Editor—It was with great interest that we read the recent paper by Oh and colleagues1 in the British Journal of Anaesthesia, which compared sugammadex with neostigmine for the reversal of rocuronium-induced neuromuscular block (NMB), focusing on the impact of these agents on 30-day unplanned readmission after major abdominal surgery. Oh and colleagues1 performed a mixed-effects logistic regression analysis of 1479 patients (sugammadex: 355; neostigmine: 1124), and found that the incidence of 30-day unplanned readmission was 34% lower in patients receiving sugammadex compared with those receiving neostigmine (odds ratio: 0.66, P=0.031).1
Sugammadex has been found to be superior to neostigmine for the reversal of rocuronium-induced NMB, with a lower risk of adverse postoperative events.2 Use of sugammadex may also increase operating room efficiency by accelerating NMB reversal and reducing the risk of residual NMB, thereby producing potential economic benefits.3, 4, 5, 6 However, it remains unclear whether sugammadex has a positive effect on healthcare expenditures during the postoperative period compared with neostigmine.7, 8 Recently, postoperative residual curarisation (PORC) was not found to be independently associated with increased hospital costs (adjusted incidence rate ratio, 1.04; P=0.22),7 despite an increased risk of unplanned ICU admission.5, 7, 9 Neostigmine, particularly if not used at the appropriate dose or time,9 may predispose to an increased risk of 30-day unplanned readmission.10 Conversely, sugammadex has the potential to reduce the risk of 30-day unplanned readmission.1 Therefore, the study by Oh and colleagues1 may represent an opportunity to further evaluate the potential benefits of sugammadex on postoperative healthcare expenditures.
Oh and colleagues1 reported a readmission rate after major abdominal surgery of 13.3% for their entire study population. This rate seems a little high, especially as 41.0% of their patients underwent urologic or gynaecologic procedures, which often have low readmission rates.1 The American College of Surgeons National Surgical Quality Improvement Program, for example, reported 30-day readmission rates of 3.8% among 25 119 women undergoing hysterectomy11 and 5.8% among 23 108 patients undergoing inpatient urologic surgery.12 However, Oh and colleagues1 provided no information about the specific types of operations, and 30-day unplanned readmission rates tend to increase with increasing surgical complexity. Wilbur and colleagues13 reported a 30-day readmission rate of 11.0% among 1605 women at an academic gynaecologic oncology service. Damle and colleagues14 found that readmission occurred in 13.7% of 70 484 patients after colorectal surgery. Ejaz and colleagues15 reported readmission in 17.2% of 4114 patients undergoing colorectal (42.8%), pancreatic (40.4%), or hepatic resection (16.9%) surgeries.
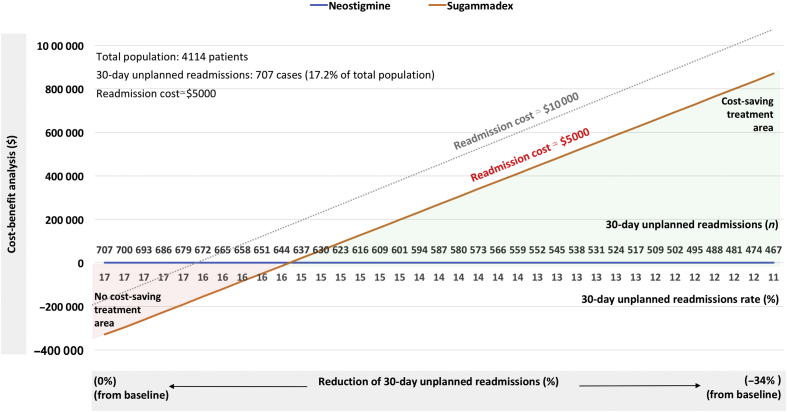
Unfortunately, Oh and colleagues1 did not provide data regarding the cost of readmission, which could be used in a cost-benefit analysis of sugammadex compared with neostigmine in their tertiary teaching hospital. However, at another tertiary care hospital, Ejaz and colleagues15 reported that readmission increased the total index hospitalisation costs by nearly $5000 ($29 312 vs $24 321; P<0.001) after abdominal surgery. Damle and colleagues14 examined data from the University Health System Consortium and found that readmission may increase the total index hospitalisation costs by nearly $13 000 ($26 917 vs $13 817; P<0.001). Both readmission costs are consistent with the second ($6493) and third ($11 514) readmission cost quartiles reported for readmission costs after major abdominal surgeries (i.e. abdominal aortic aneurysm repair, cystectomy, oesophagectomy, and pancreatectomy), based on analyses of inpatient databases for NY, IA, NC, and WA states in USA.16 Furthermore, Wilbur and colleagues13 reported a mean cost of $25 416 per readmission (total readmission-related costs during the study period were $4 523 959). Based on these reports, sugammadex may yield considerable economic benefits despite the higher direct costs of reversing moderate rocuronium-induced NMB with sugammadex compared with neostigmine.4, 5, 17 However, whether sugammadex results in potential cost savings4, 5 will depend on readmission costs and the effective reduction in 30-day unplanned readmission rates (Fig. 1).
Fig. 1.
Example of cost-saving analysis of reversal of moderate rocuronium-induced neuromuscular block. We performed a cost-benefit analysis based on the assumptions that use of sugammadex, compared with neostigmine, reduced the incidence of 30-day unplanned readmission by 34%,1 and that neostigmine did not affect the 30-day unplanned readmission rate.9, 10 Data from the study by Ejaz and colleagues15 were used for the total population number, baseline readmission number and rate, and readmission cost in our analysis. The total costs for reversal of moderate rocuronium-induced NMB using sugammadex or neostigmine were evaluated. On the basis of the potential costs of reversal drugs,4, 5 the difference in direct NMB reversal costs between sugammadex and neostigmine was assumed to be $80. Considering the total cost of readmissions and the total cost of NMB reversal for the total population, the model shows that sugammadex becomes a cost-effective treatment as the 30-day readmission rate decreases from baseline to 34% below baseline (data below the x-axis). The corresponding reduction in number of readmissions can also be determined (data above the x-axis). A treatment was considered cost-saving if the gain obtained by reducing the 30-day unplanned readmission rate was greater than the total NMB reversal cost necessary for treatment. In our model, sugammadex appears to be cost saving compared with neostigmine when it reduces the 30-day unplanned readmission rate by at least 10% (orange line) or 5% (grey dotted line) from baseline for readmission costs. NMB, neuromuscular block.
Although the statistical analysis represents an important strength of their study, it is not an RCT and thereby has the drawbacks of all retrospective observational studies.1 It is unclear whether all patients were controlled for the level of recovery of neuromuscular function after reversal drug administration. If not, the effects of PORC may not have been completely excluded.1 Kotake and colleagues18 found that after antagonism of rocuronium-induced NMB in the absence of neuromuscular monitoring (objective or subjective), a train-of-four ratio <0.9 was present with both reversal drugs, although it was less frequent with sugammadex (five of 117 patients [4.3%]) than with neostigmine (26 of 109 patients [23.9%]) (P<0.001). PORC may predispose to an increased risk of unplanned ICU or hospital admission.9, 10 In a controlled setting (e.g. train-of-four ratio ≥0.9 via accelomyographic monitoring), reduction of the rate of 30-day unplanned readmission after major abdominal surgery with sugammadex compared with neostigmine may be less than that reported by Oh and colleagues.1 Hence, in an uncontrolled setting, sugammadex may have a favourable economic impact1; however, in a controlled setting with correct dosing of reversal drugs to obtain complete reversal of NMB based on the level of measured NMB, this benefit may be less relevant.
In a recent retrospective evaluation, after propensity matching patients undergoing colorectal surgery, no significant difference was found between 30-day readmission rates after sugammadex or neostigmine.19 Oh and colleagues1 found that sugammadex not only reduced 30-day unplanned readmission rates by 34%, but also shortened hospital length of stay by 20% and reduced related hospital charges by 24%, compared with neostigmine. Taken together, these results provide further support for the potential economic benefits of sugammadex. While their findings also suggest that sugammadex potentially improves patient care and healthcare expenditures, these findings require confirmation by additional, controlled studies.
Declarations of interest
The authors are supported only by departmental funds. MC has received payments for lectures from Merck Sharp & Dohme (MSD), Italy. ADC and GI have no interests to disclose.
References
- 1.Oh T.K., Oh A.Y., Ryu J.H. Retrospective analysis of 30-day unplanned readmission after major abdominal surgery with reversal by sugammadex or neostigmine. Br J Anaesth. 2019;122:370–378. doi: 10.1016/j.bja.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Hristovska A.M., Duch P., Allingstrup M., Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8:CD012763. doi: 10.1002/14651858.CD012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueckmann B., Sasaki N., Grobara P. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115:743–751. doi: 10.1093/bja/aev104. [DOI] [PubMed] [Google Scholar]
- 4.Paton F., Paulden M., Chambers D. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105:558–567. doi: 10.1093/bja/aeq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carron M., Baratto F., Zarantonello F., Ori C. Sugammadex for reversal of neuromuscular blockade: a retrospective analysis of clinical outcomes and cost-effectiveness in a single center. Clinicoecon Outcome Res. 2016;8:43–52. doi: 10.2147/CEOR.S100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insinga R.P., Joyal C., Goyette A., Galarneau A. A discrete event simulation model of clinical and operating room efficiency outcomes of sugammadex versus neostigmine for neuromuscular block reversal in Canada. BMC Anesthesiol. 2016;16:114. doi: 10.1186/s12871-016-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabitz S.D., Rajaratnam N., Changani K. The effects of postoperative residual neuromuscular blockade on hospital costs and intensive care unit admission: a population-based cohort study. Anesth Analg Adv Access Published January. 2019;8 doi: 10.1213/ANE.0000000000004028. [DOI] [PubMed] [Google Scholar]
- 8.Ünal D.Y., Baran İ., Mutlu M., Ural G., Akkaya T., Özlü O. Comparison of sugammadex versus neostigmine costs and respiratory complications in patients with obstructive sleep apnoea. Turk J Anaesthesiol Reanim. 2015;43:387–395. doi: 10.5152/TJAR.2015.35682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose S., Xu X., Eikermann M. Does reversal of neuromuscular block with sugammadex reduce readmission rate after surgery? Br J Anaesth. 2019;122:294–298. doi: 10.1016/j.bja.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Thevathasan T., Shih S.L., Safavi K.C. Association between intraoperative non-depolarising neuromuscular blocking agent dose and 30-day readmission after abdominal surgery. Br J Anaesth. 2017;119:595–605. doi: 10.1093/bja/aex240. [DOI] [PubMed] [Google Scholar]
- 11.Merkow R.P., Ju M.H., Chung J.W. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313:483–495. doi: 10.1001/jama.2014.18614. [DOI] [PubMed] [Google Scholar]
- 12.Stone B.V., Cohn M.R., Donin N.M. Evaluation of unplanned hospital readmissions after major urologic inpatient surgery in the era of accountable care. Urology. 2017;109:94–100. doi: 10.1016/j.urology.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Wilbur M.B., Mannschreck D.B., Angarita A.M. Unplanned 30-day hospital readmission as a quality measure in gynecologic oncology. Gynecol Oncol. 2016;143:604–610. doi: 10.1016/j.ygyno.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Damle R.N., Cherng N.B., Flahive J.M. Clinical and financial impact of hospital readmissions after colorectal resection: predictors, outcomes, and costs. Dis Colon Rectum. 2014;57:1421–1429. doi: 10.1097/DCR.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 15.Ejaz A., Gonzalez A.A., Gani F., Pawlik T.M. Effect of index hospitalization costs on readmission among patients undergoing major abdominal surgery. JAMA Surg. 2016;151:718–724. doi: 10.1001/jamasurg.2015.5557. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs B.L., He C., Li B.Y. Variation in readmission expenditures after high-risk surgery. J Surg Res. 2017;213:60–68. doi: 10.1016/j.jss.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaouter C., Mion S., Palomba A., Hemmerling T.M. A short update on sugammadex with a special focus on economic assessment of its use in North America. J Anesth Clin Res. 2017;8:740. [Google Scholar]
- 18.Kotake Y., Ochiai R., Suzuki T. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117:345–351. doi: 10.1213/ANE.0b013e3182999672. [DOI] [PubMed] [Google Scholar]
- 19.Chae Y.J., Joe H.B., Oh J., Lee E., Yi I.K. Thirty-day postoperative outcomes following sugammadex use in colorectal surgery patients; retrospective study. J Clin Med. 2019;8:97. doi: 10.3390/jcm8010097. [DOI] [PMC free article] [PubMed] [Google Scholar]