Abstract
Recent findings have revealed that aberrant miR-125a-5p expression is involved in the development of atherosclerosis. The present study aimed to investigate the precise mechanism of microRNA (miR)-125a-5p in atherosclerosis. Human vascular smooth muscle cells (HVSMCs) were treated with 20 µg/ml oxidized low-density lipoprotein (ox-LDL) for 24 h and were employed as in vitro models of atherosclerosis. Reverse transcription quantitative (RT-qPCR) assays were used to detect miR-125a-5p levels. Immunofluorescence analysis was conducted to assess α-smooth muscle actin (α-SMA) expression. Western blotting and RT-qPCR assays were performed to measure the expression levels of NACHT, LRR and PYD domains-containing protein 3 (NLRP3), apoptosis associated speck-like protein (ASC), caspase-1, active interleukin (IL)-1β and C-C motif chemokine 4-like (CCL4). Furthermore, the association between miR-125a-5p and CCL4 was assessed using a double luciferase analysis. In addition, VSMCs were transfected with miR-125a-5p mimics (30 nM), miR-125a-5p inhibitor (100 nM) or small interfering RNA against CCL4 (si-CCL4, 50 pM), respectively to further investigate the function of miR-125a-5p in ox-LDL-treated HVSMCs. The present study found that the expression levels of miR-125a-5p were significantly downregulated in HVSMCs, whereas the expression levels of α-SMA, NLRP3, ASC, caspase-1, IL-1β and CCL4 were markedly upregulated following ox-LDL treatment. Overexpression of miR-125a-5p in the absence of ox-LDL treatment decreased NLRP3, IL-1β and CCL4 expression, whereas inhibition of miR-125a-5p exhibited the opposite effects. The results of double luciferase analysis confirmed that CCL4 was a direct target of miR-125a-5p. Moreover, transfection of si-CCL4 into HVSMCs significantly decreased the ox-LDL-induced expression of NLRP3, ASC, caspase-1 and IL-1β proteins. Taken collectively, the results of the present study suggested that miR-125a-5p could negatively regulate the NLRP3 inflammasome by targeting CCL4 in ox-LDL-treated HVSMCs. The data provide new insight to the inhibition of atherosclerosis progression.
Keywords: miR-125a-5p, atherosclerosis, human vascular smooth muscle cells, NACHT, LRR and PYD domains-containing protein 3, C-C motif chemokine 4-like
Introduction
Atherosclerosis is associated with several cardiovascular complications including myocardial infarction, coronary heart disease, cerebral infarction, diabetes and dyslipidemia (1,2). It is a chronic inflammatory arterial disease causing serious medical and socioeconomic problems (1,2). Chronic vascular inflammation plays an important and understudied role in the initiation, development and progression of atherosclerosis (3,4). Vascular smooth muscle cells (VSMCs) are a dominant cellular constituent of arteries and play key roles in the development of atherosclerosis (5,6). The inflammatory response to vascular injury promotes the dedifferentiation of VSMCs, which causes a switch of these cells from the quiescent ‘contractile’ phenotype to the ‘proinflammatory’ phenotype (5). The dedifferentiated cells release cytokines and cause upregulation of multiple inflammasome components, which in turn promotes chemotaxis of monocytes/macrophages, thus accelerating the process of atherogenesis (7,8). Therefore, the investigation of the underlying molecular mechanism involved in the inflammation of VSMCs may be of potential scientific interest.
MicroRNAs (miR/miRNA) are small RNA molecules that contribute to mRNA degradation or transcriptional inhibition by direct interaction with the 3′-untranslated region (3′UTRs) of their target mRNAs. miRNAs regulate gene expression at the post-transcriptional level and have emerged as potential critical regulators of diverse biological and pathological processes (9,10). Accumulating evidence has shown that miRNAs play an important role in the processes of atherogenesis (11,12). For example, a recent study demonstrated that the expression levels of miR-20a were repressed in human aortic endothelial cells treated with oxidized LDL (ox-LDL), whereas the overexpression of miR-20a reduced the production of reactive oxygen species and the development of inflammatory injuries (13). A clinical trial conducted by Hao et al (14) indicated that miR-125a-5p levels were downregulated in atherosclerotic plaques of coronary atherosclerosis patients. Decreased expression of miR-125a-5p in patients with arteriosclerosis was also observed in another clinical trial (6). This evidence suggests that miR-125a-5p plays an important role in atherosclerosis. However, the underlying molecular mechanism of miR-125a-5p in the progression of atherosclerosis is still poorly understood.
The inflammasome of NACHT, LRR and PYD domains-containing protein 3 (NLRP3) is composed of NLRP3, apoptosis associated speck-like protein (ASC) and caspase-1. This complex triggers the conversion of pro-caspase-1 to active caspase-1. The active form of caspase-1 can convert the cytokine precursor pro-interleukin (IL)-1β into mature and biologically active IL-1β, which can trigger an inflammatory response (15,16). The present study aimed to explore the role of miR-125a-5p in atherosclerosis and to identify novel targets that may be clinically useful. The data indicated that miR-125a-5p was significantly downregulated in ox-LDL-treated human VSMCs (HVSMCs). Overexpression of miR-20a negatively regulated NLRP3 inflammasome by targeting the C-C motif chemokine ligand 4 (CCL4) and resulted in the protection of HVSMCs from ox-LDL-induced inflammatory injury.
Materials and methods
Cell culture and treatment
HVSMCs (American Type Culture collection; ATCC® CRL-1999™) were cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), in a humidified atmosphere with 5% CO2 at 37°C. The cells were treated with ox-LDL (20 µg/ml) for 24 h.
Transfection
The cells were seeded in a six-well plate at a density of 4×105 cells/ml for 24 h prior to transfection. The miR-125a-5p mimics (5′-UCCCUGAGACCCUUUAACCUGUGA-3′), the miR-125a-5p (5′-UCACAGGUUAAAGGGUCUCAGGGA-3′) inhibitor and the corresponding negative control [NC (5′-UUGUACUACACAAAAGUACUG-3′)] samples were synthesized by Shanghai GenePharma, Co., Ltd., and transfected into the VSMCs using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The final concentrations of miR-125a-5p mimics, NC mimics, miR-125a-5p inhibitor and NC inhibitor were 30, 30, 100 and 100 nM, respectively. The si-CCL4 (5′-GCCACAACCUGUCACUCAATT-3′, cat. no. stB0007785C-1-5; Guangzhou RiboBio, Co., Ltd.) and si-NC (5′-UUCUCCGAACGUGUCACGUUU-3′, cat. no. siN05815122147-1-5; Guangzhou RiboBio, Co., Ltd.) were transfected into the cells with a final concentration of 50 pM. Further experiments were performed at 48 h after transfection.
Immunofluorescence
HVSMCs were seeded on coverslips at a density of 4×105 cells per ml in a six-well plate for 24 h. At 24 h following ox-LDL treatment, the cells were fixed in 4% formaldehyde at 25°C for 15 min and blocked in 5% normal goat serum (Invitrogen; Thermo Fisher Scientific, Inc.)/0.3% Triton™ X-100 for 1 h at room temperature. The cells were incubated with anti-α-smooth muscle actin (cat. no. 34105; 1:50; Cell Signaling Technology, Inc.) antibody at 4°C overnight and subsequently with a fluorescein-conjugated mouse anti-rabbit IgG (cat. no. sc-2359; 1:500; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Following staining with DAPI (D9542; Sigma-Aldrich; Merck KGaA) for 5 min at room temperature, the samples were imaged using a laser scanning confocal microscope (Leica Microsystems GmbH) at 200× magnification.
ELISA
The culture medium of the ox-LDL-treated HVSMCs was collected. The levels of IL-1β were measured by IL-1β ELISA kits (cat. no. ab100562; Abcam) according to the manufacturer's protocols.
Western blotting
Total proteins were extracted from cells using RIPA lysis buffer (Beyotime Institute of Biotechnology). The protein concentration was measured using the bicinchoninic acid kit (Bio-Rad Laboratories, Inc.). The protein samples (30 µg/lane) were separated on 10% SDS-PAGE gels and blotted onto polyvinylidene difluoride membranes (EMD Millipore Corp.). The membranes were blocked in 5% non-fat milk for 1 h at room temperature, followed by overnight incubation at 4°C with primary antibodies as follows: NLRP3 (cat. no. 13158; 1:1,000; Cell Signaling Technology, Inc.), ASC (cat. no. 13833; 1:1,000; Cell Signaling Technology, Inc.), caspase-1 (cat. no. 3866; 1:1,000; Cell Signaling Technology, Inc.), IL-1β (cat. no. 12703; 1:1,000; Cell Signaling Technology, Inc.), α-SMA (cat. no. 68463; 1:1,000; Cell Signaling Technology, Inc.), CCL4 (cat. no. ab106028; 1:1,000; Abcam) and GAPDH (cat. no. sc-81545; 1:2,000; Santa Cruz Biotechnology, Inc.), respectively. Then membranes were further probed with horse radish peroxidase-conjugated secondary antibody rabbit anti-mouse (1:10,000; cat. no. sc-358914; Santa Cruz Biotechnology, Inc.) or mouse anti-rabbit (1:10,000; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) at 4°C overnight and immunoblots were visualized using an ECL Plus chemiluminescence kit (Amersham Biosciences). The relative protein expression was analyzed using ImageJ software 1.4 (National Institute of Health).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cells was extracted using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as described in the manufacturer's protocol. The TaqMan microRNA reverse transcription kit (Takara Bio, Inc.) combined with specific stem-loop primers and Prime Script™ RT reagent kit (Takara Biotechnology Co., Ltd.) were used to obtain cDNA from miR-125a-5p and mRNA molecules respectively, according to the manufacturer's protocol. Reaction conditions were as follows: 37°C for 15 min, followed by incubation at 85°C for 5 sec. RT-qPCR was performed using a Perfect Real Time SYBR Premix Ex Taq kit (Takara Bio, Inc.) with an ABI 7500 thermal cycler (Thermo Fisher Scientific, Inc.) under the following conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The experiments were repeated at least three times. Quantitative measurements were analyzed by the 2−ΔΔCq method (17). The expression of U6 and GAPDH was used as internal control for the expression of miRNA and mRNA molecules, respectively. The primers sequences used were the following: NLRP3, forward, 5′-GCGCCTCAGTTAGAGGATGT-3′, reverse: 5′-ACCAGCTACAAAAAGCATGGA-3′; ASC, forward: 5′-TGGATGCTCTGTACGGGAAG-3′, reverse: 5′-CCAGGCTGGTGTGAAACTGAA-3′; caspase-1, forward: 5′-TTTCCGCAAGGTTCGATTTTCA-3′, reverse: 5′-GGCATCTGCGCTCTACCATC-3′; IL-1β, forward: 5′-GGGCCTCAAGGAAAAGAATC-3′, reverse: 5′-TTCTGCTTGAGAGGTGCTGA-3′; CCL4, forward: 5′-GCTTTTCTTACACTGCGAGGA-3′, reverse: 5′-CCAGGATTCACTGGGATCAG-3′; GAPDH, forward:5′-AAGGTGAAGGTCGGAGTCAAC-3′, reverse: 5′-GGGGTCATTGATGGCAACAATA-3′; U6, stem-loop primer: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGG-3′, forward: 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′. miR-125a-5p, stem-loop primer: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCACAG′, forward: 5′-AGCGCGTCCCTGAGACCCTTTAAC-3′ and reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′.
Dual-luciferase reporter assays
TargetScan Human (www.targetscan.org) was used to predict that CCL4 is the potential target of miR-125a-5p. The mutant type of the CCL4 3′UTR sequence was constructed using a GeneArt™ Site-Directed Mutagenesis System (cat. no. A13282; Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer protocol. The wild type or mutant type of CCL4 3′UTR was then cloned into the firefly luciferase reporter vector with the pGL3-promoter (Promega Corp.) to generate the recombinant vector pGL3-CCL4-3′UTR wild type (3′UTR-WT) or pGL3-CCL4-3′UTR mutant type (3′UTR-MUT). 293T cells (ATCC) were cultured in 24 well plates for 24 h and co-transfected with 50 ng 3′UTR-WT (or 3′UTR-MUT) vector and 20 µM of miR-125a-5p (or NC) mimics. The transfections were performed using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as indicated by the manufacturer's protocol. At 48 h following transfection, the Dual-Luciferase Reporter Assay System (Promega Corp.) and Turner DesignsTD-20/20 luminometer (Promega Corp.) were used to determine the luciferase activity normalized to Renilla.
Statistical analysis
The data are presented as the mean ± standard deviation. The differences between two groups were analyzed using the Student's t-test. The comparisons among the multiple groups of data were analyzed by one-way analysis of variance followed by the Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant difference. All data were analyzed using SPSS 13.0 software (SPSS, Inc.).
Results
Ox-LDL treatment increases NLRP3 expression and decreases miR-125a-5p expression levels in HVSMCs
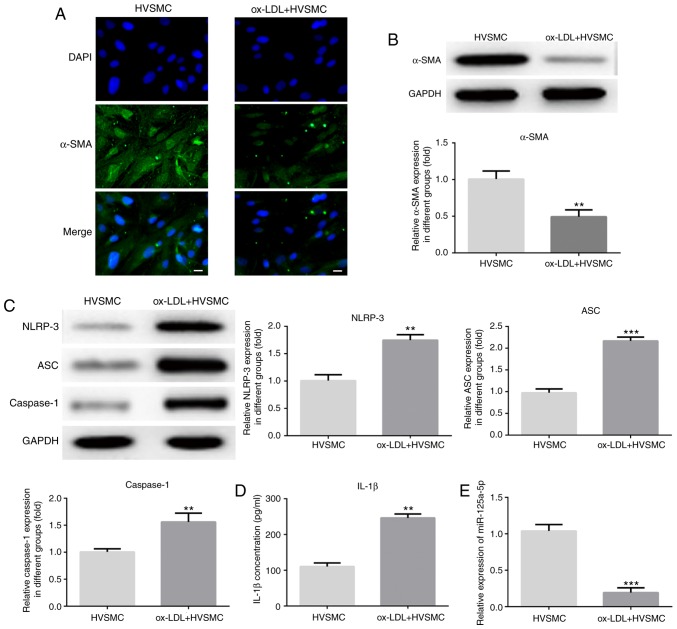
The levels of the VSMC differentiation marker α-SMA were measured by immunofluorescence and western blot assays in order to observe the phenotypic alterations of HVSMCs induced by ox-LDL. The results indicated that ox-LDL significantly downregulated α-SMA expression (P<0.01; Fig. 1A and B). The production and release of the inflammatory mediators by VSMCs played a critical role in atherogenesis. The data suggested that ox-LDL treatment significantly increased NLRP3, ASC, caspase-1 and IL-1β expression levels (P<0.01; Fig. 1C and D). Furthermore, the results of the RT-qPCR assay indicated that the miR-125a-5p levels were significantly decreased in ox-LDL-treated HVSMCs (P<0.01; Fig. 1E).
Figure 1.
Ox-LDL treatment increases and decreases NLRP3 and miR-125a-5p expression levels, respectively in HVSMCs. The cells were treated with ox-LDL (20 µg/ml) for 24 h. α-SMA levels was measured by (A) immunofluorescence and (B) western blot assays. The expression levels of (C) NLRP3, ASC, caspase-1 and (D) IL-1β were detected using western blotting and ELISA, respectively. (E) Reverse transcription quantitative-PCR assays were used to detect miR-125a-5p levels. Scale bar, 50 µm. **P<0.01 and ***P<0.001 vs. HVSMCs. HVSMC, human vascular smooth muscle cells; miR, microRNA; IL, interleukin; ox-LDL, oxidised low-density lipoprotein; NLRP3, NACHT, LRR and PYD domains-containing protein 3; α-SMA, smooth muscle actin; ASC, apoptosis associated speck-like protein.
miR-125a-5p is responsible for the ox-LDL induced expression of NLRP3 and IL-1β
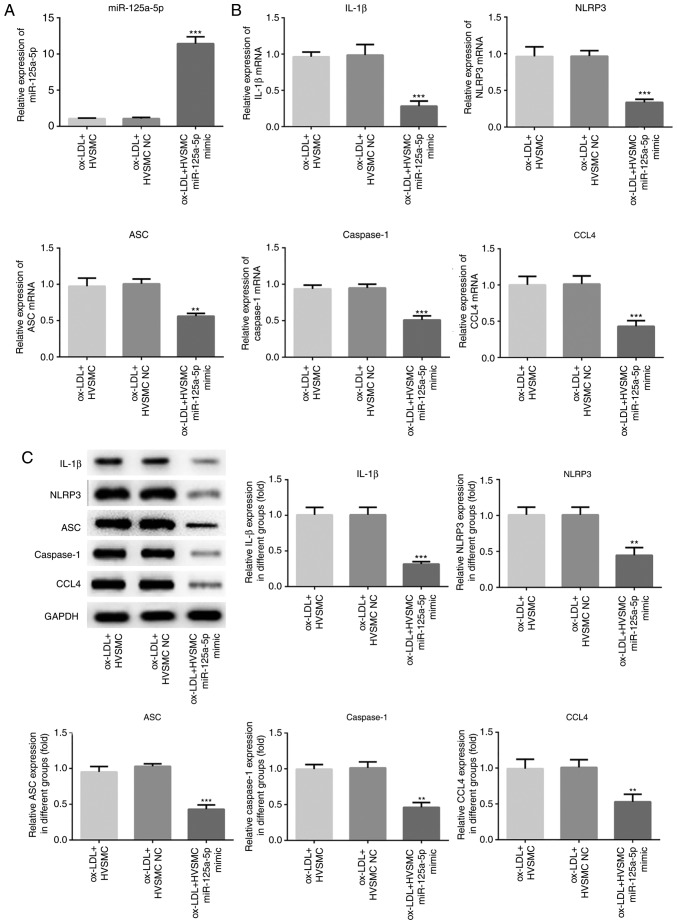
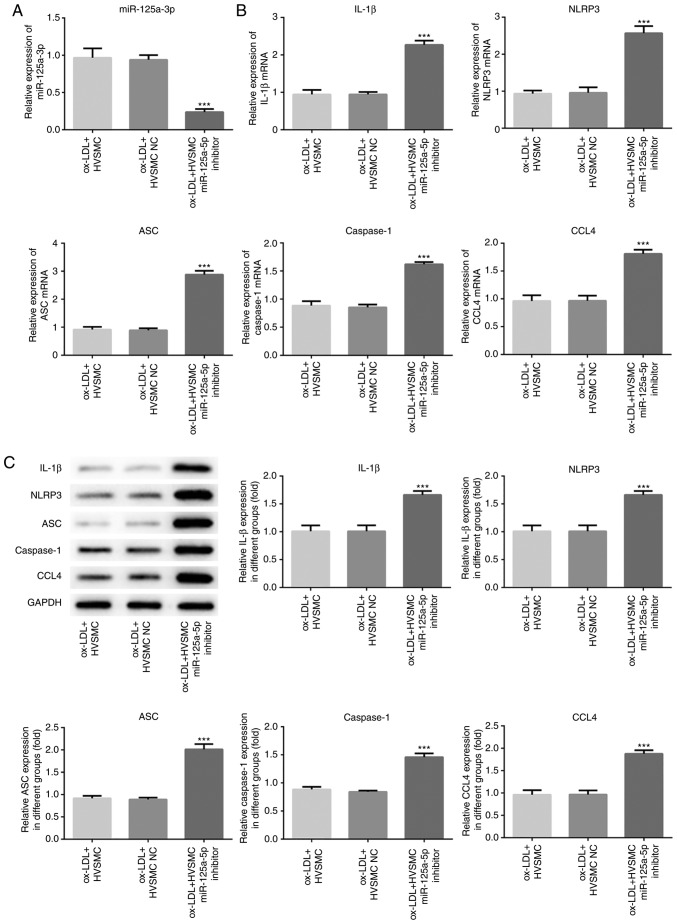
To identify the role of miR-125a-5p in the progression of atherosclerosis, miR-125a-5p mimics and miR-125a-5p inhibitor were used to cause upregulation and downregulation of miR-125a-5p expression levels, respectively, in ox-LDL-treated HVSMCs (Figs. 2 and 3). Overexpression of miR-125a-5p significantly inhibited the expression levels of NLRP3, ASC, caspase-1 and IL-1β in HVSMCs (P<0.01; Fig. 2B and C). In contrast to the miR-125a-5p mimics, the expression levels of NLRP3, ASC, caspase-1 and IL-1β were significantly increased in the miR-125a-5p inhibitor group compared with those noted in the NC group (P<0.001; Fig. 3B and C). The data suggested that miR-125a-5p is, at least partly, responsible for the induction of NLRP3 and IL-1β expression that was mediated by ox-LDL treatment.
Figure 2.
Overexpression of miR-125a-5p inhibited the expression levels of NLRP3, ASC, caspase-1, IL-1β and CCL4 in HVSMCs. The cells were trasfected with miR-125a-5p mimics and NC mimics, and treated with ox-LDL treatment. (A) The miR-125a-5p levels were detected by RT-qPCR. (B) RT-qPCR and (C) western blot assays were performed to analyse the NLRP3, IL-1β and CCL4 mRNA and protein expression levels. **P<0.01 and ***P<0.001 vs. ox-LDL + HVSMCs. HVSMC, human vascular smooth muscle cells; miR, microRNA; IL, interleukin; ox-LDL, oxidised low-density lipoprotein; NLRP3, NACHT, LRR and PYD domains-containing protein 3; α-SMA, smooth muscle actin; RT-q, reverse transcription-quantitative; CCL4, C-C motif chemokine 4-like; ASC, apoptosis associated speck-like protein.
Figure 3.
Downregulation of miR-125a-5p increases the expression levels of NLRP3, ASC, caspase-1, IL-1β and CCL4 in HVSMCs. The cells were trasfected with miR-125a-5p inhibitor and NC inhibitor, and treated with ox-LDL. (A) The miR-125a-5p levels were detected by RT-qPCR. (B) RT-qPCR and (C) western blot assays were performed to analyse NLRP3, ASC, caspase-1, IL-1β and CCL4 mRNA and protein expression levels. ***P<0.001 vs. ox-LDL + HVSMCs. HVSMC, human vascular smooth muscle cells; miR, microRNA; IL, interleukin; ox-LDL, oxidised low-density lipoprotein; NLRP3, NACHT, LRR and PYD domains-containing protein 3; α-SMA, smooth muscle actin; RT-q, reverse transcription-quantitative; CCL4, C-C motif chemokine 4-like; NC, negative control; ASC, apoptosis associated speck-like protein.
CCL4 is a direct target gene of miR-125a-5p
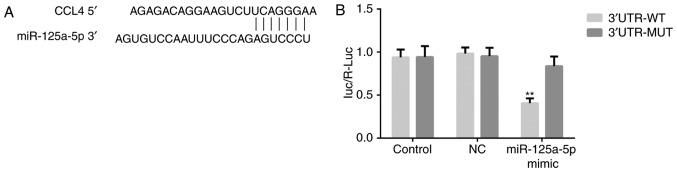
TargetScan Human 7.1 (www.targetscan.org) was used to investigate whether CCL4 is a potential target of miR-125a-5p. RT-qPCR and western blot assays were performed to detect the expression levels of CCL4 mRNA and protein, respectively, and the results indicated that overexpression of miR-125a-5p significantly decreased CCL4 expression levels (P<0.01; Fig. 2B and C), whereas downregulation of miR-125a-5p exhibited the opposite effects (P<0.001; Fig. 3B and C). Furthermore, a dual-luciferase reporter assay was performed. Transfection of miR-125a-5p mimics and the CCL4 3′UTR-WT vector significantly reduced luciferase activity compared with transfection of NC mimics and the CCL4 3′UTR-WT vector in HVSMCs (P<0.01; Fig. 4). However, no significant change was noted in the cells that were transfected with the CCL4 3′UTR-WT vector (Fig. 4). The results indicated that miR-125a-5p could directly bind to the 3′UTR of CCL4.
Figure 4.
CCL4 is a direct target gene of miR-125a-5p. (A) The putative miR-125a-5p targeted sequences in the 3′-UTR of CCL4 mRNA are shown. (B) Dual-luciferase reporter assay was performed to analyse the relative luciferase activity in 293T cells. **P<0.01 vs. NC. CCL4, C-C motif chemokine 4-like; NC, negative control; miR, microRNA; UTR, untranslated region; WT, wild-type; Mut, mutant.
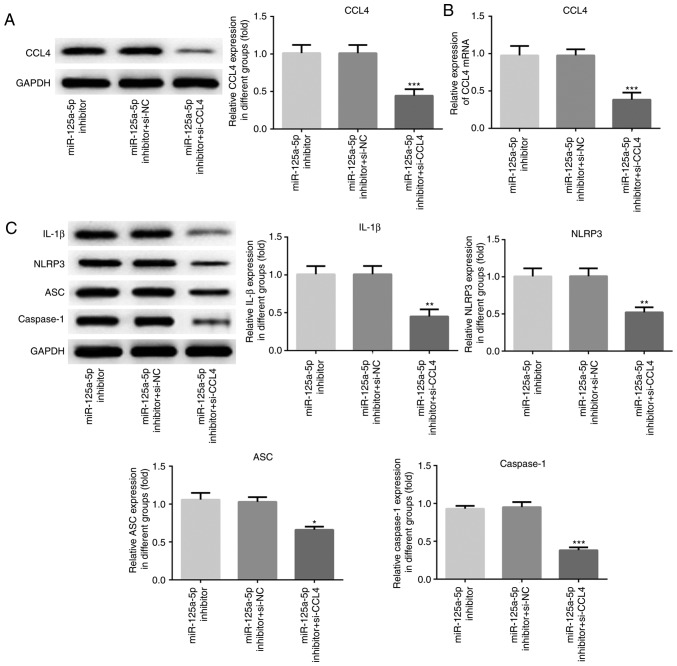
Knockdown of CCL4 attenuates miR-125a-5p inhibitor induced NLRP3 and IL-1β expression
In order to further explore the role of CCL4 in ox-LDL-induced HVSMCs, the cells were co-transfected with si-CCL4 or si-NC and the miR-125a-5p inhibitor (Fig. 5). The results of the western blot assays indicated that the expression levels of CCL4 were significantly downregulated in HVSMCs transfected with si-CCL4 compared with those of the si-NC group (P<0.001; Fig. 5A). Moreover, silencing of CCL4 significantly decreased the expression levels of NLRP3, ASC, caspase-1 and IL-1β in HVSMCs transfected with the miR-125a-5p inhibitor (P<0.05; Fig. 5C). These results demonstrated that knockdown of CCL4 could attenuate activation of the NLRP3 inflammasome and the production of its downstream substrate IL-1β that was mediated by the inhibition of miR-125a-5p.
Figure 5.
Knockdown of CCL4 attenuated the induction of NLRP3, ASC, caspase-1 and IL-1β expression by the miR-125a-5p inhibitor. human vascular smooth muscle cells were co-transfected with si-CCL4 and miR-125a-5p inhibitor for 48 h. (A) Western blot and (B) reverse transcription-quantitative PCR assays were performed to analyse CCL4 mRNA and protein expression levels. (C) The NLRP3, ASC, caspase-1 and IL-1β expression levels were detected by western blot assays. *P<0.05, **P<0.01 and ***P<0.001 vs. miR-125a-5p inhibitor. CCL4, C-C motif chemokine 4-like; NC, negative control; IL, interleukin; miR, microRNA; si, small interfering; NLRP3, NACHT, LRR and PYD domains-containing protein 3; ASC, apoptosis associated speck-like protein.
Discussion
Ox-LDL can regulate the expression of inflammatory factors and enhance the cellular inflammatory response (17,18). During that process, vascular damage is induced, which leads to the development of atherosclerosis (18,19). Accumulating evidence has suggested that the inflammatory response to vascular injury promotes the dedifferentiation of VSMCs, which accelerates the process of atherogenesis (3,4). In the present study, the data demonstrated that ox-LDL addition upregulated the expression levels of α-SMA, which is one of the VSMC differentiation marker genes. In addition, the expression of NLRP3 and its downstream gene IL-1β was upregulated in ox-LDL-treated HVSMCs. This result was consistent with the study by Li et al (20), which demonstrated that ox-LDL treatment increased the expression levels of NLRP3 and of its downstream genes (caspase-1, IL-1β and IL-18) in a dose-dependent manner. Inflammation plays a key role in the stages of atherogenesis. A variety of inflammatory cytokines have been associated with atherosclerosis, including IL-1β (21). IL-1β can promote the inflammatory response and enhance VSMC proliferation and migration (22). A recent randomized, placebo-controlled trial indicated that the monoclonal antibody canakinumab, which directly targets and neutralizes IL-1β, was associated with reduced risk of adverse cardiovascular events compared with that noted for the placebo subjects (23). In addition, the activation of the NLRP3 inflammasome is a powerful mediator of the inflammatory response that contributes to the VSMC phenotypic transformation and proliferation (23–25). It is therefore believed that the NLRP3 inflammasome activation is involved in the pathogenesis of atherosclerosis (24–26).
miR-125a-5p has been reported to be involved in numerous cellular functions by the regulation of multiple signaling pathways. For example, Natalia et al (27) suggested that miR-125a-5p promotes migration of cervical cancer cells via targeting the Microtubule-Affinity-Regulating Kinase1. In addition, overexpression of miR-125a-5p inhibits cell proliferation and migration by blocking the p38/JNK/ERK signaling pathway in salivary adenoid cystic carcinoma. In the present study, it was suggested that miR-125a-5p, which was downregulated following ox-LDL treatment, was responsible for the increased expression of NLRP3 and IL-1β in HVSMCs. Downregulation of miR-125a-5p in atherosclerotic patients was observed in previous studies (6,14). However, these reports did not elucidate the underlying molecular pathways of miR-125a-5p that were involved in atherogenesis. In the current study, miR-125a-5p mimics and miR-125a-5p inhibitor were transfected into ox-LDL-treated HVSMCs in order to cause upregulation and downregulation of miR-125a-5p expression, respectively. The results demonstrated that miR-125a-5p may be involved in the process of the inflammatory response and in the development of atherogenesis by the regulation of the NLRP3 inflammasome. In addition, TargetScan Human (www.targetscan.org) was used to predict whether CCL4 is the potential target of miR-125a-5p. CCL4 is a member of the chemokine superfamily, which is rapidly induced following vascular damage. CCL4 is involved in the regulation of the inflammatory response (28,29). The results of the dual-luciferase reporter assay suggested that CCL4 is a direct target gene of miR-125a-5p. Furthermore, HVSMCs were co-transfected with si-CCL4 and the miR-125a-5p inhibitor. The knockdown of CCL4 by siRNA attenuated the miR-125a-5p inhibitor-induced production of NLRP3 and IL-1β. Taken collectively, the results demonstrated that ox-LDL could induce NLRP3 and IL-1β expression by upregulating the expression levels of CCL4.
In conclusion, the current study suggested that ox-LDL treatment significantly downregulated miR-125a-5p levels and induced NLRP3, and IL-1β expression by upregulating the expression levels of the target gene of miR-125a-5p, CCL4. miR-125a-5p caused a negative regulation on the NLRP3 inflammasome by targeting CCL4 in ox-LDL treated HVSMCs. The data revealed that miR-125a-5p may be involved in the inflammatory response and the regulation of atherogenesis, indicating its potential application in atherosclerosis therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JW and ZX designed the experiment. JW, QW, JY and XC carried out data acquisition, data analysis and statistical analysis together. Moreover, JW and ZX carried out literature search and manuscript editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes Metab Res Rev. 2015;31:113–126. doi: 10.1002/dmrr.2558. [DOI] [PubMed] [Google Scholar]
- 3.Meijles DN, Pagano PJ. Nox and inflammation in the vascular adventitia. Hypertension. 2016;67:14–19. doi: 10.1161/HYPERTENSIONAHA.115.03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii S. Atherosclerosis, chronic inflammation, and thrombosis: In search of the missing link in laboratory medicine. Rinsho Byori. 2015;63:605–611. (In Japanese) [PubMed] [Google Scholar]
- 5.Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 2015;214:33–50. doi: 10.1111/apha.12466. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Shao S, Geng H, Yu Y, Wang C, Liu Z, Yu C, Jiang X, Deng Y, Gao L, Zhao J. Expression profiles of six circulating microRNAs critical to atherosclerosis in patients with subclinical hypothyroidism: A clinical study. J Clin Endocrinol Metab. 2014;99:E766–E774. doi: 10.1210/jc.2013-1629. [DOI] [PubMed] [Google Scholar]
- 7.Hasanov Z, Ruckdeschel T, Konig C, Mogler C, Kapel SS, Korn C, Spegg C, Eichwald V, Wieland M, Appak S, Augustin HG. Endosialin promotes atherosclerosis through phenotypic remodeling of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2017;37:495–505. doi: 10.1161/ATVBAHA.116.308455. [DOI] [PubMed] [Google Scholar]
- 8.Byon CH, Han T, Wu J, Hui ST. Txnip ablation reduces vascular smooth muscle cell inflammation and ameliorates atherosclerosis in apolipoprotein E knockout mice. Atherosclerosis. 2015;241:313–321. doi: 10.1016/j.atherosclerosis.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss EG. MicroRNAs: Hidden in the genome. Curr Biol. 2002;12:R138–R140. doi: 10.1016/S0960-9822(02)00708-X. [DOI] [PubMed] [Google Scholar]
- 10.Duarte FV, Palmeira CM, Rolo AP. The emerging role of mitomiRs in the pathophysiology of human disease. Adv Exp Med Biol. 2015;888:123–154. doi: 10.1007/978-3-319-22671-2_8. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson CJ, Lao KH, Zeng L. The salient role of microRNAs in atherogenesis. J Mol Cell Cardiol. 2018;122:98–113. doi: 10.1016/j.yjmcc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Lucas T, Bonauer A, Dimmeler S. RNA therapeutics in cardiovascular disease. Circ Res. 2018;123:205–220. doi: 10.1161/CIRCRESAHA.117.311311. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Li W, Zhang Y, Yang J. MicroRNA-20a protects human aortic endothelial cells from Ox-LDL-induced inflammation through targeting TLR4 and TXNIP signaling. Biomed Pharmacother. 2018;103:191–197. doi: 10.1016/j.biopha.2018.03.129. [DOI] [PubMed] [Google Scholar]
- 14.Hao L, Wang XG, Cheng JD, You SZ, Ma SH, Zhong X, Quan L, Luo B. The up-regulation of endothelin-1 and down-regulation of miRNA-125a-5p, −155, and −199a/b-3p in human atherosclerotic coronary artery. Cardiovasc Pathol. 2014;23:217–223. doi: 10.1016/j.carpath.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Di X, Tang X, Di X. Montelukast inhibits oxidized low-density lipoproteins (ox-LDL) induced vascular endothelial attachment: An implication for the treatment of atherosclerosis. Biochem Biophys Res Commun. 2017;486:58–62. doi: 10.1016/j.bbrc.2017.02.125. [DOI] [PubMed] [Google Scholar]
- 19.Karim MR, Rahman M, Islam K, Mamun AA, Hossain S, Hossain E, Aziz A, Yeasmin F, Agarwal S, Hossain MI, et al. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci. 2013;135:17–25. doi: 10.1093/toxsci/kft130. [DOI] [PubMed] [Google Scholar]
- 20.Li WL, Hua LG, Qu P, Yan WH, Ming C, Jun YD, Yuan LD, Nan N. NLRP3 inflammasome: A novel link between lipoproteins and atherosclerosis. Arch Med Sci. 2016;12:950–958. doi: 10.5114/aoms.2016.61356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. IL-1β enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul Pharmacol. 2015;72:108–117. doi: 10.1016/j.vph.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Aday AW, Ridker PM. Antiinflammatory therapy in clinical care: The CANTOS trial and beyond. Front Cardiovasc Med. 2018;5:62. doi: 10.3389/fcvm.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074. doi: 10.1038/cddis.2017.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, Liu Q. Role of the NLRP3 inflammasome in the transient release of IL-1beta induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lord) 2015;12:30. doi: 10.1186/s12950-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi Y, Kurita-Ochiai T, Kobayashi R, Suzuki T, Ando T. Activation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated atherosclerosis. Pathog Dis. 2015;73:ftv011. doi: 10.1093/femspd/ftv011. [DOI] [PubMed] [Google Scholar]
- 27.Natalia MA, Alejandro GT, Virginia TJ, Alvarez-Salas LM. MARK1 is a novel target for miR-125a-5p: Implications for cell migration in cervical tumor cells. Microma. 2018;7:54–61. doi: 10.2174/2211536606666171024160244. [DOI] [PubMed] [Google Scholar]
- 28.Verite J, Janet T, Julian A, Chassaing D, Page G, Paccalin M. Peripheral blood mononuclear cells of Alzheimer's disease patients control CCL4 and CXCL10 levels in a human blood brain barrier model. Curr Alzheimer Res. 2017;14:1215–1228. doi: 10.2174/1567205014666170417110337. [DOI] [PubMed] [Google Scholar]
- 29.Edsfeldt A, Grufman H, Asciutto G, Nitulescu M, Persson A, Nilsson M, Nilsson J, Gonçalves Circulating cytokines reflect the expression of pro-inflammatory cytokines in atherosclerotic plaques. Atherosclerosis. 2015;241:443–449. doi: 10.1016/j.atherosclerosis.2015.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.