Abstract
Background
Both the adductor canal block (ACB) and local infiltration analgesia (LIA) are effective analgesic techniques after anterior cruciate ligament (ACL) reconstruction, but they have never been compared head-to-head. This randomised controlled triple-blinded trial tested the hypothesis that ACB provides superior analgesia to LIA after ACL reconstruction, with additional focus on postoperative functional outcomes.
Methods
Of 104 enrolled ACL reconstruction patients receiving general anaesthesia, 52 were randomly allocated to either ACB under ultrasound guidance or LIA. For each intervention, ropivacaine 0.5%, 20 ml was injected. Postoperative pain treatment followed a predefined protocol with i.v. patient-controlled morphine, paracetamol, and ibuprofen. The primary outcome was cumulative i.v. morphine consumption at 24 h after operation. Secondary pain-related outcomes included resting and dynamic pain scores (numeric rating scale out of 10) measured 2, 24, and 48 h after operation and cumulative i.v. morphine consumption 2 and 48 h after operation. Early function-related outcomes evaluated were quadriceps strength, walking distance, and range of motion, all measured 24 and 48 h after operation. Late function-related outcomes were concentric quadriceps strength, single-hop test, triple-hop test, cross-over test, and Y balance test, measured at 4 and 8 postoperative months.
Results
Cumulative i.v. morphine consumption at 24 h was similar between groups (ACB group: 17.1 mg [95% confidence interval, CI: 13.1, 21.2]; LIA group: 17.7 mg [95% CI: 13.2, 22.6], P=0.84). Similarly, no differences between groups were seen in the secondary pain- or function-related outcomes.
Conclusions
ACB and LIA result in equivalent postoperative opioid consumption with similar impact on postoperative pain scores and functional outcomes.
Clinical trial registration
Keywords: anterior cruciate ligament reconstruction, local infiltration analgesia, postoperative analgesia, regional anaesthesia, ropivacaine
Editor's key points.
-
•
The analgesic technique producing optimal postoperative pain relief and functional outcomes after anterior cruciate ligament repair has not been established.
-
•
In a single centre randomised controlled trial, adductor canal block was compared with local anaesthetic infiltration.
-
•
Morphine consumption, resting and dynamic pain scores, and postoperative nausea and vomiting were comparable between groups, and neither technique affected early or late functional outcomes.
-
•
Both techniques provided similar postoperative analgesia with minimal negative impact on functional outcomes after anterior cruciate ligament reconstruction.
Early rehabilitation after anterior cruciate ligament (ACL) reconstruction is of paramount importance with multimodal analgesia, inclusive of local anaesthetic administration, a critical element.1 However, a range of options exist for local anaesthetic administration including femoral nerve block,2, 3 adductor canal block (ACB)4, 5 or periarticular infiltration of the knee joint, generally termed local infiltration analgesia (LIA),6, 7 with the optimal approach unknown. Femoral nerve block has been incriminated in cases of impaired postoperative mobility and falls, although robust evidence has suggested the opposite may be true.8 Given this concern, alternative approaches have been explored, with ACB providing equivalent analgesia to femoral nerve block, while preserving quadriceps muscle strength after ACL reconstruction.9 Similarly, LIA does not seem to impact quadriceps strength.10
A recent meta-analysis compared the analgesic benefit of peripheral nerve block with LIA after ACL reconstruction and concluded that peripheral nerve block was superior.10 Two major points were highlighted: first, all included trials compared LIA with femoral nerve block and none to ACB; and second, despite their intent to investigate the impact of each technique on functional outcomes, no conclusions could be drawn because of the absence of functional outcomes reported among included trials.
As no trial has compared ACB with LIA for pain- and function-related outcomes after ACL reconstruction, we undertook this randomised controlled triple-blinded trial and tested the hypothesis that ACB provides superior analgesia to LIA after ACL reconstruction, with a detailed secondary focus on postoperative functional outcomes.
Methods
Recruitment and randomisation
This trial was approved by the Ethics Committee of the Lausanne University Hospital (Commission d’Ethique Romande, protocol number 193-15) and was prospectively registered on clinicaltrials.gov (NCT02524652). The present report follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines.11 All patients aged 18 yr or older and under elective primary ACL reconstruction between November 2015 and January 2018 at Lausanne University Hospital were eligible to participate in this study. Exclusion criteria were: existing femoral nerve deficit or pre-existing peripheral neuropathy, chronic pain condition, pregnancy, or contraindications to peripheral nerve block (e.g. allergy to local anaesthetics, coagulopathy, infection in the area). After providing written informed consent, participating patients were randomly allocated on the day of surgery to either the ACB group or the LIA group using a computer-generated randomisation table in blocks of 10. Assignments were concealed in a sealed opaque envelope.
Intraoperative procedure
After application of routine monitors in the operating theatre, subjects received a standard general anaesthetic. Anaesthesia was induced using fentanyl 1–2 μg kg−1 i.v. and propofol 2–4 mg kg−1 i.v. Tracheal intubation was facilitated with rocuronium 0.6 mg kg−1 i.v. Maintenance of anaesthesia was via inhaled sevoflurane 1.6–2.4 vol% in a 40:60 mixture of oxygen and air. Positive pressure ventilation was initiated with tidal volume and rate adjusted to maintain end-tidal PCO2 of 4.7–5.3 kPa. Fentanyl 25–50 μg i.v. was administered as needed to treat increases in arterial pressure or HR >15% above preinduction baseline values. As per our routine institutional practice, all subjects received magnesium sulphate 50 mg kg−1 i.v.12 and dexamethasone 0.15 mg kg−1 i.v.,13 and ondansetron 4 mg i.v. and droperidol 1 mg i.v. for multimodal analgesia and antiemetic prophylaxis, respectively. All surgical operations were performed with a tourniquet (pressure at 280 mm Hg) by a single surgeon (RM) who harvested gracilis and semitendinosis hamstring tendons for single bundle ACL reconstruction. In addition to arthroscopic portals, a two-incision approach was used to drill tunnels for tibial and femoral fixation of the graft, as previously described.1 Muscle relaxation was antagonised with neostigmine 50 μg kg−1 and glycopyrrolate 5–10 μg kg−1 at the end of surgery.
Regional procedure
After completion of surgery and before emergence from anaesthesia, an experienced staff regional anaesthesiologist or a directly supervised regional anaesthesia fellow performed an ACB for subjects allocated to this group with ultrasound (US) guidance. The mid-thigh site was sterilised with a solution of chlorhexidine 2% in isopropyl alcohol 70% (v/v). Under sterile conditions, a high-frequency linear array transducer (13–6 MHz, SonoSite S-Nerve; SonoSite Inc., Bothell, WA, USA) was placed on the medial aspect of the mid-thigh to see the superficial femoral nerve in short axis. The skin was infiltrated with 3 ml lidocaine 1%, and a 19-gauge 100-mm insulated facet tip needle (SonoLong NanoLine cannula; Pajunk® GmbH, Geisingen, Germany) was inserted in-plane with the US beam, in a lateral to medial direction. The needle tip was advanced under direct US guidance to the supero-lateral corner of the femoral artery, just below the sartorius muscle. As the saphenous nerve is commonly difficult to identify, the needle was targeted to the triangular hyperechoic region lateral to the artery, bounded by the sartorius muscle superiorly, and the vastus medialis laterally.9 A small amount of dextrose 5%, 1–2 ml was used for needle tip hydrolocation at the discretion of the operator. Once the needle tip was satisfactorily positioned, 20 ml of ropivacaine 0.5% was injected, in slow 5 ml increments, with intermittent aspiration to prevent intravascular injection. Adequate spread of local anaesthetic solution around the saphenous nerve was observed in a caudo-cephalad direction.
For subjects allocated to the LIA group, the surgeon performed periarticular infiltration of local anaesthetic at the end of surgery as described.10 Briefly, a first injection (8 ml) was performed in the empty space left by the harvest of the gracilis and semitendinosis tendons, a second injection (5 ml) in the iliotibial band through the surgical exposure used for femoral tunnel drilling, and finally, subcutaneous tissue was infiltrated (7 ml). The subjects, research assistant and physiotherapist collecting the data, and the statistician were all blinded to group allocation.
Postoperative procedure
After surgery, subjects were brought to phase 1 recovery, where pain (numeric rating scale [NRS] >3 or patient request for analgesia) was treated with morphine 1–2 mg i.v. every 10 min as needed. Subjects were provided morphine i.v. patient-controlled analgesia (PCA) with boluses of 2 mg available every 10 min. Both groups received instruction on the use of PCA and a visual analogue scale for pain and satisfaction measurement. All subjects received our institutional standard multimodal analgesia of paracetamol 1 g every 6 h, and ibuprofen 400 mg every 8 h. Antiemetic medications on the ward included ondansetron 4 mg i.v. and metoclopramide 10 mg i.v. On the morning of postoperative day 2, PCA was discontinued.
Outcomes
The primary outcome was i.v. morphine consumption at 24 h after operation. Secondary outcomes were divided into pain, early functional outcomes, and late functional outcomes. Pain-related outcomes included cumulative i.v. morphine consumption at 2 and 48 h; resting and dynamic pain scores (NRS, 0–10) at 2, 24, and 48 h after operation; and the incidences of postoperative nausea and vomiting, at 2, 24, and 48 h. Early function-related outcomes were quadriceps strength (ordinal scale of 1–5, with 5 being the maximal developed strength compared with the opposite side), walking distance (m), and range of motion (°), all measured at 24 and 48 h after operation. Late function-related outcomes were concentric quadriceps strength (limb symmetry index [LSI], calculated as the mean score of the involved limb divided by the mean score of the uninvolved limb, expressed in percentage)14 for single-hop test (LSI, %),15 triple-hop (LSI, %),15 cross-over (LSI, %),15 agility (s),16 Y balance17 tests, ACL Return to Sport After Injury (ACL-RSI) scale,18 and the International Knee Documentation Committee scale (IKDC) score19 measured at 4 and 8 postoperative months. Any procedure-related complication, such as haematoma, infection, persistent new paraesthesia or new hypaesthesia, neuropathic pain, leg weakness, or signs of chondrolysis, were sought at the postoperative surgical visits.
Sample size calculation
Based on pilot data, the mean cumulative consumption of i.v. morphine at 24 h was 23 mg with a standard deviation of 12 mg for patients who received ACB. Assuming a 30% increase in the LIA group, an alpha error of 0.05 and a power of 80%, we calculated that 47 subjects would be required for each group (total 94) to detect a difference. Allowing for a 10% drop-out rate, we planned to recruit a total of 104 subjects.
Statistical analysis
Data were analysed on an intention-to-treat basis. Categorical variables are presented as frequencies, ordinal variables as medians and inter-quartile range, and continuous variables as means with 95% confidence intervals (95% CI). Continuous parametric and non-parametric data were compared using the Student's t-test and Mann–Whitney U-test, respectively. Categorical and dichotomous data were compared using the Fisher's exact test or Pearson test as appropriate. Significance was considered at P<0.05 based for two-tailed probability. Statistical analysis was performed using the JMP 9 statistical package (SAS Institute, Cary, NC, USA).
Results
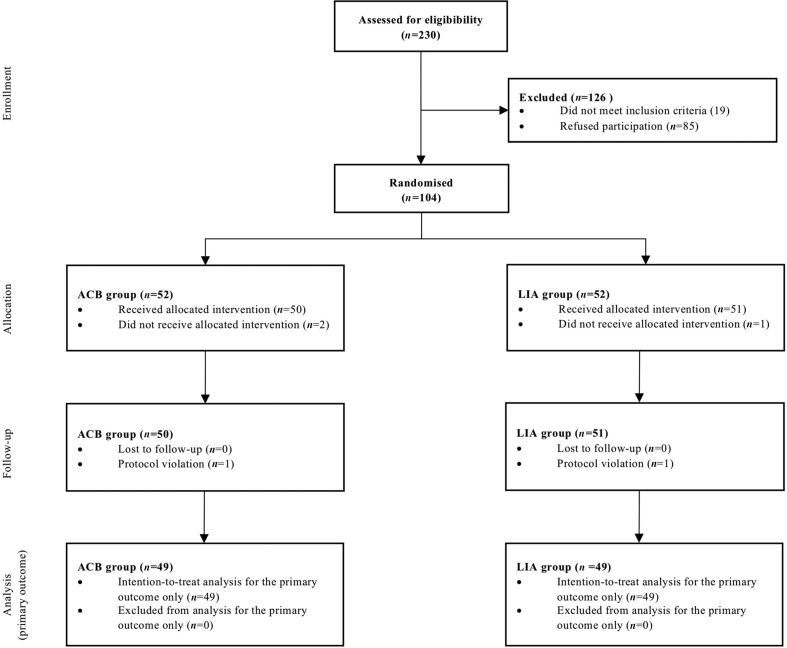
Of 104 subjects recruited, 98 completed the protocol to measurement of the primary outcome. Figure 1 describes the flow of subjects during the trial and Table 1 presents subject characteristics.
Fig. 1.
Flow of subjects through trial. ACB, adductor canal block; LIA, local infiltration analgesia.
Table 1.
Subject characteristics and clinical data presented as mean with 95% confidence interval or absolute number as appropriate. ACB, adductor canal block; LIA, local infiltration analgesia.
| ACB group | LIA group | P-value | |
|---|---|---|---|
| Sex (male/female) | 38/11 | 32/17 | 0.11 |
| Age (yr) | 29 (26, 31) (range: 18-53) | 28 (25, 30) (range: 18-45) | 0.47 |
| Height (cm) | 177 (174, 179) | 175 (172, 178) | 0.44 |
| Weight (kg) | 76 (72, 80) | 76 (72, 79) | 0.99 |
| ASA physical status (1/2) | 29/20 | 28/21 | 0.84 |
| Duration of surgery (min) | 134 (125, 142) | 137 (128, 146) | 0.54 |
| Duration of anaesthesia (min) | 192 (184, 201) | 195 (185, 205) | 0.63 |
Cumulative i.v. morphine consumption at 24 h after operation was similar between groups (ACB group: 17.1 mg [95% CI: 13.1, 21.2]; LIA group: 17.7 mg [95% CI: 13.2, 22.6], P=0.84). Secondary pain-related outcomes were also similar between groups (Table 2), with the exception of resting pain scores at 48 h after operation, which favoured the ACB group.
Table 2.
Pain-related outcomes. Data are presented as mean with 95% confidence interval or absolute number as appropriate. ACB, adductor canal block; LIA, local infiltration analgesia; NRS, numeric rating scale; PONV, postoperative nausea and vomiting.
| ACB group | LIA group | P-value | |
|---|---|---|---|
| 2 Postoperative hours | |||
| Resting pain score (NRS, 0–10) | 2.4 (1.9, 2.9) | 2.6 (2.1, 3.1) | 0.52 |
| Morphine consumption (i.v., mg) | 8.0 (6.6, 9.4) | 8.8 (7.2, 10.4) | 0.49 |
| Presence of PONV (yes/no) | 1/48 | 2/47 | 0.46 |
| 24 Postoperative hours | |||
| Resting pain score (NRS, 0–10) | 1.0 (0.57, 1.43) | 1.4 (0.9, 1.9) | 0.22 |
| Dynamic pain score (NRS, 0–10) | 2.2 (1.5, 2.9) | 2.5 (1.9, 3.1) | 0.54 |
| Morphine consumption (i.v., mg) | 17.2 (13.1, 21.3) | 18.1 (13.6, 22.7) | 0.75 |
| Presence of PONV (yes/no) | 11/38 | 10/39 | 0.46 |
| 48 Postoperative hours | |||
| Resting pain score (NRS, 0–10) | 0.8 (0.5, 1.2) | 1.5 (1.0, 2.0) | 0.03 |
| Dynamic pain score (NRS, 0–10) | 1.8 (1.2, 2.3) | 2.3 (1.7, 2.9) | 0.14 |
| Morphine consumption (i.v., mg) | 31.2 (24.7, 38.9) | 39.7 (29.3, 50.2) | 0.21 |
| Presence of PONV (yes/no) | 4/45 | 5/44 | 0.21 |
Regarding early (Table 3) and late functional outcomes (Table 4), there were no differences between groups, except for the agility test at 4 postoperative months and ACL-RSI score at 8 postoperative months, which favoured the LIA group. No haematoma, infection, persistent new paraesthesia or new hypaesthesia, neuropathic pain, leg weakness, or chondrolysis were reported after surgery.
Table 3.
Early functional-related outcomes. Data are presented as mean with 95% confidence interval or absolute number as appropriate. ACB, adductor canal block; LIA, local infiltration analgesia.
| ACB group | LIA group | P-value | |
|---|---|---|---|
| 24 Postoperative hours | |||
| Quadriceps muscle strength (1–5) | 2.6 (2.4, 2.8) | 2.6 (2.4, 2.8) | 0.92 |
| Walking distance (m) | 58.7 (50.7, 66.8) | 70.4 (60.6, 80.2) | 0.07 |
| Range of motion (degree) | 70.6 (66.7, 76.9) | 71.8 (66.7, 76.9) | 0.72 |
| 48 Postoperative hours | |||
| Quadriceps muscle strength (1–5) | 2.7 (2.5, 2.9) | 2.7 (2.5, 2.9) | 0.80 |
| Walking distance (m) | 75.1 (66.6, 83.6) | 78.1 (66.6, 89.5) | 0.68 |
| Range of motion (degree) | 78.4 (74.5, 82.4) | 74.5 (69.3, 79.7) | 0.23 |
Table 4.
Late functional-related outcomes. Data are presented as mean with 95% confidence interval. Limb symmetry index (LSI) is calculated as the mean score of the involved limb divided by the mean score of the uninvolved limb, with the result multiplied by 100 (LSI%). ACB, adductor canal block; ACL-RSI scale, Anterior Cruciate Ligament Return to Sport After Injury scale; IKDC, International Knee Documentation Committee scale, LIA, local infiltration analgesia.
| ACB group | LIA group | P-value | |
|---|---|---|---|
| 4 Postoperative months | |||
| Concentric quadriceps strength (LSI, %) | 75.9 (71.7, 80.1) | 74.1 (69.6, 78.7) | 0.58 |
| Single-hop test (LSI, %) | 84.4 (79.3, 89.5) | 79.4 (74.0, 89.5) | 0.17 |
| Triple-hop test (LSI, %) | 87.7 (83.8, 91.7) | 83.3 (79.7, 86.8) | 0.09 |
| Cross-over test (LSI, %) | 87.0 (81.8, 92.2) | 84.4 (80.7, 88.2) | 0.42 |
| Y balance test (LSI, %) | 95.0 (92.3, 97.8) | 91.6 (88.8, 94.4) | 0.08 |
| Agility test (s) | 6.0 (5.7, 6.3) | 6.4 (6.2, 6.6) | 0.03 |
| ACL-RSI scale | 51.9 (45.9, 58.0) | 59.1 (51.6, 66.6) | 0.14 |
| IKDC score | 60.1 (56.0, 64.2) | 61.1 (57.0, 65.2) | 0.74 |
| 8 Postoperative months | |||
| Concentric quadriceps strength (LSI, %) | 85.6 (82.2, 89.0) | 85.9 (81.1, 90.6) | 0.93 |
| Single-hop test (LSI, %) | 91.1 (87.2, 94.9) | 92.0 (85.9, 98.1) | 0.78 |
| Triple-hop test (LSI, %) | 91.8 (87.3, 96.2) | 92.2 (88.5, 96.0) | 0.87 |
| Cross-over test (LSI, %) | 93.0 (88.9, 97.0) | 93.7 (89.4, 98.0) | 0.82 |
| Y balance test (LSI, %) | 96.5 (94.1, 99.0) | 94.4 (91.7, 97.0) | 0.23 |
| Agility test (s) | 5.8 (5.6, 6.1) | 6.1 (5.8, 6.3) | 0.21 |
| ACL-RSI scale | 47.7 (39.7, 55.7) | 63.3 (56.5, 70.0) | <0.01 |
| IKDC score | 64.1 (58.0, 70.1) | 64.8 (59.8, 69.8) | 0.84 |
Discussion
This randomised controlled triple-blinded trial suggests that the analgesic efficacy after ACL reconstruction provided by ACB is similar to that provided by LIA. Morphine consumption, resting and dynamic pain scores, and postoperative nausea and vomiting at 2 and 24 h after operation were comparable between groups, and neither analgesic technique impacted either early or late functional outcomes. As both ACB and LIA are believed to have a reduced impact on quadriceps muscle strength compared with the femoral nerve block, we felt it was of paramount importance to properly compare functional outcomes after ACL reconstruction alongside analgesic impact. The differences observed in resting pain scores at 48 h after operation, the agility test at 4 postoperative months, and the ACL-RSI score at 8 postoperative months appear to most likely represent type 1 errors given that these differences were not observed at other time points or in related functional outcomes. Although this study was not powered to detect a difference in any specific functional outcome, we feel that the wide range of outcomes examined at 24 and 48 h, and the long-term postoperative measures are unique in such studies. These findings suggest that the choice between the two analgesic techniques does not impact functional recovery.
Recently, three systematic reviews published by a single group explored the efficacy of different regional analgesic techniques:4, 7, 20 femoral nerve block vs placebo (five trials included),20 femoral nerve block and LIA vs LIA (three trials included),20 ACB vs placebo (two trials included),4 ACB vs femoral nerve block (three trials included),4 and LIA vs control (11 trials included).7 They concluded that for ACL reconstruction, LIA provides effective postoperative pain relief and could be recommended,7 unlike ACB or femoral nerve block which resulted in conflicting results compared with placebo. This latter conclusion is limited by the small number of included articles (from two4 to five20) or the absence of quantitative analysis.20 An additional meta-analysis including 11 trials showed that femoral nerve block was superior to LIA in reducing pain scores and opioid consumption during the first 24 h after operation, suggesting this approach as part of a standardised perioperative protocol.10 Despite this suggestion, no trials have compared LIA with ACB,4, 10 so ACB could be an interesting alternative to femoral nerve block because of sparing of the quadriceps muscle weakness.9 Our trial fills this knowledge gap and demonstrates that these two techniques represent equivalent analgesic and functional options.
There are notable limitations to our study. First, no assessment of the block quality was performed in order to avoid unmasking the group allocation blinding. We cannot therefore prove with certainty that the ACB was effective. However, postprocedural verification of appropriate local anaesthetic spread around the saphenous nerve by US ensured that the ACB was reliably performed according to standards.5 In addition, we did not perform a formal intra-articular injection. True intra-articular injection after ACL reconstruction has been associated with cases of chondrolysis that necessitated partial knee arthroplasty in patients <30 yr old.21 This concern led to development of the peri-articular injection technique for LIA that was used in our study. Of note, intra-articular injection was inferior to the femoral nerve block in a previous meta-analysis,10 further suggesting that injection within the joint may not be the optimal approach to LIA. Finally, we elected not to combine local anaesthetic with other adjuncts in either the LIA or ACB solutions to allow for greater homogeneity between groups, and to avoid off-label administration of medications without robust evidence.6 Some of these potential agents are included in our standard multimodal analgesic regimen, including magnesium sulphate and dexamethasone, which were administered i.v. in line with other RCT.22, 23 Administration of adjunct medications periarticularly or perineurally represents an unknown impact on our findings and an opportunity for further investigation.
In conclusion, ACB and LIA provide similar postoperative analgesia after ACL reconstruction, with an equivalent impact on early and late functional outcomes. Therefore, these two techniques represent equivalent analgesic options after ACL reconstruction.
Authors' contributions
Study design: KS, RM, EA.
Submission of the ethics protocol: KS.
Statistical analysis: KS, EA.
Patient recruitment: RM.
Manuscript editing: RM, JL, ADS.
Manuscript writing: KRK.
Study registration, data interpretation, manuscript preparation: EA.
Approved the final manuscript: all authors.
Declarations of interest
EA received an honorarium from B. Braun Medical AG. The other authors declared that they have no conflict of interest.
Funding
Swiss Academy for Anaesthesia Research (SACAR), Lausanne, Switzerland; B. Braun Medical AG; and Swiss National Science Foundation (grant number: 32003B_169974/1 to EA).
Handling editor: H.C. Hemmings Jr
Editorial decision: 17 April 2019
Footnotes
This article is accompanied by an editorial: Anterior cruciate ligament repair and peripheral nerve blocks: time to change our practice? by Ramlogan et al., Br J Anaesth 2019:123:e186–e188, doi: 10.1016/j.bja.2019.05.028
References
- 1.Stebler K., Martin R., Kirkham K.R., Küntzer T., Bathory I., Albrecht E. Electrophysiological study of femoral nerve function after a continuous femoral nerve block for anterior cruciate ligament reconstruction: a randomized, controlled single-blind trial. Am J Sports Med. 2017;45:578–583. doi: 10.1177/0363546516669715. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht E., Morfey D., Chan V. Single-injection or continuous femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res. 2014;472:1384–1393. doi: 10.1007/s11999-013-3192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht E., Niederhauser J., Gronchi F. Transient femoral neuropathy after knee ligament reconstruction and nerve stimulator-guided continuous femoral nerve block: a case series. Anaesthesia. 2011;66:850–851. doi: 10.1111/j.1365-2044.2011.06836.x. [DOI] [PubMed] [Google Scholar]
- 4.Sehmbi H., Brull R., Shah U.J. Evidence basis for regional anesthesia in ambulatory arthroscopic knee surgery and anterior cruciate ligament reconstruction: Part II: adductor canal nerve block-a systematic review and meta-analysis. Anesth Analg. 2019;128:223–238. doi: 10.1213/ANE.0000000000002570. [DOI] [PubMed] [Google Scholar]
- 5.Jæger P., Jenstrup M.T., Lund J. Optimal volume of local anaesthetic for adductor canal block: using the continual reassessment method to estimate ED95. Br J Anaesth. 2015;115:920–926. doi: 10.1093/bja/aev362. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht E., Guyen O., Jacot-Guillarmod A., Kirkham K.R. The analgesic efficacy of local infiltration analgesia vs femoral nerve block after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2016;116:597–609. doi: 10.1093/bja/aew099. [DOI] [PubMed] [Google Scholar]
- 7.Yung E.M., Brull R., Albrecht E., Joshi G.P., Abdallah F.W. Evidence basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction: Part III: local instillation analgesia-a systematic review and meta-analysis. Anesth Analg. 2019;128:426–437. doi: 10.1213/ANE.0000000000002599. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht E., Kirkham K.R. Optimising day-case analgesia for anterior cruciate ligament reconstruction - a reply. Anaesthesia. 2018;73:515–516. doi: 10.1111/anae.14266. [DOI] [PubMed] [Google Scholar]
- 9.Abdallah F.W., Whelan D.B., Chan V.W. Adductor canal block provides noninferior analgesia and superior quadriceps strength compared with femoral nerve block in anterior cruciate ligament reconstruction. Anesthesiology. 2016;124:1053–1064. doi: 10.1097/ALN.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 10.Kirkham K.R., Grape S., Martin R., Albrecht E. Analgesic efficacy of local infiltration analgesia vs. femoral nerve block after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Anaesthesia. 2017;72:1542–1553. doi: 10.1111/anae.14032. [DOI] [PubMed] [Google Scholar]
- 11.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht E., Kirkham K.R., Liu S.S., Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia. 2013;68:79–90. doi: 10.1111/j.1365-2044.2012.07335.x. [DOI] [PubMed] [Google Scholar]
- 13.Baeriswyl M., Kirkham K.R., Jacot-Guillarmod A., Albrecht E. Efficacy of perineural vs systemic dexamethasone to prolong analgesia after peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2017;119:183–191. doi: 10.1093/bja/aex191. [DOI] [PubMed] [Google Scholar]
- 14.Barfod K.W., Feller J.A., Hartwig T., Devitt B.M., Webster K.E. Knee extensor strength and hop test performance following anterior cruciate ligament reconstruction. Knee. 2019;26:149–154. doi: 10.1016/j.knee.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Gustavsson A., Neeter C., Thomeé P. A test battery for evaluating hop performance in patients with an ACL injury and patients who have undergone ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:778–788. doi: 10.1007/s00167-006-0045-6. [DOI] [PubMed] [Google Scholar]
- 16.Królikowska A., Ł Sikorski, Czamara A., Reichert P. Effects of postoperative physiotherapy supervision duration on clinical outcome, speed, and agility in males 8 months after anterior cruciate ligament reconstruction. Med Sci Monit. 2018;24:6823–6831. doi: 10.12659/MSM.912162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers H., Christopherson Z., Butler R.J. Relationship between the lower quarter Y-balance test scores and isokinetic strength testing in patients post-ACL reconstruction. Int J Sports Phys Ther. 2018;13:152–159. [PMC free article] [PubMed] [Google Scholar]
- 18.Bohu Y., Klouche S., Lefevre N., Webster K., Herman S. Translation, cross-cultural adaptation and validation of the French version of the anterior cruciate ligament-return to Sport after injury (ACL-RSI) scale. Knee Surg Sports Traumatol Arthrosc. 2015;23:1192–1196. doi: 10.1007/s00167-014-2942-4. [DOI] [PubMed] [Google Scholar]
- 19.Noorduyn J.C.A., van de Graaf V.A., Mokkink L.B. Responsiveness and minimal important change of the IKDC of middle-aged and older patients with a meniscal tear. Am J Sports Med. 2019;47:364–371. doi: 10.1177/0363546518812880. [DOI] [PubMed] [Google Scholar]
- 20.Vorobeichik L., Brull R., Joshi G.P., Abdallah F.W. Evidence basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction: Part I-femoral nerve block. Anesth Analg. 2019;128:58–65. doi: 10.1213/ANE.0000000000002854. [DOI] [PubMed] [Google Scholar]
- 21.Noyes F.R., Fleckenstein C.M., Barber-Westin S.D. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Jt Surg Am. 2012;94:1448–1457. doi: 10.2106/JBJS.K.01333. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht E., Bathory I., Fournier N., Jacot-Guillarmod A., Farron A., Brull R. Reduced hemidiaphragmatic paresis with extrafascial compared with conventional intrafascial tip placement for continuous interscalene brachial plexus block: a randomized, controlled, double-blind trial. Br J Anaesth. 2017;118:586–592. doi: 10.1093/bja/aex050. [DOI] [PubMed] [Google Scholar]
- 23.Palhais N., Brull R., Kern C. Extrafascial injection for interscalene brachial plexus block reduces respiratory complications compared with a conventional intrafascial injection: a randomized, controlled, double-blind trial. Br J Anaesth. 2016;116:531–537. doi: 10.1093/bja/aew028. [DOI] [PubMed] [Google Scholar]