Abstract
Wnt3a is a crucial modulator of bone metabolism through the canonical Wnt/β-catenin signaling pathway in bone-forming osteoblasts. We previously reported that the expression of osteocalcin is stimulated by triiodothyronine (T3) at least in part through the activation of p38 mitogen-activated protein (MAP) kinase but not p44/p42 MAP kinase in osteoblast-like MC3T3-E1 cells. In the present study, we investigated the effect of Wnt3a on the T3-induced osteocalcin expression in these cells. Wnt3a suppressed the release of osteocalcin induced by T3. The inhibitory effect of Wnt3a was dose-dependent between 0.3 and 30 ng/ml. SB216763, an inhibitor of glycogen synthase kinase-3β, that reduces the phosphorylation of β-catenin, inhibited the T3-induced osteocalcin release. Wnt3a, as well as SB216763, reduced the expression of osteocalcin mRNA induced by T3. The transcriptional activity induced by T3, assessed by a luciferase activity, was also suppressed by both Wnt3a and SB216763. In contrast, Wnt3a did not markedly affect the T3-stimulated phosphorylation of p38 MAP kinase. These results suggested that Wnt3a downregulates the T3-stimulated osteocalcin expression in MC3T3-E1 cells, and the suppressive effect of Wnt3a is independent of p38 MAP kinase.
Keywords: Wnt3a, triiodothyronine, osteocalcin, osteoblast
Introduction
Bone remodeling, an essential and strictly regulated process for maintaining the mass and the strength of skeletal tissue in adults, is known to be initiated by osteoclastic bone resorption, followed by osteoblastic bone formation (1). In bone formation stage, the Wnt/β-catenin signaling pathway, called the canonical Wnt signaling pathway, plays a crucial role in the expression of target genes, including Runx2, a pivotal element of bone formation (2). Wnts form a complex by binding to Frizzled receptors and low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6), which leads to inactivation via the phosphorylation of glycogen synthase kinase-3β (GSK-3β), resulting in the stabilization of β-catenin and promoting its translocation into the nucleus (3). Nuclear β-catenin up-regulates the transcription of target genes, including Runx2 (2). It Constitutively-activated β-catenin is known to potently induce the up-regulation of Runx2, resulting in osteogenesis (2). Thus, the canonical Wnt signaling pathway is an essential positive regulator of osteogenesis in mammals.
Regarding osteoblast functions, we previously reported that Wnt3a upregulates the synthesis of vascular endothelial growth factor stimulated by fibroblast growth factor-2 (FGF-2), transforming growth factor-β (TGF-β) and prostaglandin F2α (PGF2α) but downregulates the interleukin-6 (IL-6) synthesis stimulated by tumor necrosis factor-α (TNF-α) in osteoblast-like MC3T3-E1 cells (4–7).
Osteocalcin is a highly differentiated osteoblast secretary phenotype and the most abundant non-collagenous protein (8). Osteocalcin is post-translationally modified by vitamin K-dependent γ-carboxylation, which is known to be a bone Gla-protein (8). An increase in bone formation is reportedly observed in osteocalcin-deficient mice without impaired bone resorption (9). Thus, osteoblast-producing osteocalcin is considered to play a pivotal role in the regulation of bone formation. In addition, uncarboxylated osteocalcin reportedly regulates energy metabolism by stimulating the secretion of insulin from β cells of pancreatic islets and up-regulating the insulin sensitivity of target organs (10). Thus, bone may function as an endocrine organ through osteocalcin release.
Thyroid hormone plays an essential role as a regulator of bone metabolism as well as whole-body metabolism. An excess of thyroid hormone, namely hyperthyroidism, is well known to boost the bone metabolic turnover and increase the ratio of bone resorption to bone formation, leading to osteoporosis and subsequently increased risk of bone fracture (11). The bone mineral density actually decreases in patients with hyperthyroidism (12). Thyroid hormone receptor is a member of the nuclear receptor superfamily (13), and its actions are chiefly mediated through its binding to specific nuclear receptors, with the complex of receptor and hormone complex activating the transcription of corresponding genes (14). In osteoblasts, thyroid hormone induces alkaline phosphatase and modulates cell proliferation (15). We previously showed that triiodothyronine (T3) elicits the expression of osteocalcin via p38 mitogen-activated protein (MAP) kinase at least in part, and the adenylyl cyclase-cAMP system regulates the osteocalcin expression via the suppression of p38 MAP kinase activation in osteoblast-like MC3T3-E1 cells (16,17). However, the exact mechanism behind thyroid hormone-induced expression of osteocalcin including a role of Wnt signaling remains to be elucidated.
In the present study, we investigated the effect of Wnt3a on the T3-stimulated osteocalcin expression in osteoblast-like MC3T3-E1 cells.
Materials and methods
Materials
T3 and SB216763 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Wnt3a was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The mouse osteocalcin enzyme-linked immunosorbent assay (ELISA) kit was purchased from Alfa Aesar, Thermo Fisher Scientific (Heysham, Lancashire, UK). Antibodies against phospho-specific β-catenin, β-catenin, phospho-specific p38 MAP kinase and p38 MAP kinase were purchased from Cell Signaling Technology, Inc., (Biverly, MA, USA). Antibodies against GAPDH were purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA, USA). Other materials and chemicals were purchased from commercial sources. T3 was dissolved in 0.1 M NaOH, and SB216763 was dissolved in DMSO. The maximum concentration of NaOH or SB216763 was 10 µM or 0.1%, respectively, which did not affect the assay for osteocalcin, reverse transcription-polymerase chain reaction (RT-PCR) or western blotting.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from newborn mouse calvaria (18) were maintained as previously described (19). In brief, the cells were cultured in α-minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were seeded into 35-mm diameter dishes (5×104 cells/dish) or 90-mm diameter dishes (2×105 cells/dish) in α-MEM containing 10% FBS. After 5 days, the medium was exchanged for α-MEM containing 0.3% FBS. The cells were used for experiments following a 48 h incubation period at 37°C.
Assay for osteocalcin
The cultured cells were stimulated by 10 nM of T3 or vehicle in 1 ml of α-MEM containing 0.3% FBS for the indicated periods. When indicated, the cells were pretreated with various dose of Wnt3a or SB216763 for 60 min. The conditioned medium was collected at the end of incubation, and the osteocalcin concentration in the medium was then measured using the mouse osteocalcin ELISA kit according to the manufacturer's protocol.
RT-quantitative PCR (RT-qPCR)
The cultured cells were pretreated with 30 ng/ml of Wnt3a, 10 µM of SB216763 or vehicle for 60 min, and then stimulated by 10 nM of T3 or vehicle in α-MEM containing 0.3% FBS for 48 h. Total RNA was isolated and reverse transcribed into complementary DNA using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and Omniscript Reverse Transcriptase kit (Qiagen Inc., Valencia, CA, USA), respectively. RT-qPCR was performed in capillaries using a LightCycler system with the LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics, Basel, Switzerland). Sense and antisense primers were synthesized based on the reports of Zhang et al (20) for mouse osteocalcin and Simpson et al for mouse GAPDH (21). The amplified products were determined using a melting curve analysis according to the system protocol. The Bglap2 mRNA levels were normalized to those of GAPDH mRNA.
Luciferase reporter assay
A reporter plasmid, pDR4 (thyroid hormone response element)-Luc was purchased from Stratagene (La Jolla, CA, USA). The cultured cells were pretreated with 30 ng/ml of Wnt3a, 10 µM of SB216763 or vehicle at 6 h after transfection with the pDR4-Luc reporter plasmid (1 µg/dish) using UniFector transfection reagent (B-Bridge International, Mountain View, CA, USA). After pretreatment (60 min) with Wnt3a, the cells were stimulated by 10 nM of T3 or vehicle for 48 h. Samples were lysed using passive lysis buffer (Promega Corp., Madison, WI, USA) and collected with a cell scraper. The luciferase activity of the cell lysates was measured using a dual luciferase reporter assay system (Promega Corp., Madison, WI, USA) according to the manufacture's protocol. The cells were cotransfected with pRL-CMV (Renilla luciferase; 0.1 µg/dish) as an internal standard to normalize transfection efficiency.
Western blot analysis
Western blotting was performed as previously described (22). In brief, the cultured cells were treated with various doses of SB216763 for 120 min or pretreated with various doses of Wnt3a for 60 min. The Wnt3a-pretreated cells were then stimulated by 10 nM of T3 in α-MEM containing 0.3% FBS for 2 h. The cells were washed twice with phosphate-buffered saline and then lysed, homogenized and sonicated in a lysis buffer containing 62.5 mM Tris-HCL, pH6.8, 3% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol, and 10% glycerol. SDS-polyacrylamide gel electrophoresis (PAGE) was performed according to Laemmli (23) on 10% polyacrylamide gel. The protein was fractionated and transferred onto an Immun-Blot PVDF membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% fat-free dry milk in Tris-buffered saline-Tween (TBS-T; 20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) for 1 h before incubation with primary antibodies. Phospho-specific β-catenin antibodies, β-catenin antibodies, phospho-specific p38 MAP kinase antibodies, p38 MAP kinase antibodies and GAPDH antibodies were used as primary antibodies, and peroxidase-labeled antibodies raised in goat against rabbit IgG (KPL, Inc., Gaitherburg, MD, USA) were used as secondary antibodies. The primary and secondary antibodies were diluted at 1:1,000 with 5% fat-free dry milk in TBS-T. The peroxidase activity on the PVDF sheet was visualized on X-ray film by an ECL Western blotting detection system.
Statistical analysis
The data were analyzed by an analysis of variance followed by Bonferroni's method for multiple comparisons between pairs, and P<0.05 was considered to indicate a statistically significant difference. All data are presented as the mean ± standard error of the mean of triplicate determinations from three independent cell preparations.
Results
Effects of Wnt3a and SB216763 on the T3-stimulated osteocalcin release in MC3T3-E1 cells
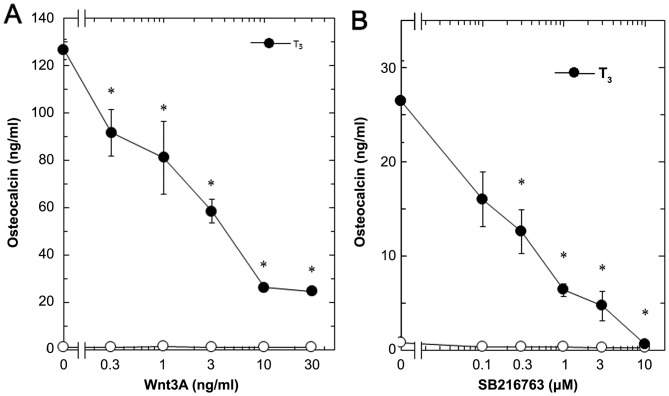
In our previous study (16), we showed that T3-stimulated osteocalcin release could be detected after 48 to 96 h of stimulation in osteoblast-like MC3T3-E1 cells. We therefore first examined the effect of Wnt3a on the release of osteocalcin stimulated by T3 in these cells. Regarding the working concentrations of Wnt3a, we previously reported that Wnt3a at a dose of 10 to 30 ng/ml caused a remarkable amplification in the VEGF synthesis induced by FGF-2, TGF-β and PGF2α in osteoblast-like MC3T3-E1 cells (4–6).
Wnt3a by itself did not affect the basal levels of osteocalcin, but significantly reduced the release of osteocalcin stimulated by T3 (Fig. 1). The suppressive effect of Wnt3a on the T3-stimulated osteocalcin release was dose-dependent in between 0.3 and 30 ng/ml (Fig. 2A). The maximum inhibitory effect of Wnt3a observed at 30 ng/ml was approximately 80% in the T3-effect of osteocalcin release in these cells. The effects of Wnt3a at doses of 10 and 30 ng/ml on the T3-induced release of osteocalcin were similar. It is unlikely that we would have been able to obtain a further inhibitory effect of Wnt3a at a dose higher than 30 ng/ml.
Figure 1.
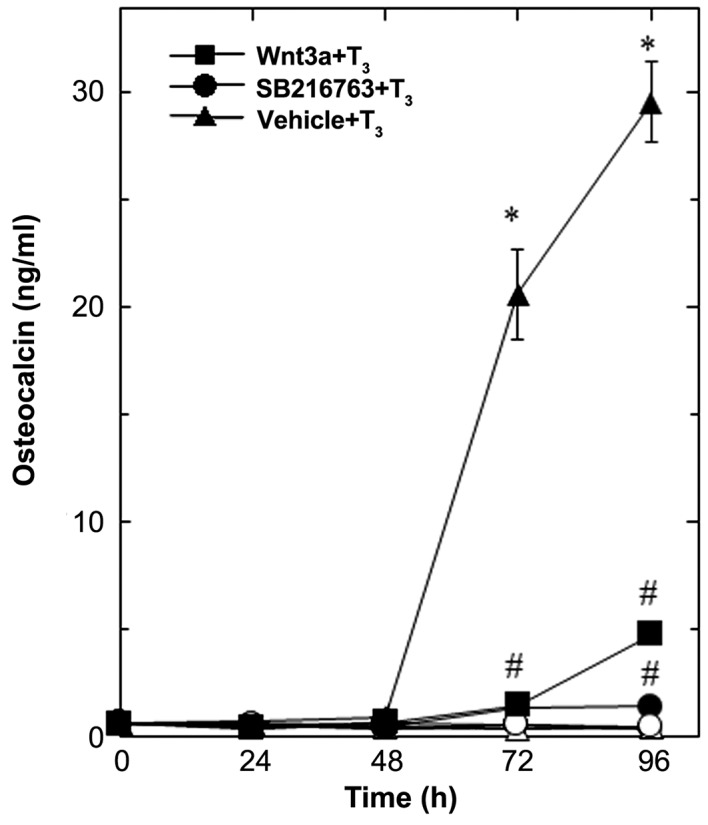
Effects of Wnt3a on the T3-stimulated osteocalcin release in MC3T3-E1 cells. The cultured cells were pretreated with 30 ng/ml of Wnt3a (■,□), 10 µM of SB216763 (•, ◦) or vehicle (▲,∆) for 60 min, and subsequently stimulated with 10 nM of T3 (■,•,▲) or vehicle (□,◦,∆) for the indicated periods. Osteocalcin concentrations in the culture medium were determined by an ELISA. Each value represents the mean ± SEM of triplicate determinations from three independent cell preparations. Each line binding the closed boxes (■) and the closed circles (•) are the experimental groups indicating Wnt3a or SB216763, respectively, relative to the control line (T3 alone) binding the closed triangles (▲) *P<0.05 vs. the value of the control; #P<0.05 vs. the value of T3 alone. T3, triiodothyronine.
Figure 2.
Dose-dependent effects of Wnt3a and SB216763 on the T3-stimulated osteocalcin release in MC3T3-E1 cells. The cultured cells were pretreated with various doses of (A) Wnt3a or (B) SB216763 for 60 min, and subsequently stimulated by 10 nM of T3 (•) or vehicle (◦) for 96 h. Each line binding the closed circles (•) are the experimental groups indicating (A) Wnt3a or (B) SB216763. Osteocalcin concentrations in the culture medium were determined by an ELISA. Each value represents the mean ± SEM of triplicate determinations from three independent cell preparations. *P<0.05 vs. the value of T3 alone. T3, triiodothyronine.
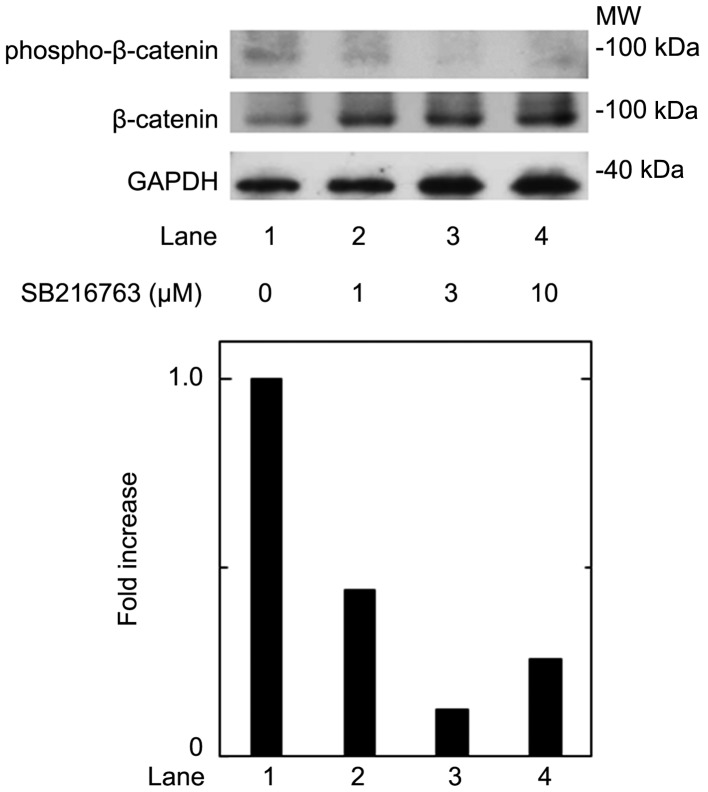
Wnt3a stimulation has been established to induce the inactivation of GSK-3β, resulting in the stabilization of β-catenin and the promotion of its translocation into the nucleus, called the canonical Wnt pathway (3). To elucidate the effect of GSK-3β inhibition, we examined the effect of SB216763, a selective inhibitor of GSK-3β (24), on the T3-stimulated osteocalcin release in osteoblast-like MC3T3-E1 cells. As for the working concentrations of SB216763, we previously reported that SB216763 at a dose of 10 µM remarkably enhanced the VEGF synthesis stimulated by FGF-2, TGF-β and PGF2α in these cells (4–6). SB216763, which by itself hardly affected the release of osteocalcin, significantly inhibited the T3-stimulated release of osteocalcin as well as Wnt (Fig. 1). The inhibitory effect of SB216763 was dose-dependent between 0.1 µM and 10 µM (Fig. 2B). SB216763 at 10 µM almost completely inhibited the release of osteocalcin stimulated by T3 in these cells. We confirmed that SB216763 truly reduced the phosphorylation of β-catenin, the functional substrate of GSK-3β (3), in a dose-dependent manner between 1 and 10 µM (Fig. 3). We also confirmed that neither Wnt3a nor SB216763 affected the cell numbers of MC3T3-E1 cells with or without T3 up to 96 h (data not shown).
Figure 3.
Effects of SB216763 on the phosphorylation of β-catenin in MC3T3-E1 cells. The cultured cells were treated with various doses of SB216763 for 120 min. The cell extracts were subjected to SDS-PAGE with subsequent western blotting with antibodies against phospho-specific β-catenin, β-catenin or GAPDH. The histogram shows the quantitative representations of the levels of phosphorylated-β-catenin after normalization with respect to GAPDH obtained from a laser densitometric analysis. The levels were expressed as the fold increase to the basal levels presented as lane 1. The representative result of triplicate independent experiments are presented.
Effects of Wnt3a and SB216763 on the T3-induced expression of Bglap2 mRNA in MC3T3-E1 cells
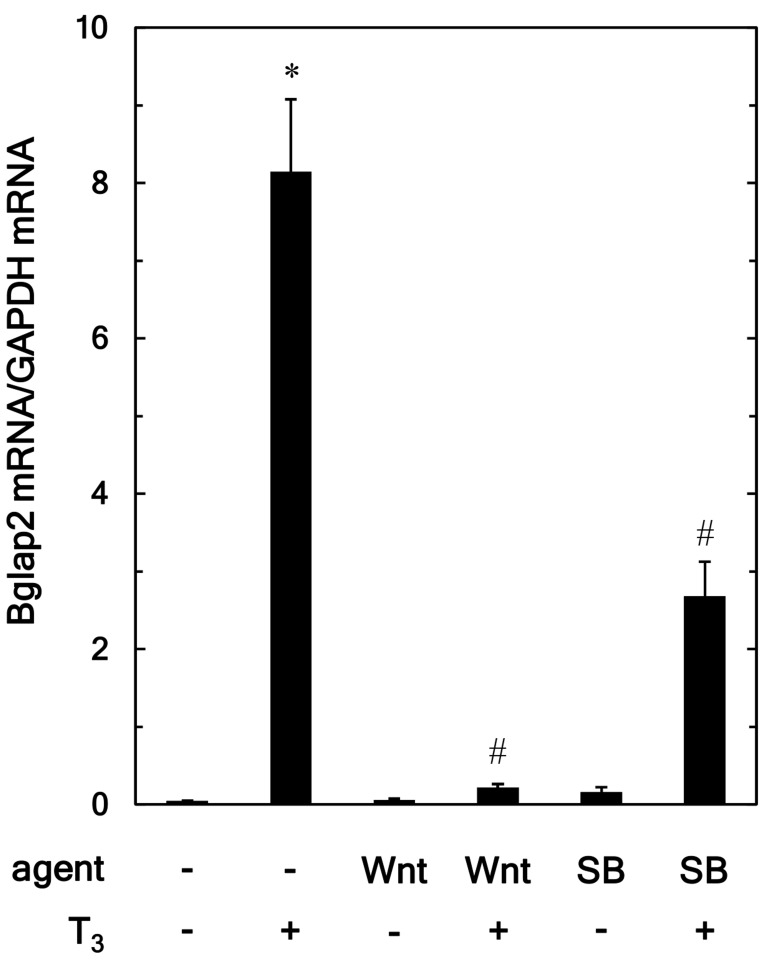
To elucidate whether the suppressive effect of Wnt3a on the osteocalcin release stimulated by T3 was elicited through transcriptional events, we examined the effect of Wnt3a on the Bglap2 mRNA expression induced by T3, using RT-qPCR. Wnt3A significantly attenuated the expression of Bglap2 mRNA induced by T3, whereas, Wnt3a by itself had little effect on the expression levels (Fig. 4). SB216763, which alone had little effect on the levels of Bglap2 mRNA, also suppressed the T3-stimulated upregulation of Bglap2 mRNA expression (Fig. 4).
Figure 4.
Effects of Wnt3a and SB on the T3-induced expression of Bglap2 mRNA in MC3T3-E1 cells. The cultured cells were pretreated with 30 ng/ml of Wnt3a, 10 µM of SB or vehicle for 60 min and subsequently stimulated by 10 nM of T3 or vehicle for 48 h. The respective total RNA was subsequently isolated and transcribed into cDNA. The expression of Bglap2mRNA and GAPDH mRNA were quantified by reverse transcription-quantitative PCR. The Bglap2 mRNA levels were normalized to those of GAPDH mRNA. Each value represents the mean ± SEM of triplicate determinations from three independent cell preparations. *P<0.05 vs. the value of the control; #P<0.05 vs. the value of T3 alone. SB, SB216763; T3, triiodothyronine.
Effects of Wnt3a and SB216763 on the T3-stimulated transactivation activity of the thyroid hormone-responsive element in MC3T3-E1 cells
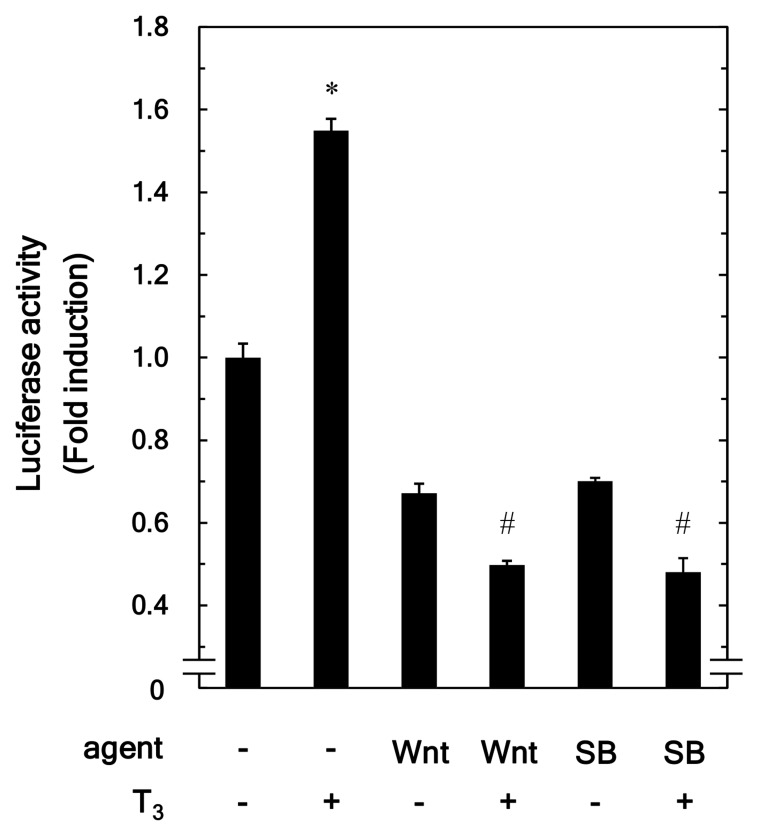
Using a luciferase reporter assay, we further examined the effect of Wnt3a on the T3-stimulated transactivation of thyroid hormone response element in osteoblast-like MC3T3-E1 cells. Wnt3a significantly reduced the T3-upregulated transactivation activity (Fig. 5). In addition, SB216763 markedly suppressed the transactivation activity stimulated by T3 (Fig. 5).
Figure 5.
Effects of Wnt3a and SB on the T3-stimulated transactivation activity of the thyroid hormone response element in MC3T3-E1 cells. The cultured cells were pretreated with 30 ng/ml of Wnt3a, 10 µM of SB or vehicle for 60 min at 6 h after the transfection with the pDR4-Luc reporter plasmid (1 µg/dish). The cells were then stimulated by 10 nM of T3 or vehicle for 48 h. The measurement of the luciferase activity of the cell lysates were performed using a dual luciferase reporter assay system. Each value represents the mean ± SEM of triplicate determinations from three independent cell preparations. *P<0.05 vs. the value of the control; #P<0.05 vs. the value of T3 alone. SB, SB216763; T3, triiodothyronine.
Effect of Wnt3a on the T3-stimulated phosphorylation of p38 MAP kinase in MC3T3-E1 cells
We previously reported that p38 MAP kinase is involved in the T3-stiumulated osteocalcin expression in osteoblast-like MC3T3-E1 cells (16). Therefore, we further investigated the effect of Wnt3a on the phosphorylation of p38 MAP kinase stimulated by T3 in these cells. We confirmed that T3 significantly upregulated p38 MAP kinase phosphorylation (Fig. 6, lane 1 vs. lane 2). However, Wnt3a hardly affected the phosphorylation at a dose up to 50 ng/ml (Fig. 6, lane 2 vs. lane 5). Wnt3a at a dose up to 50 ng/ml had little effect on the phosphorylation of p38 MAP kinase induced by T3. Wnt3a likely had little effect on the phosphorylation at a dose up to 50 ng/ml, which was sufficient to suppress the release of osteocalcin induced by T3.
Figure 6.
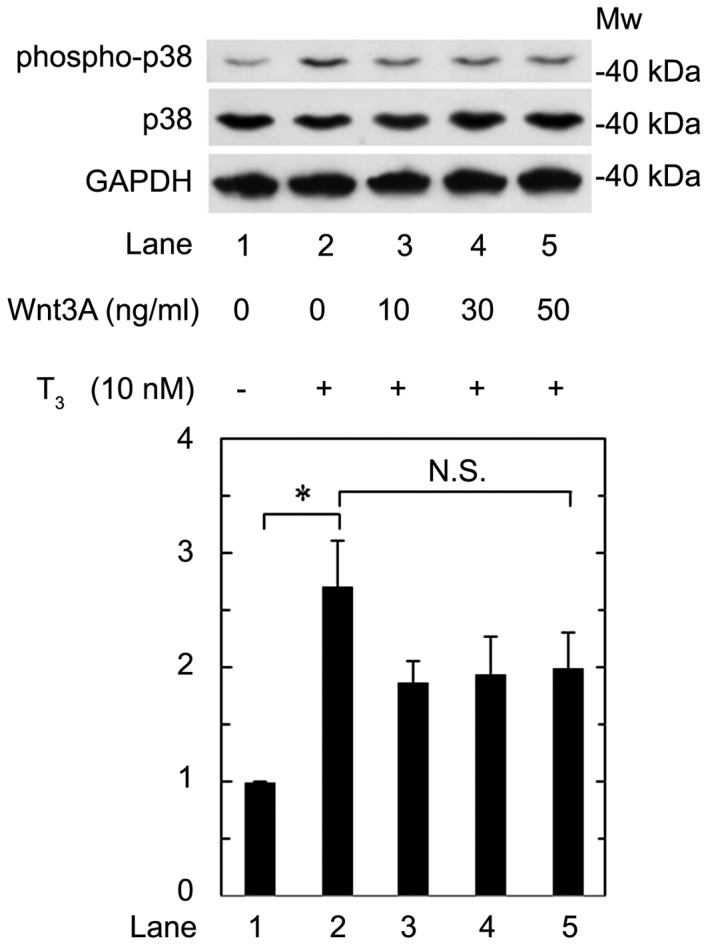
Effect of Wnt3a on the T3-induced phosphorylation of p38 MAP kinase in MC3T3-E1 cells. The cultured cells were pretreated with various doses of Wnt3a for 60 min, and then stimulated by 10 nM of T3 for 2 h. The cell extracts were subjected to SDS-PAGE with subsequent western blotting with antibodies against phospho-specific p38 MAP kinase, p38 MAP kinase or GAPDH. The histogram shows the quantitative representations of the levels of phosphorylated-p38 MAP kinase after normalization with respect to GAPDH obtained from laser densitometric analysis. The levels were expressed as the fold increase to the basal levels presented as lane 1. Each value represents the mean ± SEM of triplicate determinations from three independent cell preparations. *P<0.05. N.S., not significant; MW, molecular weight; T3, triiodothyronine; MAP, mitogen-activated protein.
Discussion
In the present study, we clearly demonstrated that Wnt3a reduced the osteocalcin release stimulated by T3 in osteoblast-like MC3T3-E1 cells. We also showed that Wnt3a suppressed the Bglap2 mRNA expression induced by T3 in these cells. These results suggest that Wnt3a suppressed the gene transcription of osteocalcin stimulated by T3 or potentiate the degradation of Bglap2 mRNA in osteoblasts.
Thyroid hormone is established to belong nuclear receptor superfamily, and its biological functions are mediated through the activation of the transcription of target genes, including osteocalcin, via the binding of hormone and specific nuclear receptor (13). Thus, using a luciferase reporter assay, we examined the effect of Wnt3a on the transactivation activity of thyroid hormone response element stimulated by T3 and found that Wnt3a truly reduced the T3-stimulated transactivation activity in these cells. Therefore, it is probable that Wnt3a downregulates the osteocalcin expression stimulated by T3, and the effect is exerted through gene transcription in osteoblast-like MC3T3-E1 cells. To our knowledge, this is the first report to clearly demonstrate the suppressive effect of Wnt3a on the osteocalcin expression in osteoblast-like cells.
Wnts is well known to comprise a complex of Fizzled receptors and LRP5/6, resulting in the inactivation of GSK-3β and the accumulation of β-catenin, which translocates into the nucleus and upregulates the transcription of target genes, in a process known as the Wnt canonical pathway (2,3). To clarify the suppressive effect of Wnt3a on the osteocalcin expression stimulated by T3, we examined the effects of SB216763, a selective inhibitor of GSK-3β (20), on the osteocalcin release and the Bglap2 mRNA expression stimulated by T3 in osteoblast-like MC3T3-E1 cells. We found that SB216763 actually mimicked the suppressive effects of Wnt3a on both the osteocalcin release and the Bglap2 mRNA expression levels stimulated by T3 in these cells. We confirmed that SB216763 truly reduced the phosphorylation of β-catenin. It is most likely that the effects of SB216763 presented here resulted from the inhibition of GSK-3β activity in these cells. SB216763 appears to exert a small inhibitory effect on the up-regulation of Bglap2 (osteocalcin) mRNA expression induced by T3 stimulation compared with Wnt3a, while suppressing the T3-stimulated transactivation activity of thyroid hormone response element, similar to Wnt3a. The mRNA degradation might be upregulated in Wnt3a-treated cells.
In addition, we confirmed that SB216763 as well as Wnt3a truly inhibited the T3-stimulated transactivation activity of the thyroid hormone response element in osteoblast-like MC3T3-E1 cells. It is likely that the inactivation of GSK-3β and, in turn, the activation of the Wnt canonical pathway caused the downregulation of the osteocalcin expression stimulated by T3 in these cells. Therefore, it is most likely that Wnt3a downregulates the T3-induced osteocalcin expression through the canonical pathway in osteoblast-like cells. Treatment of Wnt3a or SB216763 alone evidently reduced the transactivation activity of the thyroid hormone response element. It is likely that the upregulation of β-catenin resulting from the inhibition of GSK-3β causes deterioration of the transactivation activity of the thyroid hormone response element without T3 in osteoblast-like MC3T3-E1 cells.
Regarding the signaling mechanism of T3 in osteoblasts, we previously showed that p38 MAP kinase is involved, at least in part, in the T3-stimulated osteocalcin expression in osteoblast-like MC3T3-E1 cells (16). We therefore additionally examined the effect of Wnt3a on the phosphorylation of p38 MAP kinase induced by T3; however, this was hardly affected by Wnt3a in these cells. It is unlikely that the suppressive effect of Wnt3a on the osteocalcin expression stimulated by T3 is mediated through the regulation of p38 MAP kinase in osteoblasts. Taking our present findings into account as a whole, it is most likely that Wnt3a downregulates the T3-induced osteocalcin expression through the canonical pathway without affecting p38 MAP kinase in osteoblast-like cells.
The Wnt/β-catenin signaling pathway is known to play a role in osteogenesis (2). We previously reported that Wnt3a upregulates the syntheses of vascular endothelial growth factor stimulated by FGF-2, TGF-β and PGF2α, but downregulates the IL-6 synthesis stimulated by TNF-α in osteoblast-like MC3T3-E1 cells (4–7). These findings prompted us to speculate that Wnt3a exerts pleiotropic effects on the osteoblastic functions, leading to the promotion of osteogenesis. However, it is widely accepted that osteocalcin, a mature osteoblast phenotype, is specifically produced by these cells and placed in the bone matrix during the process of bone formation (3). The Gla residues in osteocalcin are essential for its function, and Gla-carboxylated osteocalcin becomes attached to hydroxyapatite with a high affinity and thereby supports calcification (3). In addition, the bone mass and strengty in osteocalcin-deficient mice have been reported to be more highly expressed than in wild-type mice, namely, osteocalcin has been reported to play a regulatory role in bone formation (4). T3 definitely plays a pivotal role in physiological bone metabolism. Therefore, our present findings showing the downregulation by Wnt3a of T3-induced osteocalcin in osteoblast-like cells may suggest that Wnt3a promotes osteogenesis through relief of the regulation of bone formation by osteocalcin, at least in part. These findings might also support a novel therapeutic strategy for bone metabolic diseases, such as osteoporosis and distress of bone fracture repair, by modulating the Wnt/β-catenin signaling pathway. Further investigations will be required to clarify the exact roles of Wnt3a in bone metabolism.
In conclusion, our present findings strongly suggest that Wnt3a downregulates T3-induced osteocalcin expression through canonical pathways in osteoblasts without affecting p38 MAP kinase.
Acknowledgements
The authors would like to thank Mrs Yumiko Kurokawa (Department of Pharmacology, Gifu University Graduate School of Medicine) for her skillful technical assistance.
Funding
The present study was supported in part by Grant-in-Aid for Scientific Research (grant nos. 19591042 and 26462289) from the Ministry of Education, Science, Sports and Culture of Japan, the Research Funding for Longevity Sciences (grant no. 28-9, 29-12) from National Center for Geriatrics and Gerontology (Obu, Japan).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KF, TO, OK and HT conceived and designed the experiments. KF, TK, GS, WK and RM-N performed the experiments. KF, TK, GS, RM-N, OK and HT analyzed the data. KF, TO, OK and HT wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/S1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 2.Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- 3.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 4.Tokuda H, Adachi S, Matsushima-Nishiwaki R, Kato K, Natsume H, Otsuka T, Kozawa O. Enhancement of basic fibroblast growth factor-stimulated VEGF synthesis by Wnt3a in osteoblasts. Int J Mol Med. 2011;27:859–864. doi: 10.3892/ijmm.2011.644. [DOI] [PubMed] [Google Scholar]
- 5.Natsume H, Tokuda H, Matsushima-Nishiwaki R, Kato K, Yamakawa K, Otsuka T, Kozawa O. Wnt3a upregulates transforming growth factor-β-stimulated VEGF synthesis in osteoblasts. Cell Biochem Funct. 2011;29:373–377. doi: 10.1002/cbf.1759. [DOI] [PubMed] [Google Scholar]
- 6.Kondo A, Tokuda H, Mizutani J, Matsushima-Nishiwaki R, Kozawa O, Otsuka T. Wnt3a upregulates prostaglandin F2α-stimulated vascular endothelial growth factor synthesis in osteoblasts. Mol Med Rep. 2012;6:421–425. doi: 10.3892/mmr.2012.916. [DOI] [PubMed] [Google Scholar]
- 7.Natsume H, Tokuda H, Adachi S, Matsushima-Nishiwaki R, Kato K, Minamitani C, Otsuka T, Kozawa O. Wnt3a regulates tumor necrosis factor-α-stimulated interleukin-6 release in osteoblasts. Mol Cell Endocrinol. 2011;331:66–72. doi: 10.1016/j.mce.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 9.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 10.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogakos AI, Duncan Bassett JH, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503:129–136. doi: 10.1016/j.abb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk-a meta-analysis. Thyroid. 2003;13:585–593. doi: 10.1089/105072503322238854. [DOI] [PubMed] [Google Scholar]
- 13.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasono K, Sato K, Han DC, Fujii Y, Tsushima T, Shizume K. Stimulation of alkaline phosphatase activity by thyroid hormone in mouse osteoblast-like cells (MC3T3-E1): A possible mechanism of hyperalkaline phosphatasia in hyperthyroidism. Bone Miner. 1988;4:355–363. [PubMed] [Google Scholar]
- 16.Ishisaki A, Tokuda H, Yoshida M, Hirade K, Kunieda K, Hatakeyama D, Shibata T, Kozawa O. Activation of p38 mitogen-activated protein kinase mediates thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Cell Endocrinol. 2004;214:189–195. doi: 10.1016/j.mce.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Kanno Y, Ishisaki A, Yoshida M, Nakajima K, Tokuda H, Numata O, Kozawa O. Adenylyl cyclase-cAMP system inhibits thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Cell Endocrinol. 2005;229:75–82. doi: 10.1016/j.mce.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozawa O, Tokuda H, Miwa M, Kotoyori J, Oiso Y. Cross-talk regulation between cyclic AMP production and phosphoinositide hydrolysis induced by prostaglandin E2 in osteoblast-like cells. Exp Cell Res. 1992;198:130–134. doi: 10.1016/0014-4827(92)90158-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Yang N, Shi XM. Regulation of mesenchymal stem cell osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ) J Biol Chem. 2008;283:4723–4729. doi: 10.1074/jbc.M704147200. [DOI] [PubMed] [Google Scholar]
- 21.Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: A versatile approach to quantitative PCR. Mol Vis. 2000;6:178–183. [PubMed] [Google Scholar]
- 22.Kato K, Ito H, Hasegawa K, Inaguma Y, Kozawa O, Asano T. Modulation of the stress-induced synthesis of hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells. J Neurochem. 1996;66:946–950. doi: 10.1046/j.1471-4159.1996.66030946.x. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/S1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.