Perioperative goal-directed haemodynamic therapy (GDT) is a protocolised treatment strategy aimed at optimisation of global cardiovascular dynamics, including oxygen delivery to tissues and organ perfusion pressure. This is achieved by titrating fluids, vasopressors, and inotropes to predefined physiological target values of haemodynamic variables.1 First proposed in the 1980s by Shoemaker and colleagues,2 perioperative GDT has been refined, further developed, and widely tested in clinical trials over the past two decades. Numerous RCTs comparing perioperative GDT with standard of care have been performed in many fields of perioperative medicine including abdominal, cardiothoracic, vascular, orthopaedic, liver, and general high-risk surgery.3 The results of these trials are summarised in several meta-analyses that provide substantial evidence that perioperative GDT can improve postoperative outcomes in patients having surgery.4, 5, 6, 7
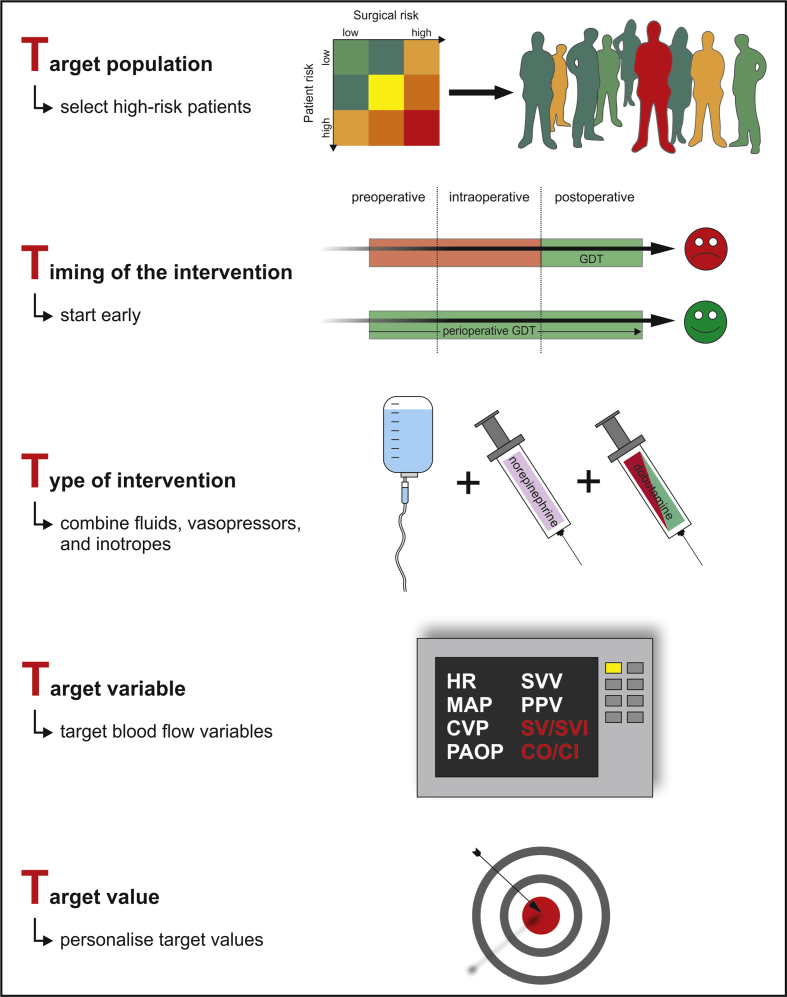
However, despite this evidence, even in high-resource settings, perioperative GDT is still poorly adopted in routine clinical practice. Perioperative therapeutic interventions are often performed according to subjective criteria rather than structured treatment algorithms based on advanced haemodynamic monitoring.8, 9, 10, 11 This might in part be because of the fact that the term ‘goal-directed therapy’ is poorly defined and is used to describe widely differing and heterogeneous treatment strategies.3, 12 To be widely adopted in clinical practice, the somewhat vague concept of perioperative GDT and its indications need to be clearly defined based on available evidence. We suggest the ‘5 Ts’ to specifically describe the concept and framework of perioperative GDT: target population, timing of the intervention, type of intervention, target variable, and target value (Fig. 1).
Fig. 1.
The ‘5 Ts’ of perioperative goal-directed haemodynamic therapy. CI, cardiac index; CO, cardiac output; CVP, central venous pressure; GDT, goal-directed haemodynamic therapy; PAOP, pulmonary artery occlusion pressure; PPV, pulse pressure variation; SV, stroke volume; SVI, stroke volume index; SVV, stroke volume variation.
Target population
Not all surgical patient populations benefit from perioperative GDT. For perioperative GDT to be effective, the target population needs to be at substantial risk for postoperative complications. As only ∼10% of all surgical patients account for >80% of postoperative deaths,13 identifying these high-risk patients before surgery is key to enable treating them using perioperative GDT. Although there are multiple scores for predicting the risk of postoperative complications,14 defining the ‘high-risk patient’ can be subjective and complex because multiple patient- and surgery-related factors contribute to the risk of adverse postoperative outcomes. Indicators for high patient risk include age, cardiovascular or pulmonary comorbidities, and impaired preoperative functional capacity.14 Surgical risk depends on the urgency, extent, invasiveness, and complexity of the procedure, and is especially high in patients having emergency, major abdominal, cardiac, or vascular surgery.14 Perioperative GDT reduces postoperative morbidity and mortality compared with standard care mainly in high-risk patients having major surgery (i.e. in patients with high patient risk, high surgical risk, or both).4, 5 To decide whether perioperative GDT is indicated in a patient, it is therefore of crucial importance to consider the individual patient risk and surgery-related risk. Identifying patients in whom perioperative GDT can actually improve postoperative outcomes is the first step in using perioperative GDT wisely.
Timing of the intervention
In contrast to critical illness in patients admitted to the ICU, major surgery under general anaesthesia is ‘scheduled trauma’ that enables alterations in cardiovascular dynamics to be anticipated and treated early or even prevented.15, 16 Perioperative GDT should thus start early in the intraoperative period rather than only in the postoperative phase.5 As there is evidence that haemodynamic instability occurring before surgical incision is associated with postoperative organ dysfunction,17, 18, 19 haemodynamic optimisation should probably start even before induction of general anaesthesia. This also implies early implementation of advanced haemodynamic monitoring (e.g. arterial catheter placement in the awake patient before induction of anaesthesia and assessment of advanced haemodynamic variables in addition to routine monitoring) and treatment (e.g. BP and blood flow optimisation during and after induction of general anaesthesia before surgical incision). Perioperative GDT bears the opportunity to prevent the development of organ dysfunction rather than only treating established organ failure.15
Type of intervention
To optimise organ perfusion pressure and tissue oxygen delivery, different therapeutic interventions are used including fluids (to optimise the intravascular fluid status and thus cardiac preload), vasopressors (to optimise cardiac afterload), and inotropes (to optimise myocardial contractility). Perioperative GDT only can exhibit a sustained improvement in postoperative outcomes if both fluids and vasoactive agents are part of the treatment protocol.4, 5, 20
Target variable
Numerous haemodynamic variables have been proposed as targets to be included in algorithms for perioperative GDT.1, 3 These include basic haemodynamic variables such as HR or BP, static cardiac filling pressures (central venous pressure, pulmonary artery occlusion pressure), dynamic cardiac preload variables (pulse pressure variation, stroke volume variation), and flow-related variables such as stroke volume, cardiac output/cardiac index, or oxygen delivery. To improve patient-centred outcomes, perioperative GDT should primarily include variables reflecting blood flow as targets and not only dynamic cardiac preload variables4, 5, 21 or even static cardiac preload variables that have been shown to be unsuitable to guide fluid therapy.22, 23
Target value
Defining absolute target values or thresholds of haemodynamic variables used in perioperative GDT is challenging. Dynamic cardiac preload variables used to predict fluid responsiveness (i.e. the probability that a fluid bolus will increase blood flow) have multiple limitations24 including a ‘grey zone’ (i.e. an ‘in-between’ range of values in which the variables are inconclusive), and have limited predictive capabilities.25 Target values of BP should probably be individualised to patients' normal BP26, 27 or personal BP profile.28 Cardiac output has a wide normal range and markedly depends on biometric factors including age29, 30, 31 and metabolic activity. To tailor target values of haemodynamic variables to the individual patient's cardiovascular physiology, functional haemodynamic monitoring, adaptive targets, personalised targets, and multiparametric approaches including physiological targets should be used.28, 32
In summary, there is substantial evidence that modern perioperative GDT, a protocolised haemodynamic optimisation strategy for titration of fluids, vasopressors, and inotropes to predefined target values of haemodynamic variables, improves postoperative patient-centred outcomes in terms of a reduction of complications and mortality. However, despite this evidence, perioperative GDT remains a poorly defined concept, and therefore is still poorly adapted to routine care. We suggest using the ‘5 Ts’ to specifically guide perioperative GDT and select the optimal target population (patients with high patient risk, high surgical risk, or both), timing of the intervention (early in the intraoperative period or even before induction of anaesthesia), type of intervention (combination of fluids and vasoactive agents), target variable (reflecting blood flow), and target value (personalised definition for each individual patient). We hope that this framework will help to facilitate implementation and adoption of perioperative GDT into clinical practice in order to improve patient outcomes.
Authors' contributions
Drafted the manuscript, created the figure, and approved the final version of the manuscript to be published: all authors.
Declaration of interests
BS collaborates with Pulsion Medical Systems SE (Feldkirchen, Germany) as a member of the medical advisory board and has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems SE. BS has received research support and honoraria for giving lectures from Edwards Lifesciences (Irvine, CA, USA). BS has received honoraria for giving lectures and travel expenses from CNSystems Medizintechnik GmbH (Graz, Austria). BS has received institutional restricted research grants, honoraria for consulting, and travel expenses from Tensys Medical Inc. (San Diego, CA, USA). BS has received institutional restricted research grants from Retia Medical LLC (Valhalla, NY, USA). BS has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). KK has no conflicts of interest to disclose. TWLS received research grants and honoraria from Edwards Lifesciences and Masimo Inc. (Irvine, CA, USA) for consulting and lecturing, and from Pulsion Medical Systems SE for lecturing.
References
- 1.Saugel B., Reuter D.A. Perioperative Goal-Directed Therapy using invasive uncalibrated pulse contour analysis. Front Med (Lausanne) 2018;5:12. doi: 10.3389/fmed.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoemaker W.C., Appel P.L., Kram H.B., Waxman K., Lee T.S. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann T., Clement R.P., Scheeren T.W.L., Saugel B., Keus F., van der Horst I.C.C. Perioperative goal-directed therapy: a systematic review without meta-analysis. Acta Anaesthesiol Scand. 2018;62:1340–1355. doi: 10.1111/aas.13212. [DOI] [PubMed] [Google Scholar]
- 4.Cecconi M., Corredor C., Arulkumaran N. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong M.A., Wang Y., Berbenetz N.M., McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? A systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:469–483. doi: 10.1097/EJA.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 6.Michard F., Giglio M.T., Brienza N. Perioperative goal-directed therapy with uncalibrated pulse contour methods: impact on fluid management and postoperative outcome. Br J Anaesth. 2017;119:22–30. doi: 10.1093/bja/aex138. [DOI] [PubMed] [Google Scholar]
- 7.Pearse R.M., Harrison D.A., MacDonald N. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad T., Beilstein C.M., Aldecoa C. Variation in haemodynamic monitoring for major surgery in European nations: secondary analysis of the EuSOS dataset. Perioper Med (Lond) 2015;4:8. doi: 10.1186/s13741-015-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearse R.M., Ackland G.L. Perioperative fluid therapy. BMJ. 2012;344:e2865. doi: 10.1136/bmj.e2865. [DOI] [PubMed] [Google Scholar]
- 10.Cannesson M., Pestel G., Ricks C., Hoeft A., Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011;15:R197. doi: 10.1186/cc10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biais M., Pearse R. Perioperative haemodynamic therapy: why are recommendations not being adopted? Anaesth Crit Care Pain Med. 2019;38:5–7. doi: 10.1016/j.accpm.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Roche A.M., Miller T.E. Goal-directed or goal-misdirected - how should we interpret the literature? Crit Care. 2010;14:129. doi: 10.1186/cc8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearse R.M., Harrison D.A., James P. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmor D., Kelly B. How to better identify patients at high risk of postoperative complications? Curr Opin Crit Care. 2017;23:417–423. doi: 10.1097/MCC.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 15.Saugel B., Michard F., Scheeren T.W.L. Goal-directed therapy: hit early and personalize! J Clin Monit Comput. 2018;32:375–377. doi: 10.1007/s10877-017-0043-x. [DOI] [PubMed] [Google Scholar]
- 16.Südfeld S., Brechnitz S., Wagner J.Y. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64. doi: 10.1093/bja/aex127. [DOI] [PubMed] [Google Scholar]
- 17.Maheshwari K., Turan A., Mao G. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–1228. doi: 10.1111/anae.14416. [DOI] [PubMed] [Google Scholar]
- 18.Sessler D.I., Bloomstone J.A., Aronson S. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–574. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Wesselink E.M., Kappen T.H., Torn H.M., Slooter A.J.C., van Klei W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Foss NB, Kehlet H. Perioperative haemodynamics and vasoconstriction – time for reconsideration? Br J Anaesth. 2019;123:100–103. doi: 10.1016/j.bja.2019.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Q.W., Tan W.C., Zhao B.C., Wen S.H., Shen J.T., Xu M. Is goal-directed fluid therapy based on dynamic variables alone sufficient to improve clinical outcomes among patients undergoing surgery? A meta-analysis. Crit Care. 2018;22:298. doi: 10.1186/s13054-018-2251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marik P.E., Cavallazzi R., Vasu T., Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 23.Marik P.E., Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 24.Michard F., Chemla D., Teboul J.L. Applicability of pulse pressure variation: how many shades of grey? Crit Care. 2015;19:144. doi: 10.1186/s13054-015-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannesson M., Le Manach Y., Hofer C.K. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology. 2011;115:231–241. doi: 10.1097/ALN.0b013e318225b80a. [DOI] [PubMed] [Google Scholar]
- 26.Futier E., Lefrant J.Y., Guinot P.G. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders R.D., Hughes F., Shaw A. Perioperative Quality Initiative consensus statement on preoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:552–562. doi: 10.1016/j.bja.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Saugel B., Vincent J.L., Wagner J.Y. Personalized hemodynamic management. Curr Opin Crit Care. 2017;23:334–341. doi: 10.1097/MCC.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 29.Saugel B., Mair S., Gotz S.Q. Indexation of cardiac output to biometric parameters in critically ill patients: a systematic analysis of a transpulmonary thermodilution-derived database. J Crit Care. 2015;30:957–962. doi: 10.1016/j.jcrc.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Cain P.A., Ahl R., Hedstrom E. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging. 2009;9:2. doi: 10.1186/1471-2342-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattermole G.N., Leung P.Y., Ho G.Y. The normal ranges of cardiovascular parameters measured using the ultrasonic cardiac output monitor. Physiol Rep. 2017;5 doi: 10.14814/phy2.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funcke S., Saugel B., Koch C. Individualized, perioperative, hemodynamic goal-directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial. Trials. 2018;19:273. doi: 10.1186/s13063-018-2620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]