Abstract
Background
Previous studies have shown that anaesthetic technique can affect outcomes of cancer surgery. We investigated the association between anaesthetic technique and patient outcomes after elective hepatectomy for hepatocellular carcinoma.
Methods
This was a retrospective single-centre cohort study of patients who received elective hepatectomy for hepatocellular carcinoma from January 2005 to December 2014. Patients were grouped according to propofol or desflurane anaesthesia. Kaplan–Meier analysis was performed and survival curves were constructed from the date of surgery to death. After propensity matching, univariable and multivariable Cox regression models were used to compare hazard ratios for death. Subgroup analyses were performed for tumour–node–metastasis staging and distant metastasis and local recurrence.
Results
A total of 492 patients (369 deaths, 75.0%) with desflurane anaesthesia and 452 (139 deaths, 30.8%) with propofol anaesthesia were eligible for analysis. After propensity matching, 335 patients remained in each group. In the matched analysis, propofol anaesthesia had a better survival with hazard ratio of 0.47 (95% confidence interval, 0.38–0.59; P<0.001). Subgroup analyses also showed significantly better survival in the absence of distant metastasis (hazard ratio, 0.47; 95% confidence interval, 0.37–0.60; P<0.001) or local recurrence (hazard ratio, 0.22; 95% confidence interval, 0.14–0.34; P<0.001) in the matched groups.
Conclusions
Propofol anaesthesia was associated with better survival in hepatocellular carcinoma patients who underwent hepatectomy. Prospective studies are warranted to evaluate the effects of propofol anaesthesia on surgical outcomes in hepatocellular carcinoma patients.
Keywords: cancer surgery, desflurane, hepatectomy, hepatocellular carcinoma, outcomes, propofol, survival
Editor's key points.
-
•
Considerable evidence suggests that anaesthetic techniques can influence cancer metastasis and outcomes after surgery.
-
•
A large single-centre retrospective study analysed the association of anaesthesia type on survival, metastasis, and local recurrence after hepatectomy for hepatocellular carcinoma.
-
•
Propofol anaesthesia was associated with longer survival and reduced distant metastasis and local recurrence compared with desflurane anaesthesia.
-
•
Prospective randomised studies are necessary to test this association.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and carries a poor prognosis.1 In Taiwan, the crude mortality rate of HCC is ∼30.21 per 100,000 person-years, making it the first and second leading cause of cancer-related mortality in males and females, respectively.2 Surgical resection is the treatment of choice for HCC in non-cirrhotic patients and in patients with compensated cirrhosis.2 However, surgical stress leads to metabolic and neuroendocrine changes, which may cause significant suppression of cell-mediated immunity and may eventually stimulate implantation of circulating tumour cells.3 This potential combination of tumour seeding and impaired immune responses increases the susceptibility of patients undergoing cancer surgery to the development of metastasis, and is associated with worse long-term outcomes. The potential role of anaesthetic drugs in the process of cancer recurrence has attracted interest.3
Growing evidence from animal and human cancer cell line studies reveal that various anaesthetics can influence the immune system in different ways.4, 5, 6, 7, 8, 9 Studies have shown that volatile anaesthetics (VAs) are proinflammatory and may alter immune processes that may increase the incidence of cancer metastases.8, 9, 10, 11, 12 In contrast, propofol appears to suppress tumour growth and to reduce the risk of metastases in mice and humans.6, 11, 12, 13, 14
Lai and colleagues1 reported that propofol anaesthesia was associated with a lower risk of cancer recurrence compared with epidural anaesthesia during percutaneous radiofrequency ablation in patients with HCC.1 However, Yan and colleagues15 showed no significant difference between propofol and sevoflurane anaesthesia in the long-term prognosis after hepatectomy in HCC patients. To our knowledge, no study has compared the effects of propofol-vs desflurane-based anaesthesia on patient outcomes after hepatectomy for HCC. We hypothesised that those patients receiving desflurane anaesthesia might have worse outcome as in our previous colon cancer study.16 Therefore, we conducted a retrospective study to assess whether the choice of desflurane vs propofol anaesthesia was associated with long-term survival, local recurrence, and distant metastasis after hepatectomy for HCC.
Methods
Study design
This was a retrospective cohort study conducted at the Tri-Service General Hospital (TSGH), Taipei, Taiwan, Republic of China.
Participants and data sources
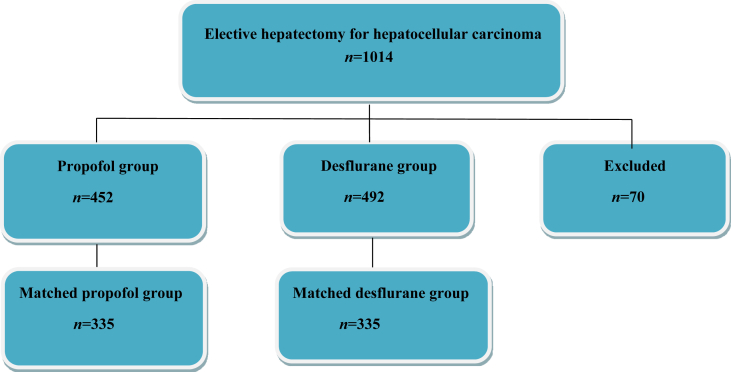
The ethics committee of TSGH approved this study and waived the need for informed consent on May 18, 2017 (TSGHIRB No: 1-106-05-089). The relevant information was retrieved from the medical records and electronic database of TSGH. From January 2005 to December 2014, 1014 patients with an ASA physical status of 2–3 who had undergone elective open hepatectomy for tumour–node–metastasis (TNM) stage I–IV HCC under propofol anaesthesia (propofol group, n=452) or desflurane anaesthesia (desflurane group, n=492) were included. The type of anaesthesia was determined according to the anaesthesiologist's preference. No isoflurane, sevoflurane, or regional analgesia was used in these patients. The exclusion criteria were combined propofol anaesthesia with inhalation anaesthesia or regional analgesia, incomplete data, age <20 yr, or undergoing liver transplantation; 70 patients were excluded from this analysis (Figure 1).
Fig 1.
Flow diagram detailing the selection of patients included in the retrospective analysis. 70 patients were excluded because of combined propofol anaesthesia with inhalation anaesthesia or regional analgesia, incomplete data, age <20 yr, or undergoing liver transplantation.
Anaesthetic technique
No premedication was given before anaesthesia induction. Routine monitoring, including noninvasive blood pressure, electrocardiography (lead II), pulse oximetry, and end-tidal carbon dioxide, and direct radial arterial blood pressure monitoring and a central venous catheter were used for each patient. Anaesthesia was induced with fentanyl, propofol, and rocuronium or cisatracurium for all patients.
In the propofol group, anaesthesia was maintained using target-controlled infusion (TCI; Fresenius Orchestra Primea; Fresenius Kabi AG, Bad Homburg, Germany) with propofol at an effect-site concentration (Ce) of 3–4 μg mL−1 in 100% oxygen at a flow rate of 0.3 L min−1. In the desflurane group, the desflurane vaporiser was set between 4 and 10 vol% in 100% oxygen at a flow of 300 mL min−1 in a closed breathing system. Repetitive bolus injections of cisatracurium and fentanyl were given as necessary.16
Maintenance of the Ce using TCI with propofol or desflurane was adjusted upward and downward by 0.2–0.5 μg mL−1 or 0.5–2 vol%, respectively, when necessary according to the haemodynamics. The end-tidal carbon dioxide level was maintained at 4.7–6.0 kPa by adjusting the ventilation rate and maintained maximum airway pressure <2.9 kPa. Patients were sent to the PACU or ICU for further care and were assessed after surgery by the anaesthesiologist in charge.16
Variables
We retrospectively collected the following patient data: anaesthetic technique; time since the earliest included patient, which served as a surrogate of calendar year; calendar period; sex; age at the time of surgery; serum hepatitis B surface antigen (HBsAg) positivity; serum hepatitis C virus (HCV) positivity; history of alcoholism; serum alpha-fetoprotein (AFP) concentration; Child–Pugh score; and model for end-stage liver disease (MELD) score. For AFP concentration, patients were grouped according to whether their AFP was >20 or ≤20 ng mL−1 because AFP > 20 ng mL−1 is associated with poor recurrence-free survival.17 The 10-yr survival in patients with multiple comorbidities was predicted using the Charlson comorbidity index (CCI) of 0 (least comorbidity) to 37 (highest). Preoperative functional status was assessed in metabolic equivalents (METs), and patients were grouped according to whether their MET was ≥4 or <4 METs because perioperative cardiac and long-term risks increase with a capacity of <4 METs.18 Other data included the ASA physical status; TNM stage of the primary tumour; Barcelona clinic liver cancer (BCLC) stage; use of preoperative radiation therapy; use of preoperative transarterial embolisation (TAE); pathological TNM stage; tumour size; tumour number; grade of surgical complications using the Clavien–Dindo classification (from 0 [no] to V [most]); intraoperative blood transfusion; postoperative chemoembolisation; postoperative retroviral therapy; presence of local recurrence; and presence of distant metastasis. These variables were chosen as potential confounders because they have been shown or posited to affect outcome.
Study sample size
The study sample included patients aged ≥20 yr who received elective hepatectomy for HCC from January 2005 to December 2014. All available patients were included: 452 in the propofol group and 492 in the desflurane group. Assuming a mortality rate of 24% with desflurane anaesthesia and 13.5% with propofol anaesthesia, and to achieve a power of 80% and a two-tailed type I error rate of α=0.05, we calculated that 213 patients were needed in each unmatched group.3
Statistical analysis
The primary endpoint was overall survival, which was compared between groups receiving propofol or desflurane as the main anaesthetic agent. Survival time was defined as the interval between the date of surgery and the date of death or August 31, 2017 for those who were censored. All data are presented as mean (standard deviation, sd) or n (%).
Patient characteristics and death rates were compared between the groups using Student's t-test or χ2 test. Survival according to the type of anaesthesia was depicted visually in a Kaplan–Meier survival curve. The relationship between type of anaesthesia (propofol or desflurane) and survival was analysed using the Cox proportional hazards model with and without adjustment for the abovementioned variables. Because significant interactions with type of anaesthesia (propofol or desflurane) were found, we also performed subgroup analyses for TNM stage and distant metastasis and local recurrence.
To ensure the comparability between propofol anaesthesia and desflurane anaesthesia before operation, propensity score (PS) matching using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA) was used to select the most similar PSs for preoperative variables (with calipers set at 0.2 sd of the logit of the PS) across propofol or desflurane in a 1:1 ratio. As AFP, TNM, and Child–Pugh score were still significantly different between two groups, further exact matching on these three variables was carried out to enhance comparability. Two-tailed P-values <0.05 were considered significant.
Results
Patient and treatment characteristics are shown in Table 1. Compared with the propofol group, the desflurane group had significantly more patients with AFP concentration >20 ng mL−1 (P<0.001), Child–Pugh Class B (P<0.001), preoperative functional status <4 METs (P<0.001), ASA score of 3 (P<0.001), intraoperative blood transfusion (P<0.001), postoperative chemoembolisation (P=0.032), and postoperative retroviral therapy (P<0.001). The MELD score (9.7±4.1 vs 8.3±3.5; P<0.001) and CCI (6.7±2.4 vs 5.8±2.0; P<0.001) were significantly higher in the desflurane group than in the propofol group. The TNM stage (P<0.001), BCLC stage (P<0.001), pathological stage (P<0.001), and grade of surgical complications (P<0.001) differed significantly between the desflurane and propofol groups. The tumour was also significantly smaller in the propofol group than in the desflurane group (P=0.005). The time since the earliest included patient, calendar period, sex, age, HBsAg, HCV, alcoholism, use of preoperative radiation therapy and TAE, and tumour number did not differ significantly between groups (Table 1).
Table 1.
Patient and treatment characteristics for overall group and matched group after propensity scoring. Data shown as mean (standard deviation, sd) or n (%). Grade of surgical complications: Clavien–Dindo classification. AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; MELD, model for end-stage liver disease; MET, metabolic equivalents; N/A not applicable; SMD,; TAE, transarterial embolisation; TNM, tumor–node–metastasis
| Variables | Overall patients |
Matched patients |
|||||
|---|---|---|---|---|---|---|---|
| Propofol (n=452) | Desflurane (n=492) | P-value | Propofol (n=335) | Desflurane (n=335) | P-value | SMD | |
| Time since the earliest included patient (yr), mean (sd) | 6.3 (2.4) | 6.1 (2.4) | 0.206 | 6.4 (2.4) | 6.2 (2.4) | 0.368 | 0.070 |
| Calendar period, n (%) | 0.570 | 0.828 | |||||
| 2005–08 | 93 (21) | 111 (23) | 63 (19) | 67 (20) | |||
| 2009–12 | 204 (45) | 227 (46) | 160 (48) | 163 (49) | |||
| 2013–5 | 155 (34) | 154 (31) | 112 (33) | 105 (31) | |||
| Male sex, n (%) | 330 (73) | 362 (74) | 0.902 | 245 (73) | 242 (72) | 0.862 | 0.020 |
| Age (yr), mean (sd) | 61 (13) | 62 (13) | 0.348 | 61 (13) | 62 (13) | 0.553 | 0.046 |
| HBsAg, n (%) | 268 (59) | 272 (55) | 0.239 | 195 (58) | 191 (57) | 0.815 | 0.020 |
| HCV, n (%) | 122 (27) | 138 (28) | 0.771 | 88 (26) | 89 (27) | 1.000 | 0.007 |
| Alcoholism, n (%) | 62 (14) | 82 (17) | 0.152 | 52 (16) | 55 (16) | 0.833 | 0.024 |
| AFP, n (%) | <0.001 | 1.000 | 0 | ||||
| ≤20 | 286 (63) | 184 (37) | 169 (50) | 169 (50) | |||
| >20 | 166 (37) | 308 (63) | 166 (50) | 166 (50) | |||
| Child–Pugh score, n (%) | <0.001 | 1.000 | 0 | ||||
| A (mild) | 395 (87) | 357 (73) | 278 (83) | 278 (83) | |||
| B (moderate) | 57 (13) | 135 (27) | 57 (17) | 57 (17) | |||
| MELD score, mean (sd) | 8.3 (3.5) | 9.7 (4.1) | <0.001 | 8.7 (3.9) | 8.7 (3.3) | 0.915 | 0.008 |
| Charlson comorbidityindex, mean (sd) | 5.8 (2.0) | 6.7 (2.4) | <0.001 | 6.0 (2.1) | 6.2 (2.3) | 0.148 | 0.089 |
| Functional status, n (%) | <0.001 | ||||||
| <4 MET | 152 (34) | 225 (46) | N/A | N/A | |||
| ≥4 MET | 300 (66) | 267 (54) | N/A | N/A | |||
| ASA physical status, n (%) | <0.001 | 0.337 | 0.080 | ||||
| 2 | 300 (66) | 267 (54) | 217 (65) | 204 (61) | |||
| 3 | 152 (34) | 225 (46) | 118 (35) | 131 (39) | |||
| TNM stage of primary tumor, n (%) | <0.001 | 1.000 | 0 | ||||
| I | 226 (50) | 162 (33) | 151 (45) | 151 (45) | |||
| II | 96 (21) | 112 (23) | 70 (21) | 70 (21) | |||
| III | 108 (24) | 184 (37) | 92 (28) | 92 (28) | |||
| IV | 22 (5) | 34 (7) | 22 (7) | 22 (7) | |||
| BCLC stage, n (%) | <0.001 | 0.899 | 0.046 | ||||
| 0 | 49 (11) | 27 (6) | 29 (9) | 24 (7) | |||
| A | 270 (60) | 245 (50) | 194 (58) | 195 (58) | |||
| B | 112 (25) | 185 (38) | 91 (27) | 93 (28) | |||
| C | 21 (5) | 35 (7) | 21 (6) | 23 (7) | |||
| Preoperative radiation therapy, n (%) | 5 (1) | 15 (3) | 0.065 | 5 (2) | 5 (2) | 1.000 | 0 |
| Preoperative TAE, n (%) | 63 (14) | 87 (18) | 0.138 | 47 (14) | 56 (17) | 0.392 | 0.075 |
| Tumor size (cm), mean (sd) | 4.6 (3.5) | 5.3 (3.6) | 0.005 | 4.9 (3.7) | 4.8 (3.5) | 0.704 | 0.029 |
| Tumor number, n (%) | 0.579 | 0.527 | 0.057 | ||||
| 1 | 379 (84) | 420 (85) | 278 (83) | 285 (85) | |||
| >1 | 73 (16) | 72 (15) | 57 (17) | 50 (15) | |||
| Intraoperative blood transfusion, n (%) | 162 (36) | 297 (60) | <0.001 | 138 (41) | 172 (51) | 0.011 | N/A |
| Grade of surgical complications, n (%) | <0.001 | 0.611 | N/A | ||||
| 0 | 375 (83) | 312 (63) | 261 (78) | 247 (74) | |||
| I | 56 (12) | 114 (23) | 53 (16) | 60 (18) | |||
| II | 18 (4) | 56 (11) | 18 (5) | 24 (7) | |||
| III | 3 (1) | 10 (2) | 3 (1) | 4 (1) | |||
| Pathologic TNM stage, n (%) | <0.001 | 0.937 | N/A | ||||
| I | 217 (48) | 154 (31) | 151 (45) | 143 (43) | |||
| II | 108 (24) | 118 (24) | 73 (22) | 76 (23) | |||
| III | 105 (23) | 186 (38) | 89 (27) | 94 (28) | |||
| IV | 22 (5) | 34 (7) | 22 (7) | 22 (7) | |||
| Postoperative chemoembolisation, n (%) | 20 (5) | 39 (8) | 0.032 | 18 (5) | 25 (8) | 0.344 | N/A |
| Postoperative retrovival therapy, n (%) | 63 (14) | 122 (26) | <0.001 | 49 (15) | 72 (22) | 0.027 | N/A |
| Local recurrence, n (%) | 171 (38) | 345 (70) | <0.001 | 137 (41) | 236 (70) | <0.001 | N/A |
| Distant metastasis, n (%) | 25 (6) | 78 (16) | <0.001 | 25 (8) | 43 (13) | 0.03 | N/A |
| All-cause mortality, n (%) | 139 (31) | 369 (75) | <0.001 | 129 (30) | 343 (73) | <0.001 | N/A |
| Cancer specific mortality, n (%) | 129 (30) | 343 (73) | <0.001 | 115 (35) | 204 (63) | <0.001 | N/A |
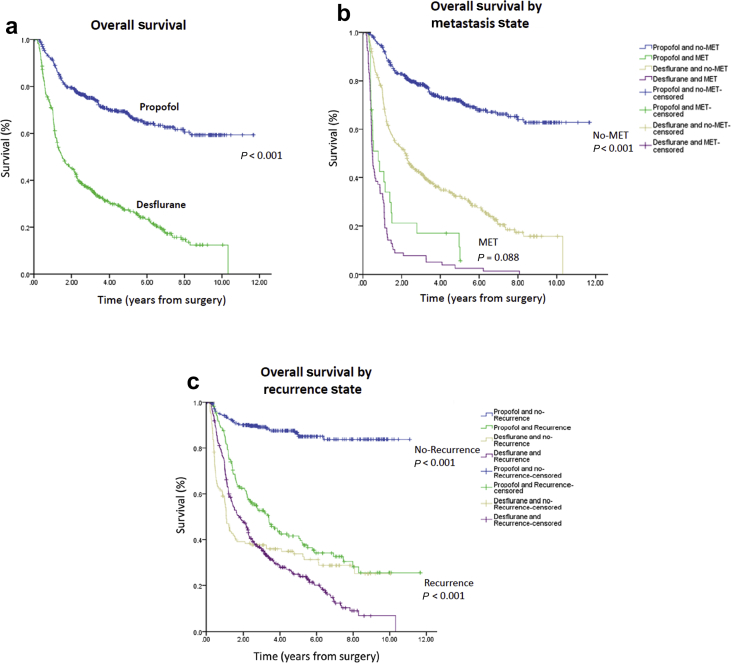
Overall mortality was significantly higher in the desflurane group (75.0%) than in the propofol group (30.8%) during follow-up (P<0.001). Moreover, the cancer-specific mortality rate was also significantly higher in the desflurane group (73.0%) than in the propofol group (30.0%) during follow-up (P<0.001). A higher percentage of patients in the desflurane group (70.1%) exhibited local recurrence compared with the propofol group (37.8%; P<0.001). The presence of distant metastasis was also significantly higher in the desflurane group (15.9%) than in the propofol group (5.5%) during follow-up (P<0.001) (Table 1). Kaplan–Meier survival curves for the two types of anaesthesia are shown in Figure 2a.
Fig 2.
(a) Overall survival curves from the date of surgery by anaesthesia type. (b) Overall survival curves from the date of surgery by presence (or not) of metastasis. (c) Overall survival curves from the date of surgery by presence (or not) of recurrence. MET, metastasis.
Overall mortality associated with use of propofol or desflurane anaesthesia for hepatectomy is shown in Table 2. Overall survival from the date of surgery grouped according to anaesthesia type and other variables was compared separately in a univariable Cox model and subsequently in a multivariable Cox regression model. Other variables that significantly increased the risk of death after the multivariable analysis were a longer time since the earliest included patient, higher AFP level, higher MELD score, higher TNM stage, use of preoperative radiation therapy, higher grade of surgical complications (except grade II and III), and use of postoperative retroviral therapy (Table 2). Patients who received propofol anaesthesia exhibited better overall survival than those who received desflurane anaesthesia (overall survival 69.2% vs 25.0%, respectively; the crude hazard ratio (HR) was 0.28 (95% confidence interval [CI], 0.23–0.34; P<0.001). This finding did not change substantially in the multivariable analysis after adjustment for the time since the earliest included patient, HBsAg, alcoholism, AFP level, Child–Pugh score, MELD score, CCI, ASA score, TNM stage, preoperative radiation therapy, tumour size, intraoperative blood transfusion, grade of surgical complications, and surgeons (HR, 0.32; 95% CI, 0.26–0.39; P<0.001) (Table 2).
Table 2.
Cox proportional hazards regression for mortality: univariable and multivariable models for overall patients. Adjusted HRs were adjusted by those variables were significant in the univariable analyses and surgeons (n=12). Three variables were excluded from the multivariable because they were highly correlated with other variables (functional status with ASA and BCLC stage and pathologic TNM stage with TNM stage). AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; CI, confidence interval; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HR, hazard ratio; MELD, model for end-stage liver disease; MET, metabolic equivalents; TAE, transarterial embolisation; TNM, tumour–node–metastasis
| Variables | Univariable |
Multivariable |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Anaesthesia, propofol (ref: desflurane) | 0.28 (0.23–0.34) | <0.001 | 0.32 (0.26–0.39) | <0.001 |
| Time since the earliest included patient (yr) | 1.05 (1.01–1.09) | 0.010 | 1.10 (1.06–1.15) | <0.001 |
| Female (ref: male) | 0.92 (0.75–1.12) | 0.392 | ||
| Age (yr) | 1.01 (1.01–1.02) | 0.501 | ||
| HBsAg (ref: no) | 0.81 (0.68–0.97) | 0.018 | 0.80 (0.62–1.02) | 0.075 |
| HCV (ref: no) | 1.03 (0.85–1. 25) | 0.768 | ||
| Alcoholism (ref: no) | 1.44 (1.15–1. 81) | 0.002 | 1.09 (0.82–1.43) | 0.558 |
| AFP > 20 (ref: ≤20) | 4.90 (4.01–6.00) | <0.001 | 3.21 (2.57–4.01) | <0.001 |
| Child–Pugh score, B (ref: A) | 2.21 (1.82–2.69) | <0.001 | 0.74 (0.46–1.17) | 0.193 |
| MELD score | 1.09 (1.07–1. 12) | <0.001 | 1.07 (1.02–1.12) | 0.002 |
| Charlson comorbidity index | 1.27 (1.23–1.32) | <0.001 | 1.02 (0.96–1.08) | 0.554 |
| Functional status, ≥4 METs (ref: <4 METs) | 0.57 (0.48–0.68) | <0.001 | ||
| ASA physical status 3, (ref: 2) | 1.77 (1.49–2.11) | <0.001 | 1.18 (0.95–1.47) | 0.146 |
| TNM stage of primary tumour (ref: I) | ||||
| II | 2.34 (1.81–3.04) | <0.001 | 2.13 (1.63–2.78) | <0.001 |
| III | 4.32 (3.44–5.41) | <0.001 | 2.72 (2.05–3.61) | <0.001 |
| IV | 16.9 (12.1–23.6) | <0.001 | 12.2 (7.66–19.5) | <0.001 |
| BCLC stage (ref: 0) | ||||
| A | 1.94 (1.21–3.10) | 0.006 | ||
| B | 5.76 (3.60–9.21) | <0.001 | ||
| C | 19.6 (11.5–33.2) | <0.001 | ||
| Preoperative radiation therapy (ref: no) | 3.25 (2.02–5.21) | <0.001 | 2.26 (1.36–3.76) | 0.002 |
| Preoperative TAE (ref: no) | 1.07 (0.85–1. 36) | 0.566 | ||
| Pathologic TNM stage (ref: I) | ||||
| II | 2.16 (1.66–2.82) | <0.001 | ||
| III | 4.84 (3.84–6.10) | <0.001 | ||
| IV | 17.7 (12.6–24.8) | <0.001 | ||
| Tumour size | 1.12 (1.10–1.14) | <0.001 | 1.01 (0.98–1.04) | 0.553 |
| Tumour number >1 (ref: 1) | 1.17 (0.92–1.46) | 0.204 | ||
| Intraoperative blood transfusion (ref: no) | 3.28 (2.72–3.95) | <0.001 | 1.21 (0.93–1.57) | 0.166 |
| Grade of surgical complications (ref: 0) | ||||
| I | 2.33 (1.88–2.87) | <0.001 | 1.43 (1.03–1.99) | 0.031 |
| II | 3.94 (3.02–5.15) | <0.001 | 0.96 (0.58–1.60) | 0.879 |
| III | 4.77 (2.67–8.53) | <0.001 | 1.57 (0.73–3.37) | 0.252 |
| Postoperative chemoembolisation (ref: no) | 1.73 (1.28–2.33) | <0.001 | 1.23 (0.89–1.70) | 0.214 |
| Postoperative retroviral therapy (ref: no) | 2.21 (1.82–2.68) | <0.001 | 1.36 (1.04–1.77) | 0.025 |
| Postoperative recurrence (ref: no) | 2.78 (2.28–3.39) | <0.001 | ||
| Postoperative metastasis (ref: no) | 5.68 (4.53–7.12) | <0.001 | ||
Because of the significant differences in baseline characteristics between groups, we used the PS from logistic regression and exact matching to adjust the baseline characteristics and choice of therapy between groups. After matching, 335 pairs were formed. All standardised mean differences for the variables were <0.1 (Table 1).
Subgroup analyses for presence of distant metastasis, local recurrence, TNM stage, and disease progression
Patients without distant metastasis who received propofol had better survival than those who received desflurane. For patients with no distant metastasis, the crude HR was 0.27 (95% CI, 0.22–0.34; P<0.001), the PS-adjusted HR was 0.39 (95% CI, 0.32–0.49; P<0.001), and the PS-matched HR was 0.47 (95% CI, 0.37–0.60; P<0.001). Accordingly, patients who received propofol anaesthesia had lower metastasis-free mortality than those who received desflurane anaesthesia. For patients with distant metastasis, the crude HR was 0.66 (95% CI, 0.41–1.07; P=0.088), the PS-adjusted HR was 0.60 (95% CI, 0.37–0.98; P=0.040), and the PS-matched HR was 0.73 (95% CI, 0.43–1.23; P=0.224) (Table 3; Figure 2b).
Table 3.
Subgroup analyses for presence of distant metastasis, local recurrence, TNM stage, and disease progression. CI, confidence interval; HR, hazard ratio; PS, propensity score; TNM, tumour–node–metastasis
| Stratified variable | Anaesthesia | Crude HR (95% CI) | P-value | P=value (interaction) | PS-adjusted HR (95% CI) | P=value | PS-matched HR (95% CI) | P=value | |
|---|---|---|---|---|---|---|---|---|---|
| Non-stratified | |||||||||
| Desflurane | 1.00 | 1.00 | 1.00 | ||||||
| Propofol | 0.28 (0.23–0.34) | <0.001 | 0.41 (0.33–0.50) | <0.001 | 0.47 (0.38–0.59) | <0.001 | |||
| Metastasis | 0.002 | ||||||||
| No | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.27 (0.22–0.34) | <0.001 | 0.39 (0.32–0.49) | <0.001 | 0.47 (0.37–0.60) | <0.001 | |||
| Yes | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.66 (0.41–1.07) | 0.088 | 0.60 (0.37–0.98) | 0.040 | 0.73 (0.43–1.23) | 0.224 | |||
| Recurrence | <0.001 | ||||||||
| No | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.12 (0.09–0.18) | <0.001 | 0.28 (0.19–0.42) | <0.001 | 0.22 (0.14–0.34) | <0.001 | |||
| Yes | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.59 (0.47–0.74) | <0.001 | 0.71 (0.56–0.89) | 0.003 | 0.98 (0.76–1.27) | 0.884 | |||
| TNM stage | 0.909 | ||||||||
| TNM: I+II | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.27 (0.21–0.36) | <0.001 | 0.39 (0.29–0.53) | <0.001 | 0.41 (0.29–0.56) | <0.001 | |||
| TNM: III+IV | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.32 (0.24–0.42) | <0.001 | 0.40 (0.30–0.52) | <0.001 | 0.47 (0.35–0.64) | <0.001 | |||
| Cancer-specific mortality | |||||||||
| Desflurane | 1.00 | 1.00 | 1.00 | ||||||
| Propofol | 0.38 (0.31–0.47) | <0.001 | 0.60 (0.48–0.74) | <0.001 | 0.55 (0.44–0.69) | <0.001 | |||
| Disease progression | |||||||||
| Local recurrence | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.31 (0.26–0.37) | <0.001 | 0.39 (0.32–0.47) | <0.001 | 0.42 (0.34–0.52) | <0.001 | |||
| Distant metastasis | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.13 (0.08–0.20) | <0.001 | 0.41 (0.25–0.69) | 0.001 | 0.25 (0.15–0.41) | <0.001 | |||
| Local recurrence + Distant metastasis | Desflurane | 1.00 | 1.00 | 1.00 | |||||
| Propofol | 0.32 (0.27–0.38) | <0.001 | 0.43 (0.36–0.52) | <0.001 | 0.44 (0.36–0.54) | <0.001 | |||
Patients without local recurrence who received propofol had better survival than those who received desflurane. For patients with no local recurrence, the crude HR was 0.12 (95% CI, 0.09–0.18; P<0.001), the PS-adjusted HR was 0.28 (95% CI, 0.19–0.42; P<0.001), and the PS-matched HR was 0.22 (95% CI, 0.14–0.34; P<0.001). Accordingly, patients who received propofol anaesthesia had lower recurrence-free mortality than those who received desflurane anaesthesia. For patients with local recurrence, the crude HR was 0.59 (95% CI, 0.47–0.74; P<0.001), the PS-adjusted HR was 0.71 (95% CI, 0.56–0.89; P=0.003), and the PS-matched HR was 0.98 (95% CI, 0.76–1.27; P=0.884) (Table 3; Figure 2c). Although PS matching showed that propofol anaesthesia provided better outcomes in TNM I+II and III+IV patients, there was no significant interaction between type of anaesthesia and TNM stage (P=0.909) (Table 3).
Patients who received propofol anaesthesia had lower cancer-specific mortality than those who received desflurane anaesthesia: crude HR was 0.38 (95% CI, 0.31–0.47; P<0.001), PS-adjusted HR was 0.60 (95% CI, 0.48–0.74; P<0.001), and PS-matched HR was 0.55 (95% CI, 0.44–0.69; P<0.001). Patients who received propofol anaesthesia had less local recurrence than those who received desflurane anaesthesia: crude HR was 0.31 (95% CI, 0.26–0.37; P<0.001), PS-adjusted HR was 0.39 (95% CI, 0.32–0.47; P<0.001), and PS-matched HR was 0.42 (95% CI, 0.34–0.52; P<0.001). Patients who received propofol anaesthesia had less distant metastasis than those who received desflurane anaesthesia: crude HR was 0.13 (95% CI, 0.08–0.20; P<0.001), PS-adjusted HR was 0.41 (95% CI, 0.25–0.69; P=0.001), and PS-matched HR was 0.25 (95% CI, 0.15–0.41; P<0.001). Patients who received propofol anaesthesia had less local recurrences and distant metastases than those who received desflurane anaesthesia: crude HR was 0.32 (95% CI, 0.27–0.38; P<0.001), PS-adjusted HR was 0.43 (95% CI, 0.36–0.52; P<0.001), and PS-matched HR was 0.44 (95% CI, 0.36–0.54; P<0.001).
In summary, HCC patients without distant metastasis or local recurrence had better outcomes for propofol anaesthesia vs desflurane anaesthesia. Patients who received desflurane anaesthesia had higher cancer-specific mortality and poor disease progression (such as local recurrence, distant metastases, or local recurrence and distant metastases) than those who received propofol anaesthesia.
Discussion
Our major finding is that propofol anaesthesia for hepatectomy in HCC patients was associated with better survival and lower rates of local recurrence and distant metastasis compared with desflurane anaesthesia. We found significantly better survival in patients who received propofol anaesthesia in the absence of distant metastasis or local recurrence. These results are consistent with previous studies of propofol-based anaesthesia that reported better outcomes after surgery for gastrointestinal cancers, such as oesophageal, gastric, or colon cancer, compared with VAs.19, 20
Surgical excision is the mainstay of treatment for potentially removable solid tumours. However, surgery can inhibit important host defences and promote the development of metastasis. The 5-yr recurrence rate remains at 60% after hepatectomy for HCC.15 Postoperative metastasis and recurrence play important roles in survival and prognosis; therefore, research on HCC has focused on finding ways to improve overall patient survival by reducing metastasis and recurrence.15 The likelihood of tumour metastasis depends on the balance between the metastatic potential of the tumour and the anti-metastatic host defences, of which cell-mediated immunity and natural killer cell function in particular, are critical components.21 Growing evidence from studies of animal and human cancer cell lines shows that various anaesthetics or anaesthetic techniques affect the immune system in different ways4, 5, 6, 7, 8, 9 and may therefore influence cancer patient survival or risk of recurrence.6, 8, 9, 10, 11
In this study, we found an approximately 50% lower death rate with propofol than with desflurane anaesthesia in patients after hepatectomy for HCC. We previously reported that propofol anaesthesia was associated with a lower incidence of postoperative recurrence and metastasis compared with desflurane anaesthesia in colon cancer surgery.16 Similarly, Lai and colleagues1 reported that propofol anaesthesia, but not epidural anaesthesia, reduced recurrence of HCC. However, a recent retrospective analysis (n=185) found no significant difference in overall survival of HCC patients between propofol anaesthesia and sevoflurane anaesthesia.15 Similarly, Lai and colleagues1 reported no difference in overall survival between propofol anaesthesia and epidural anaesthesia after percutaneous radiofrequency ablation in HCC patients. There are few studies on the effects of the type of anaesthesia in HCC patients; further study is warranted to understand the effects of the type of anaesthesia on tumour recurrence and metastasis after hepatectomy in patients with HCC.
We also found that a longer time since the earliest operation was associated with poor survival after hepatectomy for HCC; this may reflect the state of art of HCC treatment. However, further investigation is needed to determine why a longer time since the earliest included patient influenced survival. We found that a higher AFP level was associated with poor survival after open liver resection for HCC, in agreement with a previous study.22 We also found that a higher MELD score was associated with poor survival after open liver resection for HCC, also consistent with a previous study.23 We found that a higher TNM stage was associated with poor survival after hepatectomy for HCC patients, as observed previously.15 In addition, we found that preoperative radiation therapy was associated with poor survival after hepatectomy for HCC. This may reflect the use of preoperative radiation therapy for portal vein tumour thrombus in our hospital and that the prognosis for portal vein tumour thrombus was poor.24 We also found that a higher grade of surgical complications (Clavien–Dindo class) was associated with poor survival after hepatectomy for HCC, which may reflect greater surgery-related morbidity and mortality.25 Finally, we found that postoperative retroviral therapy was associated with poor survival after hepatectomy for HCC. This may reflect that we often use postoperative retroviral therapy in liver cirrhosis patients because of the policy of national health insurance in our country, and liver cirrhosis—not antiretroviral therapy—predicts clinical outcome of HCC.26
Laboratory data provide support for the effect of propofol on HCC cancer growth and survival through several different pathways.27, 28, 29 Using human HCC cell lines, Liu and colleagues27 reported that propofol inhibited the proliferation, migration, and invasion of HCC cells via downregulation of miR-374a. Zhang and colleagues28 reported that propofol decreased HCC cell invasion, partly through downregulation of matrix metalloproteinase 9 (MMP-9) expression by miR-199a. Liu and colleagues29 also found that propofol inhibited tumour growth and expression of MMP-2 and vascular endothelial growth factor proteins in hepatoma xenografts in mice in a dose-dependent manner. Taken together, these findings suggest that propofol anaesthesia may be an effective drug for use in HCC, although further clinical studies are warranted.
Nishiwada and colleagues30 have shown that sevoflurane can increase proliferation of human HCC cells. A recent study suggested that isoflurane anaesthesia increased expression insulin-like growth factor (IGF).31 Overexpression of IGF contributed to cell cycle progression and inhibition of apoptosis, and may increase cancer cell survival, as noted in many cancers including HCC and ovarian cancer.31, 32, 33 Sevoflurane anaesthesia is also associated with angiogenesis after primary breast cancer surgery.34 Taken together, these reports on cancer cell lines suggest that administration of VAs may promote cancer cell growth, whereas propofol has the opposite (beneficial) effect by inhibiting cancer cell growth.3, 11, 12
This study has important limitations. First, the study was retrospective and patients were not randomly allocated. Patient characteristics such as AFP level, Child–Pugh score, MELD score, CCI, TNM stage, tumour size, grade of surgical complications, and intraoperative blood transfusion differed significantly between groups, and these variables may affect each other. We adjusted for these confounding factors in our analysis, but we cannot avoid the possibility of residual effects caused by unmeasured confounders. Second, different VAs may have different effects on HCC. We analysed only desflurane because it is the most frequently used agent in our hospital. Third, NSAIDs have been linked to better survival in HCC populations.35 In our hospital, we do not routinely use NSAIDs during hepatectomy because of the risk of life-threatening complications such as peptic ulceration.36 Third, opioids can affect long-term survival after resection of HCC37; however, information about opioid use, especially for postoperative pain control, was incomplete in the medical records used in our study. The prescription of opioids was similar to that used in our previously reported open liver surgery,38 and we presumed there was no significant difference between groups in the use of opioids in the current study. Finally, PS matching showed that propofol anaesthesia provided better outcomes in TNM I+II and III+IV patients; however, we found no significant interaction effect between the type of anaesthesia and TNM stage. The TNM system is useful for staging in surgical candidates39; however, the system does not consider parameters related to liver reserve and may be less discriminatory in patients with more advanced-stage disease.40 Therefore, propofol anaesthesia in HCC patients receiving liver resection might be limited in patients with poor liver function with poor outcome, although further investigation is necessary.
In conclusion, during open hepatectomy for HCC, use of propofol anaesthesia was associated with longer survival than that of desflurane. Patients given propofol anaesthesia had significantly less distant metastasis and local recurrence.
Authors' contributions
Study design: HCL, ZFZ.
Conduct of the study: HCL, ZFZ.
Data analysis: MSL, KTL, YHH, CSW, SMC.
Assistance in data analysis (propensity score matching): CL.
Data collection: KTL, YHH, CSW, SMC.
Preparation of the manuscript: MSL.
Writing of the manuscript: HCL, ZFZ.
All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare that they have no conflict of interests.
Funding
Ministry of Science and Technology (MOST 107-2314-B-016 -048), Taiwan, Republic of China.
Acknowledgements
The authors thank the Cancer Registry Group of Tri-Service General Hospital for clinical data support.
Handling editor: H.C. Hemmings Jr
Editorial decision: 22 April 2019
References
- 1.Lai R., Peng Z., Chen D. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesth Analg. 2012;114:290–296. doi: 10.1213/ANE.0b013e318239c2e3. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group Management consensus guideline for hepatocellular carcinoma: 2016 updated by the taiwan liver cancer association and the gastroenterological society of taiwan. J Formos Med Assoc. 2018;117:381–403. doi: 10.1016/j.jfma.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Wigmore T.J., Mohammed K., Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 4.Inada T., Kubo K., Kambara T. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can J Anesth. 2009;56:222–229. doi: 10.1007/s12630-008-9035-0. [DOI] [PubMed] [Google Scholar]
- 5.Inada T., Yamanouchi Y., Jomura S. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954–959. doi: 10.1111/j.1365-2044.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 6.Kushida A., Inada T., Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol. 2007;29:477–486. doi: 10.1080/08923970701675085. [DOI] [PubMed] [Google Scholar]
- 7.Loop T., Dovi-Akue D., Frick M. Volatile anesthetics induce caspase dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Melamed R., Bar-Yosef S., Shakhar G. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro J., Jersky J., Katzav S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68:678–685. doi: 10.1172/JCI110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moudgil G.C., Singal D.P. Halothane and isoflurane enhance melanoma tumour metastasis in mice. Can J Anaesth. 1997;44:90–94. doi: 10.1007/BF03014331. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Kang S.H., Kim Y. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69:126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enlund M., Berglund A., Andreasson K. The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119:251–261. doi: 10.3109/03009734.2014.922649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilliland H.E., Armstrong M.A., Carabine U. The choice of anesthetic maintenance technique influences the antiinflammatory cytokine response to abdominal surgery. Anesth Analg. 1997;85:1394–1398. doi: 10.1097/00000539-199712000-00039. [DOI] [PubMed] [Google Scholar]
- 14.Mammoto T., Mukai M., Mammoto A. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–170. doi: 10.1016/s0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 15.Yan T., Zhao J.J., Bi X.Y. Effect of different anesthetic techniques on the prognosis of hepatocellular carcinoma after hepatectomy. Zhonghua Yi Xue Za Zhi. 2017;97:1719–1723. doi: 10.3760/cma.j.issn.0376-2491.2017.22.009. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z.F., Lee M.S., Wong C.S. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129:932–941. doi: 10.1097/ALN.0000000000002357. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Fiel M.I., Blank S. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer. 2013;109:573–581. doi: 10.1038/bjc.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eagle K.A., Berger P.B., Calkins H. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines (committee to update the 1996 guidelines on perioperative cardiovascular evaluation for noncardiac surgery) J Am Coll Cardiol. 2002;39:542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 19.Jun I.J., Jo J.Y., Kim J.I. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020. doi: 10.1038/s41598-017-14147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sessler D.I., Riedel B. Anesthesia and cancer recurrence: context for divergent study outcomes. Anesthesiology. 2019;130:3–5. doi: 10.1097/ALN.0000000000002506. [DOI] [PubMed] [Google Scholar]
- 21.Snyder G.L., Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 22.Ma W.J., Wang H.Y., Teng L.S. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. doi: 10.1186/1477-7819-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teh S.H., Christein J., Donohue J. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Takizawa D., Kakizaki S., Sohara N. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. 2007;52:3290–3295. doi: 10.1007/s10620-007-9808-2. [DOI] [PubMed] [Google Scholar]
- 25.Bolliger M., Kroehnert J.A., Molineus F. Experiences with the standardized classification of surgical complications (Clavien–Dindo) in general surgery patients. Eur Surg. 2018;50:256–261. doi: 10.1007/s10353-018-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M.N., Hwang S.G., Kim B.K. Liver cirrhosis, not antiviral therapy, predicts clinical outcome in cohorts with heterogeneous hepatitis B viral status. Gut Liver. 2019;13:197–205. doi: 10.5009/gnl18204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S.Q., Zhang J.L., Li Z.W. Propofol inhibits proliferation, migration, invasion and promotes apoptosis through down-regulating miR-374a in hepatocarcinoma cell lines. Cell Physiol Biochem. 2018;49:2099–2110. doi: 10.1159/000493814. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Zhang D., Wu G.Q. Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression. Hepatobiliary Pancreat Dis Int. 2013;12:305–309. doi: 10.1016/s1499-3872(13)60048-x. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Zhang N., Cao Q. The effects of propofol on the growth behavior of hepatoma xenografts in Balb/c mice. Biomed Pharmacother. 2017;90:47–52. doi: 10.1016/j.biopha.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Nishiwada T., Kawaraguchi Y., Uemura K. Effect of sevoflurane on human hepatocellular carcinoma HepG2 cells under conditions of high glucose and insulin. J Anesth. 2015;29:805–808. doi: 10.1007/s00540-015-2025-9. [DOI] [PubMed] [Google Scholar]
- 31.Luo X., Zhao H., Hennah L. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2014;114:831–839. doi: 10.1093/bja/aeu408. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Yao M., Zheng W. Insulin-like growth factor I receptor: a novel target for hepatocellular carcinoma gene therapy. Mini Rev Med Chem. 2019;19:272–280. doi: 10.2174/1389557518666181025151608. [DOI] [PubMed] [Google Scholar]
- 33.Clayton P.E., Banerjee I., Murray P.G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 34.Looney M., Doran P., Buggy D.J. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor β in women undergoing anesthesia and surgery for breast cancer. Anesthesiology. 2010;113:1118–1125. doi: 10.1097/ALN.0b013e3181f79a69. [DOI] [PubMed] [Google Scholar]
- 35.Tao Y., Li Y., Liu X. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:2695–2709. doi: 10.2147/CMAR.S167560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong C.P., Blower A.L. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987;28:527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao L., Chang Y., Lin W. Long-term survival after resection of hepatocelluar carcinoma: a potential risk associated with the choice of postoperative analgesia. Anesth Analg. 2014;118:1309–1316. doi: 10.1213/ANE.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 38.Lu C.H., Yeh C.C., Huang Y.S. Hemodynamic and biochemical changes in liver transplantation: a retrospective comparison of desflurane and total intravenous anesthesia by target-controlled infusion under auditory evoked potential guide. Acta Anaesthesiol Taiwan. 2014;52:6–12. doi: 10.1016/j.aat.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Choi S.B., Lee J.G., Kim K.S. The prognosis and survival analysis according to seven staging systems of hepatocellular carcinoma following curative resection. Hepatogastroenterology. 2008;55:2140–2145. [PubMed] [Google Scholar]
- 40.Chan S.L., Mo F.K., Johnson P.J. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–347. doi: 10.1111/j.1440-1746.2010.06329.x. [DOI] [PubMed] [Google Scholar]