Abstract
The effect of mild hypothermia on the expression of interleukin (IL)-10 and IL-18 in neonates with hypoxic ischemic encephalopathy was investigated. A retrospective analysis was performed on 97 neonates with hypoxic-ischemic encephalopathy (HIE) admitted to the neonatal intensive care unit of Xuzhou Children's Hospital, Xuzhou Medical University from September 2016 to August 2018. Of these patients, 54 with mild hypothermia were included in the experimental group (moderate + severe), and 43 patients who received conventional therapy were included in the control group (moderate + severe). The effect of mild hypothermia on IL-10 and IL-18 expression levels was analyzed, and the relationship between IL-10, IL-18 and disease severity in children with hypoxic ischemic encephalopathy was analyzed. The concentration of IL-10 in the experimental group was significantly lower than that in the control group at 1, 2, 3 and 5 days (P<0.05). The concentration of IL-18 in the experimental group was significantly lower than that in the control group at the 2, 3 and 5 days (P<0.05). NBNA scores of the experimental group were significantly higher than those of the control group at 14 and 28 days after birth (P<0.05). Before treatment, the expression levels of IL-10 and IL-18 in severe children were significantly higher than those in moderate children (P<0.05). IL-10 was positively correlated with the severity of the disease (r=0.521, P<0.05), and IL-18 was also positively correlated with the severity of the disease (r=0.616 P<0.05). Mild hypothermia treatment can effectively improve the neurological function of children with HIE, reduce the expression of IL-10 and IL-18 in serum, and inhibit the inflammatory response. Therefore, the expression of IL-10 and IL-18 was positively correlated with the severity of disease in children with HIE, and could be used as an indicator to judge the severity of HIE.
Keywords: mild hypothermia, neonates with hypoxic ischemic encephalopathy, IL-10, IL-18
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a neonatal disease that is very common in perinatal period. HIE is mainly due to neonatal brain damage caused by partial or complete hypoxia in the brain under intrauterine distress. The pathogenesis of the disease is still unknown, so there is no effective treatment plan (1). Clinical studies have revealed that approximately 20% of children with HIE die in the neonatal period. Even if they survive, some patients will have severe neurological dysfunction and other sequelae (2). The effective treatment of HIE has been controversial for a long time, and the efficacy and safety of most therapeutic drugs have yet to be verified by further animal experiments (3).
Non-pharmaceutical hypothermia treatment is currently the only safe treatment that has been proven to have a good effect on neonatal HIE, and it is generally considered that the optimal treatment time is 6 h after birth (4). However, recent studies (5) have shown that children with HIE undergoing hypothermia within 24 hours of birth can also reduce the risk of death within 18 months. Some studies exploring the pathogenesis of HIE have indicated that inflammatory response also plays an important role in neonatal HIE (6). As an anti-inflammatory cytokine, interleukin (IL)-10 has a certain protective effect on hypoxic ischemic encephalopathy because of its anti-inflammatory properties (7). IL-l8 also has both anti-inflammatory and pro-inflammatory effects. It acts as an anti-inflammatory factor to protect brain tissue. At the same time, it acts as a pro-inflammatory factor and may induce IL-2, IL-1B and other inflammatory factors to cause brain damage (8,9).
However, studies on the effects of mild hypothermia on IL-10 and IL-18 in children with HIE are rare. This study explored the therapeutic effect of mild hypothermia on serum IL-10 and IL-18 in children with HIE. The aim was to investigate the efficacy of hypothermia therapy in the treatment of children with HIE.
Patients and methods
General information
A retrospective analysis of 97 children with HIE admitted to Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou, China) from September 2016 to August 2018 was conducted. There were 51 male patients and 46 female patients with an average gestational age of 37.95±1.73 weeks. Among them, 54 patients with mild hypothermia were included in the experimental group (moderate + severe), and 43 patients who received conventional therapy were included in the control group (moderate + severe). There were no significant differences in sex and gestational age between the two groups (P>0.05; Table I). Inclusion criteria: Clinical diagnosis of neonatal hypoxic ischemic encephalopathy, in line with the diagnostic criteria of the 4th edition of Practical Neonatology, 2011, with moderate to severe degree, hospitalized within 24 h after birth. Exclusion criteria: children with complex congenital heart disease; children with congenital diaphragmatics; children with refractory pneumothorax; active bleeding, grade II and IV intracranial hemorrhage, platelet ≤100×109/l and children with bleeding tendency; parents who gave up treatment during treatment. Family members of all the children agreed to participate and signed the informed consent. This experiment was approved by the Ethics Committee of Xuzhou Children's Hospital, Xuzhou Medical University.
Table I.
Comparison of general data between two groups of children [n (%)].
| Items | Experimental group (n=54) | Control group (n=43) | t/χ2 value | P-value |
|---|---|---|---|---|
| Sex | 0.062 | 0.803 | ||
| Male | 29 (53.70) | 22 (51.16) | ||
| Female | 25 (46.30) | 21 (48.84) | ||
| Gestational age (weeks) | 0.029 | 0.866 | ||
| ≤37 | 21 (38.89) | 16 (37.21) | ||
| >37 | 33 (61.11) | 27 (62.79) | ||
| Weight (kg) | 3.31±0.79 | 3.30±0. 81 | 0.061 | 0.951 |
| HIE level | 0.000 | 0.986 | ||
| Moderate | 34 (62.96) | 27 (62.79) | ||
| Severe | 20 (37.04) | 16 (37.21) |
HIE, hypoxic-ischemic encephalopathy.
Treatment
Children in the control group were treated routinely after birth, including maintaining airway patency, maintaining ambient temperature, keeping blood pressure and blood glucose at a normal level, sedation treatment, intracranial pressure reduction, anticonvulsant treatment, improving ventilation and brain function protection. In the experimental group, the ALLon hypothermia thermoregulatory system (MTRE, Israel) was used for systemic hypothermia treatment. Anus temperature of the child was tested, and the core temperature was maintained at 34.0±0.4°C for 72 h, then the treatment was stopped and the child was rewarmed.
Detection of serum IL-10 and IL-18 concentrations in the two groups of children
Peripheral venous blood (2 ml) was taken at 0 h, 1, 2, 3 and 5 days after hypothermia treatment in the experimental group and after admission in the control group. Blood was centrifuged at 3,000 × g for 5 min at 4°C to collect serum and the serum was stored at −80°C before use. Concentrations of IL-10 and IL-18 were determined by enzyme linked immunosorbent assay (ELISA).
Observation indicators
i) Concentrations of IL-10 and IL-18 at 0 h, 1, 2, 3 and 5 days after treatment were tested and compared. ii) Neonatal behavioral neurological score (NB-NA) (10) was calculated at 7, 14, and 28 days after birth. iii) Severity of disease was analyzed and its correlation with IL-10 and IL-18 was explored.
Statistical analysis
In this study, SPSS18.0 [Bizinsight (Beijing) Information Technology Co., Ltd.] was used for statistical analyses. Chi-square test was used for comparisons of enumeration data, and measurement data were expressed as mean ± standard deviation. Comparison between the two groups was analyzed by t-test, and comparison among multiple groups was analyzed by ANOVA with Dunnetts post hoc test. Comparison of different time-points was performed by repeated measures ANOVA. Pearson's correlation analysis test was used for correlation between IL-8, IL-10 and severity of the disease. P<0.05 was considered to indicate a statistically significant difference.
Results
IL-10 and IL-18 concentrations at different time-points after treatment in both groups
No significant difference was observed in the concentration of IL-10 between the two groups at 0 h (P>0.05), and IL-10 in the groups decreased gradually after treatment, but the concentration of IL-10 in the experimental group was lower than that of the control group at days 1, 2, 3 and 5 (P<0.05). There was no significant difference in the concentration of IL-18 between the two groups at 0 h (P>0.05). The concentration of IL-18 in the experimental group began to decrease at day 1, and was significantly lower than that in the control group at days 2, 3 and 5, the difference was statistically significant (P<0.05). The concentration of IL-18 in the control group gradually increased at days 1, 2 and 3, and peaked at day 3, then gradually decreased, and the concentration of IL-18 at day 5 was significantly lower than that at 0 h and days 1, 2 and 3, the difference was statistically significant (P<0.05) (Tables II and III).
Table II.
Changes in IL-10 concentration at different time-points (pg/ml).
| Time points | Experimental group (n=54) | Control group (n=43) | t value | P-value |
|---|---|---|---|---|
| 0 h | 316.53±45.67 | 317.19±46.23 | 0.070 | 0.944 |
| 1 day | 221.43±29.36 | 305.21±31.67 | 13.48 | <0.001 |
| 2 days | 185.42±26.18 | 275.19±29.33 | 15.90 | <0.001 |
| 3 days | 143.31±16.79 | 246.27±25.82 | 23.69 | <0.001 |
| 5 days | 132.11±13.47 | 193.31±21.35 | 17.21 | <0.001 |
| F value | 373.2 | 130.5 | – | – |
| P-value | <0.001 | <0.001 | – | – |
IL, interleukin.
Table III.
Changes in IL-18 concentration at different time-points (pg/ml).
| Time points | Experimental group (n=54) | Control group (n=43) | t value | P-value |
|---|---|---|---|---|
| 0 h | 117.45±21.36 | 118.21±22.42 | 0.170 | 0.865 |
| 1 day | 126.39±23.11 | 131.65±27.35 | 1.026 | 0.307 |
| 2 days | 81.54±18.23 | 154.49±33.79 | 13.96 | <0.001 |
| 3 days | 69.51±24.35 | 181.47±42.36 | 16.84 | <0.001 |
| 5 days | 61.19±15.72 | 78.40±18.95 | 4.889 | <0.001 |
| F value | 105.9 | 123.8 | – | – |
| P-value | <0.001 | <0.001 | – | – |
IL, interleukin.
NBNA scores of the two groups at different time-points after birth
No significant difference in NBNA scores was observed between the two groups at 7 days (P>0.05). With the prolongation of birth time, the NBNA score of children increased gradually, but the NBNA scores of the experimental group were significantly higher than those of the control group at 14 and 28 days after birth, and the difference was statistically significant (P<0.05) (Table IV).
Table IV.
NBNA scores of two groups at different time-points after birth.
| Time points | Experimental group (n=54) | Control group (n=43) | t value | P-value |
|---|---|---|---|---|
| 7 days | 32.94±1.21 | 33.02±1.19 | 0.326 | 0.745 |
| 14 days | 38.14±1.33 | 34.95±1.31 | 11.81 | <0.001 |
| 28 days | 39.57±1.29 | 36.11±1.13 | 13.85 | <0.001 |
| F value | 402.7 | 71.28 | – | – |
| P-value | <0.001 | <0.001 | – | – |
Correlation between severity of disease and expression of IL-10 and IL-18 in the two groups
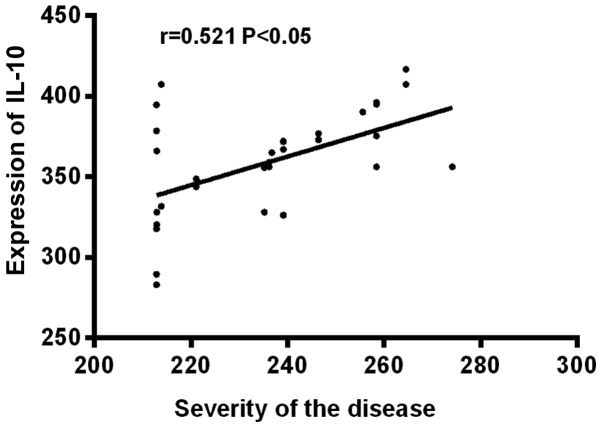
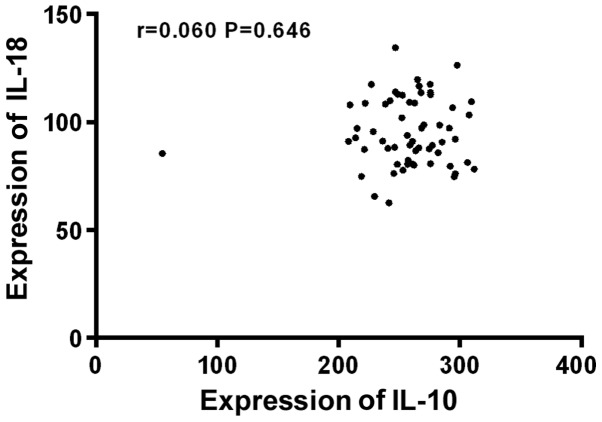
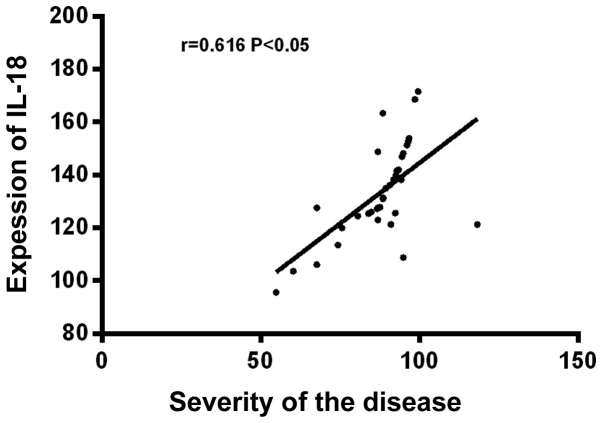
Before treatment, the expression levels of IL-10 and IL-18 were significantly higher in severe children than in moderate children, the difference was statistically significant (P<0.05). IL-10 was positively correlated with the severity of the disease (r=0.521, P<0.05), and IL-18 was also positively correlated with the severity of the disease (r=0.616, P<0.05), but there was no significant correlation between IL-10 and IL-18 (P>0.05) (Table V and Figs. 1–3).
Table V.
Expression levels of serum IL-10 and IL-18 in children with different severity before treatment.
| Factors | Moderate (n=61) | Severe (n=36) | t value | P-value |
|---|---|---|---|---|
| IL-10 | 256.15±33.92 | 357.23±23.65 | 15.75 | <0.001 |
| IL-18 | 95.23±14.49 | 129.55±19.73 | 9.829 | <0.001 |
IL, interleukin.
Figure 1.
Correlation analysis between severity of disease and expression of IL-10 in serum. IL-10 was positively correlated with the severity of HIE (r=0.521, P<0.05). IL, interleukin; HIE, hypoxic-ischemic encephalopathy.
Figure 3.
Correlation analysis between IL-10 and IL-18 in serum of children with HIE. There was no significant correlation between IL-10 and IL-18 (P>0.05). IL, interleukin; HIE, hypoxic-ischemic encephalopathy.
Discussion
HIE is a neonatal brain injury disease characterized by changes in muscle tone or hypotension, convulsions and other clinical symptoms, and serious illness can lead to death in children (11). As one of the most effective methods for the treatment of HIE, sub-hypothermia treatment reduces the temperature of the body by 2–5°C, thereby reducing the level of adenosine triphosphate (ATP) in the brain cells and reducing lactic acid accumulation, which in turn reduces the levels of excitatory neurotransmitters, inflammatory mediators and other cytotoxins, so as to protect the nerves (12). Studies have shown that the inflammatory response plays a very important role in the pathological process of HIE (13).
In our study, levels of IL-10 and IL-18 at different time-points after treatment were first tested. The results showed no significant difference in concentration of IL-10 between the two groups at 0 h (P>0.05), but the concentration of IL-10 in the experimental group was lower than that of the control group at 1, 2, 3 and 5 days (P<0.05). The concentration of IL-18 in the experimental group began to decrease at 1 day, and the concentration of IL-18 in the experimental group at the 2, 3 and 5 days was significantly lower than that in the control group (P<0.05). The concentration of IL-18 in the control group gradually increased at 1, 2 and 3 days, and the peak was reached at 3 days, then gradually decreased, and the concentration of IL-18 at 5 days was significantly lower than that at 0 h and 1, 2 and 3 days (P<0.05). The data suggest that hypothermia treatment can inhibit the inflammatory response in children with HIE. Studies have shown that IL-18 is an inflammatory factor with diverse biological activities, it is not only involved in the body's immune response, but also participates in the development of anti-allergic diseases (14). Some studies have revealed that IL-18 is elevated at 12 h after cerebral hypoxia-reperfusion injury in neonatal mice, which is due to the body's immune response to ischemia and hypoxia injury (15). Some studies (16) have shown that hypothermia treatment can inhibit the excessive initiation of IL-18 after trauma in children with HIE, and regulate the inflammatory response of the body, thus avoiding further aggravation of brain damage, which is consistent with some of our results. However, to the best of our knowledge, there is no relevant study on the effect of hypothermia therapy on the expression of serum IL-10 in children with HIE, but some studies (17) indicate that IL-10 is highly expressed in HIE rats, which is also consistent with our data.
NBNA score is a sensitive indicator of early brain injury in children with HIE (18), so we also compared the NBNA scores at different time-points after birth in the two groups. No significant difference in NBNA scores was observed between the two groups at 7 days (P>0.05). With the prolongation of birth time, the NBNA score of children increased gradually, but NBNA scores were significantly higher in the experimental group than in the control group at 14 and 28 days after birth (P<0.05). These data suggest that hypothermia therapy can effectively protect the child's nerves and promote brain function recovery in children. Some studies (19) have also shown that the NBNA score of the treatment group was significantly higher than that of the control group when investigating the efficacy of hypothermia therapy in the treatment of children with HIE, which also confirmed our conclusion. Finally, we also analyzed the correlation between the severity of the disease and the expression of IL-10 and IL-18 in the two groups. It was observed that expression levels of IL-10 and IL-18 were significantly higher in severe children than in moderate children before treatment (P<0.05). IL-10 and IL-18 were both positively correlated with the severity of the disease. These data indicate that IL-10 and IL-18 gradually increase with the severity of HIE, suggesting that IL-10 and IL-18 can be used as indicators of early clinical judgement of the severity of HIE children. Previous studies (20) have shown that serum IL-10 levels increase with the severity of HIE. A previous study (16) also indicated that the expression level of IL-18 in the serum of children with moderate to severe HIE was significantly higher than that of children with mild HIE. The above studies were consistent with our conclusions.
In summary, mild hypothermia treatment can effectively improve the neurological function of children with HIE, reduce the expression of IL-10 and IL-18 in the serum of children, and inhibit inflammatory response. The expression of IL-10 and IL-18 was positively correlated with the severity of the disease in children with HIE, and could be used as a clinical indicator for judging the severity of HIE children.
Figure 2.
Correlation analysis between severity of disease and expression of IL-18 in serum. IL-18 was positively correlated with the severity of HIE (r=0.616, P<0.05). IL, interleukin; HIE, hypoxic-ischemic encephalopathy.
Acknowledgements
Not applicable.
Funding
This study was supported by 2016 Jiangsu Province Maternal and Child Health Scientific Research Project (no. F201610).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
HW wrote the manuscript. HW, GL, XY and QL performed the research. GL collected and analyzed the data of this study. ZL was responsible for ELISA. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou, China). Patients who participated in this research had complete clinical data. Parents of the child patients signed an informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zalewska T, Jaworska J, Ziemka-Nalecz M. Current and experimental pharmacological approaches in neonatal hypoxic- ischemic encephalopathy. Curr Pharm Des. 2015;21:1433–1439. doi: 10.2174/1381612820999141029162457. [DOI] [PubMed] [Google Scholar]
- 2.Wang KY, Singer HS, Crain B, Gujar S, Lin DD. Hypoxic-ischemic encephalopathy mimicking acute necrotizing encephalopathy. Pediatr Neurol. 2015;52:110–114. doi: 10.1016/j.pediatrneurol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, Cilio MR. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–562. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Liaison Committee on Resuscitation, corp-author. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 2: Adult basic life support. Resuscitation. 2005;67:187–201. doi: 10.1016/j.resuscitation.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, Parikh NA, Ambalavanan N, Pedroza C, Pappas A, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: A randomized clinical trial. JAMA. 2017;318:1550–1560. doi: 10.1001/jama.2017.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai JCY, Rocha-Ferreira E, Ek CJ, Wang X, Hagberg H, Mallard C. Immune responses in perinatal brain injury. Brain Behav Immun. 2017;63:210–223. doi: 10.1016/j.bbi.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, Li YY. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci. 2014;18:905–909. [PubMed] [Google Scholar]
- 8.Tsutsumi N, Kimura T, Arita K, Ariyoshi M, Ohnishi H, Yamamoto T, Zuo X, Maenaka K, Park EY, Kondo N, et al. The structural basis for receptor recognition of human interleukin-18. Nat Commun. 2014;5:5340. doi: 10.1038/ncomms6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galasso JM, Liu Y, Szaflarski J, Warren JS, Silverstein FS. Monocyte chemoattractant protein-1 is a mediator of acute excitotoxic injury in neonatal rat brain. Neuroscience. 2000;101:737–744. doi: 10.1016/S0306-4522(00)00399-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Zheng CX, Peng SF, Zhou HY, Su ZY, He L, Ai T. Evaluation of urinary S100B protein level and lactate/creatinine ratio for early diagnosis and prognostic prediction of neonatal hypoxic-ischemic encephalopathy. Neonatology. 2010;97:41–44. doi: 10.1159/000227292. [DOI] [PubMed] [Google Scholar]
- 11.Nespeca M, Giorgetti C, Nobile S, Ferrini I, Simonato M, Verlato G, Cogo P, Carnielli VP. Does whole-body hypothermia in neonates with hypoxic-ischemic encephalopathy affect surfactant disaturated-phosphatidylcholine kinetics? PLoS One. 2016;11:e0153328. doi: 10.1371/journal.pone.0153328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao W, Xu H, Ding J, Huang H. Mild hypothermia therapy for moderate or severe hypoxicischemic encephalopathy in neonates. Iran J Public Health. 2018;47:64–69. [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Y, He M, Zhou X, Liu J, Hou N, Bin T, Zhang Y, Li T, Chen J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci Rep. 2016;6:18587. doi: 10.1038/srep18587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka H, Narita M, Teramoto S, Saikai T, Oashi K, Igarashi T, Abe S. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121:1493–1497. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 15.Hedtjärn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CQ, Xia YF, Yuan YX, Li L, Qiu XL. Effects of selective head cooling with mild hypothermia on serum levels of caspase-3 and IL-18 in neonates with hypoxic-ischemic encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:690–692. (In Chinese) [PubMed] [Google Scholar]
- 17.Youn YA, Kim SJ, Sung IK, Chung SY, Kim YH, Lee IG. Serial examination of serum IL-8, IL-10 and IL-1Ra levels is significant in neonatal seizures induced by hypoxic-ischaemic encephalopathy. Scand J Immunol. 2012;76:286–293. doi: 10.1111/j.1365-3083.2012.02710.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu XD, Zhang J, Yan CH, Shen XM. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–238. doi: 10.1016/j.envres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Li Z, Yang X, Liu J, Wang W, Liu G. SBDPs and Tau proteins for diagnosis and hypothermia therapy in neonatal hypoxic ischemic encephalopathy. Exp Ther Med. 2017;13:225–229. doi: 10.3892/etm.2016.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Mazary AA, Nasif KA, Abdel-Hakeem GL, Sherif T, Farouk E, El-Gezawy EM. Adiponectin, leptin and insulin levels at birth and in early postnatal life in neonates with hypoxic ischemic encephalopathy. J Diabetes Metab Disord. 2015;14:87. doi: 10.1186/s40200-015-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.