Abstract
Background
The long-term use of opioid analgesics is limited by the development of unwanted side-effects, such as tolerance. The molecular mechanisms of morphine anti-nociceptive tolerance are still unclear. The mitochondrial calcium uniporter (MCU) is involved in painful hyperalgesia, but the role of MCU in morphine tolerance has not been uncharacterised.
Methods
Rats received intrathecal injection of morphine for 7 days to induce morphine tolerance. The mechanical withdrawal threshold was measured using von Frey filaments, and thermal latency using the hotplate test. The effects of an MCU inhibitor, antisense oligodeoxynucleotide against cyclic adenosine monophosphate response element (CRE)-binding protein (CREB) or cytoplasmic polyadenylation element-binding protein 1 (CPEB1) in morphine tolerance were examined.
Results
Spinal morphine tolerance was associated with an increased expression of neuronal MCU, phospho-CREB (pCREB), and CPEB1 in the spinal cord dorsal horn. MCU inhibition increased the mechanical threshold and thermal latency, and reduced the accumulation of mitochondrial calcium in morphine tolerance. Intrathecal antisense oligodeoxynucleotide against CREB or CPEB1 restored the anti-nociceptive effects of morphine compared with mismatch oligodeoxynucleotide in von Frey test and hotplate test. Chromatin immunoprecipitation with quantitative PCR assay showed that CREB knockdown reduced the interaction of pCREB with the ccdc109a gene (encoding MCU expression) promoter and decreased the MCU mRNA transcription. RNA immunoprecipitation assay suggested that CPEB1 binds to the MCU mRNA 3′ untranslated region. CPEB1 knockdown decreased the expression of MCU protein.
Conclusions
These findings suggest that spinal MCU is regulated by pCREB and CPEB1 in morphine tolerance, and that inhibition of MCU, pCREB, or CPEB1 may be useful in preventing the development of opioid tolerance.
Keywords: calcium, CPEB, CREB, mitochondrial calcium uniporter, morphine, opioid tolerance, opioids
Editor's key points.
-
•
The molecular mechanisms of morphine tolerance are unclear.
-
•
The role of the mitochondrial calcium uniporter (MCU) in morphine tolerance was characterised in a rat model of morphine tolerance induced by intrathecal morphine injection for 1 week.
-
•
Morphine tolerance was associated with increased expression of neuronal MCU, phospho-CREB, and CPEB1 in the spinal cord dorsal horn.
-
•
Inhibition of the MCU and CREB or CPEB1 knockdown increased mechanical threshold and thermal latency, indicating that the spinal MCU is regulated by pCREB and CPEB1 in morphine tolerance.
-
•
Inhibition of the MCU, pCREB, or CPEB1 provide potential novel targets for preventing development of opioid tolerance.
Consumption of prescription opioids has been growing.1, 2 However, long-term opioid use is limited by the development of unwanted side-effects, such as tolerance, hyperalgesia, and dependence/addiction, which contribute to the current epidemic of opioid abuse and overdose-related deaths in the USA.2, 3, 4 Tolerance to opioid analgesia is described as reduced responsiveness to the analgesia produced by opioids. Previous evidence showed that morphine tolerance induces neurotoxicity in spinal neurones5; however, the molecular mechanism of morphine tolerance is not clear.
The mitochondrial calcium uniporter (MCU) is a critical protein of the inner mitochondrial membrane mediating Ca2+ uptake into the matrix.6 MCU is involved in the induction of painful hyperalgesia and spinal long-term potentiation (LTP).7 Chronic morphine induces a facilitated LTP.8 However, the role of MCU in morphine tolerance is not clear. The transcription factor, cyclic adenosine monophosphate response element (CRE)-binding protein (CREB), has been shown to regulate neural plasticity.9 Short- and long-term morphine exposure alter phosphorylated CREB (pCREB) expression in different brain regions.10 Chronic morphine raises pCREB levels in the lumbar dorsal root ganglion and the dorsomedial nucleus of the hypothalamus.10, 11 Repeated morphine exposure causes neuroadaptations in neurones through the activation of CREB.12 Cytoplasmic polyadenylation element-binding proteins (CPEB) are mRNA-binding proteins associated with protein translation that play an important role in development, health, and disease.13 Recent evidence shows that CPEB is involved in hyperalgesia priming and pain chronification.14, 15, 16 Of the four CPEB protein subtypes (CPEB1–4),17 CPEB1 is involved in anxiety and chronic pain.18 Similar cellular and intracellular changes occur in the spinal cord after peripheral nerve injury and morphine tolerance19; however, the role of MCU or CPEB1 in morphine tolerance remains unclear.
pCREB binds at the promoter of the ccdc109a gene (encoding MCU expression) and stimulates its expression.20 However, the relationship of MCU and pCREB or CPEB1 in morphine tolerance is not clear. Here, we report that pCREB mediates the expression of MCU transcription in an epigenetic manner, and that CPEB1 mediates the expression of spinal MCU protein.
Methods
Animals
Before the beginning of the study, male 7–8-week-old Sprague Dawley rats (weight: 210–230 g) obtained from Charles River Laboratories, Wilmington MA USA were housed (one to three per cage) for ∼1 week. The rats freely received access to food and water, and were maintained on a 12:12 light:dark schedule at 21°C and 60% humidity. The University of Miami Institutional Animal Care and Use Committee approved the housing conditions and experimental procedures. All procedures were in accordance with relevant aspects of the Animal Research: Reporting of In Vivo Experiments and National Institutes of Health guidelines. An intrathecal catheter was implanted through an incision in the atlanto-occipital membrane, and advanced 8.5 cm caudally to position its tip at the level of the lumbar enlargement, as described21 (see Supplementary Methods and Results for details).
Anti-nociception testing
We used calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA) to determine the mechanical withdrawal threshold serially to the hind paw in ascending order of strength, as described.21 Experimenters were blinded to the groups during behavioural tests. Hotplate thermal latency was measured with a hotplate apparatus (IITC Life Science Inc., Woodland Hills, CA, USA). The anti-nociceptive effects of morphine were represented as a percentage of the maximum possible effect (%MPE) using the formula %MPE=([test–baseline]/[cut-off–baseline])×100% (see Supplementary Methods and Results for details).
Quantitative real time polymerase chain reaction
Rat lumbar L4/5 spinal cord dorsal horn (SCDH) tissue was collected and RNeasy Mini Kit (Qiagen, Germantown, MD, USA) was used for total RNA isolation. One microgram of RNA was converted into cDNA, and then real-time polymerase chain reaction (PCR) was performed (see Supplementary Methods and Results for details).
Western immunoblots
Rat SCDH tissue or B35 cells (American Type Culture Collection (ATCC), Manassas, VA) were lysed, homogenised, and sonicated with 1× RIPA protein lysis buffer containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Proteins were denatured and transferred onto a polyvinylidene difluoride (PVDF) membrane, and the membrane was incubated with primary antibodies overnight at 4°C, including rabbit polyclonal anti-MCU, mouse monoclonal anti-pCREB, goat anti-CPEB1, mouse anti-VDAC1, and mouse monoclonal anti-β-actin. The PVDF membrane was incubated with secondary antibodies, and then developed in chemiluminescence solution (see Supplementary Methods and Results for details).
Chromatin immunoprecipitation with quantitative PCR
For chromatin immunoprecipitation with quantitative PCR (ChIP-qPCR), SCDH tissue was homogenised and fixed with formaldehyde 1% for 10 min, and 2.5 M glycine was used to stop the reaction. Fixed tissue was washed and resuspended with sodium dodecyl sulphate (SDS) lysis buffer 250 μl (50 mM Tris-HCl [pH 8.0], 10 mM ethylenediamine tetra-acetic acid, and SDS 1%), as described22 (see Supplementary Methods and Results for details).
RNA immunoprecipitation with quantitative PCR
B35 cells were treated with recombinant tumour necrosis factor alpha (rTNFα) (10 ng ml−1) or vehicle,22 cross linked with formaldehyde 1%, lysed in lysis buffer, and immunoprecipitated with anti-CPEB1 antibody (see Supplementary Methods and Results for details). RNA was extracted and converted into cDNA, and then real-time PCR was performed with Fast SYBR Green Master Mix (Applied Biosystems, Grand Island, NY, USA).
Immunohistochemistry
Immunostaining was carried out, as described.23 On Day 7 of chronic morphine, 1.5 h after the last morphine dose, the rats were perfused with paraformaldehyde (PFA) 4% in 0.1 M phosphate-buffered saline. The spinal cord was postfixed and cryoprotected. Immunostaining of glial fibrillary acidic protein (GFAP), OX42, NeuN, pCREB, MCU, or CPEB1 in the SCDH was performed (see Supplementary Methods and Results for details).
Mitochondrial Ca2+ imaging in the spinal dorsal horn
Rhod-2/AM (a mitochondrial Ca2+ indicator; Thermo Fisher Scientific, Waltham, MA) was dissolved in dimethylsulphoxide 2% in saline to a final concentration of 33 μM. Rhod-2 (30 μl) was injected intrathecally, as described previously24 (see Supplementary Methods and Results for details).
Drugs
Morphine sulphate was purchased from West-Ward Pharmaceuticals (Eatontown, NJ, USA). MCU antagonist ruthenium 360 (Ru360) was purchased from VWR (Radnor, PA, USA) and dissolved in saline. The sequences of rat antisense CREB oligodeoxynucleotides (ODNs), 5′-TGGTCATCTAGTCACCGGTG-3′, and mismatch ODN (mmODN), 5′-GACCTCAGGTAGTCGTCGTT-3′ (synthesised by Sigma-Aldrich), were designed, as reported.25, 26 The sequences of rat CPEB1 antisense ODN, 5′-CATACACCACTCCACCAAATAG-3′, and mmODN, 5′-AATAGAACACACCACCTGATAC-3′, were purchased from Sigma-Aldrich.
Data analysis
Behavioural data were analysed by two-way analysis of variance (anova) with repeated measures subjected to Bonferroni testing. Statistical significance was determined by t-test or one-way anova with post hoc test after Fisher's protected least significant difference (PLSD) (StatView 5, SAS Institute Inc., Cary, NC). Data are presented as mean (standard error of the mean), with P-values of <0.05 considered statistically significant.
Results
Role of spinal MCU in morphine tolerance
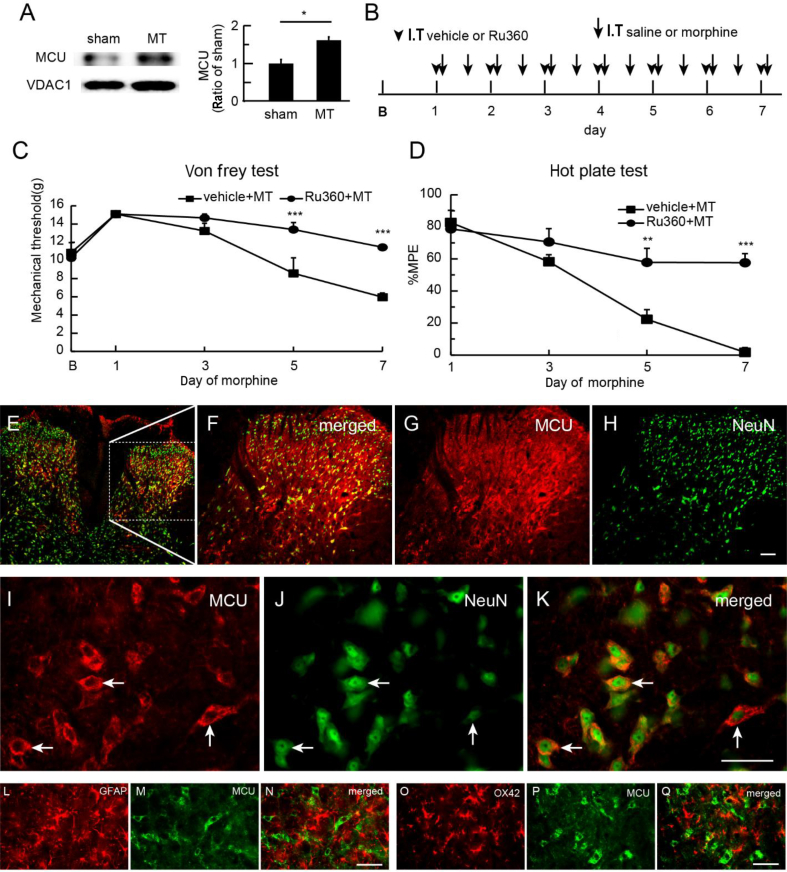
To examine the role of the MCU in morphine tolerance, we harvested SCDH after the last morphine on Day 7 for immunoblotting. Morphine tolerance increased the expression of MCU in the SCDH compared with sham; P<0.05; t-test; n=5–6 (Fig. 1a). We then examined whether MCU inhibition reduced the spinal morphine tolerance. Ru360, an MCU inhibitor, specifically blocks Ca2+ uptake by MCU.27 We injected Ru360 (50 μM) intrathecally 30 min before the morning morphine injection for 7 days (Fig. 1b), and tested the mechanical threshold and thermal latency before and 60 min after the morning morphine injection on Days 1, 3, 5, and 7. Ru360 up-regulated the mechanical withdrawal threshold compared with vehicle (saline) (Fig. 1c). The mechanical withdrawal threshold in the Ru360 group was higher than in the control on Days 5 and 7 (P<0.001 vs vehicle; two-way anova Bonferroni tests; n=6) (Fig. 1c). In the hotplate test, thermal-latency MPE was higher in the Ru360 group than that in the saline vehicle group on Day 5 or 7 (P<0.01, or P<0.001 vs vehicle, respectively; two-way anova Bonferroni tests; n=6) (Fig. 1d). Neither Ru360 nor vehicle changed the basic mechanical threshold and thermal latency in control rats (Supplementary Fig. S1). The immunoreactivity (IR) of MCU in SCDH co-localised with NeuN (a neuronal marker; Fig. 1e–k), but not with GFAP (a marker of astrocytes; Fig. 1l–n) or OX42 (a marker of microglia; Fig. 1o–q), suggesting that MCU was expressed in SCDH neurones, but not glia, in morphine-tolerant rats.
Fig. 1.
Effect of mitochondrial calcium uniporter (MCU) inhibition on morphine tolerance. (a) We harvested spinal cord dorsal horn (SCDH) 1 h after the last morphine dose on Day 7 in morphine-tolerant rats. Western blots demonstrated that morphine tolerance increased the expression of MCU in the SCDH compared with sham (P<0.05; t-test; n=5–6). (b) Scheme for intrathecal administration. Morphine tolerance was induced by intrathecal injection of morphine (arrow; twice a day); sham group was saline. Ruthenium 360 (Ru360) or vehicle (arrowhead) was administered every morning 30 min before morphine or saline (sham). (c) Intrathecal MCU inhibitor, Ru360, significantly up-regulated the mechanical withdrawal threshold compared with vehicle (F(4,40)interaction=6.62, P<0.001; F(4,40)main effect time=23.51, P<0.0001; F(1,10)main effect treatment=12.53, P<0.01; two-way anova repeated measures; n=6). The mechanical withdrawal threshold in the Ru360 group was higher than that in the vehicle group on Days 5 and 7 (***P<0.001 vs vehicle; two-way anova Bonferroni tests). (d) In the hotplate test, there was a significant difference in maximum possible effect (MPE) (%) of thermal latency between Ru360 and saline (F(3,30)interaction=9.47; P<0.001; F(3,30)main effect time=29.45; P<0.0001; F(1,10)main effect treatment=25.47; P<0.001; two-way anova repeated measures; n=6). MPE (%) in the Ru360 group was higher than that for vehicle on Day 5 or 7 (**P<0.01; ***P<0.001 vs vehicle; two-way anova Bonferroni tests). (e–h) Low magnification of MCU immunoreactivity (IR) (MCU-IR) images. (i–k) High-magnification images display that MCU-IR co-localised with NeuN, but not with (l–n) glial fibrillary acidic protein (GFAP) or (o–q) OX42; scale bar: 50 μm. MT, morphine anti-nociceptive tolerance.
MCU inhibition reduced spinal cord dorsal horn neurone mitochondrial calcium
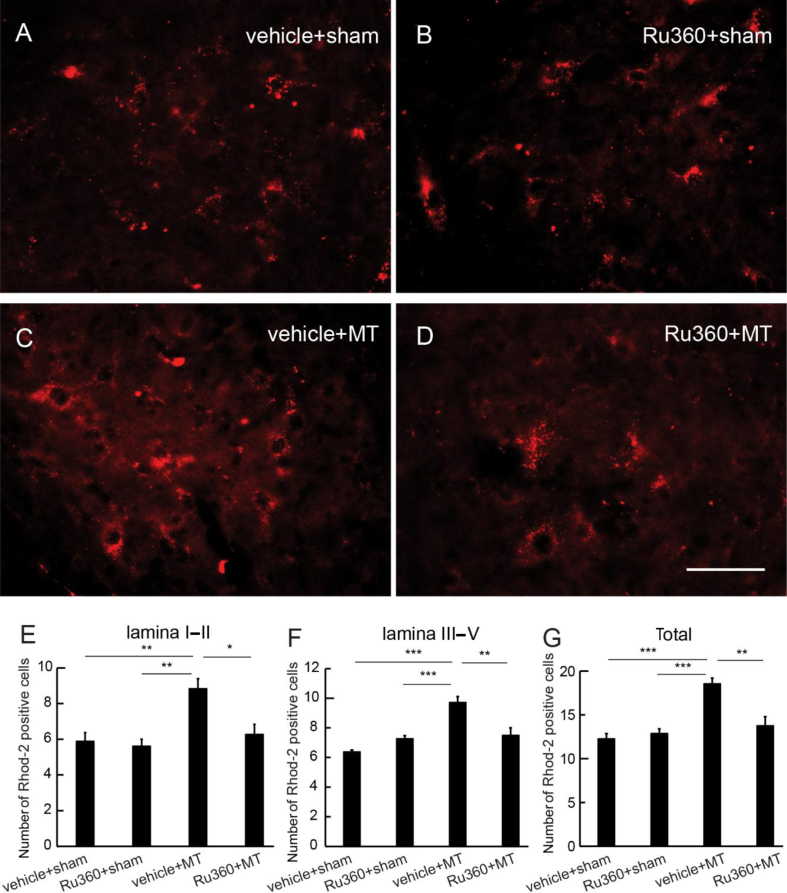
We examined whether MCU inhibition reduced the spinal mitochondrial Ca2+ using a Rhod-2 assay.24 Ru360 (50 μM) was injected intrathecally every morning 30 min before the morphine injection for 7 days. Half an hour after the last morphine dose, Rhod-2 was injected intrathecally, the animals were perfused 70 min after Rhod-2, and the spinal cord was harvested for imaging. Representative images of Rhod-2-positive cells in vehicle+sham, Ru360+sham, vehicle+morphine anti-nociceptive tolerance (MT), or Ru360+MT are shown in Fig 2. There was a significant increase in Rhod-2-positive cell number in SCDH Laminae I and II in the vehicle+MT group compared with that in the vehicle+sham or Ru360+sham groups (P<0.01; one-way anova; Fig. 2e); Rhod-2-positive cells in the Ru360+MT group were lower than in the vehicle+MT SCDH Laminae I and II (P<0.05; one-way anova; Fig. 2e). Similarly, there was an increase in Rhod-2-positive cells in SCDH Laminae III–V (Fig. 2f) and total amount of Rhod-2-positive cells in Laminae I–V (Fig. 2g) in the vehicle+MT group compared with the vehicle+sham or Ru360+sham group. There were fewer Rhod-2-positive cells in the Ru360+MT group than in the vehicle+MT group in Laminae III–V (Fig. 2f) or in total Rhod-2-positive cells in Laminae I–V (Fig. 2g).
Fig. 2.
Effect of mitochondrial calcium uniporter inhibition on mitochondrial calcium in spinal cord dorsal horn (SCDH) neurones. Ruthenium 360 (Ru360) (50 μM) was injected intrathecally every morning 30 min before morphine injection once a day for 7 days. At 30 min after the last morphine, Rhod-2/AM was intrathecally injected for Rhod-2 imaging. (a–d) Representative image of Rhod-2 in the SCDH in (a) saline+sham, (b) Ru360+sham, (c) saline+morphine anti-nociceptive tolerance (MT), and (d) Ru360+MT groups. (e) There was a significant increase in Rhod-2-positive cells at SCDH Laminae I and II in the vehicle+MT group compared with the vehicle+sham or Ru360+sham groups (**P<0.01; one-way anova; n=6); Rhod-2-positive cells in the Ru360+MT group was lower than that in the vehicle+MT group in SCDH Laminae I and II (P<0.05; one-way anova; n=6). Similarly, there was a significant increase in Rhod-2-positive cells in the vehicle+MT group compared with the vehicle+sham or Ru360+sham group in (f) Laminae III–V or (g) Laminae I–V (***P<0.001; one-way anova; n=6). (f) Rhod-2-positive cells in Ru360+MT group were fewer than in the vehicle+MT group in SCDH Laminae III–V (**P<0.01; one-way anova; n=6). (g) Total Rhod-2-positive cells in Laminae I–V in the Ru360+MT group was lower than in the vehicle+MT group (**P<0.01; one-way anova; n=6).
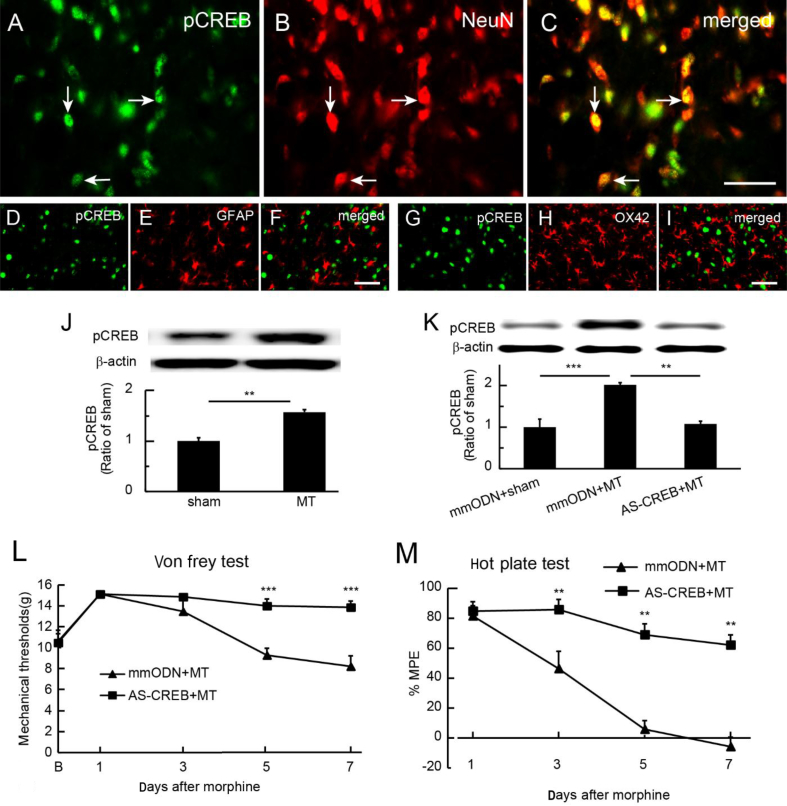
Knockdown of spinal CREB reduced morphine tolerance
Chronic morphine exposure increases pCREB expression in various brain regions.10 Immunostaining showed that spinal pCREB co-localised with NeuN (Fig. 3a–c), but not with GFAP (Fig. 3d–f) or OX42 (Fig. 3g–i), in morphine-tolerant rats. Western immunoblots showed that the expression of pCREB was up-regulated in the SCDH in the morphine tolerant compared with the sham group; P<0.01; t-test; n=6 (Fig. 3j). To determine if knockdown of CREB using antisense ODN against CREB (AS-CREB; 50 μg) reduced the spinal morphine tolerance, we intrathecally administered AS-CREB or mmODN 30 min before each morning morphine injection for 7 days. Using western immunoblots, we found that spinal pCREB in the mmODN+MT group was higher than in the sham group; P<0.001; one-way anova with post hoc Fisher's PLSD; n=4–5 (Fig. 3k); in the AS-CREB+MT group, pCREB was lower than in the mmODN+MT group; P<0.01; one-way anova with post hoc Fisher's PLSD; n=4–5 (Fig. 3k). We tested the mechanical threshold and thermal latency 60 min after morphine injection on Days 1, 3, 5, and 7. Intrathecal AS-CREB caused an elevation of mechanical threshold compared with mmODN (Fig. 3l). The mechanical threshold in the AS-CREB group was higher than that in the mmODN group on Days 5 and 7; P<0.001 vs mmODN; two-way anova Bonferroni tests; n=6 (Fig. 3l). Intrathecal AS-CREB increased MPE in thermal latency compared with mmODN (Fig. 3m). Thermal latency in the AS-CREB group was higher than in the mmODN group on Day 5 or 7; P<0.01 vs mmODN; two-way anova Bonferroni tests; n=6 (Fig. 3m). In sham rats receiving repeatedly intrathecal saline for 7 days, there was no significant difference between intrathecal mmODN and AS-CREB injections on basic mechanical threshold and thermal latency (Supplementary Fig. S2).
Fig. 3.
Effect of knockdown of transcriptional factor, cyclic adenosine monophosphate response element (CRE)-binding protein (CREB), on spinal morphine tolerance. (a–c) Immunostaining showed that phosphorylated cyclic adenosine monophosphate response element-binding protein (pCREB) co-localised with NeuN, but not with (d–f) glial fibrillary acidic protein (GFAP) or (g–i) OX42 in spinal cord dorsal horn (SCDH) in morphine-tolerant rats, suggesting neuronal pCREB activity in morphine tolerance. (j) Western immunoblots showed that morphine tolerance up-regulated the expression of pCREB in the SCDH compared with the sham group (**P<0.01; t-test; n=6). (k) Expression of spinal pCREB in the mismatch ODN (mmODN)/morphine anti-nociceptive tolerance (MT) group was higher than in the sham group (***P<0.001; one-way analysis of variance [anova] with post hoc Fisher's protected least significant difference [PLSD]; n=4–5); pCREB in the AS-CREB+MT group was significantly lower than that in the mmODN+MT group (**P<0.01; one-way anova with post hoc Fisher's PLSD; n=4–5). (l) Intrathecal AS-CREB caused an elevation of mechanical withdrawal threshold compared with mmODN (F(4,40)main interaction=6.70, P<0.001; F(4,40)main effect time=15.08, P<0.0001; F(1, 10)=22.93, P<0.001; two-way anova repeated measures; n=6). The mechanical withdrawal threshold in the AS-CREB group was higher than in the mmODN group on Days 5 and 7 (***P<0.001 vs mmODN; two-way anova Bonferroni tests; n=6). (m) Intrathecal AS-CREB increased %MPE in thermal latency compared with mmODN (F(3,40)main effect interaction=9.08, P<0.001; F(3,30)main effect time=26.41, P<0.0001; F(1, 10)main effect treatment= 55.29, P<0.0001; two-way anova repeated measures; n=6). Thermal latency in the AS-CREB group was higher than that in the mmODN group on Day 5 or 7 after ODN (**P<0.01 vs mmODN; two-way anova Bonferroni tests; n=6).
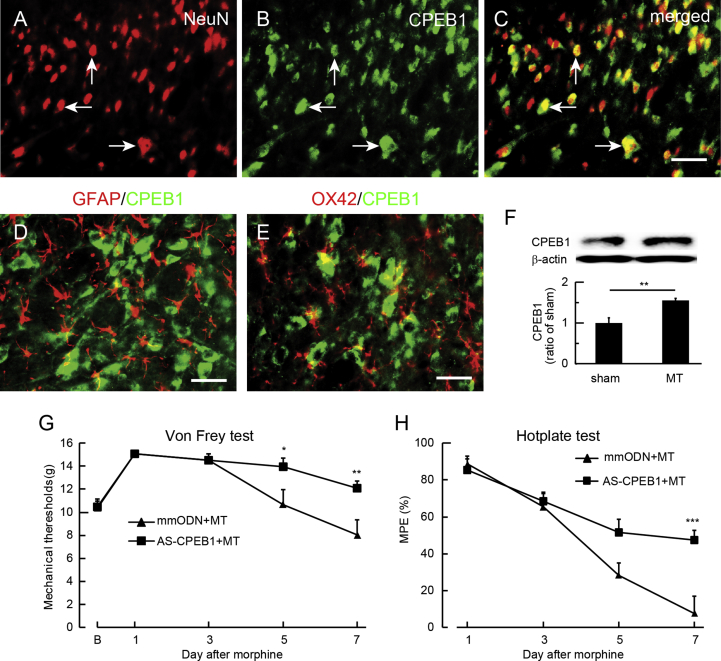
Role of spinal CPEB in morphine tolerance
CPEB is a sequence-specific RNA-binding protein at the mRNA 3′ untranslated region (UTR) regulating mRNA translation that modulates neuronal synaptic plasticity.28 CPEB is responsible for induction of hyperalgesia priming in the chronification of pain,14 and CPEB1 is involved in anxiety and chronic pain.18 To examine the role of CPEB in morphine tolerance, double immunostaining showed that CPEB1 IR co-localised with NeuN (Fig. 4a–c), but not with GFAP (Fig. 4d) or OX42 (Fig. 4e). Western blots showed that morphine tolerance increased the expression of CPEB1 in SCDH compared with sham treatment (P<0.01; t-test; n=4; Fig. 4f). To further verify the role of CPEB1 in morphine tolerance, we examined the effect of CPEB1 knockdown using CPEB1 antisense ODN (AS-CPEB). AS-CPEB1 or mmODN (40 μg) was injected intrathecally 30 min before each morning morphine injection for 7 days followed by testing of mechanical threshold and thermal latency 60 min after the injection on Days 1, 3, 5, and 7. Intrathecal AS-CPEB1 caused an elevation of mechanical withdrawal threshold compared with mmODN (Fig. 4g). The mechanical withdrawal threshold in the AS-CPEB1+MT group was higher than in the mmODN+MT group on Days 5 and 7 (P<0.05; two-way anova Bonferroni tests; Fig. 4g). Intrathecal AS-CPEB increased MPE in the hotplate test compared with mmODN (Fig. 4h). Thermal MPE in the AS-CPEB1+MT group was higher than in the mmODN+MT group on Day 7 (P<0.001 vs mmODN+MT; two-way anova Bonferroni tests; Fig. 4h). Neither AS-CPEB1 nor mmODN changed the basic mechanical threshold or thermal latency in sham rats (Supplementary Fig. S3).
Fig. 4.
Effect of spinal cytoplasmic polyadenylation element-binding protein 1 (CPEB1) on morphine tolerance. Double immunostaining showed that the immunoreactivity (IR) of CPEB1-IR co-localised with (a–c) NeuN, but not with (d) glial fibrillary acidic protein (GFAP) or (e) OX42. (f) Western immunoblots showed that morphine tolerance increased the expression of CPEB1 in the spinal cord dorsal horn compared with sham (**P<0.01; t-test; n=4). (g) Intrathecal AS-CPEB1 caused an elevation of mechanical withdrawal threshold compared with mismatch oligodeoxynucleotide (mmODN) (F(4,40)interaction=4.30, P<0.01; F(4,40) main effect time=22.13, P<0.0001; F(1,10) main effect treatment=5.27, P<0.05; two-way analysis of variance [anova] repeated measures). The mechanical withdrawal threshold in the AS-CPEB1+morphine anti-nociceptive tolerance (MT) group was higher than in the mmODN+MT group 5 and 7 days after ODN (*P<0.05, ** P<0.01 vs mmODN+MT; two-way anova Bonferroni tests). (h) Intrathecal AS-CPEB1 increased thermal latency using hotplate test compared with mmODN F(3,30)interaction=5.90, P<0.01; F(3,30) main effect time=43.68, P<0.0001; F(1,10)main effect treatment=6.78, P<0.05; two-way anova repeated measures). The mechanical withdrawal threshold in the AS-CPEB1+MT group was higher than in the mmODN+MT group on Day 7 (***P<0.001 vs mmODN+MT; two-way anova Bonferroni tests).
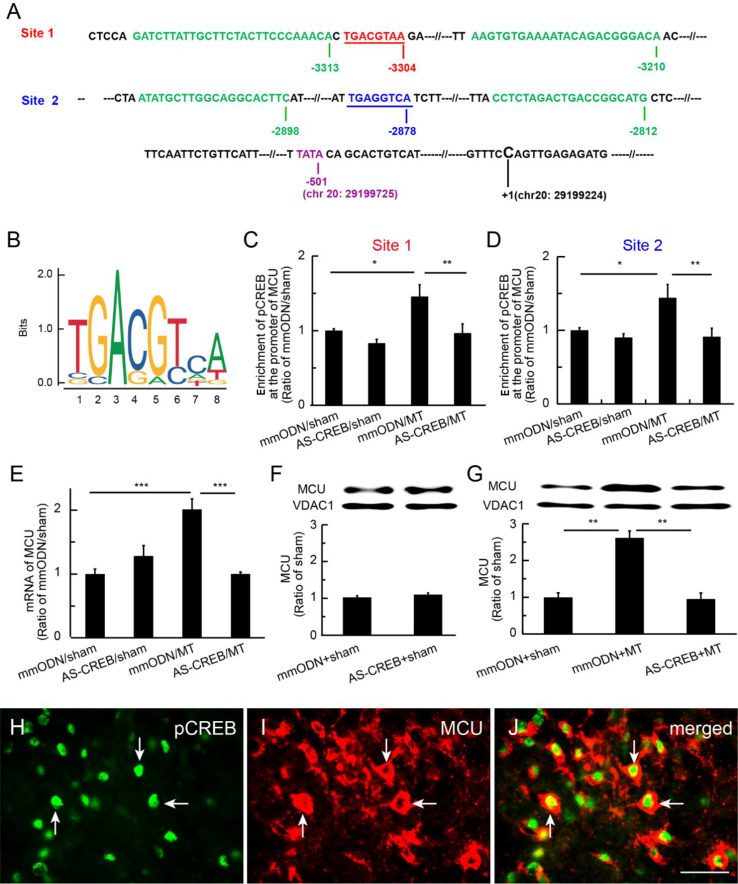
pCREB binding mediated MCU transcription
The activation of pCREB induces MCU expression through binding to the mcu gene promoter to drive mRNA transcription in human cultured HeLa cells.20 To further clarify the role of pCREB in mediating MCU expression in rats in vivo, we analysed the alignment of rat mcu gene promoter regions. The two putative CRE-binding areas, Site 1 (TGACGTAA)29 and Site 2 (TGAGGTCA)30, 31; the ChIP-qPCR primer areas of the gene at rat chromosome 20 (accession number: Genbank Location, Chromosome 20, NC_005119.4 (29037452..29199224); NCBI Reference Sequence: NC_005119.4); and the TATA box (a DNA sequence in the promoter region of genes) of the gene are shown in Fig 5a, and the motif of sequence logo for CREB-binding sites based on the JASPAR data sets (http://jaspar.genereg.net/matrix/MA0018.2) is shown in Fig 5b. We used the ChIP-qPCR assay to determine if pCREB mediated gene expression in the SCDH in an epigenetic manner. One hour after the last injection of morphine, lumbar SCDH was harvested for ChIP-qPCR, which showed that enrichment of pCREB at Site 1 or 2 on the mcu promoter in the mmODN+MT group was increased compared with the mmODN+sham group (P<0.05; one-way anova; Fig. 5c and d), and that enrichment of pCREB at Site 1 or 2 in the AS-CREB+MT group was lower than in the mmODN+MT group (P<0.01; one-way anova; Fig. 5c and d). RT–PCR revealed that MCU mRNA in the mmODN+MT group was higher than in the mmODN+sham group, and that MCU mRNA in the AS-CREB+MT group was lower than in the mmODN+MT group (Fig. 5e). Western immunoblots showed that there was no difference in the expression of MCU protein between mmODN+sham and AS-CREB+sham groups (Fig. 5f). However, MCU protein expression in the mmODN+MT group was higher than in the mmODN+sham group; MCU in the AS-CREB+MT group was lower than in the mmODN+MT group (P<0.01; one-way anova; Fig. 5g). These results suggest that pCREB modulates mcu gene expression in an epigenetic manner in morphine tolerance. Double immunostaining showed that pCREB was co-localised with MCU in the SCDH of morphine-tolerant rats (Fig. 5h–j).
Fig. 5.
Phosphorylated cyclic adenosine monophosphate response element-binding protein (pCREB) bound mcu gene promoter regions in the spinal cord dorsal horn (SCDH) and mediated mitochondrial calcium uniporter (MCU) transcription. (a) We analysed the alignment of rat MCU gene promoter regions, and found two putative CRE-binding areas, Site 1 (TGACGTAA)29 and Site 2 (TGAGGTCA),30, 31 and chromatin immunoprecipitation with quantitative PCR (ChIP-qPCR) primer areas of the MCU gene at rat chromosome 20 (accession number: NC_005119; NCBI Reference Sequence: NC_005119.4). The TATA box of mcu gene was shown. The first base C (chr 20: 29199224) of the first intron of the mcu gene was referred to as number +1. (b) Motif of the CREB-binding sites based on the JASPAR data sets (http://jaspar.genereg.net/matrix/MA0018.2). (c and d) ChIP-qPCR assay showed that enrichment of pCREB at CRE Site 1 or 2 of the mcu gene promoter region in the mismatch oligodeoxynucleotide (mmODN)+morphine anti-nociceptive tolerance (MT) group was increased compared with the mmODN+sham group (*P<0.05; one-way analysis of variance [anova]; n=5). Enrichment of pCREB at Site 1 or 2 on mcu gene promoter in the AS-CREB+MT group was lower than that in the mmODN+MT group (**P<0.01; one-way anova; post hoc Fisher's protected least significant difference [PLSD]; n=5). (e) RT–PCR showed increased MCU mRNA expression (***P<0.001; one-way anova; post hoc Fisher's PLSD test; n=5). (f and g) Western immunoblots showed no differences in expression of MCU protein between mmODN+sham and AS-CREB+sham groups (f). (g) MCU protein expression increased (**P<0.01; one-way anova). (h–j) Double immunostaining showed that pCREB was co-localised with MCU in the SCDH in morphine-tolerant rats; scale bar: 50 μm.
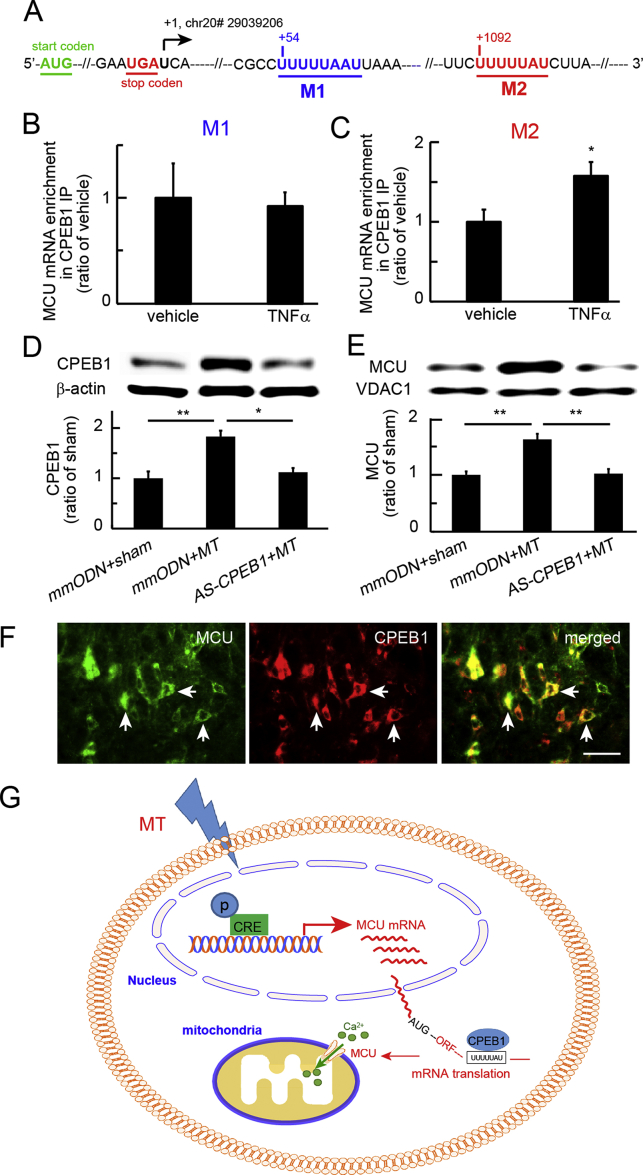
CPEB1 binding to MCU mRNA 3′ UTR in vitro
Transcription of the mcu gene is mediated by pCREB binding to the mcu promoter in HeLa cells.20 To verify whether CPEB1 regulates MCU mRNA translation to protein, we analysed the alignment of rat MCU mRNA 3′ UTR (immediately following the translation stop codon), and observed that there were two CPE motifs, UUUUUAAU32 (M1) and CPE consensus sequence UUUUUAU13, 33 (M2) (Fig. 6a; Supplementary Fig. S4). Expression of MCU and CPEB1 was induced in rat neuronal B35 cells treated with recombinant TNFα for 3 h, as described.22 Using an RNA immunoprecipitation (RNA-IP) assay, we observed that MCU mRNA expression in CPEB1 immunoprecipitates was not increased at the M1 CPE sequence (Fig. 6b); however, MCU mRNA in CPEB1 immunoprecipitates was increased at the M2 CPE consensus sequence (Fig. 6c). We verified that expression of MCU and CPEB1 proteins was up-regulated by rTNFα treatment (Supplementary Fig. S5).
Fig. 6.
Cytoplasmic polyadenylation element-binding protein 1 (CPEB1) on the mitochondrial calcium uniporter (MCU) mRNA. (a) We analysed the alignment of rat CPEB1 motifs at the MCU mRNA 3′ untranslated region (UTR) and found two putative CPE-binding areas, motif 1, UUUUUAAU32 (M1), and motif 2, CPE consensus sequence, UUUUUAU13, 33 (M2). AUG, translation start codon, and UGA, translation stop codon, are shown. The first base U (chr20# 29039206) following UGA was referred to as number +1. (b) To induce the expression of MCU and CPEB1, we used rat neuronal B35 cells treated with recombinant tumour necrosis factor alpha (rTNFα) for 3 h. Using an RNA immunoprecipitation assay, we observed that MCU mRNA in CPEB1 immunoprecipitation was not increased at the M1 CPE sequence, and that (c) MCU mRNA in CPEB1 immunoprecipitation was increased at the M2 CPE consensus sequence. (d) Morphine-tolerant rats were treated with antisense oligodeoxynucleotide against CPEB1. Western immunoblots showed a significant increase in CPEB1 in the mismatch oligodeoxynucleotide (mmODN)+morphine anti-nociceptive tolerance (MT) group compared with the sham group (**P<0.01; one-way analysis of variance [anova] with post hoc Fisher's protected least significant difference [PLSD] test; n=4–5). Spinal CPEB1 in the AS-CPEB1+MT group was lower than in the mmODN+MT group (*P<0.05; one-way anova with post hoc Fisher's PLSD test; n=4–5). (e) Western immunoblots showed that MCU in the spinal cord dorsal horn (SCDH) was increased in the mmODN+MT group compared with sham rats treated with mmODN; MCU in the SCDH of the AS-CPEB+MT group was lower than in the mmODN+MT group (**P<0.01; one-way anova with post hoc Fisher's PLSD; n=4–5). (f) Double immunostaining showed that CPEB1 immunoreactivity was co-localised with MCU; scale bar: 50 μm. (g) The proposed signalling pathways mediated by phosphorylated cyclic adenosine monophosphate response element-binding protein (pCREB) and CPEB1. Morphine tolerance evoked neuronal activity to induce phosphorylation of CREB. pCREB binds to CRE sites of the mcu gene promoter to induce MCU transcriptional expression. Up-regulated CPEB1 may regulate MCU translation. CPE motifs in MCU mRNA 3′ UTRs reside in a complex containing CPEB1.13 CPEB1 binds to the MCU mRNA 3′ UTR to regulate MCU mRNA translation. AUG, mRNA translation start codon; ORF, open reading frame of the stretch of codons between AUG and a stop codon.
Knockdown of spinal CPEB1 reduced the expression of spinal MCU protein in morphine tolerance
We determined the effect of spinal CPEB1 knockdown by CPEB1 antisense ODN on protein expression of CPEB1 and MCU in spinal morphine tolerance. SCDH was collected on Day 7 at 1 h after the last morphine injection for western immunoblot analysis. CPEB1 expression in the mmODN+MT group increased compared with the sham group (P<0.01; one-way anova with post hoc Fisher's PLSD test; n=4–5). Spinal CPEB1 in the AS-CPEB1+MT group was lower than in the mmODN+MT group (P<0.05; one-way anova with post hoc Fisher's PLSD test; n=4–5; Fig. 6d). MCU protein expression was increased in the mmODN+MT group compared with the sham group treated with mmODN (P<0.01; one-way anova with post hoc Fisher's PLSD; n=4–5; Fig. 6e). MCU in the AS-CPEB1+MT group was lower than in the mmODN+MT group (P<0.01; one-way anova with post hoc Fisher's PLSD; n=4–5; Fig. 6e). Double immunostaining showed that CPEB1 IR co-localised with MCU (Fig. 6f).
Discussion
We found that: (i) spinal morphine tolerance increased the expression of MCU, pCREB, and CPEB1 in the SCDH; (ii) MCU inhibition decreased the behavioural response to morphine tolerance and mitochondrial Ca2+ accumulation; (iii) CREB or CPEB1 knockdown reduced the morphine-tolerance behavioural response; (iv) pCREB regulated the mcu gene transcription in an epigenetic manner; (v) CPEB1 binds MCU mRNA 3′ UTR; and (vi) knockdown of CPEB1 down-regulated the MCU protein expression. The present findings suggest that spinal MCU was regulated by pCREB and CPEB1 in spinal morphine tolerance.
Chronic use of opioid analgesics is often hampered by the development of unwanted side-effects, such as tolerance and dependence, contributing to the current epidemic of opioid abuse and overdose-related deaths.34 The molecular mechanisms of morphine are still unclear. Chronic morphine treatment induces neuro-inflammation in a manner analogous to endotoxin.35 Opioid-induced neurotoxic consequences involve neuronal apoptosis.5 Chronic opioid use often leads to altered gene activation and increased oxidative stress affecting protein expression and ion channel function. N-methyl-D-aspartate receptor-mediated activation of intracellular signalling may also be involved in the development of opioid tolerance.36 Spinal reactive oxygen species contribute to chronic morphine effects.37, 38 Emerging evidence suggests a critical role for epigenetic mechanisms in opioid abuse.12 Gene transcriptional factors play a pivotal role in learning, memory, and drug abuse.12, 39 Chronic morphine induced the upregulation of spinal pCREB.36 However, the molecular modulation at specific genes in spinal morphine tolerance is still not clear.
Mitochondrial Ca2+ is involved in many functions from control of metabolism and ATP production to regulation of cell death. The activity of the MCU as a highly selective ion channel controls the mitochondrial Ca2+ influx.40 MCU, which was identified genetically in 2011,41, 42 enhances mitochondrial Ca2+ uptake upon overexpression.42 We observed that the MCU inhibitor, Ru360, reduced the behavioural response to morphine tolerance. Consistent with MCU expression controlling mitochondrial Ca2+ uptake in cultured cells20 and in mouse SCDH,24 we used Rhod-2/AM (a mitochondrial Ca2+ indicator) fluorescence assay to show that morphine tolerance increased the Ca2+ accumulation in mitochondria, and that intrathecal Ru360 decreased the mitochondrial Ca2+ in morphine-tolerant rats, the first demonstration of a role of MCU in morphine tolerance.
CREB is involved in learning, memory, and drug addiction,43 and is thought to be an important mediator in chronic morphine treatment.44, 45 The number of neurones expressing pCREB is increased in morphine-exposed cultured neurones, and in morphine-tolerant rats the number of pCREB-expressing neurones in the lumbar dorsal horn ganglion is also increased.11 Previous studies showed that morphine tolerance increased pCREB in the spinal cord.46, 47 We found that chronic morphine treatment increased pCREB in the SCDH in line with these findings.46, 47
CPEB plays an important role in modulating synaptic plasticity, learning, and memory.28 Polyadenylation of mRNA initiates translation, which is regulated by CPEB.28, 48 CPEB plays an important role in the induction of hyperalgesic priming, raising the possibility of a role in the chronification of pain.14 The CPEB family of RNA-binding proteins is composed of four members in vertebrates (CPEB1–4). CPEB1 is essential to the regulation of mitochondrial energy production in neurones,13 and stabilises the binding of activated cytoplasmic polyadenylation specific factor that attracts poly(A) polymerase to catalyse poly(A) elongation. The polyadenylated poly(A) tail is involved in the activation of protein translation. Knockdown of CPEB expression by intrathecal antisense ODN can prevent the induction of hyperalgesic priming,49 and a similar mechanism is shared in chronic opioid treatment.50 We found that chronic morphine tolerance increased the expression of CPEB1 in SCDH neurones, and that knockdown of CPEB1 reduced morphine tolerance, which is consistent with previous studies showing that CPEB1 is involved in hypernociception.14
Protein expression is controlled by extensive transcriptional or translational processing. Recent studies have implicated epigenetic modulation in brain reward regions after drug abuse or stress.51 However, little is known about MCU regulation through gene transcription and translation in morphine tolerance. CREB phosphorylation modulates gene transcription that is dependent on the activation of CRE at the promoters.52 In human HeLa cells, CREB binds the mcu gene promoter and initiates gene transcription.20 Our bioinformatic analysis revealed at least two putative CREB-binding sites in the rat mcu promoter location, and we found that morphine tolerance increased the enrichment of pCREB on mcu gene promoter regions, which was reduced by knockdown of CREB, suggesting that pCREB regulates mcu gene transcription.
CPEB1 is a sequence-specific mRNA-binding protein that controls mRNA translation to protein.53 CPEB binds CPE, a uridine-rich sequence element (consensus UUUUUAU motif) of the 3′ UTR of target mRNAs.33, 54 We found that MCU mRNA contains CPEs in its 3′ UTR and that CPEB1 was enriched on the MCU mRNA 3′ UTR in the cultured neurones, suggesting that CPEB1 mediates MCU mRNA translation and increases MCU protein expression. Knockdown of CPEB1 expression prevented the up-regulation of MCU protein, suggesting that CPEB1 plays an important role of MCU translation. However, the detailed mechanism of CPEB1 regulation of MCU translation is still unclear.
In conclusion, we provide evidence that spinal morphine tolerance increased MCU, pCREB, and CPEB1; that pCREB mediated MCU expression in an epigenetic manner; and that CPEB1 likely contributed to MCU protein regulation in morphine tolerance. The results also suggest that an MCU inhibitor, or agents blocking pCREB or CPEB1 upregulation may be useful in preventing the development of opioid tolerance, an issue of significant clinical relevance.
Authors' contributions
Study conception/design: KT, HY, JG, SH.
Data acquisition: KT, HY, JG, SH, DI, SL, TI, YK.
Data analysis/interpretation: all authors.
Writing paper: KT, HY, JG, SH, CD, TK.
Approving final version of the paper: all authors.
Declaration of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Authors thank Kentaro Hayashi’s assistant in preparing the paper.
Handling editor: H.C. Hemmings Jr
Editorial decision: 07 May 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.05.027.
Funding
National Institutes of Health (R01NS066792, DA026734, R01DA34749, R01DA047089, and R01DA047157) to SH; and the Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, FL, USA.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.O’Brien P.L., Karnell L.H., Gokhale M., Kenneth Pack B.S., Campopiano M., Zur J. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223–230. doi: 10.1016/j.drugalcdep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Volkow N.D., McLellan A.T. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 3.Frenk S.M., Porter K.S., Paulozzi L.J. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS Data Brief. 2015;1–8 [PubMed] [Google Scholar]
- 4.Miller M., Barber C.W., Leatherman S. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–615. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 5.Mao J., Sung B., Ji R.R., Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22:7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J.C., Parks R.J., Liu J. The in vivo biology of the mitochondrial calcium uniporter. Adv Exp Med Biol. 2017;982:49–63. doi: 10.1007/978-3-319-55330-6_3. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.Y., Lee K.Y., Lu Y. Mitochondrial Ca2+ uptake is essential for synaptic plasticity in pain. J Neurosci. 2011;31:12982–12991. doi: 10.1523/JNEUROSCI.3093-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko S.W., Wu L.J., Shum F., Quan J., Zhuo M. Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol Brain. 2008;1:2. doi: 10.1186/1756-6606-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrot M., Olivier J.D., Perrotti L.I. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren X., Lutfy K., Mangubat M. Alterations in phosphorylated CREB expression in different brain regions following short- and long-term morphine exposure: relationship to food intake. J Obes. 2013;2013:764742. doi: 10.1155/2013/764742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W., Zheng W.H., Powell K., Jhamandas K., Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- 12.Koo J.W., Mazei-Robison M.S., LaPlant Q. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci. 2015;18:415–422. doi: 10.1038/nn.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivshina M., Lasko P., Richter J.D. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu Rev Cell Dev Biol. 2014;30:393–415. doi: 10.1146/annurev-cellbio-101011-155831. [DOI] [PubMed] [Google Scholar]
- 14.Bogen O., Alessandri-Haber N., Chu C., Gear R.W., Levine J.D. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari L.F., Bogen O., Alessandri-Haber N., Levine E., Gear R.W., Levine J.D. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience. 2012;222:392–403. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari L.F., Bogen O., Levine J.D. Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33:11002–11011. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.H., Panicker L.M., Seigneur E.M. Cytoplasmic polyadenylation element binding protein is a conserved target of tumor suppressor HRPT2/CDC73. Cell Death Differ. 2010;17:1551–1565. doi: 10.1038/cdd.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue J., Wang X.S., Guo Y.Y. Anxiolytic effect of CPEB1 knockdown on the amygdala of a mouse model of inflammatory pain. Brain Res Bull. 2018;137:156–165. doi: 10.1016/j.brainresbull.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Mayer D.J., Mao J., Holt J., Price D.D. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmughapriya S., Rajan S., Hoffman N.E. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci Signal. 2015;8:ra23. doi: 10.1126/scisignal.2005673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W., Ouyang H., Zheng X. Glial TNFα in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi H., Liu S., Kashiwagi Y. Phosphorylated CCAAT/enhancer binding protein beta contributes to rat HIV-related neuropathic pain, in vitro and in vivo studies. J Neurosci. 2018;38:555–574. doi: 10.1523/JNEUROSCI.3647-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao S., Liu S., Zheng X. The role of TNFα in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology. 2011;36:664–676. doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.Y., Lee K.Y., Lu Y. Mitochondrial Ca2+ uptake is essential for synaptic plasticity in pain. J Neurosci. 2011;31:12982–12991. doi: 10.1523/JNEUROSCI.3093-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W., Hatzis C., Eisenach J.C. Intrathecal injection of cAMP response element binding protein (CREB) antisense oligonucleotide attenuates tactile allodynia caused by partial sciatic nerve ligation. Brain Res. 2003;988:97–104. doi: 10.1016/s0006-8993(03)03348-1. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari L.F., Bogen O., Reichling D.B., Levine J.D. Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci. 2015;35:495–507. doi: 10.1523/JNEUROSCI.5147-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen N.X., Armache J.P., Lee C. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature. 2018;559:570–574. doi: 10.1038/s41586-018-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter J.D. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Taupenot L., Mahata S.K., Wu H., O’Connor D.T. Peptidergic activation of transcription and secretion in chromaffin cells. Cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP) J Clin Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikuyama S., Shimura H., Hoeffler J.P., Kohn L.D. Role of the cyclic adenosine 3′,5′-monophosphate response element in efficient expression of the rat thyrotropin receptor promoter. Mol Endocrinol. 1992;6:1701–1715. doi: 10.1210/mend.6.10.1333054. [DOI] [PubMed] [Google Scholar]
- 31.Berhane K., Boggaram V. Identification of a novel DNA regulatory element in the rabbit surfactant protein B (SP-B) promoter that is a target for ATF/CREB and AP-1 transcription factors. Gene. 2001;268:141–151. doi: 10.1016/s0378-1119(01)00417-6. [DOI] [PubMed] [Google Scholar]
- 32.Paris J., Richter J.D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990;10:5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox C.A., Sheets M.D., Wickens M.P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989;3:2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- 34.Cheatle M.D. Prescription opioid misuse, abuse, morbidity, and mortality: balancing effective pain management and safety. Pain Med. 2015;16 doi: 10.1111/pme.12904. S3–8. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Loram L.C., Ramos K. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim G., Wang S., Zeng Q., Sung B., Yang L., Mao J. Expression of spinal NMDA receptor and PKCγ after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle T., Bryant L., Muscoli C. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett. 2010;483:85–89. doi: 10.1016/j.neulet.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muscoli C., Cuzzocrea S., Ndengele M.M. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestler E.J. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 40.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 41.Baughman J.M., Perocchi F., Girgis H.S. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko S.W., Jia Y., Xu H. Evidence for a role of CaMKIV in the development of opioid analgesic tolerance. Eur J Neurosci. 2006;23:2158–2168. doi: 10.1111/j.1460-9568.2006.04748.x. [DOI] [PubMed] [Google Scholar]
- 44.Lane-Ladd S.B., Pineda J., Boundy V.A. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guitart X., Thompson M.A., Mirante C.K., Greenberg M.E., Nestler E.J. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem. 1992;58:1168–1171. doi: 10.1111/j.1471-4159.1992.tb09377.x. [DOI] [PubMed] [Google Scholar]
- 46.Li X., Clark J.D. Morphine tolerance and transcription factor expression in mouse spinal cord tissue. Neurosci Lett. 1999;272:79–82. doi: 10.1016/s0304-3940(99)00559-5. [DOI] [PubMed] [Google Scholar]
- 47.Fakurazi S., Rahman S.A., Hidayat M.T., Ithnin H., Moklas M.A., Arulselvan P. The combination of mitragynine and morphine prevents the development of morphine tolerance in mice. Molecules. 2013;18:666–681. doi: 10.3390/molecules18010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villalba A., Coll O., Gebauer F. Cytoplasmic polyadenylation and translational control. Curr Opin Genet Dev. 2011;21:452–457. doi: 10.1016/j.gde.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari L.F., Bogen O., Levine J.D. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araldi D., Ferrari L.F., Levine J.D. Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain. 2017;158:1204–1216. doi: 10.1097/j.pain.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller E.A., Hamilton P.J., Burek D.D. Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J Neurosci. 2016;36:4690–4697. doi: 10.1523/JNEUROSCI.0013-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlezon W.A., Jr., Duman R.S., Nestler E.J. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Afroz T., Skrisovska L., Belloc E., Guillen-Boixet J., Mendez R., Allain F.H. A fly trap mechanism provides sequence-specific RNA recognition by CPEB proteins. Genes Dev. 2014;28:1498–1514. doi: 10.1101/gad.241133.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hake L.E., Mendez R., Richter J.D. Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol Cell Biol. 1998;18:685–693. doi: 10.1128/mcb.18.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.