Abstract
This study explored the effect of pidotimod combined with azithromycin on children with mycoplasma pneumonia and the expression of interleukin-10 (IL-10) and granulocyte colony-stimulating factor (G-CSF) in serum. The clinical data of 149 children with mycoplasma pneumonia from May 2014 to May 2018 in Zhangqiu District Maternal and Child Health Care Hospital were collected. Among them, 70 children treated with azithromycin sequential therapy were the control group, and 79 children treated with the combination of pidotimod and azithromycin were the observation group. Double antibody sandwich enzyme-linked immunosorbent assay (ELISA) was used to determine the expression levels of IL-10 and G-CSF in serum before and after treatment. Pearson's correlation coefficient was used to analyze the correlation between IL-10 and G-CSF in serum. The total effective rate in the observation group (94.94%) was significantly higher than that in the control group (81.43%) (P<0.05). There was no significant difference in the expression levels of IL-10 and G-CSF between the two groups before treatment (P>0.05). The expression levels of IL-10 and G-CSF in the two groups after treatment were significantly lower than those before treatment (P<0.05). After treatment, the expression levels of IL-10 and G-CSF in serum in the observation group were significantly lower than those in the control group. There was a significant positive correlation between the expression levels of IL-10 and G-CSF before and after treatment in the observation group (P<0.05), and a significant positive correlation between the expression levels of IL-10 and G-CSF before and after treatment in the control group (P<0.05). Compared with sequential treatment with azithromycin alone, pidotimod combined with azithromycin significantly reduced the expression levels of IL-10 and G-CSF in serum of children with mycoplasma pneumonia, improved the curative effect and reduced the occurrence of adverse reactions, which has high application value in clinic.
Keywords: pidotimod, azithromycin, mycoplasma pneumonia in children, IL-10, G-CSF
Introduction
Mycoplasma pneumonia is an atypical bacterial pneumonia that can damage human organs and systems through local respiratory tract infections (1). Mycoplasma pneumonia is one of the most common community-acquired pneumonia (CAP) among children and adolescents (2), accounting for 40% of CAP in children and 18% of hospitalized patients. It has a high infectious rate. Once the patients are infected, the condition becomes more serious and the course of disease also becomes longer. If not treated in time, it may lead to a series of complications and seriously affect the physical and mental health of the children (3). At present, the treatment of this disease is mainly drug therapy (4). Azithromycin is a macrolide antibiotic with good tolerance and long half-life, which can shorten the course of treatment (5). Pidotimod is a new peptide immunomodulator, which, not only promotes the non-specific immune response, but also promotes the specific immune response, acting on the different stages of the immune response (6). It can also activate the immune system to eliminate pathogenic microorganisms (7). At present, there is little research on the combination of the two drugs on the treatment of mycoplasma pneumonia. Although it is now widely used, the mechanism of its action is still unclear.
Interleukin-10 (IL-10) is an important factor involved in airway inflammation in asthma (8). IL-10 is an anti-inflammatory medium that reduces host response through T cells, resists excessive inflammatory responses caused by pro-inflammatory factors, and reduces body damage (9,10). In recent years, it has been shown that IL-10 may play an important role in the occurrence and development of Mycoplasma pneumoniae asthmatic attack (11). Granulocyte colony-stimulating factor (G-CSF) is produced by macrophage, T cell, epithelium and fibroblast activated by IL-1, IL-6 and TNF-α. It is a glycoprotein that plays an important role in anti-infective non-immune cellular immune response and can enhance granulocyte maturation and regulate the differentiation and proliferation of medulla progenitor cells (12). The production of G-CSF is mainly stimulated and regulated by bacteria and endotoxin. When the body is infected, the level of G-CSF is significantly upregulated (13). At present, the effect of pidotimod combined with azithromycin on the expression levels of IL-10 and G-CSF in serum of children with mycoplasma pneumonia needs to be further studied.
In this study, the efficacy of pidotimod combined with azithromycin in the treatment of mycoplasma pneumonia in children and the expression levels of IL-10 and G-CSF in serum before and after treatment were detected in order to provide a more sufficient theoretical basis for the treatment of mycoplasma pneumonia in children.
Patients and methods
General materials
The clinical data of 149 children with mycoplasma pneumonia admitted to Zhangqiu District Maternal and Child Health Care Hospital (Jinan, China) from May 2014 to May 2018 were collected. Among them, 70 children were treated with azithromycin sequential therapy as control group (38 males and 32 females; mean age: 5.86±1.34 years; course of disease: 8.19±1.82 days), and 79 children with pidotimod combined with azithromycin as observation group (44 males and 35 females; mean age: 5.98±1.27 years; course of disease: 8.23±1.79 days).
Inclusion criteria: Patients with complete clinical data and good compliance; patients finally diagnosed by biochemical and imaging examinations; none of the patients had been treated with hormone or immunomodulators.
Exclusion criteria: Patients allergic to drugs used in this study; patients with other respiratory diseases; patients with family history of mental illness; patients with liver disease or abnormal liver functions, who had used other antibiotics in the course of treatment; patients with cardiovascular disease and viral pneumonia, congenital malformations, bacterial pneumonia and tuberculosis.
This study was approved by the Ethics Committee of Zhangqiu District Maternal and Child Health Care Hospital and subjects' experimental contents were described in detail. Patients who participated in this research had complete clinical data. The signed informed consents were obtained from the patients or the guardians.
Treatment method
Patients in both groups were given routine treatment on the basis of medication. Control group: azithromycin sequential therapy was used; intravenous drip of 10 mg·kg−1·day−1 of azithromycin (Jiangsu Jichuan Pharmaceutical Co., Ltd., SFDA approval no. H20030782) for 3–5 days; changed to 10 mg·kg−1·day−1 of oral azithromycin tablet (Zhejiang Asia-Pacific Pharmaceutical Co., Ltd., SFDA approval no. H10980289) after patient's condition was stable. The patients were treated for 3 days and stopped for 4 days, which was a course. The patients were administered the course again for 3 consecutive days, then stopped for four days for the next course of treatment. The patients needed to take 2 to 4 consecutive courses of treatment. Corticosteroids were given to patients with pulmonary dilatation and pulmonary interstitial fibrosis.
Observation group: Pidotimod was given orally on the basis of the control group (Jiangsu Wu Chinese Medicine Group Co., Ltd. Suzhou Pharmaceutical Factory, SFDA approval no. H20030463), 0.4 g/time, twice a day for 2 weeks.
During the course of treatment, the clinical efficacy and adverse reactions of the two groups were closely observed.
Index detection
Fasting venous blood (2 ml) was collected 24 h before treatment and 2 weeks after treatment, and was centrifuged for 10 min with 2,300 × g at 4°C. The supernatant was then collected and placed at −80°C for testing, avoiding repeated freezing and thawing. The expression levels of IL-10 and G-CSF in serum were measured by double antibody sandwich enzyme-linked immunosorbent assay (ELISA). BS-1101 enzyme label analyzer, purchased from Beijing Linmao Technology Co., Ltd., was used. IL-10 and G-CSF ELISA kits were purchased from Shanghai Yuanmu Biotechnology Co., Ltd. (art. no. YM-S0066, YM-S0040). Standard sample (50 µl) was added to the reaction well coated with enzyme. Sample diluent (40 µl) was added to the sample well and then 10 µl of sample to be tested was added (sample dilution multiple ×5). During the procedure, touching the well wall was avoided, followed by gentle agitation. The reaction well was sealed with the sealing plate membrane, and then incubated in a water bath or thermostat at 37°C for 30 min. Then the sealing membrane was carefully opened, the liquid was discarded, drying with water-absorbing paper, and each reaction well was filled with washing fluid, after which the membrane was left to stand for 30 sec. This step was repeated five times and then the membrane was patted dry. Enzyme reagent (50 µl) was added to each well, except the blank well (for the blank control well the same steps as above were carried out, but without enzyme reagent and sample), and incubated at 37°C. After 30 min, the membrane was washed. Substrate A and B (50 µl) was added to each well and the mixture was kept in the dark for 15 min at 37°C. Termination fluid (50 µl) was added to each well and a blank well was used as zero. The optical density value (OD value) of each well was detected at the wavelength of 450 nm in 25 min. The expression levels of IL-10 and G-CSF in serum were then calculated.
Observation indices
Chest X-ray inflammatory absorption time, fever clearance time, disappearance time of cough and pulmonary rales were recorded in the two groups. Therapeutic efficacy and adverse reactions between the two groups were compared. The changes of IL-10 and G-CSF levels in serum before and after treatment in the two groups were observed. The correlation of IL-10 and G-CSF in serum was analyzed.
Therapeutic evaluation criteria (14) were classified as ineffective, markedly effective and cure. Ineffective: after 2 weeks of treatment, chest X-ray was aggravated or did not improve, and the symptoms and signs of the children were aggravated or did not improve. Markedly effective: Within 2 weeks of treatment, chest X-ray showed that most of the absorbed shadows were significantly reduced, and the symptoms and signs of the children were improved. Cure: within 2 weeks, Chest X-ray examination and blood routine examination showed normal, the symptoms and signs of the children returned to normal. Total effective rate = cure rate + markedly effective rate.
Statistical analysis
SPSS20.0 (IBM Corp.) was used for statistical analysis. Enumeration data were expressed as [n (%)]. Chi-square test was used for inter-group comparison. Measurement data were expressed as mean ± SD. Independent sample t-test was used for comparison between groups. Paired t-test was used for intra-group comparison. Pearson's correlation coefficient was used for bivariate normal distribution data. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of clinical data between two groups
The general clinical data of the two groups of children were collected (Table I). There was no significant difference in sex, age, course of disease, average weight, anorexia, severity of illness, leukocytes, platelets, fibrinogen and glutamic-oxaloacetic transaminase between observation and control group (P>0.05).
Table I.
Comparison of clinical data between two groups (mean ± SD)/[n (%)].
| Factors | Observation group (n=79) | Control group (n=70) | χ2/t value | P-value |
|---|---|---|---|---|
| Sex | 0.030 | 0.863 | ||
| Male | 44 (55.70) | 38 (54.29) | ||
| Female | 35 (44.30) | 32 (45.71) | ||
| Average age (years) | 5.98±1.27 | 5.86±1.34 | 0.561 | 0.576 |
| Age (years) | 0.019 | 0.891 | ||
| <5 | 42 (53.16) | 38 (54.29) | ||
| ≥5 | 37 (46.84) | 32 (45.71) | ||
| Course of disease (days) | 8.23±1.79 | 8.19±1.82 | 0.135 | 0.893 |
| Average weight (kg) | 18.27±0.65 | 18.19±0.72 | 0.713 | 0.477 |
| Anorexia | 0.013 | 0.911 | ||
| Yes | 66 (83.54) | 58 (82.86) | ||
| No | 13 (16.46) | 12 (17.14) | ||
| Severity of illness | 0.014 | 0.993 | ||
| Mild | 23 (29.11) | 21 (30.00) | ||
| Moderate | 48 (60.76) | 42 (60.00) | ||
| Severe | 8 (10.13) | 7 (10.00) | ||
| Leukocytes (109/l) | 10.79±7.68 | 10.83±7.92 | 0.031 | 0.975 |
| Platelets (109/l) | 292.19±110.28 | 290.18±109.82 | 0.111 | 0.912 |
| Fibrinogen (g/l) | 3.39±0.96 | 3.42±0.89 | 0.197 | 0.844 |
| Glutamic-oxaloacetic transaminase (U/l) | 40.38±27.29 | 42.28±28.53 | 0.415 | 0.679 |
SD, standard deviation.
Comparison of time for improvement of symptoms between two groups
The time for improvement of symptoms of the two groups were collected, as shown in Table II. Chest X-ray inflammatory absorption time, fever clearance time, disappearance time of cough and pulmonary rales in observation group were significantly shorter than those in control group (P<0.05).
Table II.
Comparison of time for improvement of symptoms of the two groups (mean ± SD)/(days).
| Groups | Chest X-ray inflammatory absorption time | Fever clearance time | Disappearance time of cough | Disappearance time of pulmonary rales |
|---|---|---|---|---|
| Observation (n=79) | 8.29±2.02 | 3.17±0.76 | 7.24±1.68 | 9.89±1.97 |
| Control (n=70) | 10.12±2.98 | 5.01±0.67 | 10.06±1.77 | 11.03±2.07 |
| χ2 value | 4.430 | 15.590 | 9.972 | 3.442 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of therapeutic effects between two groups
The therapeutic effects of the two groups were collected. After treatment, in the observation group, 65 cases were cured, 10 cases were markedly effective, 4 cases were ineffective, and the total effective rate was 94.94%. In the control group, 40 cases were cured, 17 cases were markedly effective and 13 cases were ineffective, and the total effective rate was 81.43%. The total effective rate after treatment in the observation group was significantly higher than that in the control group (χ2=9.280, P<0.05) (Table III).
Table III.
Comparison of therapeutic effects between two groups [n (%)].
| Groups | Cure | Markedly effective | Ineffective | Total effective rate |
|---|---|---|---|---|
| Observation (n=79) | 65 (82.28) | 10 (12.66) | 4 (5.06) | 94.94% |
| Control (n=70) | 40 (57.14) | 17 (24.29) | 13 (18.57) | 81.43% |
| χ2 value | 9.280 | |||
| P-value | 0.002 |
Expression levels of IL-10 and G-CSF in serum
The expression levels of IL-10 and G-CSF in serum before and after treatment in the two groups were detected. There was no significant difference in expression levels of IL-10 and G-CSF in serum between the two groups before treatment (P>0.05). After treatment, the expression levels of IL-10 and G-CSF in the two groups were significantly lower than those before treatment, and the difference was statistically significant (P<0.05). After treatment, the levels of IL-10 and G-CSF in serum of the observation group were significantly lower than those of the control group (Table IV).
Table IV.
Expression levels of IL-10 and G-CSF in serum (mean ± SD).
| Groups | IL-10 (pg/ml) | G-CSF (pg/ml) |
|---|---|---|
| Observation (n=79) | ||
| Before treatment | 21.78±2.09 | 158.98±17.78 |
| After treatment | 6.21±0.87 | 46.32±7.27 |
| t value | 58.910 | 53.420 |
| P-value | <0.001 | <0.001 |
| Control (n=70) | ||
| Before treatment | 21.97±2.17 | 160.98±16.87 |
| After treatment | 13.56±1.12a | 79.43±6.85a |
| t value | 29.950 | 36.400 |
| P-value | <0.001 | <0.001 |
P<0.05 compared with the observation group after treatment.
Comparison of adverse reactions between the two groups
The adverse reactions of the two groups were collected, as shown in Table V. The incidence of adverse reactions in observation group (7.60%) was significantly lower than that in control group (20.00%) (t=5.980, P<0.05).
Table V.
Comparison of adverse reactions between the two groups [n (%)].
| Groups | Nausea and vomiting | Erythra | Diarrhoea | Abdominal Pain | Gastrointestinal reaction | Incidence |
|---|---|---|---|---|---|---|
| Observation (n=79) | 2 (2.53) | 0 (0.00) | 2 (2.53) | 1 (1.27) | 1 (1.27) | 7.60% |
| Control (n=70) | 5 (7.14) | 1 (1.43) | 3 (4.29) | 2 (2.86) | 3 (4.29) | 20.00% |
| χ2 value | 5.980 | |||||
| P-value | 0.015 |
Correlation of IL-10 and G-CSF expression levels in serum
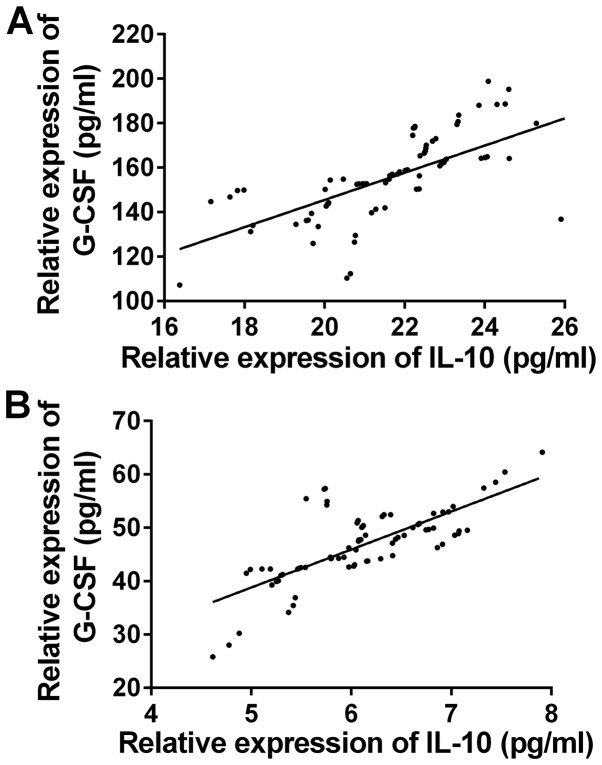
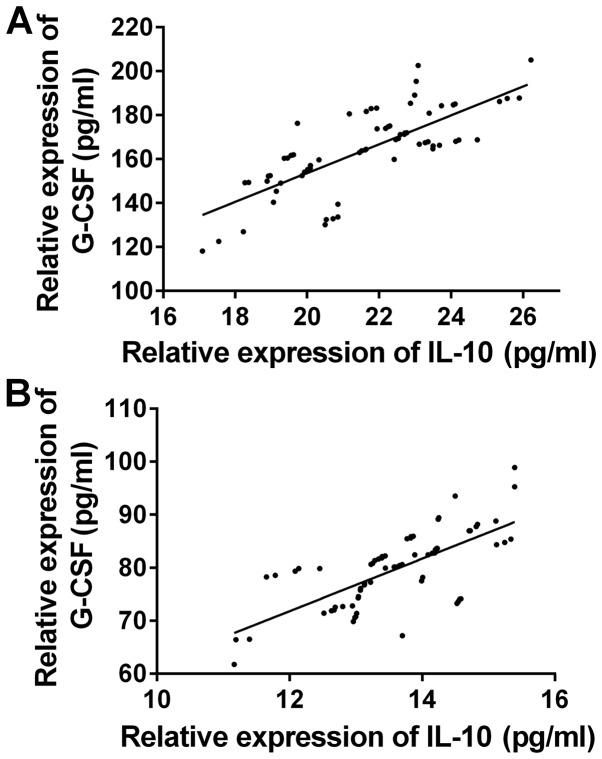
The correlation of the expression levels of IL-10 and G-CSF between the two groups before and after treatment was observed (Fig. 1). There was a significant positive correlation between the expression levels of IL-10 and G-CSF before and after treatment in the observation group (r=0.6713, 0.7387, P<0.05) (Fig. 2). There was a significant positive correlation between the expression levels of IL-10 and G-CSF before and after treatment in the control group (r=0.7547, 0.7034, P<0.05).
Figure 1.
Correlation of the expression levels of IL-10 and G-CSF in serum in observation group. Pearson's correlation analysis showed that there was a significant positive correlation between the expression levels of IL-10 and G-CSF (A) before and (B) after treatment in the observation group (r=0.6713, 0.7387, P<0.05).
Figure 2.
Correlation of the expression levels of IL-10 and G-CSF in serum in control group. Pearson's correlation analysis showed that there was a significant positive correlation between the expression levels of IL-10 and G-CSF (A) before and (B) after treatment in the control group (r=0.7547, 0.7034, P<0.05).
Discussion
Mycoplasmal pneumonia is very common in clinical pediatrics, it can occur in all seasons, and is mostly subacute onset, mainly showing cough, pharynx pain, headache and other clinical symptoms (15). The results of epidemiological studies show that the incidence of the disease has been increasing year by year (16). Clinical treatment of Mycoplasma pneumoniae is mainly based on oxygen inhalation, asthma relief, cough relief, atomization inhalation, and combined with antibiotics (17). Azithromycin is the first choice for the treatment of Mycoplasma pneumoniae pneumonia in children and can effectively achieve the role of bacteriostatic and bacteriological cleaning (18,19). Children with mycoplasma pneumonia not only have mycoplasma infection, but also have obvious airway hyperreactivity and inflammatory reaction. Simple anti-infective therapy can control the condition of children to a certain extent, but its therapeutic effect is limited, children are prone to recurrence after treatment, affecting their prognosis (20). Pidotimod is an immunologically active synthetic dipeptide molecule, which can activate the proliferation and expression of a variety of lymphocytes and regulate Th17/CD4 CD25 Treg balance in children with mycoplasma pneumonia. Pidotimod enhances specific immune function by stimulating T helper cells, promoting the production of IFN-γ and IL-2. Non-specific immune function can be enhanced by the activation of phagocytes and natural killer cells (21,22).
IL-10 is a class of Th2 type anti-inflammatory cytokine produced by innate immune cells and acquired immune cells, playing an important role in the immune regulation of the body, and its main immunomodulatory effect is achieved by inhibiting inflammatory reaction (23,24). It can inhibit the antigen presentation function of macrophages, the response of Th1 cells, and the immune response of the body by down-regulating the costimulatory signal of CD28 (25). Some studies have shown that IL-10 is related to the severity and treatment outcome of Mycoplasma pneumoniae pneumonia in children, and can be used in the diagnosis of this disease (26). The biological function of G-CSF is to regulate the proliferation, differentiation and mature release of neutral granulocyte progenitor cells, inhibit cell apoptosis and enhance its function by binding to effector surface-specific receptowr (G-CSFR), thus to aggravate inflammatory injury (27). In vitro studies have shown that G-CSF promotes the proliferation and migration of tumor cells by binding to G-CSFR and activating multiple cellular signal transduction pathways such as JAK2/STAT3 and ERK1/2 (28–30). Some findings have shown that the level of G-CSF in the diagnosis of pneumonia in children has a certain clinical value (31).
The time for improvement of symptoms of the two groups were recorded in this study. The results showed that chest X-ray inflammatory absorption time, fever clearance time, disappearance time of cough and pulmonary rales in observation group were significantly shorter than those in control group. Zhao et al showed that the clinical symptoms, the duration of signs and the length of hospitalization in patients treated with pidotimod granules combined with azithromycin sequential therapy were significantly lower than those treated with Azithromycin sequential therapy alone (32). Results of that study were similar to those obtained in this study. All the results showed that pidotimod combined with azithromycin could significantly improve the clinical symptoms of children with mycoplasma pneumonia. The total effective rate of children in the observation group (94.94%) was significantly higher than that in the control group (81.43%), indicating that pidotimod combined with azithromycin had a good curative effect in the treatment of mycoplasma pneumonia in children. The results are similar to those of Waites et al and they suggest that the treatment of mycoplasma pneumonia with pidotimod can significantly increase the expression of Foxp3 gene and the proportion of Treg cells in children with mycoplasma pneumonia, and enhance the immunity (33). Studies have shown that (34) G-CSF levels are low in normal healthy people, and when bacterial infections occur, G-CSF levels rise rapidly, and with the control of infection conditions, the level of G-CSF in vivo also decreased to normal.
It is reported that IL-10 is highly expressed in mycoplasma pneumonia and may be involved in the pathogenesis of mycoplasma pneumonia (35). The expression levels of IL-10 and G-CSF in serum before and after treatment in the two groups were detected. There was no significant difference in expression levels of IL-10 and G-CSF in serum between the two groups before treatment. After treatment, the expression levels of IL-10 and G-CSF in the two groups were significantly lower than those before treatment, the levels of IL-10 and G-CSF in serum of the observation group were significantly lower than those of the control group (P<0.05). The results showed that pidotimod combined with azithromycin could significantly decrease the expression levels of IL-10 and G-CSF in serum, effectively kill pathogenic microorganisms and inhibit or resist the growth of pathogenic bacteria; it can also inhibit the release of inflammatory transmitters to reduce the expression of inflammatory factors. The study of Lei et al on the mechanism of sequential treatment of azithromycin combined with Tanreqing on mycoplasma pneumonia in children found that the treatment could significantly downregulate the levels of serum sTREM-1, CK, G-CSF and IL-10, so as to achieve therapeutic effect (36). In the present study, pidotimod also played this role, and the effect of combined drugs was better. Our results showed that the incidence of adverse reactions in the observation group was significantly lower than that in the control group. The results are similar to those of Hakansson et al, suggesting that pidotimod combined with azithromycin is safe in the treatment of mycoplasma pneumonia in children (37). At present, there are few studies on the correlation between IL-10 and G-CSF. The present study showed that there was a significant positive correlation between the expression levels of IL-10 and G-CSF in serum before and after treatment in both groups, suggesting that G-CSF may have the function of promoting inflammation.
The effect of pidotimod combined with azithromycin on the expression levels of IL-10 and G-CSF in children with mycoplasma pneumonia was comprehensively described in this study. However, due to the bias of data collection, there are some limitations. Whether IL-10 and G-CSF can be used to evaluate the efficacy and prognosis of drugs needs to be further studied.
In conclusion, compared with sequential treatment with azithromycin alone, pidotimod combined with azithromycin in the treatment of mycoplasma pneumonia in children can significantly reduce the levels of IL-10 and G-CSF, improve the efficacy, reduce the occurrence of adverse reactions, and has high application value in clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
HS and LL were responsible for ELISA. XL and LS contributed to the analysis of observation indexes. HS wrote the manuscript. The final version was read and adopted by all the authors. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhangqiu District Maternal and Child Health Care Hospital (Jinan, China) and subjects' experimental contents were described in detail. Patients who participated in this research had complete clinical data. The signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gong L, Zhang C-L, Zhen Q. Analysis of clinical value of CT in the diagnosis of pediatric pneumonia and mycoplasma pneumonia. Exp Ther Med. 2016;11:1271–1274. doi: 10.3892/etm.2016.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L. Comparison of the effect of erythromycin combined with azithromycin sequential therapy and azithromycin in treatment of mycoplasma pneumonia in children. China Med Her. 2016;13:173–176. (In Chinese) [Google Scholar]
- 4.Bajantri B, Toolsie O, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae pneumonia: Walking pneumonia can cripple the susceptible. J Clin Med Res. 2018;10:891–897. doi: 10.14740/jocmr3592w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: A potentially severe infection. J Clin Med Res. 2018;10:535–544. doi: 10.14740/jocmr3421w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Kwon JH, Lee JY, Lee JS, Ryu JM, Kim SH, Lim KS, Kim WY. Clinical features of Mycoplasma pneumoniae coinfection and need for its testing in influenza pneumonia patients. J Thorac Dis. 2018;10:6118–6127. doi: 10.21037/jtd.2018.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X, Zhu Y, Zhang Y, Chen J, Rong L, Zhao X. Assessment of levels of D-dimer and interferon-γ in pediatric patients with Mycoplasma pneumoniae pneumonia and its clinical implication. Exp Ther Med. 2018;16:5025–5030. doi: 10.3892/etm.2018.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur S, Fuchs A, Bielicki J, Van Den Anker J, Sharland M. Antibiotic use for community-acquired pneumonia in neonates and children: WHO evidence review. Paediatr Int Child Health. 2018;38(Suppl 1):S66–S75. doi: 10.1080/20469047.2017.1409455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 10.Medjo B, Atanaskovic-Markovic M, Nikolic D, Radic S, Lazarevic I, Cirkovic I, Djukic S. Increased serum interleukin-10 but not interleukin-4 level in children with Mycoplasma pneumoniae pneumonia. J Trop Pediatr. 2017;63:294–300. doi: 10.1093/tropej/fmw091. [DOI] [PubMed] [Google Scholar]
- 11.Akashi Y, Hayashi D, Suzuki H, Shiigai M, Kanemoto K, Notake S, Ishiodori T, Ishikawa H, Imai H. Clinical features and seasonal variations in the prevalence of macrolide-resistant Mycoplasma pneumoniae. J Gen Fam Med. 2018;19:191–197. doi: 10.1002/jgf2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in neutropenia. J Immunol. 2015;195:1341–1349. doi: 10.4049/jimmunol.1500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liongue C, Ward AC. Granulocyte colony-stimulating factor receptor mutations in myeloid malignancy. Front Oncol. 2014;4:93. doi: 10.3389/fonc.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutow P, Lingner S, Laudeley R, Glage S, Hoymann HG, Dittrich AM, Fehlhaber B, Müller M, Braun A, Klos A. Severity of allergic airway disease due to house dust mite allergen is not increased after clinical recovery of lung infection with Chlamydia pneumoniae in mice. Infect Immun. 2013;81:3366–3374. doi: 10.1128/IAI.00334-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, et al. Pediatric Acute Lung Injury, Sepsis Investigators (PALISI) Network PICFlu Study Investigators Innate immune function and mortality in critically ill children with influenza: A multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trikha P, Carson WE., III Signaling pathways involved in MDSC regulation. Biochim Biophys Acta. 2014;1846:55–65. doi: 10.1016/j.bbcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang H, Liu Y, Mo L. Clinical study of using additives qianjin weijing decoction in treating children with phlegm and blood stasis mutual junction type mycoplasma pneumonia. J Sichuan Trad Med. 2017;7:81–83. [Google Scholar]
- 18.Fleming-Dutra KE, Demirjian A, Bartoces M, Roberts RM, Taylor TH, Jr, Hicks LA. Variations in antibiotic and azithromycin prescribing for children by geography and specialty-United States, 2013. Pediatr Infect Dis J. 2018;37:52–58. doi: 10.1097/INF.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He HX, Zhang XQ. Clinical observation on self-made yiqi xiaozhi decoction and azithromycin in the sequential treatment of mycoplasma pneumonia in children. J Sichuan Trad Med. 2018;7:43–46. [Google Scholar]
- 20.Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, Wang Y, Yuan W, Ma J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7:130–140. doi: 10.1007/s13238-015-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvatore CM, Techasaensiri C, Tagliabue C, Katz K, Leos N, Gomez AM, McCracken GH, Hardy RD. Tigecycline therapy significantly reduces the concentrations of inflammatory pulmonary cytokines and chemokines in a murine model of Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2009;53:1546–1551. doi: 10.1128/AAC.00979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taher TE, Bystrom J, Ong VH, Isenberg DA, Renaudineau Y, Abraham DJ, Mageed RA. Intracellular B lymphocyte signalling and the regulation of humoral immunity and autoimmunity. Clin Rev Allergy Immunol. 2017;53:237–264. doi: 10.1007/s12016-017-8609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavropoulos A, Varna A, Zafiriou E, Liaskos C, Alexiou I, Roussaki-Schulze A, Vlychou M, Katsiari C, Bogdanos DP, Sakkas LI. IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNγ-producing T cells. Clin Immunol. 2017;184:33–41. doi: 10.1016/j.clim.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Morris KT, Castillo EF, Ray AL, Weston LL, Nofchissey RA, Hanson JA, Samedi VG, Pinchuk IV, Hudson LG, Beswick EJ. Anti-G-CSF treatment induces protective tumor immunity in mouse colon cancer by promoting protective NK cell, macrophage and T cell responses. Oncotarget. 2015;6:22338–22347. doi: 10.18632/oncotarget.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, You X, Peng Z, Zhang H, Gao S, Zeng Y, Yu M, Zhu C. Mycoplasma pneumoniae capsular polysaccharides bind to DC-SIGN and promote the secretion of IL-10. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:10–13. (In Chinese) [PubMed] [Google Scholar]
- 26.Zhang X, Ma X, An H, Xu C, Cao W, Yuan W, Ma J. Upregulation of microRNA-125b by G-CSF promotes metastasis in colorectal cancer. Oncotarget. 2017;8:50642–50654. doi: 10.18632/oncotarget.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilbert TW, Denlinger LC. Role of infection in the development and exacerbation of asthma. Expert Rev Respir Med. 2010;4:71–83. doi: 10.1586/ers.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211–1220. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi H, Kimura T, Yuki N, Yoshioka A. An adult case of recurrent Guillain-Barré syndrome with anti-galactocerebroside antibodies. Intern Med. 2018;57:409–412. doi: 10.2169/internalmedicine.8941-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty A, White SM, Guha S. Granulocyte colony-stimulating receptor promotes beta1-integrin-mediated adhesion and invasion of bladder cancer cells. Urology. 2006;68:208–213. doi: 10.1016/j.urology.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 31.He J, Liu M, Ye Z, Tan T, Liu X, You X, Zeng Y, Wu Y. Insights into the pathogenesis of Mycoplasma pneumoniae (Review) Mol Med Rep. 2016;14:4030–4036. doi: 10.3892/mmr.2016.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao JL, Wang X, Wang YS. Relationships between Th1/Th2 cytokine profiles and chest radiographic manifestations in childhood Mycoplasma pneumoniae pneumonia. Ther Clin Risk Manag. 2016;12:1683–1692. doi: 10.2147/TCRM.S121928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. 2017;30:747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong YC, Yeo MS, Kim JH, Lee HB, Oh JW. Mycoplasma pneumoniae infection affects the serum levels of vascular endothelial growth factor and interleukin-5 in atopic children. Allergy Asthma Immunol Res. 2012;4:92–97. doi: 10.4168/aair.2012.4.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Xiao Y, Zhang M, Ao T, Lang S, Wang J. Serum inflammatory markers in patients with adenovirus respiratory infection. Med Sci Monit. 2018;24:3848–3855. doi: 10.12659/MSM.910692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei WT, Lin HH, Tsai MC, Hung HH, Cheng YJ, Liu SJ, Lin CY, Yeh TL. The effects of macrolides in children with reactive airway disease: A systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:3825–3845. doi: 10.2147/DDDT.S183527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakansson AP, Orihuela CJ, Bogaert D. Bacterial-host interactions: Physiology and pathophysiology of respiratory infection. Physiol Rev. 2018;98:781–811. doi: 10.1152/physrev.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.