Abstract
Background
Intense pain can last several days after tonsillectomy. It is often undertreated and improved analgesic strategies that can be safely used at home are needed.
Methods
We conducted a systematic review and meta-analysis on the effectiveness of systemic medications used for post-tonsillectomy pain in adult and adolescent (13 yr old) patients. Studies were identified from PubMed, the Cochrane Library, and by hand searching reference lists from studies and review articles. Randomised, double-blind, placebo-controlled studies reporting on pain intensity or use of rescue analgesia were included.
Results
Twenty-nine randomised controlled trials representing 1816 subjects met the inclusion criteria. Follow-up time was ≤24 h in 15 studies, in which the majority were taking nonsteroidal anti-inflammatory drugs. Thirteen studies were suitable for meta-analysis. In pooled analysis, paracetamol, dexamethasone, and gabapentinoids reduced pain intensity on the day of operation. In individual studies, ketoprofen, ibuprofen, lornoxicam, parecoxib, rofecoxib, indomethacin and dextromethorphan reduced pain intensity, need for rescue analgesics, or both on the day of operation. Oral celecoxib for 2 postoperative weeks or i.v. ketamine on the day of operation were not effective at the studied doses. Dexamethasone in multiple doses provided analgesia beyond 1 postoperative day. Pain was moderate to strong in both study and control groups during the first postoperative week.
Conclusions
Single analgesics and dexamethasone provide only a weak to moderate effect for post-tonsillectomy pain on the day of operation and thus a multimodal analgesic strategy is recommended. Short follow-up times and clinical heterogeneity of studies limit the usefulness of results.
Keywords: adolescent, adult, analgesics, dexamethasone, multimodal analgesia, postoperative pain, tonsillectomy
Editor's key points.
-
•
The authors performed a systematic review and meta-analysis of pain management after tonsillectomy. Data were scarce and postoperative follow-up was short in the majority of studies.
-
•
Paracetamol, NSAIDs, dexamethasone, gabapentinoids, and dextromethorphan showed weak to moderate analgesic effects on the day of operation.
-
•
The authors suggest that multimodal analgesia is needed after tonsillectomy, and that further research on post-tonsillectomy pain is required, with follow-up over at least 1 week.
Tonsillectomy is one of the most common procedures in ear, nose, and throat surgery. It is usually performed as a day-stay procedure.1 Pain is the most common reason for physician contact after discharge, indicating that post-tonsillectomy pain is intense and undertreated.2 Patients need analgesics that are effective and can be used safely at home.
Adults have a different pattern of pain compared with children; this is related to the different indications and techniques of tonsillectomy.3, 4 In adults, the surgery is usually performed for chronic infection with scarred tonsils that requires dissection with coagulation, thus causing intense and longer-lasting pain.5 In children, the indication is usually hypertrophy or recurrent acute infections with smaller changes in tonsil tissues than in adults.6
Multimodal analgesia has become the standard of care in postoperative pain management. Combinations of analgesics with different sites or modes of action are commonly used to improve analgesia and to reduce the doses of individual analgesics (especially opioids) and to reduce adverse effects. Adverse effects of opioids, such as sedation and respiratory depression, are particularly dangerous when the surgery has been performed along the respiratory tract. The benefits of analgesics and dexamethasone used for post-tonsillectomy pain require review. Systematic reviews on various analgesics for post-tonsillectomy pain have been published for paediatric and adolescent patients.7 Paracetamol (alone and in combination with ibuprofen) for acute postoperative pain has been analysed in systematic reviews that have included some tonsillectomy studies.8, 9, 10 Also, the effects of NSAIDs on the risk for post-tonsillectomy haemorrhage (PTH) have been studied widely.11, 12, 13 Reviews on the effect of dexamethasone on post-tonsillectomy morbidity have mainly focused on the risk for PTH and postoperative nausea and vomiting (PONV); we found one review that included pain as a clinical endpoint in adults.14 Additionally, one study reviewed the effect of dexamethasone for post-tonsillectomy pain only.15 Reviews on gabapentinoids for acute pain after general surgery, post-tonsillectomy, and after head-neck surgery have been published.16, 17, 18 However, the effect of various analgesics on post-tonsillectomy pain in adults has not been systemically reviewed, to our knowledge.
We performed a systematic review and meta-analysis of published studies to assess the effect of systemic analgesics in the treatment of post-tonsillectomy pain in adult and adolescent patients. Randomised, double-blind, placebo-controlled studies reporting at least one analgesic outcome (such as incidence or intensity of pain) or use of rescue analgesics were included.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were followed in performing and reporting the review.19
Search strategy
The following electronic databases were searched to identify published or ongoing RCTs: Pubmed, Ovid MEDLINE, The Cochrane Central Register of Controlled Trials (CENTRAL), and Cochrane Database of Systematic Reviews (CDSR). We used the following search terms: (tonsillectomy or tonsillectomy* or tonsillotomy or post tonsillectomy) AND (pain or pain* or analgesia or analgesic* or narcotics). The search retrieved only a few studies on dexamethasone. Therefore, targeted searches were made using the following terms: (tonsillectomy or tonsillectomy* or tonsillotomy or post tonsillectomy) AND (dexamethasone* or corticosteroids*). We searched trial registries www.clinicaltrials.gov and www.eudraCT.com for unpublished trials. We hand-searched reference lists of included studies and identified reviews for additional potential trials. The search was performed in February 2017 and conducted by one author (KT). Three reviewers (KT, AT, KH) independently screened all studies for eligibility by titles and abstracts, and a decision was made on whether to obtain full-text publications. These studies were then inspected for relevance and a decision was made on whether to include or exclude them. Disagreements between reviewers were resolved by consensus.
Inclusion criteria
Types of studies: double-blind placebo-controlled randomised studies (RCTs) of systemic analgesics for post-tonsillectomy pain in adults or adolescents. Only publications in English were included.20 Studies published before 1980 were excluded, as operation techniques have evolved and hence the study settings would not be comparable with those in more recent years.
Types of participants: adults and adolescents (≥13 yr old) undergoing tonsillectomy for any indication as inpatient or outpatient. Studies on paediatric patients and mixed studies of adult and paediatric patients were excluded. Studies of less than 10 participants in a group were excluded.
Types of intervention: administration of systemic (oral, rectal, i.m., or i.v.) analgesics (paracetamol, NSAIDs, gabapentin, pregabalin, dextromethorphan, ketamine) and dexamethasone for prevention or treatment of pain. Studies on topical, infiltration, and regional analgesics were excluded.
Types of outcome measures: the primary outcome was incidence or intensity of pain, or both; secondary outcomes were the use of rescue analgesia and adverse effects. Studies that did not report at least one analgesic outcome measure were excluded.
Data collection
We developed a data abstraction table, pilot-tested it on five randomly selected studies, and refined it accordingly. Data collection from included studies was performed by one author (KT) and reviewed by two other authors (AT, KH). Disagreements were resolved by discussion between three authors (HKT, AT, KH). A fourth author (VKK) was consulted if no agreement was reached.
The following data on study participants were extracted: age, number of patients, ASA status (ASA Physical Status Classification System), number and reason for dropouts, type and technique of operation, type of anaesthesia (local or general, whether analgesics were used during anaesthesia), type of intervention (type, dose, frequency, and timing of analgesics [preoperative, intraoperative, or postoperative]), additional and rescue analgesics, method of pain measurement and by whom (patient or observer), type of comparator (placebo), type of outcome measure (pain intensity before and after medication, rescue analgesics, return to daily activities, adverse effects), length of follow-up, and study design (randomisation, blinding of randomisation, and intervention).
Quality assessment and risk of bias in and across individual studies
Three authors (HKT, KH, AT) independently assessed the validity and potential bias of included studies by evaluating the adequacy of randomisation and concealment of allocation (sequence generation), blinding of patients, healthcare providers, data collectors, and outcome assessors, intention-to-treat, and for incomplete outcome data and selective outcome reporting. The Cochrane risk of bias tool was used for assessment of methodological quality of included studies.21 Publication bias of included studies was determined by a funnel plot.
Statistical analysis and synthesis
Data were analysed by calculating the mean difference with the corresponding 95% confidence intervals (CI). We performed meta-analyses of included studies when a group of studies in which clinical heterogeneity was sufficiently small could be identified. Data were analysed using Review Manager (RevMan; version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Statistical heterogeneity was analysed with a χ2 test and I2 was calculated. Pooled meta-analyses were not performed in case clinical heterogeneity was too great or reporting of outcome measures varied excessively within a group of analgesics. Instead, narrative synthesis was used to compare studies qualitatively by using significant differences reported in original studies. In the overall interpretation of the results, we consider that ‘no more than mild pain’ (less than 3/10 in the pain scale) is an acceptable result in clinical practice (moderate pain 3–6/10, strong/severe pain 6–10/10).22
Results
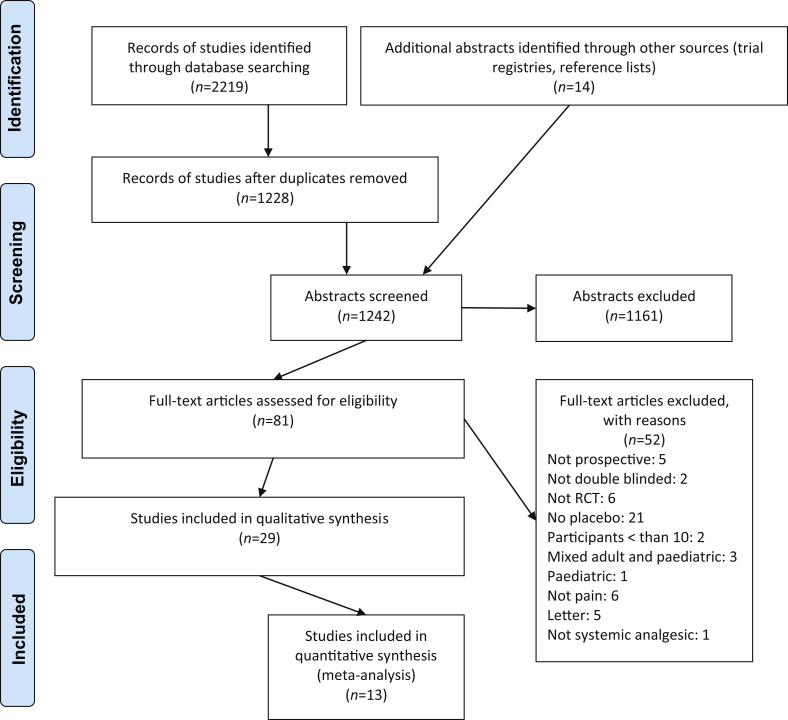
We retrieved 2219 citations from an electronic database search and an additional 14 citations from reference lists of reviews (Fig. 1). After excluding duplicates and articles that did not fulfil the inclusion criteria, full-text articles of 81 studies were assessed for eligibility, based on the title and abstract. Of these, 29 studies representing 1816 patients met the inclusion criteria, of which 13 were suitable for pooled meta-analysis.
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Characteristics of the included studies
The main characteristics and results of the included studies are shown in Table 1. A detailed description is shown in Supplementary Appendix S1. Two studies investigated paracetamol,23, 24 nine NSAIDs,25, 26, 27, 28, 29, 30, 31, 32, 33 10 dexamethasone,34, 35, 36, 37, 38, 39, 40, 41, 42, 43 three gabapentin,44, 45, 46 one pregabalin,47 two dextromethorphan,48, 49 one ketamine,50 and one oxycodone.51 Patients undergoing adenotonsillectomy were included in two studies.28, 33 In the remaining studies, all patients underwent tonsillectomy. Surgery was performed under general anaesthesia in 27 studies; in two studies the type of anaesthesia was not reported.25, 41 The surgical method was described in 19 studies.
Table 1.
Main characteristics and results of the included studies. intraop., intraoperatively; max, maximal; n, number; No., number; NS, not significant; POD, postoperative day; postop., after operation; preop., before operation; SD, standard deviation; sEMG, surface electromyography; TCI, target controlled infusion; VAS, visual analogue scale; VASr, VAS at rest; VASs, VAS on swallowing; VRS, verbal rating scale
| Reference (and year) | No. of patients, active/placebo | Study analgesics Study arm Placebo Time of administration |
Rescue analgesic, time of administration | Duration of follow-up (days) | Analgesic outcome results (study drug vs placebo) |
|---|---|---|---|---|---|
| Atef and Fawaz23 (2008) | 38/38 | Paracetamol 1 g i.v. saline i.v. Intraop., 6, 12, 18 h |
Pethidine 1 mg kg−1 i.m. if VASr>30, 0–24 h | 1 | Paracetamol reduced total dose of i.m. pethidine during 0–24 h (P<0.001), n of pethidine doses/patient 0–24 h (P<0.001) and % of patients that needed pethidine (P<0.05) Paracetamol decreased VASs and VASr at 2 and 3 h (P<0.05) |
| Salonen and colleagues24 (2009) | 39/37/38 | Paracetamol 2 g i.v. Paracetamol 1 g i.v. Saline i.v. 10 min postop. |
Oxycodone 2 mg i.v. if VASr>30 or VASs>50, 0–6 h | 6 | Proportion of patients that needed oxycodone 0–6 h NS Paracetamol 2 g reduced n of oxycodone doses/patient 0–6 h (P=0.002), paracetamol 1 g NS. Time to 1st dose of oxycodone NS. Pain intensity NS |
| Parker and colleagues25 (1986) | 44/33/33 | Ibuprofen 600 mg p.o. Acetylsalicylic acid 600 mg p.o. 30 ml syrup p.o. 0–4 times daily for 0–6 postop. days as needed |
Analgesia p.o. or i.m., dose or type unclear, hospital Paracetamol, home |
6 | Ibuprofen provided pain relief at 30 min (% of patients with pain 50% gone) (P<0.05), at 4 h ibuprofen and acetylsalicylic acid NS Analgesic consumption: ibuprofen and acetylsalicylic acid 3–4 daily, placebo less, no numerical data. Rescue analgesia: no one requested |
| Rorarius and colleagues26 (1993) | 21/21/21 | Indomethacin 50 mg i.v. Diclofenac 75 mg i.v. Saline i.v. Intraop. |
Oxycodone 3 mg i.v., 0–70 min | <1 | Indometacin reduced n of oxycodone doses/group (P=0.05). Time to 1st rescue analgesic NS. Proportion of patients that needed oxycodone NS |
| Tarkkila and Saarnivaara27 (1999) | 20/20/20/20 | Ketoprofen 100 mg i.v. Diclofenac 75 mg i.v. Ketorolac 30 mg i.v. Saline i.v. Intraop., 6, 12 h |
Oxycodone 0.05 mg kg−1 i.v., 0–2 h Oxycodone 0.1 mg kg−1 i.m., 2–24 h |
1 | All NSAIDs reduced total n of oxycodone doses/patient 0–24 h (P<0.05) Ketorolac reduced proportion of patients that requested oxycodone (P<0.05). Pain intensity NS |
| Salonen and colleagues28 (2001) | 41/40/25 | Ketoprofen 0.5 mg kg−1 i.v. bolus with ketoprofen infusion 3 mg kg−1 Saline i.v. bolus with infusion Bolus at induction (‘intra’) or in PACU (‘post’) with 24 h infusion in both |
Oxycodone 0.05 mg kg−1 i.v. if VASr≥30, 0–4 h Oxycodone 0.1 mg kg−1 i.m. if VASr≥30, 4–24 h |
1 | Proportion of patients that needed oxycodone in PACU 0–4 h NS, intra- and post-ketoprofen at ward 5–24 h reduced (P=0.002), 0–24 h NS Intra- and post-ketoprofen reduced n of oxycodone doses/patient at all time intervals vs placebo: 0–4 h (P=0.03, P=0.04, respectively), 5–24 h (P<0.01, P<0.01, respectively) and 0–24 h (P<0.01, P<0.01 respectively). Total dose of oxycodone NS |
| Naesh and colleagues29 (2005) | 20/20 | Rofecoxib 50 mg p.o. identical capsules p.o. 1.5 h preop. |
Morphine 2.5 mg i.v. until VAS<3 or patient comfortable, 0–24 h | 1 | VAS 0–24 h NS. Rofecoxib reduced strong pain (VAS>5) 0–8 h: (P=0.02) Rofecoxib reduced total dose of morphine during 0–8 h (P<0.04) Time to 1st rescue dose NS |
| Ismail and Mowafi30 (2010) | 20/19 | Lornoxicam 16 mg i.v. Saline i.v. 30 min before induction |
Solpadeine plus (paracetamol, codeine, and caffeine in ratio of 500:8:30 mg, respectively) 2 Tablets p.o. every 4 h and Tramal 50 mg i.v. if needed, 0–24 h |
1 | Lornoxicam reduced VASs and VASr at 4 h (P<0.05) and VASs at 24 h (P<0.05) Lornoxicam increased time to 1st dose of paracetamol (P<0.05) Lornoxicam reduced total dose of paracetamol (in Solpadeine) 0–12 h (P<0.05) and 0–24 h (P<0.05). None needed Tramadol |
| Mowafi and colleagues31 (2011) | 20/20 | Lornoxicam 16 mg i.v. Saline i.v. 30 min before induction |
Diclofenac 50 mg p.r., 0.5–24 h | 1 | Lornoxicam reduced VRSmax (P<0.001) and VRSr at 4 and 24 h (P<0.001) and VRSs at 4 h (P<0.03), at 24 h NS Lornoxicam reduced total dose and increased time to 1st dose of diclofenac (P<0.001, P<0.001) |
| Xie and colleagues32 (2012) | 40/40 | Parecoxib 40 mg i.v. Saline i.v. Intraop.,10 h |
PACU: fentanyl 0.2 μg kg−1 i.v. at 10 min intervals if VRS≥5 (max 0.1 mg) Ward: paracetamol 0.5 g i.v. min. 6 h intervals (max 2 g daily) |
2 | Parecoxib reduced VRSs at 2 h (P<0.001), and VRSs and VRSr at all time points (P<0.001) Parecoxib reduced proportion of patients that needed paracetamol at ward (P<0.05), fentanyl at PACU NS |
| Ng and colleagues33 ( 2017) |
40/40 | Celecoxib 200 mg×2 p.o. identical placebo capsules p.o. 1–10 POD |
PACU: morphine i.v. or oxycodone 5 mg p.o. every 6 h or when needed Home: oxycodone 5 mg p.o., max 100 mg daily |
10 | Pain intensity (overall and daily) NS. Total dose of oxycodone NS. 1st pain-free day and return to normal activities NS |
| Fields and colleagues34 (1994) | 29/29 | Dexamethasone 8 mg i.v. Saline i.v. Intraop. |
Elixir: paracetamol 250 mg with codeine 7.5 mg in 5 ml p.o., 1–7 POD | 7 | Dexamethasone decreased pain intensity at 4 h (P<0.05), from 10 pm to 7th POD NS Total and daily dose of paracetamol+codeine elixir 1–10 POD NS |
| Carr and colleagues35 (1999) | 15/14 | Dexamethasone 20 mg i.v. Saline i.v. Intraop. |
PACU: morphine i.v., pethidine i.v. Ward: codeine and paracetamol p.o. Home: codeine p.o. every 4 h or if needed and paracetamol, 1–10 POD (doses unclear) |
10 | Pain intensity (>2 VAS change) NS. N of rescue analgesic dose in PACU NS Dexamethasone reduced total dose of pethidine/patient (P=0.03), but not total dose of morphine/patient. Proportion of patients that needed rescue analgesics in PACU NS. Daily doses of codeine and paracetamol 1–10 POD NS. Number of days taken off from school or work and time until tolerating normal diet NS |
| Stewart and colleagues36 (2002) | 48/52 | Dexamethasone 8 mg i.v. at induction, 2 mg p.o. at 10 pm, 2 mg p.o. twice daily for 1–4 POD, then 2 mg daily for 5–8 POD Saline i.v. and placebo tablets p.o. Intraop. and postop. |
PACU: morphine 2 mg i.v. Home: Co-codamol (paracetamol 1g with codeine 16 mg) 2 tablets p.o. every 6 h or if needed, 1–9 POD |
9 | Dexamethasone reduced pain intensity on all 1–9 POD, except on 0 and 2nd POD, P<0.05. Dexamethasone decreased proportion of patients that needed rescue analgesics (Co-codamol >8 per day) (P=0.024), and total dose of Co-codamol/day on 2–7 POD (P<0.05). Total dose of morphine in PACU unclear |
| Al-Shehri37 (2004) | 15/15 | Dexamethasone 6 mg i.v. Saline i.v. Intraop.,8, 16 h |
None | 10 | Dexamethasone reduced VAS on 1 POD (P<0.05) and on 8 POD (P<0.05). Total dose of analgesics NS. Patients in dexamethasone group reduced their analgesic intake earlier during 2nd week; at 7, 8, and 10 POD, P unclear |
| Rujirojindakul and colleagues38 (2008) | 25/25 | Dexamethasone 20 mg i.v. Saline i.v. Intraop. |
PACU: fentanyl i.v. every 10 min if VRS>5 (dose unclear) Ward: paracetamol 10 mg kg−1 p.o. every 6 h if VRS>5 or morphine 3 mg i.v. every 1 h if VRS>5 |
2 | Dexamethasone reduced VRS at 4 h (P=0.03), other times NS Time to first rescue analgesic NS. Total dose of rescue analgesics 0–48 h NS |
| Lachance and colleagues39 (2008) | 37/49 | Dexamethasone 8 mg i.v. and 8 mg p.o. on 0 POD at home, 6 mg twice on 1 POD, 4 mg twice on 2 POD; and 2 mg twice on 3 POD Placebo in similar manner, type unclear Intraop. and postop. |
Hospital: morphine 0.1 mg kg−1 i.v. (max 4 doses) Home: hydromorphone 1 mg p.o. (max 20 mg), 0–4 POD |
7 | Dexamethasone reduced VASs on 2 POD (P=0.047), other days NS Hydromorphone consumption, NS |
| Vaiman and colleagues40 (2011) | 30/30 | Dexamethasone 20 mg i.v. Saline i.v. 20 h postop. |
Not given 14–20 h (type or route unclear) | 1 | Dexamethasone decreased VASs (postdrug vs predrug), P=0.022, while placebo did not. Dexamethasone also decreased postdrug-VASs vs placebo (P<0.05) Dexamethasone decreased muscle reactions and normalised deglutition pattern (sEMG), while placebo did not (P<0.05) |
| Vaiman and colleagues41 (2011) | 30/30/30 | Dexamethasone 20 mg i.v. infusion Oxycodone 2 mg h−1 i.v. infusion (14 mg) Saline i.v. infusion 20 h postop. Oxycodone and placebo: infusion 16–23 h postop. |
After the EMG test was performed at 24 h (type or route unclear) | 1 | Oxycodone and dexamethasone decreased VASs (postdrug vs predrug) P<0.05, while placebo did not. Oxycodone decreased postdrug-VASs vs placebo (P<0.05), dexamethasone did not. Oxycodone decreased muscle reactions (sEMG), while dexamethasone and placebo did not (P<0.05). Oxycodone and dexamethasone, in case of oedema, decreased dysphagia (P<0.05) |
| Thimmasettaiah and Chandrappa42 (2012) | 25/25/25/25 | Dexamethasone 0.5 mg kg−1 i.v. saline I.V. preop. (after inserting i.v. cannula) vs intraop. vs postop. (PACU) placebo: intraop. |
PACU: Tramadol mg kg−1 i.v. if VAS>6 | 1 | Dexamethasone decreased VAS at 6 h (P<0.05), at 12 h (P<0.001), and at 24 h (P<0.001). Preop. and intraop. groups NS. Dexamethasone reduced n of tramadol doses 0–24 h (P<0.05) |
| Khafagy and Osman43 (2013) | 43/31 | Dexamethasone 0.3 mg kg−1 i.v. (max 8 mg) Saline i.v. Intraop. |
Unclear | 7 | Dexamethasone reduced VAS on 0 POD and on 4–7 POD (P<0.05). Dexamethasone reduced overall VAS during 0–7 POD (P=0.002). 1st liquid intake NS. 1st solid food intake: dexamethasone earlier vs control group (P=0.05) |
| Mikkelsen and colleagues44 (2006) | 22/27 | Gabapentin 1200 mg p.o. 1 h preop., 600 mg p.o. twice on 0 POD, 600 mg p.o. three times on 1–5 POD Placebo in similar manner, type unclear Preop. and postop. |
Morphine 2.5 mg i.v., 0–4 h Home: ketobemidone 2.5 mg p.o., 0–5 POD |
5 | VRSr and VASs 2–4 h and 1–5 POD, NS Total dose of morphine 0–4 h NS Gabapentin reduced total dose of ketobemidone 0–24 h, on following days NS |
| Jeon and colleagues45 (2009) | 32/26 | Gabapentin 600 mg p.o. on previous evening and 600 mg p.o. 1 h preop. Similar capsule p.o. Preop. |
PCA fentanyl 20 μg bolus, diclofenac 75 mg i.m., 0–2 POD | 9 | Gabapentin reduced VASs at 2 and 4 h (P=0.04, P=0.04), on following POD NS. VASr 0–7 POD NS Gabapentin reduced total dose of PCA fentanyl (P=0.002), and total dose of diclofenac on 0–2 POD at hospital (P=0.001). Patient satisfaction NS |
| Abdelmageed and colleagues46 (2010) | 30/30 | Gabapentin 1200 mg p.o. Placebo tablets, type unclear 2 h preop. |
Pethidine 1 mg kg−1 i.m. every 6 h if VAS≥3 or if needed | 1 | Gabapentin reduced VAS at all time points: 1, 3, 6, 12, 18, 24 h (P<0.001). Gabapentin decreased worst VAS (P<0.001) Gabapentin reduced total dose of pethidine 0–24 h (P<0.001) and increased time to 1st dose of pethidine (P<0.001) |
| Mathiesen and colleagues47 (2011) | 43/45 | Pregabalin 300 mg p.o. Placebo, type unclear 1 h preop. |
Morphine 2.5 mg i.v., 0–1 h ketobemidone 2.5 mg p.o., 0–24 h | 1 | Pregabalin reduced VASs at 2 and 4 h (P=0.009, P<0.003) Pregabalin also reduced VASs mean 2–24 h (P=0.009) and VASr at 4 h (P=0.03). Pregabalin reduced total dose of ketobemidone 1–4 h (P=0.003), 1–24 h NS. Total dose of morphine 0–1 h NS |
| Kawamata and colleagues48 (1998) | 12/12/12 | Dextromethorphan 45 mg p.o. Dextromethorphan 30 mg p.o. Starch tablet p.o. 1 h preop. |
Diclofenac 50 mg p.r., 0–6 POD | 7 | Dextromethorphan 45 mg decreased VASs and VASr on all 0–6 POD vs placebo (P<0.05). Dextromethorphan 30 mg decreased VASs on 0 POD and VASr on 0, 1, and 6 POD (P<0.05). Dextromethorphan 45 mg and 30 mg reduced total dose of diclofenac/patient (P<0.05, P<0.05). Dextromethorphan increased time to 1st dose of diclofenac (P<0.05) |
| Rafiei and colleagues49 (2012) | 20/20 | Dextromethorphan 45 mg p.o. Placebo tablet p.o. 1 h preop. |
Pethidine 0.5 mg kg−1 i.v. if VAS≥3 | 1 | Dextromethorphan increased pain-free time period (P=0.002), and decreased VASs 0–24 h (P=0.047), VASr NS. Dextromethorphan increased time to 1st dose of pethidine (P=0.005) and reduced total dose of pethidine/patient (P=0.005) |
| Van Elstraete and colleagues50 (2004) | 20/20 | Ketamine 0.5 mg kg−1 i.v. bolus with ketamine 2 μg kg−1 min−1 infusion saline i.v. bolus with i.v. infusion Bolus at induction with infusion until end of procedure |
PACU: Morphine 3 mg i.v. every 5 min until VAS<30 Ward: morphine 10 mg p.o. every 4 h until VASs<30 Ketoprofen 100 mg p.o. and paracetamol 500 mg p.o., 0–24 h |
1 | Total dose of morphine/patient NS. Time to 1st morphine dose at the ward NS. VASr, VASs NS |
| Vaiman and Krakovski51 (2012) | 30/30 | Oxycodone 2 mg h−1 (14 mg) i.v. infusion Saline i.v. infusion 16–23 h postop. |
Unclear | 1 | Oxycodone decreased VASs (postdrug vs predrug), P=0.03, while placebo did not. Oxycodone decreased postdrug VASs (oxycodone vs placebo, P<0.05) and muscle reactions (sEMG), P=0.03 |
Patients in one study received dexamethasone in addition to study medication.33 Additional preoperative or postoperative analgesics were given in 13 studies, no additional analgesics were given in 10 studies, and information was not provided in six studies (Table 1). Rescue analgesics were given in 25 studies, were not allowed in one study,37 and information was not provided in three studies.34, 43, 51
Study medications were administered before operation in eight studies,29, 30, 31, 45, 46, 47, 48, 49 before operation and intraoperatively in one study,44 intraoperatively in seven studies,8, 26, 34, 35, 38, 43, 50 intraoperatively and after operation in five studies,27, 32, 36, 37, 39 after operation in seven studies,23, 24, 25, 33, 40, 41, 51 and before and after operation in one study.44 One study compared intraoperative and postoperative administration of the study medication with placebo.28 Another study compared preoperative, intraoperative, and postoperative administration of the study medication with placebo.42 There were 17 single-dose and 12 multiple-dose studies. In studies that compared more than one analgesic with placebo, only one study arm was analysed. The duration of follow-up times ranged from 70 min to 10 days (Table 2). If 4-h or 24-h data were not available, we used the datapoint closest to time. There were three multicentre studies.32, 39, 44
Table 2.
Reported pain intensity values during follow-up time. *pain intensity values not available, only need of rescue analgesics reported; ▲=pain relief; ■=VAS/VRS/NRS, ○=VASs/VRSs/NRSs; ●=VASr/VRSr/NRSr and VASs/VRSs/NRSs. NMDA, N-methyl-D-aspartate; NRS,; NRSr,; NRSs,; pod, postoperative day; VAS, visual analogue scale; VASr, VAS at rest; VASs, VAS on swallowing; VRS, verbal rating scale; VRSr, VRS at rest; VRSs, VRS on swallowing; NRS, numeric rating scale; NRSr, NRS at rest; NRSs, NRS at swallowing
| Study (and year) | 1 h | 2 h | 4 h | 12 h | 24 h | 2 pod | 3 pod | 4 pod | 5 pod | 6 pod | 7 pod | 8 pod | 9 pod | 10 pod |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paracetamol | ||||||||||||||
| Atef and Fawaz23 (2008) | ● | ● | ● | ● | ||||||||||
| Salonen and colleagues24 (2009) | ● | ● | ● | |||||||||||
| NSAIDs | ||||||||||||||
| Parker and colleagues25 (1986), ibuprofen, acetylsalicylic acid | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ||||||
| Rorarius and colleagues26 (1993), indomethacin, diclofenac | * | * | ||||||||||||
| Tarkkila and Saarnivaara27 (1999), ketoprofen | ● | ● | ||||||||||||
| Salonen and colleagues28 (2001), ketoprofen | ● | ● | ● | ● | ||||||||||
| Naesh and colleagues29 (2005), rofecoxib | ■ | ■ | ■ | |||||||||||
| Ismail and Mowafi30 (2010), lornoxicam | ● | ● | ● | ● | ● | |||||||||
| Mowafi and colleagues31 (2011), lornoxicam | ● | ● | ● | ● | ● | |||||||||
| Xie and colleagues32 (2012), parecoxib | ● | ● | ● | ● | ● | |||||||||
| Ng and colleagues33 (2017), celecoxib | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| Dexamethasone | ||||||||||||||
| Fields and colleagues34 (1994) | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| Carr and colleagues35 (1999) | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||
| Stewart and colleagues36 (2002) | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||
| Al-Shehri37 (2004) | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| Rujirojindakul and colleagues38 (2008) | ■ | ■ | ■ | ■ | ||||||||||
| Lachance and colleagues39 (2008) | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |||||||
| Vaiman and colleagues40 (2011) | ○ | |||||||||||||
| Vaiman and colleagues41 (2011) | ○ | |||||||||||||
| Thimmasettaiah and Chandrappa42 (2012) | ■ | ■ | ||||||||||||
| Khafagy and Osman43 (2013) | ■ | ■ | ■ | ■ | ■ | ■ | ||||||||
| Gabapentinoids | ||||||||||||||
| Mikkelsen and colleagues44 (2006), gabapentin | ● | ● | ● | ● | ● | ● | ||||||||
| Jeon and colleagues45 (2009), gabapentin | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Abdelmageed and colleagues46 (2010), gabapentin | ■ | ■ | ■ | |||||||||||
| Mathiesen and colleagues47 (2011), pregabalin | ● | ● | ● | |||||||||||
| NMDA antagonists | ||||||||||||||
| Kawamata and colleagues48 (1998), dextromethorphan | ● | ● | ● | ● | ● | ● | ||||||||
| Rafiei and colleagues49 (2012), dextromethorphan | ● | |||||||||||||
| Van Elstraete and colleagues50 (2004), ketamine | ● | ● | ● | ● | ● | |||||||||
| Opioids | ||||||||||||||
| Vaiman and Krakovski51 (2012), oxycodone | ○ | |||||||||||||
Pain intensity was the primary outcome in 11 studies29, 30, 31, 32, 33, 35, 36, 38, 39, 44, 47 and rescue analgesia in six studies (number of doses during follow-up,23 proportion of patients requiring rescue analgesics to maintain visual analogue scale [VAS] at rest [VASr] ≤30 mm and VAS on swallowing (VASs) ≤50 mm,24 total dose of rescue analgesics,27, 50 proportion of patients that needed rescue analgesics,28 and time to first analgesic request after discharge from operating room49). Twelve studies did not specify the primary outcome but reported data on pain intensity,34, 37, 40, 41, 42, 43, 45, 46, 48, 51 pain relief,25 and rescue analgesia.26 We converted pain intensity values to a 0–10 scale when a 0–5 scale was used in a study. Pain intensity scales of 0–6 and 0–4 were used in one dexamethasone study42 and in one gabapentin study,44 respectively; in these studies, we considered that converting values to 0–10 scales would not be appropriate, and they were excluded from pain intensity comparisons in meta-analyses.
Pain intensity was reported in all but one study26 in which the only need for rescue analgesics was evaluated (total number of doses and time to first rescue analgesic). Pain intensity was reported by the patient in 11 studies and by an observer in 10 studies. Seven studies did not report who assessed pain intensity. The criteria for administration of rescue analgesia were reported in 24 studies, of which 10 studies reported a specified pain intensity value for administration of rescue analgesia.
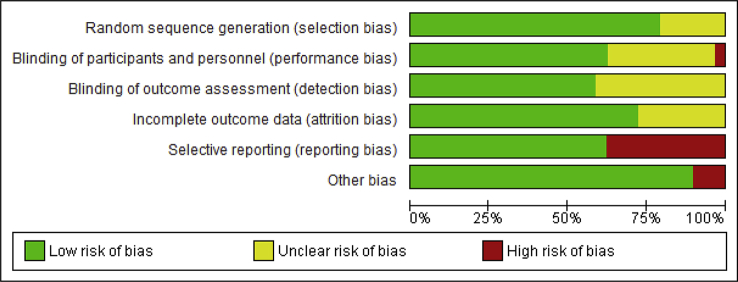
Quality and risk of bias of included studies
All included studies were randomised, double-blind, and placebo controlled. The risk of bias graph is presented in Fig. 2 and the risk of bias summary in Supplementary Appendix S2. Detailed characteristics of bias in included studies are presented in Supplementary Appendix S3.
Fig 2.
Risk of bias graph. Review authors' judgments about each risk of bias item presented as percentages across all included studies.
Pain intensity after tonsillectomy
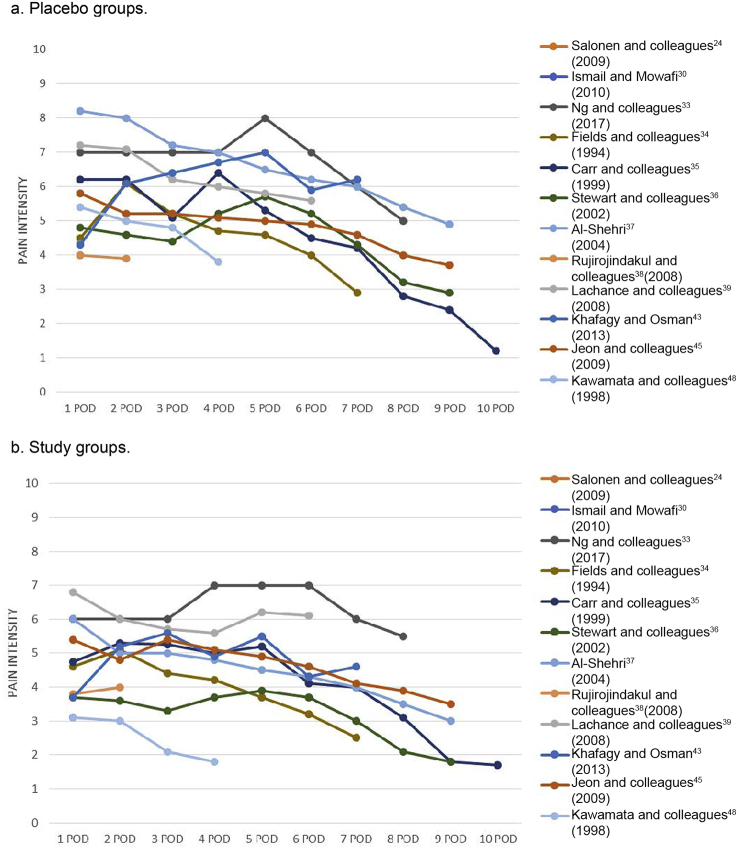
Tonsillectomy caused moderate to severe pain that lasted for several days (Fig. 3). During the first postoperative week, pain intensities (median or mean as shown in figures) in both placebo and study groups ranged from 4 to 8 (pain intensity scale 0–10). By the end of the first postoperative week (7 POD), pain intensity had decreased to less than 4/10 in the majority of study groups, whereas in the majority of placebo groups, pain intensity was still greater than 4/10 (Fig. 3b and a, respectively).
Fig 3.
Pain intensities during 1–9 postoperative days (POD). (a) Placebo groups; (b) study groups.
Randomised studies comparing paracetamol with placebo
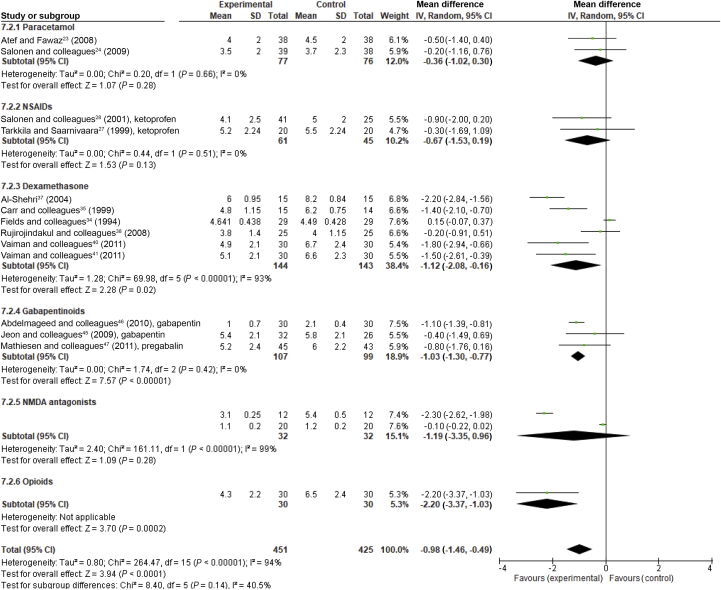
Two studies involving 153 participants23, 24 are summarised in Table 1 (Supplementary Appendix S1). The pooled estimate for intraoperatively administered paracetamol 1–2 g i.v. showed a statistically significant decrease in pain intensity (VASs) at 4 h (−0.88 [95% CI −1.66 to −0.09], P=0.03), equivalent to an 18% reduction compared with the control group. Both studies (single dose of 2 g i.v. intraoperatively24 and multiple doses up to 4 g i.v. on the day of operation23) reported a decreased need for opioids within 24 h. One study24 that reported incidence of PTH found no association between paracetamol and increased risk for PTH.
Randomised studies comparing NSAIDs with placebo
Nine studies involving 638 participants25, 26, 27, 28, 29, 30, 31, 32, 33 are summarised in Table 1 (Supplementary Appendix S1). Only two studies27, 28 reported pain intensity values as mean with standard deviation (SD) or standard error of mean (SEM was converted to SD) for which a pooled meta-analysis was possible. Intraoperative ketoprofen failed to decrease pain intensity at 2 h and at 24 h (VASs scale 0–10) (−0.82 [95% CI −2.10 to 0.45], P=0.21 and −0.67 [95% CI −1.53 to 0.19], P=0.13, respectively) (Fig. 4). Both studies reported a reduced need for rescue analgesics within 24 h.
Fig 4.
Forest plot showing the effect of perioperatively administered analgesics and dexamethasone on pain intensity at 24 h. Pain intensity values (scale 0–10) with confidence intervals (CI). Data evaluated using a random effects model.
In other NSAID studies, the reporting of pain intensities was unsuitable for meta-analysis (pain relief,25 median values instead of mean29, 30, 31, 32, 33) and the results are presented descriptively. In a study25 with oral ibuprofen 600 mg, acetylsalicylic acid 600 mg, or placebo 0–4 times daily for 6 postoperative days in patients with moderate to severe post-tonsillectomy pain, pain relief within 24 h after initial treatment was faster with ibuprofen compared with placebo. Acetylsalicylic acid did not have any effect on pain outcomes. In a study26 of diclofenac 75 mg i.v. and indomethacin 50 mg i.v. intraoperatively, indomethacin reduced the need for opioids at the PACU, while diclofenac had no effect.
Cyclooxygenase-2 selective NSAIDs, lornoxicam 16 mg i.v. before operation in two studies30, 31 and parecoxib 40 mg i.v. intraoperatively and after operation32 reduced pain and the need for rescue analgesia within 24 h. Oral rofecoxib 50 mg before operation decreased the incidence of pain (VAS>5) and the need for opioids within the first 8 h.29 Celecoxib 200 mg twice daily for 1–10 postoperative days with additional paracetamol 1 g four times daily did not reduce pain or need for opioids.33
The majority of patients given NSAIDs (74%) or placebo (85%) needed rescue analgesia within 24 h after the operation (relative risk [RR] 0.91 [95% CI 0.79–1.05], P=0.21).27, 28, 30, 31, 32 Six studies27, 28, 30, 31, 32, 33 reported incidence of PTH; none found an increased risk.
Randomised studies comparing dexamethasone with placebo
Ten studies34, 35, 36, 37, 38, 39, 40, 41, 42, 43 including 590 patients are summarised in Table 1 (Supplementary Appendix A1). Pooled estimates demonstrated that single-dose dexamethasone of 8 mg to 0.5 mg kg−1 decreased pain intensity equivalent to 23% at 4 h (−1.40 [95% CI −1.64 to −1.16], P<0.001, I2=0%) but no longer at 24 h (P=0.05) (Fig. 4, Supplementary Appendix S4). When the only multiple-dose study37 suitable for meta-analysis was included, a significant 17% reduction at 24 h was observed, although with a high heterogeneity (I2=93%). In two studies with high single doses of dexamethasone (20 mg35 and 0.5 mg kg−142), the need for rescue opioids decreased in the PACU. Reduction of pain intensity lasted beyond the first postoperative day in all multiple-dose studies and in one single-dose study.36, 37, 39, 43 The total dose of rescue analgesic (combination of paracetamol 500 mg and codeine 16 mg) was reduced over 2–7 postoperative days in a study on dexamethasone for 8 postoperative days.36
Pooled estimates demonstrated that dexamethasone was associated with less PONV during 24–48 h after operation (RR 0.41 [95% CI 0.29–0.59], P<0.001, I2=0%)35, 36, 38, 42 without increase in risk for PTH (RR 0.76 [95% CI 0.37–1.56], P=0.46, I2=0%)35, 36, 38, 42 (four studies reported risk for PTH).
Randomised studies comparing gabapentinoids with placebo
Four studies including 255 patients (three gabapentin44, 45, 46 and one pregabalin47) are summarised in Table 1 (and Supplementary Appendix S1). Pooled estimates demonstrated that gabapentinoids decreased pain intensity equivalent to 30% at 4 h (−1.58 [95% CI −2.28 to −0.88], P<0.001, I2=0%) and equivalent to 13% at 24 h (−1.03 [95% CI −1.30 to −0.77], P<0.001, I2=0%) (Fig. 4, Supplementary Appendix S5). Gabapentin 300–2400 mg on the day of operation reduced the need for rescue analgesics (opioids or diclofenac) within 24 h.44, 45, 46 Pregabalin 300 mg reduced the need for opioids within 4 h.47 In one study44 with high and multiple doses of gabapentin, adverse events were common and outweighed beneficial effects. Gabapentin decreased postoperative vomiting in pooled analysis estimation, most likely because of the decreased need for rescue opioids (RR 0.55 [95% CI 0.32–0.95], P=0.03, I2=2%).
Randomised studies comparing NMDA receptor antagonists with placebo
Three studies (116 patients) on NMDA receptor antagonists (two dextrometorphan48, 49 and one ketamine50) are summarised in Table 1 (Supplementary Appendix S1). Ketamine50 and dextromethorphan studies48, 49 were too heterogeneous to be combined in pooled analysis. Dextromethorphan 45 mg reduced VASs and the total dose of rescue analgesics within 24 h, and prolonged time to first request of rescue analgesics in both studies.48, 49 In the study of Kawamata and colleagues,48 dextromethorphan reduced pain intensity over the follow-up period of 6 postoperative days.
Ketamine as an intraoperative bolus and infusion failed to reduce pain intensity and total dose of opioids, and did not prolong the time to first analgesic requests over a follow-up period of 24 h.50 Ketamine did not increase the incidence of adverse events; nausea and sedation scores were similar between the study groups and none experienced hallucinations or nightmares.
Randomised studies comparing opioids with placebo
One study on oxycodone51 including 60 patients is summarised in Table 1 (and Supplementary Appendix S1). I.V. oxycodone 2 mg h−1 decreased pain intensity at 24 h after operation. Data on rescue analgesics or adverse events were not reported.
Discussion
Summary of evidence
The main finding of this review was the scarcity of data and short duration of follow-up in studies that investigated analgesics for post-tonsillectomy pain. This finding is clinically relevant considering the number of tonsillectomies performed yearly and the intensity of pain after the procedure. I.V. paracetamol reduced pain intensity at 3–4 h and the need for opioids ≤24 h after surgery. Ketoprofen at varying doses (100 mg or 0.5 mg kg−1 i.v.) failed to decrease pain intensity within 24 h. However, both studies reported a reduced need for opioids within 24 h. In other NSAID studies, pooled analysis was not possible because of the large heterogeneity. In individual studies, NSAIDs administered on the day of operation reduced pain intensity, the need for rescue analgesics, or both within 24 h. Celecoxib on postoperative days 1–10 had no effect on pain intensity or the need for opioids. In all NSAID studies, the majority of patients needed rescue analgesics, thus indicating that NSAIDs alone do not provide adequate analgesia for tonsillectomy patients.
Dexamethasone as a single intraoperative dose reduced pain within 4 h. When administered in multiple doses, the effect lasted beyond the first postoperative day. Gabapentinoids and dextromethorphan decreased pain intensity and need of rescue analgesia within 24 hours. Ketamine failed to show any effect at the studied dose.
Because of the high post-tonsillectomy pain intensity, the analgesic effect did not reach clinical meaningfulness when any of the analgesics or dexamethasone was used alone. PONV was reduced by dexamethasone and gabapentinoids over 24–48 h after operation because of antiemetic and opioid-sparing effects. The incidence of PTH was reported in paracetamol, NSAID and dexamethasone studies; no increase in the risk for PTH was found. Serious adverse events were not reported.
Limitations
A systematic review and meta-analysis combine data to estimate treatment effects more precisely than is possible in single studies. However, the accuracy of results of systematic reviews depends on the availability and quality of the data available. A thorough search was performed, and all relevant studies were likely included in the study. Only studies in English were included, which could limit the accuracy of results; however, no evidence of a systematic bias from the use of English language restriction in systematic review-based meta-analyses in conventional medicine has been found.20 Funnel plots of the results were drawn and examined in the review group and did not show any apparent publication bias among studies.
Studies were clinically heterogeneous with varying timing of study medications, use of rescue and additional analgesics, and duration of follow-up. Therefore, combining results in meta-analysis was possible only in selected studies and only for pain intensity. Although there were sub-optimally only two studies in some analgesic groups, we conducted a meta-analysis also for them to allow rough comparison between groups.
In the present study, 12 out of 29 included trials failed to report the primary outcome thus presenting a risk for bias.
The main limitation was the short study and follow-up time, especially in the NSAID studies. From the patient point of view, adequate pain management at home is important. At hospital, pain can be easily treated with opioids, but problems arise after discharge when strong opioids are not available. There are minimal data on optimal analgesics for post-tonsillectomy pain at home.
All studies did not report pain intensities at rest and on swallowing, thus decreasing the accuracy of the results. Pain on movement (swallowing) should be reported, as it is a major determinant of how well patients can drink and eat at home during recovery.
Comparison with results of previous studies
In a Cochrane review (that included the two studies also included in this review), a single dose of either paracetamol or propacetamol i.v. was found to provide approximately 4 h of effective analgesia for about 36% of patients with acute postoperative pain.8, 23, 24 This is consistent with our findings that paracetamol 1–2 g i.v. intraoperatively decreased pain intensity at 3–4 h. A reduced need for opioids within 24 h was reported in both studies with a cumulative dose of paracetamol 4 g i.v.23 and a single dose of paracetamol 2 g i.v.24
NSAIDs are widely used for postoperative pain and are effective for various types of surgical pain.52, 53 We found no previous reviews of NSAIDs for post-tonsillectomy pain in adults. Consistent with previous reports, NSAIDs reduced pain intensity or need for rescue analgesics in most studies.
A review on perioperative single-dose dexamethasone for postoperative pain14 (that included one study35 also included here) reported reduced pain intensity at 4 and 24 h and opioid consumption at a high dose of dexamethasone (more than 0.1 mg kg−1). A review on dexamethasone and post-tonsillectomy morbidity15 (that included five studies34, 35, 36, 37, 39 also included here) reported reduced pain intensity on the first postoperative day with high-dose (>10 mg) steroids (P<0.001). Our results in the present review are comparable. We did not observe an increased risk for PTH associated with the use of dexamethasone. Two recent studies (a register study and a systematic review) indicate an increased risk for reoperation as a result of PTH in children, but not in adults.54, 55
Previous reviews on perioperative gabapentinoids have found a reduction of postoperative pain, opioid consumption, and opioid-related adverse effects.5, 18 A recent systematic review that analysed the benefit of pregabalin for acute pain reported a minimal opioid-sparing effect in studies with low overall risk of bias; this review included two tonsillectomy studies, of which one was also included in our analysis.47 We found similar analgesic effects in our study; gabapentinoids reduced pain at 4 and 24 h after operation in pooled analysis. However, regarding observed pain reduction at 24 h, one study46 with an unclear overall risk for bias was responsible for the majority of the effect (60% weight). When an analysis that excluded this study was performed, there was no significant reduction in pain intensity. Gabapentin 300–1200 mg before operation reduced the need for opioids within 4 and 24 h44, 45, 46 and pregabalin 300 mg before operation reduced this need within 4 h.47 Previous results on adverse effects associated with gabapentinoids compared with placebo are conflicting.5, 18 In the present study, the risk of adverse effects was not increased except in one high-dose study, where dizziness, vomiting, and gait disturbance were more frequent in the study group.44
Dextromethorphan is an NMDA receptor antagonist and is a widely used antitussive medication. Dextromethorphan has been shown to potentiate the antinociceptive effects of opiates and prevent pain sensitivity and opioid tolerance without significant side-effects.56, 57 A review of 28 studies on perioperative dextromethorphan (including one study48 also included here) revealed a reduced need for opioids and considered perioperative dextromethorphan as a safe adjuvant agent to opioid analgesia.57 In our study, dextromethorphan 45 mg before operation decreased pain intensity over 24 h and total dose and time to first rescue analgesic in both studies.48, 49 Ketamine is a well-known NMDA receptor antagonist and when used in subanaesthetic doses can reduce opioid requirements, pain sensitivity, or both in acute postoperative pain with mild or absent adverse effects (Cochrane review 2005).58 In a systematic review (2014) in paediatric tonsillectomies, i.v. ketamine before operation showed an opioid-sparing effect and a reduction of pain intensity and time to resumption of liquid diet.59 Adverse effects such as nausea and vomiting, sedation, bad dreams, sleep pattern change, or hallucinations were absent. In the present review, the only ketamine study included failed to be effective in studied doses.
We did not find reviews on opioids for post-tonsillectomy pain. We included only one opioid study in which postoperative oxycodone infusion decreased pain intensity as expected.
Conclusions and clinical implications
This study confirmed the beneficial analgesic effects of paracetamol, NSAIDs, dexamethasone, gabapentinoids, and dextromethorphan for post-tonsillectomy pain on the day of operation. Dexamethasone in multiple doses had an analgesic effect that exceeded 24 h. However, the use of steroids with high total doses or continued after the day of operation must be done with caution considering both the risks and benefits. Based on our results, gabapentin, pregabalin, and possibly dextromethorphan could be useful at moderate doses as adjuvants to other analgesics. Ketamine in subanaesthetic doses is effective in various types of surgeries in decreasing the need for opioids and pain sensitivity. Although ketamine was not effective in the one included study in our analysis, it may have efficacy for post-tonsillectomy pain; this should be studied in further trials.
The short follow-up times and clinical heterogeneity of studies limit the usefulness of the results, which should be interpreted with caution.
Single analgesics or dexamethasone alone do not provide a clinically meaningful analgesic effect for the treatment of post-tonsillectomy pain. Multimodal analgesia is thus required. Further studies are needed to identify the best possible combinations. Pain on swallowing should be used as a primary analgesic outcome and a follow-up time of 1–2 postoperative weeks is recommended.
Authors' contributions
Study design/planning: all authors
Extraction of data: HKT, AT, KH
Data analysis: HKT, VKK
Writing paper: HKT
Revising paper: all authors
Declarations of interest
The authors declare that they have no conflicts of interest.
Handling editor: J.G. Hardman
Editorial decision: 05 April 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.04.063.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Crowson M.G., Ryan M.A., Rocke D.J., Raynor E.M., Puscas L. Variation in tonsillectomy rates by health care system type. Int J Pediatr Otorhinolaryngol. 2017;94:40–44. doi: 10.1016/j.ijporl.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Ovesen T., Kamarauskas G., Dahl M., Mainz J. Pain and bleeding are the main determinants of unscheduled contacts after outpatient tonsillectomy. Dan Med J. 2012;59:A4382. [PubMed] [Google Scholar]
- 3.Lavy J.A. Post-tonsillectomy pain: the difference between younger and older patients. Int J Pediatr Otorhinolaryngol. 1997;42:11–15. doi: 10.1016/s0165-5876(97)00107-9. [DOI] [PubMed] [Google Scholar]
- 4.Sarny S., Habermann W., Ossimitz G., Stammberger H. Significant post-tonsillectomy pain is associated with increased risk of hemorrhage. Ann Otol Rhinol Laryngol. 2012;121:776–781. doi: 10.1177/000348941212101202. [DOI] [PubMed] [Google Scholar]
- 5.Toma A.G., Blanshard J., Eynon-Lewis N., Bridger M.W. Post-tonsillectomy pain: the first ten days. J Laryngol Otol. 1995;109:963–964. doi: 10.1017/s0022215100131767. [DOI] [PubMed] [Google Scholar]
- 6.Patel H.H., Straight C.E., Lehman E.B., Tanner M., Carr M.M. Indications for tonsillectomy: a 10 year retrospective review. Int J Pediatr Otorhinolaryngol. 2014;78:2151–2155. doi: 10.1016/j.ijporl.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Hamunen K., Kontinen V. Systematic review on analgesics given for pain following tonsillectomy in children. Pain. 2005;117:40–50. doi: 10.1016/j.pain.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.McNicol E.D., Ferguson M.C., Haroutounian S., Carr D.B., Schumann R. Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev. 2016;23:CD007126. doi: 10.1002/14651858.CD007126.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong C.K.S., Seymour R.A., Lirk P., Merry A.F. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–1179. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 10.Derry C.J., Derry S., Moore R.A. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 2013;24:CD010210. doi: 10.1002/14651858.CD010210.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Møiniche S., Rømsing J., Dahl J.B., Tramèr M.R. Nonsteroidal antiinflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systematic review. Anesth Analg. 2003;96:68–77. doi: 10.1097/00000539-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Riggin L., Ramakrishna J., Sommer D.D., Koren G.A. 2013 Updated systematic review & meta-analysis of 36 randomized controlled trials; no apparent effects of non steroidal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clin Otolaryngol. 2013;38:115–129. doi: 10.1111/coa.12106. [DOI] [PubMed] [Google Scholar]
- 13.Lewis S.R., Nicholson A., Cardwell M.E., Siviter G., Smith A.F. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev. 2013;18:CD003591. doi: 10.1002/14651858.CD003591.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Oliveira G.S., Almeida M.D., Benzon H.T., McCarthy R.J. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 15.Diakos E.A., Gallos I.D., El-Shunnar S., Clarke M., Kazi R., Mehanna H. Dexamethasone reduces pain, vomiting and overall complications following tonsillectomy in adults: a systematic review and meta-analysis of randomised controlled trials. Clin Otolaryngol. 2011;36:531–542. doi: 10.1111/j.1749-4486.2011.02373.x. [DOI] [PubMed] [Google Scholar]
- 16.Tiippana E.M., Hamunen K., Kontinen V.K., Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545–1556. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 17.Hwang S.H., Park I.J., Cho Y.J., Jeong Y.M., Kang J.M. The efficacy of gabapentin/pregabalin in improving pain after tonsillectomy: a meta-analysis. Laryngoscope. 2016;126:357–366. doi: 10.1002/lary.25636. [DOI] [PubMed] [Google Scholar]
- 18.Sanders J.G., Dawes P.J. Gabapentin for perioperative analgesia in otorhinolaryngology–head and neck surgery: systematic review. Otolaryngol Head Neck Surg. 2016;155:893–903. doi: 10.1177/0194599816659042. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Morrison A., Polisena J., Husereau D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Altman D.G., Gøtzsche P.C. Cochrane bias methods group; Cochrane statistical methods group. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore R.A., Straube S., Aldington D. Pain measures and cut-offs – no worse than mild pain as a simple, universal outcome. Anesthesia. 2013;68:400–412. doi: 10.1111/anae.12148. [DOI] [PubMed] [Google Scholar]
- 23.Atef A., Fawaz A.A. Intravenous paracetamol is highly effective in pain treatment after tonsillectomy in adults. Eur Arch Otorhinolaryngol. 2008;265:351–355. doi: 10.1007/s00405-007-0451-5. [DOI] [PubMed] [Google Scholar]
- 24.Salonen A., Silvola J., Kokki H. Does 1 or 2 g paracetamol added to ketoprofen enhance analgesia in adult tonsillectomy patients? Acta Anaesthesiol Scand. 2009;53:1200–1206. doi: 10.1111/j.1399-6576.2009.02035.x. [DOI] [PubMed] [Google Scholar]
- 25.Parker D.A., Gibbin K.P., Noyelle R.M. Syrup formulations for post-tonsillectomy analgesia: a double-blind study comparing ibuprofen, aspirin and placebo. J Laryngol Otol. 1986;100:1055–1060. doi: 10.1017/s0022215100100568. [DOI] [PubMed] [Google Scholar]
- 26.Rorarius M.G., Baer G.A., Siirtola M., Lahti T., Laippala P. Effect of intravenous diclofenac or indomethacin on the emergence from anaesthesia for tonsillectomy. Acta Anaesthesiol Scand. 1993;37:616–621. doi: 10.1111/j.1399-6576.1993.tb03776.x. [DOI] [PubMed] [Google Scholar]
- 27.Tarkkila P., Saarnivaara L. Ketoprofen, diclofenac or ketorolac for pain after tonsillectomy in adults? Br J Anaesth. 1999;82:56–60. doi: 10.1093/bja/82.1.56. [DOI] [PubMed] [Google Scholar]
- 28.Salonen A., Kokki H., Tuovinen K. IV ketoprofen for analgesia after tonsillectomy: comparison of pre- and post-operative administration. Br J Anaesth. 2001;86:377–381. doi: 10.1093/bja/86.3.377. [DOI] [PubMed] [Google Scholar]
- 29.Naesh O., Niles L.A., Gilbert J.G. A randomized, placebo-controlled study of rofecoxib with paracetamol in early post-tonsillectomy pain in adults. Eur J Anaesthesiol. 2005;22:768–773. doi: 10.1017/s0265021505001274. [DOI] [PubMed] [Google Scholar]
- 30.Ismail S.A., Mowafi H.A. Preoperative peritonsillar lornoxicam infiltration is not superior to intravenous lornoxicam for pain relief following tonsillectomy in adults. Eur J Anaesthesiol. 2010;27:807–811. doi: 10.1097/EJA.0b013e32833c3101. [DOI] [PubMed] [Google Scholar]
- 31.Mowafi H.A., Telmessani L., Ismail S.A., Naguib M.B. Preoperative lornoxicam for pain prevention after tonsillectomy in adults. J Clin Anesth. 2011;23:97–101. doi: 10.1016/j.jclinane.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Xie J.R., Zhu Y.M., Zhang L.F., Pang Y.J., Yu L.N. Effect of perioperative administration of parecoxib on post-tonsillectomy pain in adults. Afr J Pharm Pharmacol. 2012;6:2141–2147. [Google Scholar]
- 33.Ng T.T., Diamantaras D., Priestley J., Redman J., De Silva N., Mahanta V. Is celecoxib a useful adjunct in the treatment of post-tonsillectomy pain in the adult population? A randomised, double-blind, placebo-controlled study. J Laryngol Otol. 2017;131:S18–S28. doi: 10.1017/S0022215116009476. [DOI] [PubMed] [Google Scholar]
- 34.Fields M., Cabraal D., Dawes P. The effect of dexamethasone on post operative pain following tonsillectomy in adults. Aust J Otolaryngol. 1994;1:426–429. [Google Scholar]
- 35.Carr M.M., Williams J.G., Carmichael L., Nasser J.G. Effect of steroids on posttonsillectomy pain in adults. Arch Otolaryngol Head Neck Surg. 1999;125:1361–1364. doi: 10.1001/archotol.125.12.1361. [DOI] [PubMed] [Google Scholar]
- 36.Stewart R., Bill R., Ullah R., McConaghy P., Hall S.J. Dexamethasone reduces pain after tonsillectomy in adults. Clin Otolaryngol Allied Sci. 2002;27:321–326. doi: 10.1046/j.1365-2273.2002.00588.x. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shehri A.M. Steroid therapy for post-tonsillectomy symptoms in adults: a randomized, placebo-controlled study. Ann Saudi Med. 2004;24:365–367. doi: 10.5144/0256-4947.2004.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rujirojindakul P., Atchariyasathian V., Uakritdathikran T., Boonyata N., Saefung B., Jitmun P. Effect of dexamethasone on postoperative pain after adult tonsillectomy. Thai J Anesthesiol. 2008;34:1–8. [Google Scholar]
- 39.Lachance M., Lacroix Y., Audet N., Savard P., Thuot F. The use of dexamethasone to reduce pain after tonsillectomy in adults: a double-blind prospective randomized trial. Laryngoscope. 2008;118:232–236. doi: 10.1097/MLG.0b013e318159a5cc. [DOI] [PubMed] [Google Scholar]
- 40.Vaiman M., Aviram E., Krakovski D., Gavriel H., Eviatar E. Electromyographic assessment of dexamethasone in treatment of post-tonsillectomy pain: randomized, placebo-controlled trial. Am J Med Sci. 2011;341:469–473. doi: 10.1097/MAJ.0b013e31820fb4f4. [DOI] [PubMed] [Google Scholar]
- 41.Vaiman M., Krakovski D., Haitov Z. Oxycodone and dexamethasone for pain management after tonsillectomy: a placebo-controlled EMG assessed clinical trial. Med Sci Monit. 2011;17:PI25–PI31. doi: 10.12659/MSM.881964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thimmasettaiah N.B., Chandrappa R.G. A prospective study to compare the effects of pre, intra and post operative steroid (dexamethasone sodium phosphate) on post tonsillectomy morbidity. J Pharmacol Pharmacother. 2012;3:254–258. doi: 10.4103/0976-500X.99428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khafagy A.H., Osman S.M. Preoperative administration of dexamethasone reduces post-tonsillectomy morbidities in adults. Egypt J Ear Nose Throat Allied Sci. 2013;14:113–117. [Google Scholar]
- 44.Mikkelsen S., Hilsted K.L., Andersen P.J. The effect of gabapentin on post-operative pain following tonsillectomy in adults. Acta Anaesthesiol Scand. 2006;50:809–815. doi: 10.1111/j.1399-6576.2006.01057.x. [DOI] [PubMed] [Google Scholar]
- 45.Jeon E.J., Park Y.S., Park S.S., Lee S.K., Kim D.H. The effectiveness of gabapentin on post-tonsillectomy pain control. Eur Arch Otorhinolaryngol. 2009;266:1605–1609. doi: 10.1007/s00405-008-0897-0. [DOI] [PubMed] [Google Scholar]
- 46.Abdelmageed W., Abdelrazik S., Nassar A., Abdelkawi M. Analgesic effects of gabapentine in tonsillectomy. Egypt J Hosp Med. 2010;38:51–58. [Google Scholar]
- 47.Mathiesen O., Jørgensen D.G., Hilsted K.L. Pregabalin and dexamethasone improves post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand. 2011;55:297–305. doi: 10.1111/j.1399-6576.2010.02389.x. [DOI] [PubMed] [Google Scholar]
- 48.Kawamata T., Omote K., Kawamata M., Namiki A. Premedication with oral dextromethorphan reduces postoperative pain after tonsillectomy. Anesth Analg. 1998;86:594–597. doi: 10.1097/00000539-199803000-00031. [DOI] [PubMed] [Google Scholar]
- 49.Rafiei M.R., Aghadavoudi O., Rezvani M., Poorqasemian M. Evaluation of preemptive analgesia with dextromethorphan gargling in patients undergoing tonsillectomy. J Res Med Sci. 2012;17:S200–S203. [Google Scholar]
- 50.Van Elstraete A.C., Lebrun T., Sandefo I., Polin B. Ketamine does not decrease postoperative pain after remifentanil-based anaesthesia for tonsillectomy in adults. Acta Anaesthesiol Scand. 2004;48:756–760. doi: 10.1111/j.1399-6576.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 51.Vaiman M., Krakovski D. EMG assessment of analgesia in treatment of posttonsillectomy pain. Clin J Pain. 2012;28:143–148. doi: 10.1097/AJP.0b013e3182272325. [DOI] [PubMed] [Google Scholar]
- 52.Barden J., Derry S., McQuay H.J., Moore R.A. Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;7:CD007355. doi: 10.1002/14651858.CD007355.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl J.B., Nielsen R.V., Wetterslev J. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58:1165–1181. doi: 10.1111/aas.12382. [DOI] [PubMed] [Google Scholar]
- 54.Plante J., Turgeon A.F., Zarychanski R. Effect of systemic steroids on post-tonsillectomy bleeding and reinterventions: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;345:e5389. doi: 10.1136/bmj.e5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki S., Yasunaga H., Matsui H., Horiguchi H., Fushimi K., Yamasoba T. Impact of systemic steroids on posttonsillectomy bleeding: analysis of 61 430 patients using a national inpatient database in Japan. JAMA Otolaryngol Head Neck Surg. 2014;140:906–910. doi: 10.1001/jamaoto.2014.2009. [DOI] [PubMed] [Google Scholar]
- 56.Price D.D., Mayer D.J., Mao J., Caruso F.S. NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J Pain Symptom Manage. 2000;19:S7–S11. doi: 10.1016/s0885-3924(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 57.Duedahl T.H., Rømsing J., Møiniche S., Dahl J.B. A qualitative systematic review of peri-operative dextromethorphan in post-operative pain. Acta Anaesthesiol Scand. 2006;50:1–13. doi: 10.1111/j.1399-6576.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 58.Bell R.F.1, Dahl J.B., Moore R.A., Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review) Acta Anaesthesiol Scand. 2005;49:1405–1428. doi: 10.1111/j.1399-6576.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 59.Cho H.K., Kim K.W., Jeong Y.M., Lee H.S., Lee Y.J., Hwang S.H. Efficacy of ketamine in improving pain after tonsillectomy in children: meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.