Abstract
Background
Trigeminal neuralgia (TN) can have a significant impact on wellbeing and quality of life. Limited data exist for treatments that improve TN pain acutely, within 24 h of administration. This systematic review aims to identify effective treatments that acutely relieve TN exacerbations.
Methods
We searched Medline and Cochrane Central Register of Controlled Trials (CENTRAL) for relevant English language publications. The reference list for all articles was searched for other relevant publications. All studies that satisfied the following PICO criteria were included: (i) Population—adults with acute exacerbation of primary TN symptoms; (ii) Intervention—any medication or intervention with the primary goal of pain relief within 24 h; (iii) Comparator—usual medical care, placebo, sham or active treatment; (iv) Outcome—more than 50% reduction in pain intensity within 24 h of administration.
Results
Of 431 studies, 17 studies were identified that reported immediate results of acute treatment in TN. The evidence suggests that the following interventions may be beneficial: local anaesthetic, mainly lidocaine (ophthalmic, nasal or oral mucosa, trigger point injection, i.v. infusion, nerve block); anticonvulsant, phenytoin or fosphenytoin (i.v. infusion); serotonin agonist, sumatriptan (s.c. injection, nasal). Other referenced interventions with very limited evidence include N-methyl-d-aspartate receptor antagonist (magnesium sulphate infusion) and botulinum toxin (trigger point injection).
Conclusions
Several treatment options exist that may provide fast and safe relief of TN. Future studies should report on outcomes within 24 h to improve knowledge of the acute analgesic TN treatments.
Keywords: acute pain, anticonvulsant, drug therapy, humans, local anaesthetic, serotonin agonist, treatment outcome, trigeminal neuralgia
Editor's key points.
-
•
Patients with trigeminal neuralgia may suffer sudden, severe exacerbations of their pain. Emergency treatment has often consisted of opioid analgesics, which have no useful impact.
-
•
The authors conducted a systematic review to determine how the symptoms of acute flare-ups of trigeminal neuralgia can be rapidly and effectively treated.
-
•
There have been few RCTs, and those that have been performed are of variable quality. Current recommendations include the use of topical, injected or i.v. lidocaine, i.v. fosphenytoin, and topical or injected sumatriptan to abort an acute attack and improve the effectiveness of oral anticonvulsants.
Trigeminal neuralgia (TN) is a painful condition that is associated with sudden, lancinating, unilateral pain attacks in the distribution of the trigeminal nerve branches. The attacks last from a few seconds up to 2 min and are often associated with typical triggers (e.g. brushing teeth, talking, eating). There is generally no pain between attacks.1 Primary or classical TN can be attributable to compression of the trigeminal nerve by a blood vessel. Secondary TN is attributable to compression by a tumour or as a result of demyelination of the nerve (e.g. multiple sclerosis).2
The annual incidence of TN is reported as 4.7–28.9 per 100 000 person years.3, 4 The age of onset is 53–60 yr and it is 1.5–1.9 times more common in women.5, 6 TN can be a disabling condition and many patients often report moderate to severe episodes of pain with associated functional, occupational, and emotional impairment and disability.7 In a study by Tolle and colleagues8 of TN patients established on pharmacological treatment, 48% reported severe episodes of pain over a 24 h period and 78% report at least one visit to their primary care physician over a 4 week period. These patients missed on average 3.9 working days per month because of pain and 34% had reduced hours or were unemployed because of the pain.8
Most treatment options in TN are symptomatic and aim to reduce the severity and frequency of attacks in the future. There is limited evidence on interventions that offer rescue analgesia in the acute phase of an exacerbation or attack. First line treatment is carbamazepine or oxcarbazepine.9 This is a very effective treatment option with large numbers reporting pain relief on initiation of treatment (98%).5 However, it is associated with many side-effects that limit its tolerability and 27% may have to stop or reduce the drug dose.5 Also, they must be titrated to effective concentrations over the course of days to weeks to avoid serious side-effects, making them ineffective as acute analgesics.
If pharmacological therapy fails or is not tolerated, interventional options include Gasserian ganglion rhizotomy and microvascular decompression. Such interventional options require image guidance, skilled staff, operating theatre access, or all three, which limits their utility as rescue options in the acute phase.
Data from Hospital Episodes Statistics in England show that not all patients admitted to hospital with acute TN undergo a surgical procedure. Patients in the UK and USA report the prevalent use of opioid analgesics from which it is inferred that the pain intensity is high.7, 10 The management of these cases is challenging and can have serious socioeconomic and healthcare utilisation implications, not to mention the negative impact on the patient.
The objective of our systematic review is to identify therapeutic strategies or interventions that effectively reduce the severity or terminate an acute exacerbation of TN. We will grade the evidence and offer recommendations that will support the care of these patients in the outpatient clinic, emergency department, or in the primary care setting.
Methods
Our review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.11 We used a PICO approach to define our study inclusion criteria. The Population of interest was adults (>18 yr old) with primary TN as per the International Classification of Headache Disorders (ICHD 2013), International Association for the Study of Pain (IASP) definition, or both, with an acute exacerbation of symptoms.1, 12 The Intervention was the administration of any medication or interventional treatment with the primary goal of acute pain relief within 24 h. The Comparator (or control group) may be usual medical care, placebo, sham, or active treatment. Because of the limited available evidence from RCTs, we reviewed all relevant studies (i.e. case reports, case series, observational studies, and RCTs). The primary Outcome measure we were interested in was pain intensity, and we deemed an effective treatment would give more than 50% pain relief within 24 h of administration. Other important outcomes we searched for included frequency of attacks, adverse effects of treatment, medication usage, healthcare utilisation, quality of life measures, and patient satisfaction scores.
Studies that reported on the use of interventions that require surgical theatre access or image guidance were excluded. Such interventions are not practical options in the pursuit of acute pain relief. Studies of medications that require days to weeks to titrate to effect were also excluded (e.g. carbamazepine, lamotrigine).
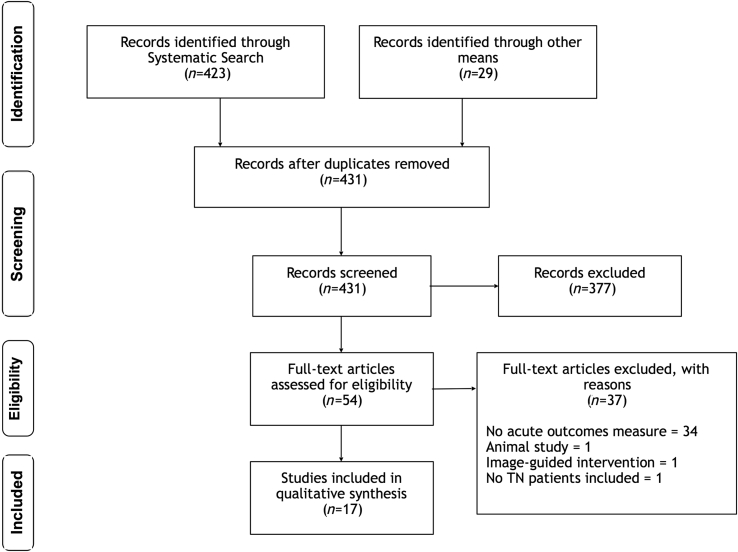
We performed a literature search for effective analgesic options in an acute TN exacerbation. We searched Medline and Cochrane Central Register of Controlled Trials (CENTRAL) for (‘trigeminal neuralgia’ OR ‘facial/trigeminal nerve pain’ OR ‘tic do*lo*re*ux*’) AND (‘acute pain’ OR ‘emergency’ OR ‘refractory’). We only included full publications in English. The reference lists for all included papers were searched for other suitable papers. The literature was reviewed by two authors independently (DMM and JMZ) and agreement on study inclusion was sought. If there was a disagreement on study inclusion, a third author (MSC) made the final decision. A PRISMA flow diagram of our search results is included below (Fig 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram details the literature search. Of 452 potentially relevant studies, 17 studies investigated or referenced the efficacy of an analgesic therapy within 24 h of administration for trigeminal neuralgia. TN, trigeminal neuralgia.
After pooling the search results, we removed duplicates. We reviewed the abstracts and selected studies that may meet inclusion criteria. We retrieved the full article for these studies and extracted data for inclusion in the review. We graded the level of evidence using the American Academy of Neurology classification scheme (Table 1).13
Table 1.
American Academy of Neurology (AAN) classification scheme requirements for therapeutic questions (https://www.aan.com/policy-and-guidelines/guidelines/about-guidelines2/).
| Class I | A randomised, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. |
| Class II | A randomised controlled clinical trial of the intervention of interest in a representative population with masked or objective outcome assessment that lacks one criteria of the additional class 1 criteria or a prospective matched cohort study with masked or objective outcome assessment in a representative population that meets Class I criteria. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. |
| Class III | All other controlled trials (including well-defined natural history controls or patients serving as their own controls) in representative populations, where outcome is independently assessed, or independently derived by objective outcome measurement. |
| Class IV | Studies not meeting Class I, II, or III criteria including consensus or expert opinion. |
We used the Cochrane Collaborations risk of bias tool to rate the level of bias in the included RCTs.14 As per the Cochrane group's recommendation, all other studies were deemed to be at ‘high’ risk of bias. Finally, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) summary of evidence tool to rate the quality of evidence and accordingly grade our recommendations.15
Results
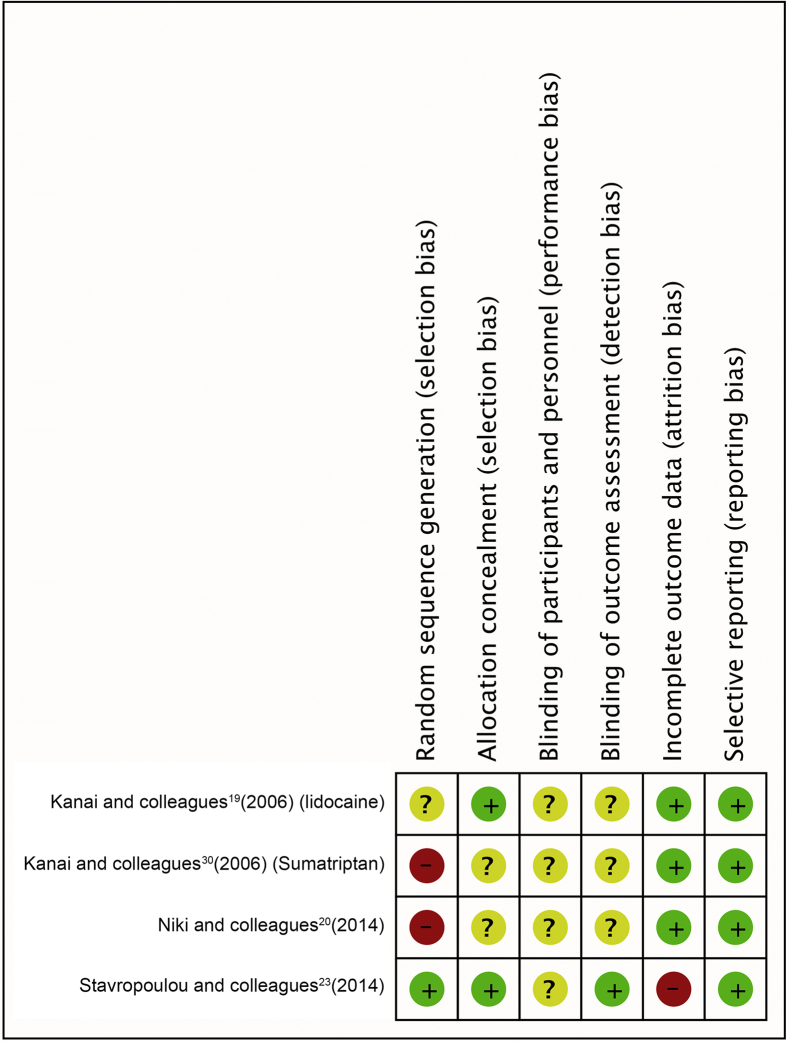
A total of 17 studies met our inclusion criteria (Fig 1). All studies investigated the effect of a pharmacological intervention, and the results are detailed below under each drug group heading. Table 2 gives an overview of the interventions and the results from each study. Figure 2 indicates the level of bias in the included RCTs. Not all the drugs mentioned in this review are available in all countries.
Table 2.
Evidence for acute analgesic treatments in trigeminal neuralgia. DN4, douleur neuropathique 4 questionnaire; GIC, global impression of change; IASP, International Association for the Study of Pain; ICHD, International Classification of Headache Disorders; IV, intravenous; MgSO4, magnesium sulphate; N, number of study patients; NRS, numerical rating scale; PE, phenytoin equivalents; PRS, pain relief scale; RCT, randomised controlled trial; SC, subcutaneous; TN, trigeminal neuralgia; V2, second division of trigeminal nerve; V3, third division of trigeminal nerve; VAS, visual analogue scale; VPR, verbal pain rating.
| Author (and year) | Drug | Study | Diagnosis | Population | N | Dosage | Route | Outcome measure(s) | Efficacy within 24 h | Side-effects (n) | Level of evidence | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zavon and Fichte17 (1991) | Proparacaine | Case report | Not specified | Classical TN Presenting for cataract surgery | 1 | 0.50% | Eye drops | Incidental reduction of TN pain | Alleviation of pain | Not specified | IV | High |
| Spaziante and colleagues16 (1992) | Proparacaine | Prospective observational | Not specified | Classical TN All divisions | 25 | 0.5% (2 drops) | Eye drops | 50% Reduction of analgesics | 15 Patients > 50% reduction (8 stopped all analgesics)—not clear at which timepoint over a 1 month period | Nil | IV | High |
| Kanai and colleagues19 (2006) | Lidocaine | RCT cross-over double-blind | ICHD | Classical TN Division: V2 Intensity on VAS>4/10 | 25 | Active: 8% 0.2 ml, Control: 0.2 ml saline | Nasal spray | VAS (15 min post-intervention), GIC (4-point scale), use of medications | Active: VAS 8.0 to 1.5, Control: 7.9 to 7.6, Duration: 4.3 h | Local irritation (stinging, burning numbness) (15), bitter taste or numb throat (1) | II | Unclear |
| Niki and colleagues20 (2014) | Lidocaine | RCT cross-over double-blind | ICHD | Classical TN Division: V2, V3 Intensity on VAS>4/10 | 24 | Active: 8% 0.2 ml, Control: saline 0.2 ml | Oral mucosa (trigger point)—applied via patients finger | NRS, GIC (4-point scale) | Active: NRS 5 to 1, Control: 5 no (change), Duration: 2.8 h | Numbness (active [9], control [3]), bitterness (1) | II | Unclear |
| Baykal and Kaplan24(2010) | Lidocaine | Retrospective observational | Not specified | Classical TN Division: V2, V3 | 13 | 5 ml 2% (100 mg) | ‘Blind’ injection of 2nd or 3rd division via mandibular notch | VAS | Complete relief 1–2 min post-injection | Hypoesthesia (4), dizziness (3), ptosis (1), insufficient block (1) | IV | High |
| Galer and colleagues21 (1993) | Lidocaine | Retrospective observational | Not specified | TN with concomitant persistent facial pain | 6 | 5 mg kg−1 h−1 | I.V. infusion over 60–90 min | VPR, VAS, PRS 1 h post-infusion | 5 Patients >70% reduction in VAS or VPR, 1 patient 30–70% reduction | Not specified | IV | High |
| Stavropoulou and colleagues23 (2014) | Lidocaine | RCT cross-over double-blind | IASP | Classical TN All divisions DN4 Score>4 VAS>3/10 | 20 | 5 mg kg−1 | I.V. infusion over 60 min | VAS, allodynia, hyperalgesia | VAS: (active) 76% reduction, (control) 40.1% reduction; at 24 h, (active) 52% reduction (control) 4% increase, decrease in evoked pain | Mild somnolence (33%), 3 patients dropped out | II | Low |
| Chaudhry and Friedman22 (2014) | Lidocaine | Case report | Not specified | TN with concomitant persistent facial pain Division: V2 |
1 | 60 mg h−1 for 4 h, 120mg h−1 for next 20 h, followed by 60 mg h−1 for next 48 h | I.V. infusion for 72 h | VPR | VPR: 10 to 0 after 1st h infusion | Not specified | IV | High |
| Arai and colleagues26 (2013) | Lidocaine+magnesium sulphate | Retrospective observational | Not specified | Classical TN Division: V2 | 9 | Lidocaine 100 mg and MgSO4 1.2 g | I.V. infusion over 60 min | NRS | NRS: 7 to 4 | Mild dizziness (2) | IV | High |
| Soleimanpour and colleagues25 (2014) | Magnesium sulphate | Case report | Not specified | Classical TN - acute presentation | 1 | 50 mg kg−1 | I.V. infusion over 30 min | VAS | VAS: 10 to 2 (30 min post-infusion) | Nil | IV | High |
| Tate and colleagues27 (2011) | Phenytoin | Case report | Not specified | Classical TN - acute presentation | 1 | 15 mg kg−1 | I.V. infusion in 2 divided doses 4 h apart | NRS | NRS: 10 to 2 after 1st infusion and 1 after 2nd infusion | Nil | IV | High |
| Cheshire29 (2001) | Fosphenytoin | Case series | Not specified | Classical TN - acute presentation, carbamazepine no longer effective | 3 | 14 (11–18) mg kg−1 (PE) over 20–180 min | I.V. infusion | Pain relief (not quantified) | Immediate pain relief post-infusion | Mild and transient dizziness, tinnitus, ataxia | IV | High |
| Vargas and Thomas28(2015) | Fosphenytoin | Case report | Not specified | Classical TN - acute presentation Division: V2+V3 | 1 | 15 mg kg−1 | I.V. infusion over 30 min | VAS | VAS: 10 to 2 | Not specified | IV | High |
| Zuniga and colleagues34 (2008) | Botulinum toxin | Prospective observational | Not specified | Classical TN All divisions | 12 | 20–50 units | S.C. trigger zones | VAS (8 weeks) | 10 Patients: relief ‘after some minutes’, 1 patient: VAS 10 to 0 in 24 h | Transient facial asymmetry (1) | IV | High |
| Kanai30 (2006) | Sumatriptan | RCT cross-over double-blind | ICHD | Classical TN All divisions Intensity on VAS>4/10 | 24 | Active: 3 mg (1 ml) Control: 1 ml saline |
S.C. | VAS (15 min post-intervention), GIC (4-point scale) | Active: Vas 8.3 to 2.4, Control: 8.5 to 8.1, Duration: 7.9 h | Mild hypertension (2), fatigue (5), nausea (2) | II | Unclear |
| Kanai32 (2006) | Sumatriptan | Prospective observational, placebo-controlled, partially blinded | ICHD | Classical TN All divisions | 15 | 3 mg (1 ml) s.c. followed by 50 mg orally twice daily for 1 week | S.C. saline, then s.c. sumatriptan, followed by oral 1 day later | VAS (15 min post-intervention) | VAS decreased by 4.0 (resting), 4.7 (touching face), 4.6 (talking) | Fatigue (4), nausea (2) | IV | High |
| Shimohata and colleagues33 (2009) | Sumatriptan | Case series | ICHD | Classical TN All divisions |
3 | 20 mg | Nasal spray | VAS | VAS: 8 to 2 (30 min) | Nil | IV | High |
Fig. 2.
Risk of bias summary—review authors' judgements about each risk of bias item for the included RCTs.
Local anaesthetic
Ophthalmic
In 1992, a prospective observational study assessed the impact of proparacaine 0.5% eye drops on 25 patients.16 This study was inspired by a letter published by Zavon and Fichte17 that reported the unintended relief of TN in a patient presenting for cataract surgery and a second patient with symptoms resistant to carbamazepine, both of whom received proparacaine 0.5% eye drops. All participants in the observational study were managed as TN cases, but the diagnostic criteria are not clear. Patients with incomplete pain relief on standard treatment or awaiting surgery were included. A positive outcome was more than 50% reduction in analgesic medications. This was reported in 15 of the 25 patients, with eight patients stopping all medications. Positive response was not limited to those with first division symptoms. The duration of follow-up was for ‘at least a month’, but the exact time frame for each patient was not documented. Also, the time to onset of pain relief in the 15 patients is not reported. No adverse events were reported.
A subsequent RCT in 47 patients with TN (primary and secondary cases) rejected the long-term benefit of proparacaine 0.5% eye drops.18 However, the first patient report was recorded at 3 days, so no acute pain reduction within the first 24 h was assessed.
Nasal
Kanai and colleagues19 published a double-blind cross-over RCT in 25 patients with second division TN. Patients were randomised to receive two sprays of lidocaine 8% (0.2 ml, 16 mg, n=13) or two sprays of saline solution (0.2 ml, n=12) in the ipsilateral nostril to the pain. After 7 days, the groups crossed over to receive the alternative treatment. Patients remained supine with their head in a neutral position for 30 min during observation, presumably to establish a sphenopalatine ganglion block.
The primary outcome was pain score (visual analogue scale [VAS]) on stimulation of a trigger zone. VAS in the lidocaine group reduced from 8.0 to 1.5, while the saline group reported a non-significant reduction from 7.9 to 7.6. Ten patients (40%) in the lidocaine group reported complete pain relief at 15 min. This positive effect of lidocaine persisted for a median of 4.3 h. Fifteen patients (60%) in the lidocaine group reported minor adverse side-effects which included stinging, burning, or numbness of the nose and eye (n=15), bitter taste, and numbness of the throat (n=1).
Oral
The same Japanese group as above conducted a study of lidocaine use in patients with primarily oral mucosa trigger points.20 They used a similar methodology to the previous study.19 They recruited 24 patients with TN in the maxillary branches, mandibular branches, or both. A dose of lidocaine spray 8%, 2 ml was applied to the patients finger and rubbed into the trigger zone of the oral mucosa (n=12). Alternatively, the patient rubbed 0.2 ml of saline into the painful zone (n=12). The numeric rating scale (NRS) in the lidocaine group reduced from five down to one. NRS in the saline group did not change from five. Eleven patients (46%) were pain free after lidocaine application. Only one patient in the saline group reported complete pain relief. The median duration of pain relief was 2.8 h (0.3, 3) in the lidocaine group.
I.V. infusion
Galer and colleagues21 reported on the use of lidocaine infusions in the management of neuropathic pain in 111 patients in a retrospective cohort study. Six of these patients were diagnosed with TN. The patients described their pain as ‘near constant lancinating pain’ which may indicate that they had classical TN with concomitant persistent facial pain (Type 2 TN). The patients received lidocaine 5 mg kg−1 h−1 infusion for 60–90 min. At 1 h post-infusion, one patient reported partial relief (30–70% reduction in pain score) and five patients reported excellent relief (more than 70% reduction in pain scores). The authors did not mention adverse side-effects.
Chaudhry and Friedman22 reported a case of lidocaine infusion relieving intractable TN symptoms from a verbal pain rating of 10/10 down to 0/10 after the first hour of infusion. The patient was on an infusion over a 72-h period, running at 60–120 mg h−1.
Stavropoulou and colleagues23 designed a cross-over double-blind RCT to assess the role of lidocaine infusion in TN. The study included patients with TN as per IASP criteria, and only patients who had a Douleur Neuropathique 4 Questionnaire (DN4) score equal to or greater than 4 (out of a maximum 10). The majority of patients had hyperalgesia and allodynia (thermal and mechanical) on examination. Each patient received four infusions (each infusion 2 days apart), two active and two control, allocated at random. The active infusion was lidocaine 5 mg kg−1 in 250 ml of dextrose 5% solution given over 1 h. The control infusion was dextrose solution 5%, 250 ml. At 1 h post-infusion, the overall reduction in pain score (VAS) was 76.4% for the active group vs 40.1% for the control group. At 24 h, pain scores reduced by 52% for the active group vs a 4% increase in pain score for the control group. The active group also reported decreased hyperalgesia and allodynia. Somnolence was the most common side-effect reported in 33% of the active infusions.
Nerve block
Baykal and Kaplan24 reported on the use of blind injection of the mandibular and maxillary branches of the trigeminal nerve at the level of the lateral pterygoid plate or the pterygopalatine fossa, respectively. Their study included 13 patients presenting with TN symptoms, the severity of which is not clearly documented. They performed a weekly injection with lidocaine 2%, 5 ml over a 5-week period (six injections). The patients were ‘completely pain free in 1–2 min’ post-injection. However, they did not report on baseline or post-intervention pain scores. They used this 5-week period to establish or titrate the carbamazepine dose. After this, one patient remained pain-free for 1 month, another two remained pain-free for 6 months, and the remaining 10 patients were pain-free for at least 12 months. Side-effects were hypoesthesia (n=4), dizziness (n=3), ptosis (n=1), and incomplete block (n=1).
N-Methyl-d-aspartate receptor antagonists
A case report details the use of a magnesium sulphate (MgSO4) infusion to control an acute exacerbation of TN.25 A 65-yr-old man presented to the emergency department with a VAS score of 10/10 and received MgSO4 30 mg kg−1 in 100 ml saline, infused over 30 min. His pain score decreased to 2/10 at 30 min post-infusion. He did not report any adverse effects. He was monitored for 4 h and was then discharged from the emergency department.
Arai and colleagues26 retrospectively reported the outcomes of nine patients with TN intractable to conventional treatment who were treated with a combined infusion of lidocaine and MgSO4. Each patient received lidocaine 100 mg mixed with MgSO4 1.2 g infused over 1 h. The mean NRS for the patients reduced from 7 down to 4 after the infusion. It is not clear how soon after the infusion the pain score was recorded. Two patients reported mild dizziness post-infusion.
Anticonvulsants
The i.v. anticonvulsants reported to be effective in the literature are phenytoin and its prodrug fosphenytoin. A case report of a 77-yr-old man with intractable TN supports the use of i.v. phenytoin.27 After admission to the emergency department, he received a phenytoin infusion of 15 mg kg−1 in two divided doses each infused over 30 min. His pain score decreased from 10/10 down to 1/10 after the infusions. This pain relief persisted for up to 3 days. No adverse effects were reported. Vargas and Thomas28 published a case report of a 53-yr-old man who presented to the emergency department with intractable TN symptoms. He received 15 mg kg−1 of fosphenytoin over 30 min. His pain score (VAS) reduced from 10/10 to 2/10 immediately after the infusion. No adverse effects were reported. The pain relief was maintained at follow-up 20 days later.
Cheshire29 reports a case series of three patients with intractable TN who responded to a fosphenytoin infusion. All three patients were previously controlled on carbamazepine, but subsequently developed an acute, unremitting phase of TN attacks that compromised their ability to eat or drink. The fosphenytoin was diluted to phenytoin sodium equivalents (PE) 5–10 mg ml−1 in dextrose 5% with saline 0.45%. During infusion, the patients were monitored with ECG and noninvasive BP observations. The first patient received PE of fosphenytoin 18 mg kg−1 over 20 min. This gave immediate pain relief which lasted 2 days. She opted for an microvascular decompression (MVD). The second and third patients received an incremental dosing schedule of 100 mg every 10 min up to a maximum of 10 doses in order to limit the required dose. Patient 2 received good pain relief after an 11 mg kg−1 dose, and this was maintained for 20 h. The patient subsequently had a trigeminal ganglion balloon compression. The third patient had 14 mg kg−1 infused in increments as above over 3 h, with immediate pain relief. This lasted for 2 days. This patient also opted for trigeminal ganglion balloon compression. All patients reported transient adverse effects including mild dizziness, tinnitus, and ataxia.
Serotonin agonists
Kanai and colleagues30 reported on the use of s.c. sumatriptan in triggered TN attacks. They used a similar methodology to the previously mentioned studies.19, 20, 31 Patients received either s.c. sumatriptan 3 mg in 1 ml (n=12) or s.c. saline 1 ml (n=12). Sumatriptan decreased VAS from 8.3 down to 2.4. There was no significant decrease in the saline group with 8.5 down to 8.1. Twelve patients (50%) were pain free after sumatriptan. The positive effect of sumatriptan lasted for a median of 7.9 h (range of 1–20 h). The reported adverse effects in the sumatriptan group included mild elevation in BP (n=2), fatigue (n=5), and nausea (n=2).
A further study by the same group in patients (n=15) with at least a 1 month exacerbation of TN symptoms demonstrated a significant decrease in VAS pain scores while resting (4.0), touching (4.7), and talking (4.6) in those who received s.c. sumatriptan (3 mg in 1 ml).32 They maintained these positive improvements with a 1 week course of oral sumatriptan 50 mg twice daily.
Nasal sumatriptan (20 mg) was effective in a case series of three patients with refractory TN reducing the mean VAS from 8 to 2 within 30 min.33 No adverse effects were reported.
Botulinum toxin
Zuniga and colleagues34 reported on the use of botulinum toxin injections in the trigger zones of patients with primary TN. The 12 patients were assessed weekly for 8 weeks in an open label study. The patients received 20–50 units in total to individual trigger points. The authors reported that 10 of the patients (83%) experienced pain relief ‘after some minutes’ of the injection. They also noted faster onset of pain relief with higher doses. However, precise details about pain scores or number of paroxysms within the first 24 h are not reported. They give details of one female patient who had a pain score (VAS) of 10 and 30–40 painful paroxysms per day. She received 40 units into a left frontotemporal trigger point, and five units each into a zygomatic and buccal trigger point. At 24 h, her pain score was 0 and she had no painful paroxysms. This level of pain relief persisted for 70 days. The same group published an RCT on botulinum toxin in primary TN.35 In the article, they refer to unpublished observations of TN pain that ‘almost remitted at the time of injection’. Other reports on botulinum toxin use in TN do not record the acute response.
Discussion
Quality of evidence
The overall quality of evidence for effective acute analgesia in TN exacerbations is very low. A number of reasons may exist for this. TN is a rare condition and the number of patients required to adequately power an RCT makes such a project very difficult. Also, the majority of TN patients respond well to conventional treatment with carbamazepine. The patient group of interest in this review is a small subset of TN patients. TN is associated with painful paroxysms that may remit for long periods of time. In observational studies it can be difficult to determine if the remission is because of treatment or simply the natural course of the condition. As such, the review includes small RCTs, observational studies, and case series/reports. In addition, many of the included studies did not specifically look at acute (emergency department or outpatient clinic) presentations, and some specifically triggered attacks to judge the acute effect of treatment,19, 20, 30, 32 while others included patients with intractable symptoms with conventional management.16, 18, 26, 34 However, all included studies reported on the degree of acute relief of TN pain within 24 h.
Very few studies in TN report outcomes within the first 24 h of starting treatment. The earliest timepoint in many studies was 7 days post-intervention, and so these were excluded. While the ultimate goal of therapy should be to reduce the intensity and frequency of TN attacks, patients would also value a therapeutic strategy that provides a fast resolution of symptoms.
Local anaesthetic
The primary agent used in the included trials was lidocaine. Like all local anaesthetic agents, it is a voltage-gated sodium channel blocker and its effect in TN is most likely attributable to its inhibition of the triggering mechanism in the ignition hypothesis, proposed by Devor and colleagues.36 Its therapeutic effect in the trials that involved local application (e.g. nasal or oral mucosa) is certainly because of blockade of the peripheral nerve sodium channels. In higher systemic doses, it can demonstrate a neuromodulatory effect by reducing C-fibre transduction of pain signals and inhibition of ectopic discharges from damaged neurons, without impacting on normal sensory function.37, 38 This would account for its effect in the i.v. infusion studies. While it has a shorter duration of action than other local anaesthetics, it is preferred because of its better safety profile in terms of cardiac and neurological toxicity.
The effect of proparacaine 0.5% eye drops in TN is equivocal. It may have an effect that is lost after a few hours, but further studies are required to establish this. This is the case in nasal and oral mucosal lidocaine which gave a median of 4.3 and 2.8 h of pain relief, respectively. The method of application used in applying the lidocaine to the oral mucosa (spray on the patients finger) was unusual,20 and in patients with mucosal trigger zones, it could be sprayed directly but using a lower concentration (e.g. 5%).
Studies investigating the effects of i.v. lidocaine included primarily TN patients with concomitant persistent facial pain (Type 2 TN). The dose of 5 mg kg−1 infused over 1 h is recognised to be effective and safe in other neuropathic pain states.39 The study by Stavropoulou and colleagues23 used the DN4 (score>4) to select patients with trigeminal neuropathic pain. However, the DN4 is not specific enough for TN and may actually select outpatients with concomitant persistent facial pain (Type 2 TN).40 As such, the role of i.v. lidocaine in classical primary TN (Type 1) is not clear. Matharu and colleagues41 reported on the use of lidocaine infusions (1.3–3.3 mg kg−1 h−1) in short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT), considered by some as TN with autonomic features. The SUNCT symptoms were significantly relieved by the infusion, but recurred soon after discontinuation.
A number of studies have investigated the utility of trigger point or distal nerve branch injection of local anaesthetic in TN patients. These included continuous infusion of bupivacaine around the peripheral nerve branches,42 ropivacaine to trigeminal trigger points either alone or in combination with gabapentin,43 peripheral nerve injection of lidocaine vs streptomycin plus lidocaine,44, 45 peripheral and proximal nerve blocks with high concentration lidocaine,46 and tetracaine with or without bupivacaine peripheral nerve injection.47, 48 While these trials reported variable long-term outcomes, none of them reported acute outcomes within the first 24 h. The Baykal paper reported on the use of more proximal nerve blocks to achieve acute pain relief while titrating carbamazepine.24 These blocks should be done with image-guidance and contrast screening for vascular uptake.
Local anaesthetic injection to trigger points offers effective but transient relief of symptoms (less than 24 h). Indeed, all the local anaesthetic interventions included in this review have a limited duration of action (2–24 h). However, this may be valuable to the patient during a period of drug titration or waiting for a more definitive treatment to be established. Importantly, the risk profile of the interventions is low, as is the cost of the medications used. Many can be performed by dental practitioners who in the UK use lidocaine 1–2%. Lidocaine infusions, on the other hand, require a nurse monitored bed for the duration of the infusion which adds to the treatment cost.
N-Methyl-d-aspartate receptor antagonists
N-Methyl-d-aspartate (NMDA) receptor activation is a key step in central sensitisation and NMDA antagonists may improve the associated symptoms.49 Central sensitisation is not believed to be a significant part of classical TN.36 The typical clinical features of central sensitisation are allodynia and hyperalgesia. These may be observed in trigeminal neuropathies, Type 2 TN, or persistent idiopathic facial pain, but should not be present in classic primary TN.
MgSO4 is a natural NMDA receptor blocker and a magnesium ion resides in the receptors central pore in normal physiological states. During periods of intense nociceptive input, the Mg ion is displaced, and the NMDA receptor can facilitate calcium influx, potentiating the nociceptive signal at the dorsal horn level in the spinal cord—one element of central sensitisation.
The included case report and case series offer very weak evidence for the use of MgSO4 in managing the acute pain of TN. In the case series, the nine patients received lidocaine 100 mg (less than 2 mg kg−1) combined with MgSO4 1.2 g (approximately 20 mg kg−1 over 1 h). The case report dose was 30 mg kg−1 infused over 30 min. The advantage of MgSO4 infusion is that it is cheap with minimal side-effects. It has proven efficacy in the management of other neuropathic pain conditions such as post-herpetic neuralgia and neuropathic back and leg pain.50, 51
Other NDMA receptor blockers have been trialled in small case series of facial pain patients. Patients with chronic facial pain received various sub-anaesthetic doses of ketamine.52 Of the seven patients, only three reported transient pain relief (1–3 days) with doses of 0.4–1.8 mg kg−1 of ketamine. All patients had pain secondary to traumatic trigeminal nerve injury rather than TN. A study of dextromethorphan (NMDA antagonist) in facial neuralgias included three patients with primary TN.53 While acute pain relief was not reported, the patients' pain control over a 14-day period was worse on the oral dextromethorphan compared with oral lorazepam (active control).
Anticonvulsants
Treatment algorithms for TN contain many anticonvulsant agents including carbamazepine, oxcarbazepine, lamotrigine, and gabapentin.9, 54 However, many of these agents are not available in i.v. form and their oral administration requires a period of cautious titration. This makes them unsuitable for the management of acute TN attacks.
The exception to the above is phenytoin, or its prodrug fosphenytoin, which is a commonly used i.v. anticonvulsant. Both drugs have been used successfully in the acute control of TN attacks. The evidence is weak, based on case reports and a small case series. Phenytoin is a use-dependent antagonist of voltage-gated sodium channels. It selectively blocks sodium channels that are opened repeatedly, a property which makes it an effective anticonvulsant.55 It also blocks voltage-gated calcium channels. These pharmacodynamic properties help to explain how it may inhibit the triggering and amplification steps in the ignition hypothesis. Fosphenytoin is a phenytoin prodrug with a better side-effect profile.56 The loading dose for both drugs is 15–18 mg kg−1 over 30 min. Similar to lidocaine infusions, the patient requires a nurse-monitored bed during the infusion. Adverse effects include dizziness and ataxia, hypotension, and rarely heart block.
The onset of pain relief in the included cases was immediate post-infusion and lasted for 1–3 days on average. This allowed the patients to consider more definitive treatment options.
Serotonin agonists
Sumatriptan is the most commonly used triptan and is a serotonin (5-hydroxytriptamine) agonist, specifically serotonin 1B and 1D receptors.57 It may be administered orally, intranasally, s.c., or rectally. It is a first line rescue agent in migraine treatment, and it is most effective when given s.c. at a dose of 6 mg.58 It may also have a role in the acute management of TN attacks.
Its mechanism of action in the termination of migraine attacks is believed to be direct vasoconstriction of dilated meningeal blood vessels, inhibition of release of vasodilatory peptides from trigeminal sensory neurons, and a reduction in pain transmission in the trigeminal dorsal horn in the pons.59, 60, 61 Sumatriptan may exert its analgesic effect in TN by reducing pain transmission in the pons. As a vasoconstrictor, it may also reduce the mechanical compression of the trigeminal nerve root by a vascular loop.
While the use of the s.c. route is more effective in migraine management, and was the chosen route in the included studies, it is more expensive than the oral option. The nasal route is a more attractive option in a patient with acute TN symptoms who cannot take oral medication or wants to avoid injections. Sumatriptan is generally well tolerated but may be associated with moderate increases in BP. It should be used with caution in hypertensive patients or those with coronary artery disease.
Botulinum toxin
Botulinum toxin is indicated as a prophylactic treatment in the management of refractory chronic migraine,62, 63 and a growing number of observational and RCT studies support its use in refractory primary TN.64 However, there is limited data on its acute analgesic effect. The included studies were not designed to investigate the acute analgesic effect of botulinum toxin in this patient group, but remark on the early onset of pain relief.
The mechanism of action of botulinum toxin in painful conditions such as chronic migraine or TN is believed to be inhibition of neuropeptide release (e.g. calcitonin gene-related peptide and substance P) from peripheral sensory nerve endings.65 This may limit peripheral sensitisation and could account for a role in reducing neural triggering in TN. However, in comparison with other treatment options, botulinum toxin is more expensive, with the National Institute of Health and Care Excellence estimating each treatment for chronic migraine may cost £349.40. A mixture of botulinum toxin and lidocaine into trigger points to manage acute exacerbations in specific cases could be trialled. The botulinum toxin prolongs the acute effect of the lidocaine, and we have had a number of positive results with this approach. As such, while the evidence is still weak for acute exacerbations, a case-by-case basis could be considered in experienced specialist centres.
Algorithm for acute management
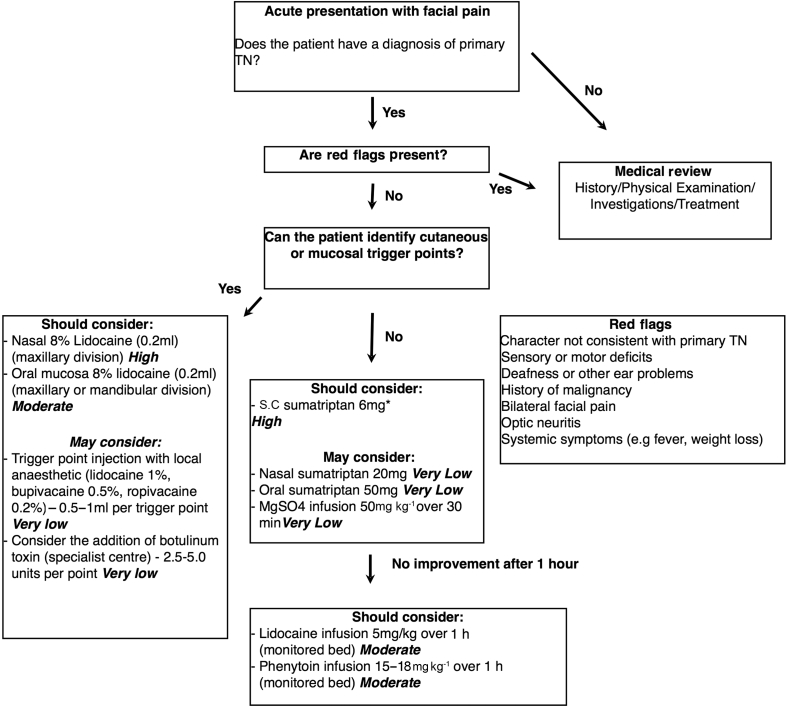
If a patient presents with an acute exacerbation of their facial pain, it is important to confirm that this is consistent with primary TN.66 If the pain is different in quality or character to their usual TN pain, or if they report red-flag symptoms, a clinical history, examination with or without investigations are warranted to rule out a secondary cause.
However, if the patient has a diagnosis of primary TN and this episode is consistent with previous attacks, then it is appropriate to treat without further investigations. The GRADE summary of evidence table gives an indication of how the data was graded (Supplementary Table S1). It is recommended to start with the intervention with the highest evidence, and lowest cost and side-effects (Fig 3). Most of the interventions are associated with immediate relief (within 1 h of completing the therapy). Therefore, it is appropriate to move swiftly through the algorithm if a treatment has not been successful.
Fig. 3.
Evidence-based treatment algorithm for the management of acute exacerbations of trigeminal neuralgia. Management of acute exacerbations should proceed in parallel with a more definitive long-term management plan. The quality of evidence is included after each intervention. The moderate- and high-quality evidence receives a ‘should consider’ recommendation. The very low- and low-quality evidence are options that the clinician ‘may consider’ if interventions with higher quality evidence are contraindicated or not available. *Sumatriptan injection pens in the UK contain 6 mg in 1 ml, and we have recommended this for convenience as opposed to the 3 mg used by the Japanese group. †These concentrations may not be available in all countries (2–5% more common). TN, trigeminal neuralgia.
Most of the interventions give less than 24 h of pain relief, so a more definitive care plan needs to be put in place according to current guidelines.9, 67 This may involve starting or titrating medications, or planning surgical interventions. The case series by Cheshire29 describes this process well with the acute management of three patients with fosphenytoin infusion while simultaneously planning more definitive management plans. Some of the studies count the early discontinuation or reduction of prophylactic medications (e.g. carbamazepine) as a positive outcome measure, and the Japanese studies even discontinued these medications 12 h before intervention.19, 20, 30 Abrupt discontinuation of a well-tolerated medication is not generally recommended and in an acute exacerbation, increasing the dose of these medications to achieve better long term control is the preferred option.
Conclusion
Trigeminal neuralgia is associated with periods of moderate to severe flare-up that can have a detrimental impact on an individual's personal and professional life. Many current treatment strategies are aimed at long-term symptom control and fail to give acute pain control (within 24 h). This may lead to work absence or hospital admission. Weak evidence exists to support the use of lidocaine, sumatriptan, phenytoin/fosphenytoin, botulinum toxin, and MgSO4 as acute rescue analgesics. Future studies of interventions in TN should consider recording the first point of onset of analgesia and the duration of effect. It may be necessary to look at N-of-one trials to get over some of the difficulties of designing RCTs of acute management.
Authors' contributions
Systematic literature search and review, study inclusion, data extraction: DMM, JMZ.
Manuscript preparation and revision: all authors.
Data retrieval: MSC, AS.
Adjudication on study inclusion: MSC.
Declaration of interest
JMZ has a consultancy with Biogen.
Funding
JMZ undertook this work at University College London and University College London Hospital Trust, UK who received a proportion of funding from the Department of Health's National Institute for Health Research (NIHR), UK Biomedical Research Centre funding scheme.
Handling editor: J.G. Hardman
Editorial decision: 03 May 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.05.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Olesen J. International classification of headache Disorders. Lancet Neurol. 2018;17:396–397. doi: 10.1016/S1474-4422(18)30085-1. [DOI] [PubMed] [Google Scholar]
- 2.Cruccu G., Finnerup N.B., Jensen T.S. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology. 2016;87:1–9. doi: 10.1212/WNL.0000000000002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall G.C., Carroll D., McQuay H.J. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002–2005. BMC Fam Pract. 2008;9:26. doi: 10.1186/1471-2296-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopman J.S., Dieleman J.P., Huygen F.J., de Mos M., Martin C.G., Sturkenboom M.C. Incidence of facial pain in the general population. Pain. 2009;147:122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano G., La Cesa S., Truini A., Cruccu G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J Headache Pain. 2014;15:34. doi: 10.1186/1129-2377-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maarbjerg S., Gozalov A., Olesen J., Bendtsen L. Trigeminal neuralgia--a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54:1574–1582. doi: 10.1111/head.12441. [DOI] [PubMed] [Google Scholar]
- 7.Zakrzewska J.M., Wu J., Mon-Williams M., Phillips N., Pavitt S.H. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158:1166–1174. doi: 10.1097/j.pain.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 8.Tolle T., Dukes E., Sadosky A. Patient burden of trigeminal neuralgia: results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Pract. 2006;6:153–160. doi: 10.1111/j.1533-2500.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 9.Cruccu G., Gronseth G., Alksne J. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15:1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 10.Zakrzewska J.M., Wu N., Lee J.Y.K., Werneburg B., Hoffman D., Liu Y. Characterizing treatment utilization patterns for trigeminal neuralgia in the United States. Clin J Pain. 2018;34:691–699. doi: 10.1097/AJP.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 12.Merskey H., Bogduk N. 2nd Edn. IASP Press; Seattle: 1994. Classification of chronic pain. Descriptors of chronic pain syndromes and definitions of pain terms. [Google Scholar]
- 13.Gross R.A., Johnston K.C. Levels of evidence: taking neurology to the next level. Neurology. 2009;72:8–10. doi: 10.1212/01.wnl.0000342200.58823.6a. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso-Coello P., Oxman A.D., Moberg J. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 16.Spaziante R., Cappabianca P., Saini M., Peca C., Mariniello G., de Divitiis E. Treatment of trigeminal neuralgia by ophthalmic anesthetic. J Neurosurg. 1992;77:159–160. doi: 10.3171/jns.1992.77.1.0159b. [DOI] [PubMed] [Google Scholar]
- 17.Zavon M.R., Fichte C.M. Trigeminal neuralgia relieved by ophthalmic anesthetic. JAMA. 1991;265:2807. doi: 10.1001/jama.265.21.2807b. [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D., Lemley T., Kestle J.R., Lunsford L.D., Fromm G.H., Jannetta P.J. The effect of single-application topical ophthalmic anesthesia in patients with trigeminal neuralgia. A randomized double-blind placebo-controlled trial. J Neurosurg. 1994;80:993–997. doi: 10.3171/jns.1994.80.6.0993. [DOI] [PubMed] [Google Scholar]
- 19.Kanai A., Suzuki A., Kobayashi M., Hoka S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97:559–563. doi: 10.1093/bja/ael180. [DOI] [PubMed] [Google Scholar]
- 20.Niki Y., Kanai A., Hoshi K., Okamoto H. Immediate analgesic effect of 8% lidocaine applied to the oral mucosa in patients with trigeminal neuralgia. Pain Med. 2014;15:826–831. doi: 10.1111/pme.12349. [DOI] [PubMed] [Google Scholar]
- 21.Galer B.S., Miller K.V., Rowbotham M.C. Response to intravenous lidocaine infusion differs based on clinical diagnosis and site of nervous system injury. Neurology. 1993;43:1233–1235. doi: 10.1212/wnl.43.6.1233. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhry P., Friedman D.I. Intravenous lidocaine treatment in classical trigeminal neuralgia with concomitant persistent facial pain. Headache. 2014;54:1376–1379. doi: 10.1111/head.12401. [DOI] [PubMed] [Google Scholar]
- 23.Stavropoulou E., Argyra E., Zis P., Vadalouca A., Siafaka I. The effect of intravenous lidocaine on trigeminal neuralgia: a randomized double blind placebo controlled trial. ISRN Pain. 2014;2014:853826. doi: 10.1155/2014/853826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baykal M., Kaplan M. Effects of oral carbamazepine with 2% lidocaine on maxillary and mandibular nerve blocks in trigeminal neuralgia. Duzce Med J. 2010;12:19–23. [Google Scholar]
- 25.Soleimanpour H., Aghamohammadi D., Ghaffarzad A., Golzari S.E., Safari S., Soleimanpour M. Novel treatment in refractory tic douloureux. J Clin Anesth. 2014;26:495–496. doi: 10.1016/j.jclinane.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Arai Y.C., Hatakeyama N., Nishihara M., Ikeuchi M., Kurisuno M., Ikemoto T. Intravenous lidocaine and magnesium for management of intractable trigeminal neuralgia: a case series of nine patients. J Anesth. 2013;27:960–962. doi: 10.1007/s00540-013-1641-5. [DOI] [PubMed] [Google Scholar]
- 27.Tate R., Rubin L.M., Krajewski K.C. Treatment of refractory trigeminal neuralgia with intravenous phenytoin. Am J Health Syst Pharm. 2011;68:2059–2061. doi: 10.2146/ajhp100636. [DOI] [PubMed] [Google Scholar]
- 28.Vargas A., Thomas K. Intravenous fosphenytoin for acute exacerbation of trigeminal neuralgia: case report and literature review. Ther Adv Neurol Disord. 2015;8:187–188. doi: 10.1177/1756285615583202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheshire W.P. Fosphenytoin: an intravenous option for the management of acute trigeminal neuralgia crisis. J Pain Symptom Manage. 2001;21:506–510. doi: 10.1016/s0885-3924(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 30.Kanai A., Saito M., Hoka S. Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache. 2006;46:577–582. doi: 10.1111/j.1526-4610.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanai A., Saito M., Hoka S. Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache. 2006;46:577–582. doi: 10.1111/j.1526-4610.2006.00405.x. discussion 583–74. [DOI] [PubMed] [Google Scholar]
- 32.Kanai A., Suzuki A., Osawa S., Hoka S. Sumatriptan alleviates pain in patients with trigeminal neuralgia. Clin J Pain. 2006;22:677–680. doi: 10.1097/01.ajp.0000210917.18536.0d. [DOI] [PubMed] [Google Scholar]
- 33.Shimohata K., Shimohata T., Motegi R., Miyashita K. Nasal sumatriptan as adjunctive therapy for idiopathic trigeminal neuralgia: report of three cases. Headache. 2009;49:768–770. doi: 10.1111/j.1526-4610.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 34.Zuniga C., Diaz S., Piedimonte F., Micheli F. Beneficial effects of botulinum toxin type A in trigeminal neuralgia. Arq Neuropsiquiatr. 2008;66:500–503. doi: 10.1590/s0004-282x2008000400012. [DOI] [PubMed] [Google Scholar]
- 35.Zúñiga C., Piedimonte F., Díaz S., Micheli F. Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol. 2013;36:146–150. doi: 10.1097/WNF.0b013e31829cb60e. [DOI] [PubMed] [Google Scholar]
- 36.Devor M., Amir R., Rappaport Z.H. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Woolf C.J., Wiesenfeld-Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C-afferent fibre evoked activity in the spinal cord. Pain. 1985;23:361–374. doi: 10.1016/0304-3959(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 38.Nagy I., Woolf C.J. Lignocaine selectively reduces C fibre-evoked neuronal activity in rat spinal cord in vitro by decreasing N-methyl-D-aspartate and neurokinin receptor-mediated post-synaptic depolarizations; implications for the development of novel centrally acting analgesics. Pain. 1996;64:59–70. doi: 10.1016/0304-3959(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 39.Challapalli V., Tremont-Lukats I.W., McNicol E.D., Lau J., Carr D.B. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005;4:Cd003345. doi: 10.1002/14651858.CD003345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanDenKerkhof E.G., Stitt L., Clark A.J. Sensitivity of the DN4 in screening for neuropathic pain syndromes. Clin J Pain. 2017 doi: 10.1097/AJP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 41.Matharu M.S., Cohen A.S., Goadsby P.J. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia. 2004;24:985–992. doi: 10.1111/j.1468-2982.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 42.Dergin G., Gocmen G., Sener B.C. Treatment of trigeminal neuralgia with bupivacaine HCL using a temporary epidural catheter and pain pump: preliminary study. J Craniomaxillofac Surg. 2012;40:124–128. doi: 10.1016/j.jcms.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Lemos L., Flores S., Oliveira P., Almeida A. Gabapentin supplemented with ropivacain block of trigger points improves pain control and quality of life in trigeminal neuralgia patients when compared with gabapentin alone. Clin J Pain. 2008;24:64–75. doi: 10.1097/AJP.0b013e318158011a. [DOI] [PubMed] [Google Scholar]
- 44.Stajcic Z., Juniper R.P., Todorovic L. Peripheral streptomycin/lidocaine injections versus lidocaine alone in the treatment of idiopathic trigeminal neuralgia. A double blind controlled trial. J Craniomaxillofac Surg. 1990;18:243–246. doi: 10.1016/s1010-5182(05)80423-8. [DOI] [PubMed] [Google Scholar]
- 45.Bittar G.T., Graff-Radford S.B. The effects of streptomycin/lidocaine block on trigeminal neuralgia: a double blind crossover placebo controlled study. Headache. 1993;33:155–160. doi: 10.1111/j.1526-4610.1993.hed3303155.x. [DOI] [PubMed] [Google Scholar]
- 46.Han K.R., Kim C., Chae Y.J., Kim D.W. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int J Clin Pract. 2008;62:248–254. doi: 10.1111/j.1742-1241.2007.01568.x. [DOI] [PubMed] [Google Scholar]
- 47.Goto F., Ishizaki K., Yoshikawa D., Obata H., Arii H., Terada M. The long lasting effects of peripheral nerve blocks for trigeminal neuralgia using high concentration of tetracaine dissolved in bupivacaine. Pain. 1999;79:101–103. doi: 10.1016/S0304-3959(98)00156-0. [DOI] [PubMed] [Google Scholar]
- 48.Radwan I.A., Saito S., Goto F. High-concentration tetracaine for the management of trigeminal neuralgia: quantitative assessment of sensory function after peripheral nerve block. Clin J Pain. 2001;17:323–326. doi: 10.1097/00002508-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Woolf C.J., Thompson S.W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 50.Yousef A.A., Al-deeb A.E. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260–266. doi: 10.1111/anae.12107. [DOI] [PubMed] [Google Scholar]
- 51.Brill S., Sedgwick P.M., Hamann W., Di Vadi P.P. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002;89:711–714. [PubMed] [Google Scholar]
- 52.Mathisen L.C., Skjelbred P., Skoglund L.A., Oye I. Effect of ketamine, an NMDA receptor inhibitor, in acute and chronic orofacial pain. Pain. 1995;61:215–220. doi: 10.1016/0304-3959(94)00170-J. [DOI] [PubMed] [Google Scholar]
- 53.Gilron I., Booher S.L., Rowan M.S., Smoller M.S., Max M.B. A randomized, controlled trial of high-dose dextromethorphan in facial neuralgias. Neurology. 2000;55:964–971. doi: 10.1212/wnl.55.7.964. [DOI] [PubMed] [Google Scholar]
- 54.Sindrup S.H., Jensen T.S. Pharmacotherapy of trigeminal neuralgia. Clin J Pain. 2002;18:22–27. doi: 10.1097/00002508-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Cranford R.E., Leppik I.E., Patrick B., Anderson C.B., Kostick B. Intravenous phenytoin: clinical and pharmacokinetic aspects. Neurology. 1978;28(9 Pt 1):874–880. doi: 10.1212/wnl.28.9.874. [DOI] [PubMed] [Google Scholar]
- 56.Boucher B.A. Fosphenytoin: a novel phenytoin prodrug. Pharmacotherapy. 1996;16:777–791. [PubMed] [Google Scholar]
- 57.Fowler P.A., Lacey L.F., Thomas M., Keene O.N., Tanner R.J., Baber N.S. The clinical pharmacology, pharmacokinetics and metabolism of sumatriptan. Eur Neurol. 1991;31:291–294. doi: 10.1159/000116756. [DOI] [PubMed] [Google Scholar]
- 58.Derry C.J., Derry S., Moore R.A. Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;5:Cd009108. doi: 10.1002/14651858.CD009108.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goadsby P.J., Edvinsson L., Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 60.Goadsby P.J. Trigeminal autonomic cephalalgias. Pathophysiology and classification. Rev Neurol. 2005;161:692–695. doi: 10.1016/s0035-3787(05)85120-3. [DOI] [PubMed] [Google Scholar]
- 61.Limmroth V., Katsarava Z., Liedert B. An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain. 2001;92:101–106. doi: 10.1016/s0304-3959(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 62.(2012) NIfHaCE. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. NICE Technology appraisal guidance [TA260].
- 63.Simpson D.M., Hallett M., Ashman E.J. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castillo-Alvarez F., Hernando de la Barcena I., Marzo-Sola M.E. Botulinum toxin in trigeminal neuralgia. Medicina Clinica. 2017;148:28–32. doi: 10.1016/j.medcli.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Aoki K.R., Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Related Disord. 2011;17:S28–S33. doi: 10.1016/j.parkreldis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Zakrzewska J.M. Differential diagnosis of facial pain and guidelines for management. Br J Anaesth. 2013;111:95–104. doi: 10.1093/bja/aet125. [DOI] [PubMed] [Google Scholar]
- 67.Zakrzewska J.M., Linskey M.E. Trigeminal neuralgia. BMJ. 2014;348:g474. doi: 10.1136/bmj.g474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.