant mesotheliomas near the heart, thoracic cavity, and diaphragm are negative for TTF‐1 (D, F, and H)
One rat in the vehicle control group and one rat in the crocidolite group, but no rats in the MWCNT‐7 group, developed adenocarcinomas. The combined incidence of adenomas and adenocarcinomas in the vehicle control, crocidolite, and MWCNT‐7 groups was 1/15, 5/19, and 1/19, respectively. There was no significant difference in the development of lung tumors between either the crocidolite or MWCNT‐7 groups and the vehicle control group. However, the incidence of epithelial type II cell hyperplasia in both the crocidolite (8/19) and MWCNT‐7 (17/19) groups was significantly higher than in the vehicle control group (1/15).
3.3. Size measurement of MWNT‐7 in the lung and mediastinal lymph node
Figure 4 shows representative scanning electron microscopic images of the lung and mediastinal lymph node of rats administered MWCNT‐7. MWCNT‐7 fibers can be seen in the cytoplasm of macrophages (Figure 4A‐D). MWCNT‐7 fibers from digested lung samples and mediastinal lymph nodes are shown in Figure 4E (lung) and F (mediastinal lymph node). A length distribution histogram is shown in Figure 5. The mean and median lengths of MWCNT‐7 were 4.8 ± 2.8 μm and 4.2 μm in the lung and 3.0 ± 1.6 μm and 2.7 μm in the mediastinal lymph node. The average length of MWCNT‐7 fibers in the mediastinal lymph node was significantly shorter than that of MWCNT‐7 fibers in the lung tissue (Figure 5, Table 3).
Figure 4.
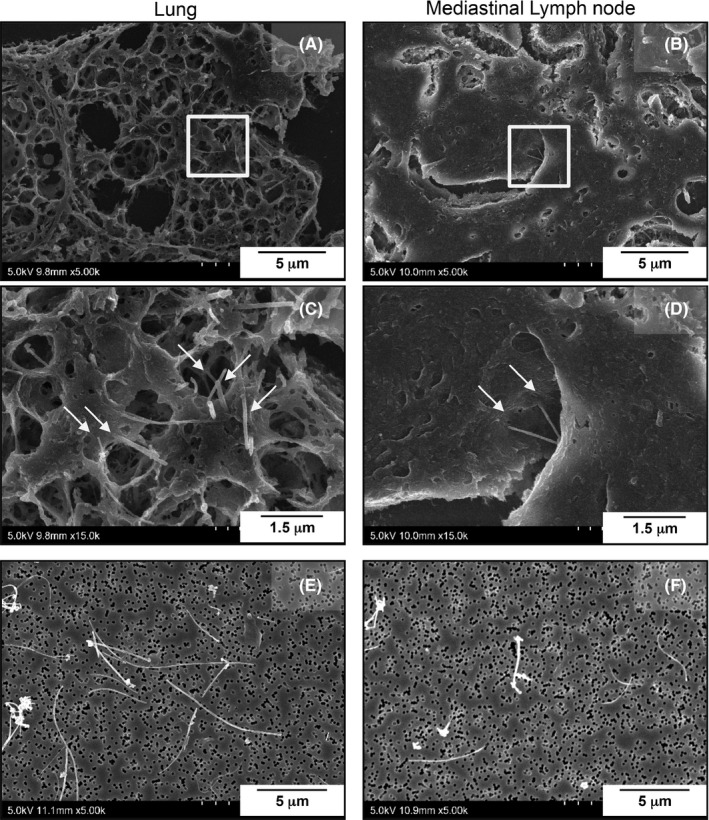
A‐D, Scanning electron microscopic images of MWCNT‐7 fibers (arrows) phagocytosed by macrophages in the lung and mediastinal lymph node. E‐F, representative images of MWCNT‐7 fibers from the lung and mediastinal lymph node after tissue digestion
Figure 5.
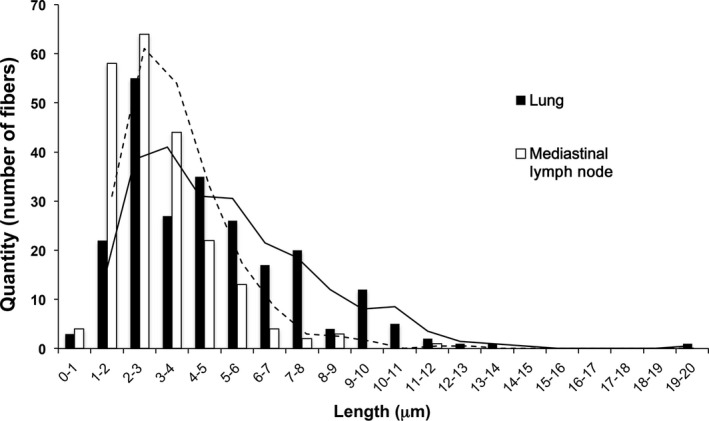
Histogram of the length distribution of MWCNT‐7 fibers in the lung (black bars) and mediastinal lymph node (white bars)
Table 3.
Mean and median diameter of MWCNT‐7 fibers in the lung and mediastinal lymph node
| Tissue | Number of fibers examined | Average size (μm) | Median size (μm) |
|---|---|---|---|
| Lung | 300 | 4.8 ± 2.8 | 4.2 |
| Mediastinal lymph node | 300 | 3.0 ± 1.6* | 2.7 |
*Different from the lung at P < 0.01.
4. DISCUSSION
In this study, we investigated the carcinogenicity of MWCNT‐7 when administered to the rat via the airway using TIPS. We found that MWCNT‐7 induced the development of malignant pleural mesothelioma. Thus, MWCNT‐7 in the lung causes development of malignant tumors in both the lung6 and pleural cavity (this study) of rats. It is also notable that under the conditions of the present study, MWCNT‐7 was a much more potent carcinogen than crocidolite asbestos.
Multi‐walled carbon nanotubes (MWCNT) are composed of multiple coaxially arranged graphene cylinders. The carbon‐carbon bonds of graphene are exclusively sp2, which make MWCNT highly stable in bodily fluids and tissues. In the present study, MWCNT‐7 fibers were readily detected in the lung and mediastinal lymph nodes 2 years after administration into the lung (see Figures 4 and 5). MWCNT‐7 fibers have a shape that allows their deposition beyond the ciliated airways and that also impedes their removal by alveolar macrophages. The fiber pathogenicity paradigm identifies biopersistent fibers that are deposited beyond the ciliated airways as possible carcinogens.1 The carcinogenicity of MWCNT‐7 in the lung and pleura is in agreement with this paradigm. However, unlike the present study, inhalation exposure to MWCNT‐7 did not cause development of pleural mesotheliomas6. Two factors could have contributed to these contrasting results. One is that using TIPS, the MWCNT‐7 is administered directly to the lung, and, consequently, the MWCNT‐7 was not filtered in the nasal passages of the exposed rats, allowing longer fibers to be deposited in the lung. Accordingly, although the average length of the MWCNT‐7 fibers found in the lungs of rats exposed by whole‐body inhalation to MWCNT‐7 was 5.8‐5.9 μm6 while the average length of the MWCNT‐7 fibers found in the rat lung in the present study was 4.8 ± 2.8 μm, approximately 25% of the fibers in the lungs of the rats administered MWCNT‐7 in the present study were longer than 6 μm (see Figure 5), and it is possible that the longer fibers were required to induce the development of mesothelioma. The second factor is that when administered by TIPS, the test material is present in the lungs of the test animal from the beginning of the experiment, and if the material is biopersistent (a crucial property of respired carcinogenic particles and fibers), carcinogenic processes promoted by the material can proceed for the entire study period. In contrast, in whole‐body inhalation studies, the test material accumulates in the lungs of the exposed animals over time and sufficient numbers of fibers may not be transported to the pleural cavity to induce the development of mesothelioma until late in the experimental period (also see Fukushima et al9). Notably, in the whole‐body inhalation study reported by Kasai et al6, MWCNT‐7 fibers did accumulate in the pleural cavity and rats did develop mesothelial hyperplastic lesions. If the study period could have been extended for an additional several months, MWCNT‐7 fibers would have continued to accumulate in the pleural cavity and it is possible that the exposed rats would have developed mesotheliomas. Thus, the extended time of exposure of the pleural mesothelium to carcinogenic levels of MWCNT‐7 fibers is likely to be a major factor in the difference in mesothelioma development in the whole‐body inhalation study reported by Kasai et al6 and our present study.
Another notable difference between the whole‐body inhalation study and our present study is that rats administered MWCNT‐7 by TIPS did not develop lung tumors. The most likely reason for this surprising result is the early deaths caused by pathologies associated with the development of malignant mesothelioma in the exposed rats. Importantly, 17 of the 19 rats in the MWCNT‐7 group developed obvious epithelial type II cell hyperplasia in the lung, and it is likely that if these rats had not died from mesothelioma they would have developed lung tumors.
The results of the present study raise an additional point: the rapid development of malignant mesothelioma and the apparently much slower development of potential lung tumors. This difference in susceptibility to tumor development can be attributed to the extremely robust defenses of the rat lung to inhaled dusts. For example, the crocidolite asbestos fiber burden required for a carcinogenic response in rats is approximately 1250 fibers/μg dry lung tissue while the fiber burden in humans that can lead to increased tumor risk is approximately 0.2 fibers/μg dry lung weight10 (also see Bignon et al11). While the short life span of the rat likely plays a role in this 6000‐fold difference in susceptibility to crocidolite asbestos, it is not surprising that in an animal such as the rat, tissue defenses in the lung, which is chronically exposed to dusts, would be robust. In contrast, defenses in the pleural cavity, which is not exposed to dusts, would be expected to be much less robust than those in the lung. Therefore, it is reasonable that carcinogenic levels of MWCNT‐7 would be lower for the pleural cavity than for the lung, and once these levels are reached, carcinogenic processes in the pleural cavity would proceed more rapidly than in the lung. In the present study, while the dose administered to the lung was expected to be carcinogenic, it was also expected that carcinogenic levels of MWCNT‐7 would accumulate in the pleural cavity in a relatively short time (see below), resulting in the rapid development of mesotheliomas in the MWCNT‐7 treated rats.
The dose of 1.5 mg/rat MWCNT‐7 is equivalent to the final amount of MWCNT‐7 that accumulated in the lungs of rats exposed to 2 mg/m3 MWCNT‐7 in the whole‐body inhalation study reported by Kasai et al (1.8 mg/lung in male rats).6 Thus, if the whole‐body inhalation study could have been extended and if this extension would have resulted in development of mesotheliomas in the exposed rats, then administration of 1.5 mg MWCNT‐7 to rats using TIPS should result in development of mesothelioma. Because we found that administration of 1.5 mg MWCNT‐7 to the rat lung did induce the development of malignant pleural mesothelioma, our results strongly suggest that the MWCNT‐7 that accumulated in the lungs of the rats exposed to MWCNT‐7 by whole‐body inhalation would have ultimately induced the development of mesotheliomas in these rats.
The 1.5 mg/rat dose did not exceed the maximum tolerated dose (MTD) for carcinogenicity studies using small rodents as defined in “Guidelines for carcinogen bioassay in small rodents”:12 There should be no more than a 10% decrease in weight compared to the control and the dose should not induce mortality, toxicity or pathologic lesions other than those related to a neoplastic response. As can be seen in Figure S1, until the rats administered MWCNT‐7 began dying from mesothelioma at week 47, the body weights of the MWCNT‐7 group were only slightly decreased compared to the body weights of the control group.
While inhalation is the most natural route of exposure to respiratory particles and fibers, this method of exposure has a few disadvantages. For obligate nose breathers, such as the rat, there is considerable filtering of inhaled particulate matter in the nasal passages, resulting in shorter fibers with smaller aerodynamic diameters being deposited beyond the ciliated airways than is the case with humans.13 Because fiber length can affect its pathogenicity1, 14, nasal filtering can have a major impact on the carcinogenicity of respirable fibers in studies with rats and mice. Another factor is the lifespan of rats and mice. While the 2‐year inhalation study reported by Kasai et al6 found that exposure to MWCNT‐7 caused lung tumors but not pleural mesotheliomas, the results of our study strongly suggest that the MWCNT‐7 fibers that had accumulated in the lungs of the exposed male rats after 2 years would have induced malignant pleural mesotheliomas if the study could have been extended for approximately 12 to 18 months. Finally, very few institutes are equipped to carry out whole‐body inhalation studies because of the cost and specialized equipment and animal facilities required for such studies. This has resulted in relatively few whole‐body inhalation studies being performed. For example, since the initial studies that demonstrated the potential carcinogenicity of MWCNT using intraperitoneal and intrascrotal injection2, 3, 4, 15 only a single whole‐body inhalation study of a single MWCNT has been carried out.6 Therefore, in the present study we used TIPS to administer MWCNT‐7 and crocidolite: TIPS administers the test material directly to the lung, bypassing the nasal passages; TIPS administers the test material at the beginning of the study, allowing carcinogenic processes to proceed for the entire study period; and TIPS uses commonly available equipment and techniques, allowing its widespread use.
In conclusion, using TIPS administration, this study demonstrated that MWCNT‐7 administered via the airway induces malignant pleural mesothelioma in rats. Our results also suggest that the MWCNT‐7 that accumulated in the lungs of the male rats in the 2‐year study reported by Kasai et al6 would probably have caused development of mesothelioma in exposed rats if it would have been possible to extend the study for approximately 12 to 18 months.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Chisato Ukai and Takako Narita for their excellent secretarial assistance.
Numano T, Higuchi H, Alexander DB, et al. MWCNT‐7 administered to the lung by intratracheal instillation induces development of pleural mesothelioma in F344 rats. Cancer Sci. 2019;110:2485–2492. 10.1111/cas.14121
Contributor Information
David B. Alexander, Email: dalexand@phar.nagoya-cu.ac.jp.
Hiroyuki Tsuda, Email: htsuda@phar.nagoya-cu.ac.jp.
REFERENCES
- 1. Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety‐by‐design. Nanomedicine (Lond). 2011;6:143‐156. [DOI] [PubMed] [Google Scholar]
- 2. Sakamoto Y, Nakae D, Fukumori N, et al. Induction of mesothelioma by a single intrascrotal administration of multi‐wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34:65‐76. [DOI] [PubMed] [Google Scholar]
- 3. Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose‐dependent mesothelioma induction by intraperitoneal administration of multi‐wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 2012;103:1440‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53 + /− mouse by intraperitoneal application of multi‐wall carbon nanotube. J Toxicol Sci. 2008;33:105‐116. [DOI] [PubMed] [Google Scholar]
- 5. Sargent LM, Porter DW, Staska LM, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi‐walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kasai T, Umeda Y, Ohnishi M, et al. Lung carcinogenicity of inhaled multi‐walled carbon nanotube in rats. Particle and Fibre Toxicology. 2016;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzui M, Futakuchi M, Fukamachi K, et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 2016;107:924‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohnishi M, Suzuki M, Yamamoto M, et al. Improved method for measurement of multi‐walled carbon nanotubes in rat lung. J Occup Med Toxicol. 2016;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukushima S, Kasai T, Umeda Y, Ohnishi M, Sasaki T, Matsumoto M. Carcinogenicity of multi‐walled carbon nanotubes: challenging issue on hazard assessment. J Occup Health. 2018;60:10‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodelsperger K, Woitowitz HJ. Airborne fibre concentrations and lung burden compared to the tumour response in rats and humans exposed to asbestos. Ann Occup Hyg. 1995;39:715‐725. [PubMed] [Google Scholar]
- 11. Bignon J, Brochard P, Brown R, et al. Assessment of the toxicity of man‐made fibres. A final report of a workshop held in Paris, France 3‐4 February 1994. Ann Occup Hyg. 1995;39:89‐106. [DOI] [PubMed] [Google Scholar]
- 12. Program NT . Guidelines for carcinogen bioassay in small rodents. National Cancer Institute Carcinogenesis Technical Report Series. 1976; 1: 1. https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr001.pdf [PubMed]
- 13. Miller FJ. Dosimetry of particles in laboratory animals and humans in relationship to issues surrounding lung overload and human health risk assessment: a critical review. Inhalation Toxicol. 2000;12:19‐57. [DOI] [PubMed] [Google Scholar]
- 14. Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagai H, Okazaki Y, Chew SH, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:E1330‐E1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


