Abstract
Background
Neuropathic pain, a type of chronic pain as a result of direct central or peripheral nerve damage, is associated with significant quality of life and functional impairment. Its underlying mechanisms remain unclear. We investigated whether ROR2, a member of the receptor tyrosine kinase-like orphan receptor (ROR) family, participates in modulation of neuropathic pain.
Methods
Thermal hyperalgesia and mechanical allodynia were measured using radiant heat and von Frey filament testing. Immunofluorescence staining was used to detect expression of ROR2 in neuronal nuclei. Fos expression was determined by immunocytochemistry. Phosphorylation status was detected by western blot and immunoprecipitation. Small interfering RNA was used to knock down ROR2 expression.
Results
ROR2 was upregulated and activated in spinal neurones after chronic constriction injury (CCI) in mice [1.3 (0.1) to 2.1 (0.1)-fold of sham, P<0.01] from Day 1–21. CCI induced significant demethylation of the CpG island in the ROR2 gene promoter [0.37 (0.06) vs 0.12 (0.03)% CpG methylation, P<0.001]. Knockdown of ROR2 in the spinal cord prevented and reversed CCI-induced pain behaviours and spinal neuronal sensitisation [Fos expression: 130 (12) vs 81 (8) cells, P<0.05; 120 (11) vs 70 (7) cells, P<0.05]. In contrast, activation of spinal ROR2 by intrathecal injection of Wnt5a induced pain behaviours and spinal neuronal sensitisation [Fos expression: 11 (1) vs 100 (12) cells, P<0.001] in wild-type mice. Furthermore, ROR2-mediated pain modulation required phosphorylation of N-methyl-D-aspartate receptor 2B subunit (GluN2B) at Ser 1303 and Tyr1472 by pathways involving protein kinase C (PKC) and Src family kinases. Intrathecal injection of GluN2B, PKC, or Src family kinase-specific inhibitors significantly attenuated Wnt5a-induced pain behaviours.
Conclusions
ROR2 in the spinal cord regulates neuropathic pain via phosphorylation of GluN2B, suggesting a potential target for prevention and relief of neuropathic pain.
Keywords: central sensitization, DNA methylation, neuropathic pain, NMDA receptors, ROR2 tyrosine kinase
Editor's key points.
-
•
The molecular mechanisms underlying neuropathic pain remain obscure, hampering development of new drugs.
-
•
The receptor tyrosine kinase (RTK) system in the spinal cord has been implicated in the development of neuropathic pain and spinal central sensitisation by modulation of N-methyl-D-aspartate receptor expression and plasticity.
-
•
The RTK-like orphan receptor (ROR), ROR2 was shown to be critical to pain hypersensitivity and spinal neuronal sensitisation in several mouse models of neuropathic pain.
-
•
Roles for ROR2 promoter methylation and N-methyl-D-aspartic acid receptor phosphorylation were identified and provide novel therapeutic opportunities for neuropathic pain.
Receptor tyrosine kinases (RTKs), a family of cell-surface receptors that have an intrinsic protein tyrosine kinase activity, are widely expressed in the CNS and involved in a variety of neurophysiological and neuropathological processes, including neuronal development, synaptic plasticity, and CNS diseases.1, 2 RTKs and their downstream signal transduction pathways play an important role in the development and persistence of neuropathic pain. For example, peripheral nerve injury induces activation and expression of the nerve growth factor/tropomyosin receptor kinase A,3 brain-derived neurotrophic factor/tropomyosin receptor kinase B,4 and glial cell line-derived neurotrophic factor/Ret5 RTK systems in the spinal cord. Intrathecal injection of inhibitors of these signalling pathways prevents and reverses neuropathic pain.6, 7 Previous studies have demonstrated that activation of the ephrinB/EphB RTK system in the spinal cord is associated with development of neuropathic pain and spinal central sensitisation by interacting with N-methyl-D-aspartate (NMDA) and toll-like receptors.8, 9 These findings suggest that activation of RTKs is a critical mechanism underlying the pathogenesis of neuropathic pain.
The receptor tyrosine kinase-like orphan receptors (RORs) are a conserved RTK family that is essential for cell migration, skeletal and nervous systems development, and oncogenesis.10, 11 There are two homologous ROR genes expressed in mammalian cells: ROR1 and ROR2. ROR1 lacks tyrosine kinase activity and is a pseudokinase. In contrast, ROR2 has an active tyrosine kinase domain and most likely functions as a genuine RTK.12 ROR2, but not ROR1, is expressed at excitatory synapses of hippocampal neurones and enhances NMDA receptor-mediated synaptic currents, suggesting that ROR2 plays an important role in synaptic plasticity.13, 14 Dysfunction of ROR2 expression is implicated in a variety of CNS diseases, such as neuronal injury, ischaemia, and Alzheimer's disease.15 Moreover, ROR2 is upregulated in several pain models, including in neuropathic pain.16, 17 However, the role of ROR2 in controlling this process is not well understood. Here, using a chronic constriction injury (CCI) of the sciatic nerve model, we examined the correlation between expression of ROR2 and neuropathic pain and the molecular mechanism of how ROR2 modulates neuropathic pain.
Methods
Additional details are provided in the Supplementary Methods.
Pain models
Adult male ICR mice were obtained and housed in the centre of Experimental Animals of Nanjing Medical University. All experimental procedures were approved by the Animal Care and Use Committee of Nanjing Medical University and were in keeping with the International Association for the Study of Pain guidelines. CCI, L5 spinal nerve ligation, and spared nerve injury models of neuropathic pain were performed as described.18 For sham-operated mice, the nerve was exposed but without ligation or transection.
Drugs and reagents
ROR2 small interfering RNA (siRNA) (5′-CGUACGCGUGGAACUGUGUTT-3′) and its scrambled negative control (5′-GUAUGCGCGUCGUAGAGUCTT-3′) were designed and synthesised by Shanghai GenePharma (Shanghai, China). The NMDA receptor antagonist MK801, selective NR2B inhibitor ifenprodil, PKC inhibitor calphostin C, Src family kinase (SFK) inhibitor SU6656, c-Jun N-terminal kinase (JNK) inhibitor SP600125, protease inhibitor cocktail, and phosphatase inhibitor cocktails 2 and 3 were purchased from Sigma (St. Louis, MO, USA). Recombinant mouse Wnt5a protein was purchased from R&D Systems (Minneapolis, MN, USA). Antibodies against ROR2, Fos, NeuN, phosphotyrosine, phosphoserine, GluN1, GluN2A, GluN2B, GluN2B–Ser 1303, GluN2B–Ser 1323, GluN2B-Tyr1336, and GluN2B-Tyr1472 were purchased from Millipore (Billerica, MA, USA). Antibody against β-actin and horseradish peroxidase (HRP)-linked goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Alexa Fluor 488- or 594-conjugated goat anti-rabbit secondary antibodies were purchased from Molecular Probes (Eugene, OR, USA). Intrathecal injection was performed with a 28 G stainless steel needle between the L5 and L6 vertebrae to deliver drugs to the subarachnoid space.19 siRNAs were mixed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) before intrathecal injection.
Experimental protocol
Mice were randomised to each model on Day 0. ROR2 expression was assessed on Days 1, 3, 7, 14, and 21. ROR2/neuronal nuclei double label and methylation status of the ROR2 promoter were assessed on Day 7. ROR2 siRNA was administrated daily for 3 days before surgery or on Days 7, 8, and 9 after surgery. Pain behavioural testing was assessed on Days 2–15. Spinal Fos expression was determined on Days 7 and 11. Phosphorylation of NMDA receptors was investigated on Day 11.
Behavioural testing
Paw withdrawal latency (PWL) to thermal stimulus and paw withdrawal threshold (PWT) to mechanical stimulus were performed as described.19 For PWL, mice were placed on a glass plate in a plastic box and allowed to adapt for 1 h. The plantar surface of the hind paw was exposed to radiant heat through the glass. Baseline values were adjusted to 12–15 s and an automatic 25 s cutoff was used to prevent injury. Values were averaged over three tests at intervals of 5 min. For PWT, mice were placed in a plastic box with a metal mesh floor. The plantar surface was stimulated, in ascending order of strength, with a series of von Frey filaments (starting with 0.31 g and ending with 4.0 g). Results were analysed using the Dixon up-and-down method.20 Behavioural testing was performed in a blinded manner.
Real-time quantitative polymerase chain reaction
Total RNA was extracted and reverse transcribed into cDNA using Trizol and oligo (dT) primers. Polymerase chain reaction (PCR) was performed with the Real-Time Detection System using SYBR Green I dye detection (Takara, Dalian, Liaoning, China). Data were analysed with the 2−ΔΔCT method.21 Primers for the selected genes were as follows: ROR1: forward 5-TGAGCCGATGAATAACATCACAA-3′, reverse 5-CAGGTGCATCATTCTTGAACCA-3′; ROR2: forward 5-ATCGACACCTTGGGACAACC-3′, reverse 5-AGTGCAGGATTGCCGTCTG-3; glyceraldehyde 3-phosphate dehydrogenase: forward 5-AGGTCGGTGTGAACGGATTTG-3, reverse 5-TGTAGACCATGTAGTTGAGGTCA-3.
Western blot and immunoprecipitation
Protein samples, prepared as described,19 were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. After blocking with non-fat milk, blots were incubated with primary antibodies against ROR2, phosphotyrosine, phosphoserine, GluN1, GluN2A, GluN2B, GluN2B–Ser 1303, GluN2B–Ser 1323, GluN2B-Tyr1336, GluN2B-Tyr1472, and β-actin overnight at 4°C. Blots were extensively washed and further incubated with HRP-conjugated secondary antibody at room temperature for 2 h, with immune complexes detected by using chemiluminescence (Pierce, Dallas, TX, USA). The intensity of each band was determined using Image J software (National Institutes of Health, Bethesda, MA, USA). For immunoprecipitation, the solubilised protein samples were preincubated with protein G/A-agarose for 6 h at 4°C and then incubated with 50 ml of protein G/A-agarose precoupled to antibody against primary antibodies for at least 3 h. The mixtures were then washed, boiled, and subjected to western blot procedures.
Immunofluorescence and immunocytochemistry
Mice were deeply anaesthetised and transcardially perfused with 4% paraformaldehyde. The spinal cord was quickly removed, postfixed with 4% paraformaldehyde, cryoprotected in 30% sucrose, and cut into 30 μm sections. After blocking and membrane permeabilisation with (phosphate buffer saline containing 5% normal goat serum and 0.3% Triton X-100), sections were incubated with antibodies against ROR2 and NeuN overnight at 4°C. On the following day, Alexa Fluor 488- and 594-conjugated secondary antibodies were added and incubated at room temperature for 2 h. Controls omitted the primary or secondary antibodies. Sections were subsequently washed and mounted for confocal imaging. Fos immunocytochemistry was performed as reported.22
Analysis of CpG islands and bisulfite sequencing
CpG (cytosine guanine dinucleotide) islands, CpG dinucleotide-rich regions, were predicted using Methprimer (http://www.urogene.org/methprimer/). Genomic DNA from the spinal cord was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Bisulphite treatment was carried out according to the manufacturer's recommendations (EZ DNA Methylation-Gold kit, Zymo, Orange, CA, USA). After PCR amplification, PCR products were purified and cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA, USA), and 20 clones from each mouse were randomly chosen for DNA sequencing. The primers for PCR were as follows: forward (5′-GTTTTTGATGGTGGGTGGTAATA-3′) and reverse (5′-CAAAAAACAAAATCCCCAACTC-3′).
Statistical analysis
Data were expressed as mean (standard deviation), and P<0.05 was considered significant. Differences between two groups were analysed using unpaired two-tailed Student's t-test. Differences between more than two groups were analysed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Behavioural data were compared by two-way repeated measures ANOVA followed by Tukey's post hoc test. Statistical analysis of differences between groups in our experiment were based on a normality test of the data.
Results
Increase of ROR2 expression and activation in the spinal cord in neuropathic pain models
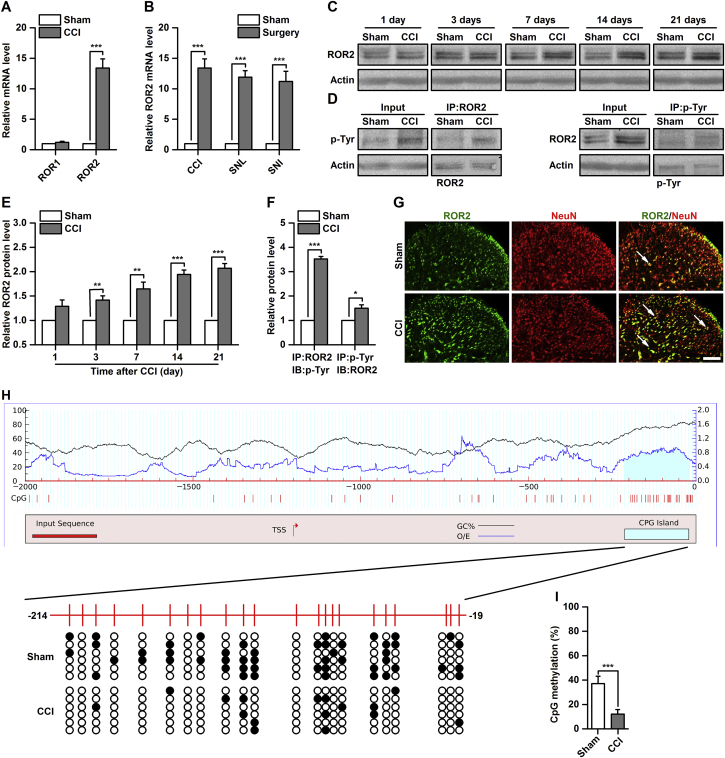
To investigate whether spinal RORs are involved in the modulation of neuropathic pain, we first examined their expression in a CCI-induced neuropathic pain model. The results from qPCR revealed that ROR2, but not ROR1, mRNA concentration was significantly increased on Day 7 after CCI [13.4 (1.5)-fold of sham, P<0.001, Fig 1a]. This increase in spinal ROR2 mRNA was also observed following sciatic nerve ligation (SNL) or sciatic nerve injury (SNI) by 11.9 (1.1)- and 11.2 (1.7)-fold of sham, respectively (P<0.001, Fig 1b). Based on the change of ROR2 mRNA induced by peripheral nerve injury, we analysed the time course of ROR2 expression in the spinal cord after CCI or sham surgery. Expression of ROR2 protein was significantly increased from Day 3–21 in CCI mice, but not in sham-operated mice by 1.3 (0.1)- to 2.1 (0.1)-fold of sham (P<0.01, Fig. 1c and e). We analysed tyrosine phosphorylation of ROR2 to indicate ROR2 activation by CCI causes in the spinal cord. Tyrosine phosphorylation of ROR2 was significantly increased on Day 7 after CCI [3.5 (0.1)-fold of sham, P<0.001, Figs 1d and f]. Immunofluorescence studies found that ROR2 was colocalised with NeuN (a neuronal marker) in the spinal cord and that ROR2 expression was increased in CCI mice compared with sham control mice (Fig 1g).
Fig 1.
Alterations in receptor tyrosine kinase-like orphan receptor (ROR) expression after chronic constriction injury (CCI). (a) ROR mRNA expression in the spinal cord on Day 7 after CCI or sham surgery. ***P<0.001, n=6. (b) ROR2 mRNA expression in the spinal cord on Day 7 after sciatic nerve ligation (SNL) or sciatic nerve injury (SNI). ***P<0.001, n=6. (c and e) time course of ROR2 protein expression in the spinal cord after CCI or sham surgery; representative western blots are shown in (c) and quantitative data are shown in (e). **P<0.01, ***P<0.001, n=6. (d and f) ROR2 activation on Day 7 after CCI or sham surgery; representative western blots are shown in (d) and quantitative data are shown in (f). *P<0.05, ***P<0.001, n=6. IP, immunoprecipitation; p-Tyr, tyrosine phosphorylation. (g) Combined ROR2 and NeuN (a neuronal marker) immunofluorescence staining in the spinal cord on Day 7 after CCI or sham surgery. Scale bar, 25 μm. (h) CpG island methylation status of ROR2 promoter. Black line indicates the GC% of input sequence; Blue line indicates the observed/expected CpG ratio (O/E) of input sequence. Left y-axis scale pertains to black line; and right y-axis scale pertains to blue line. White and black dots represent demethylated and methylated CpG dinucleotide, respectively. Each line indicates an individual sequence.
DNA methylation has recently been implicated in regulation of ROR2 expression,23, 24 so we investigated possible aberrant epigenetic regulation of ROR2 in neuropathic pain. Our analysis of the 2000 bp upstream sequence of the ROR2 gene revealed a typical CpG island (Fig 1h). By bisulfite sequencing, we found that this CpG island was significantly demethylated in CCI mice compared with the sham group [37.1 % (6.0 %) vs 12.2% (3.7 %), P<0.001, Fig 1h]. These results indicate that epigenetic modification of spinal ROR2 expression may be involved in the pathogenesis of neuropathic pain.
Knockdown of ROR2 inhibited and reversed CCI-induced pain behaviours and spinal neuronal sensitisation
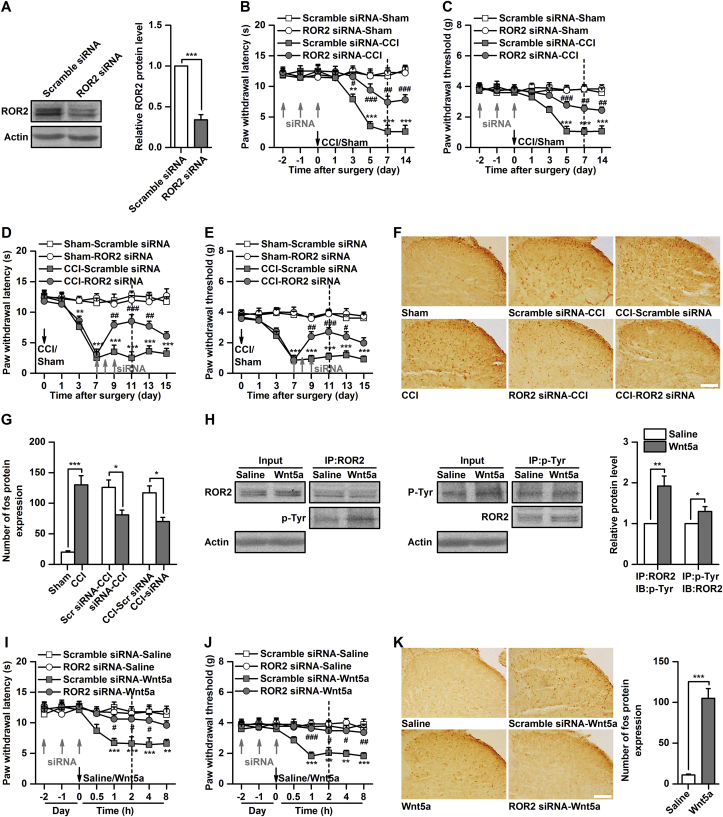
To determine whether increased ROR2 expression in the spinal cord contributes to pain behaviours and spinal neuronal sensitisation induced by CCI, we pre- or posttreated mice with ROR2 siRNA to silence ROR2 gene expression before or after CCI. The efficacy of intrathecal injection of ROR2 siRNA on protein expression was validated by western blot [0.34 (0.06)-fold of scrambled siRNA, P<0.001, Fig 2a]. Spinal administration of ROR2 siRNA, but not of scrambled control siRNA, for 3 consecutive days significantly inhibited and reversed CCI-induced thermal hyperalgesia (P<0.05, ROR2 siRNA-CCI vs scrambled siRNA-CCI; P<0.01, CCI-ROR2 siRNA vs CCI-scrambled siRNA, Fig. 2b and d) and mechanical allodynia (P<0.01, ROR2 siRNA-CCI vs scrambled siRNA-CCI; P<0.05, CCI-ROR2 siRNA vs CCI-scrambled siRNA, Fig. 2c and e). To further determine the effect of ROR2 knockdown on CCI-induced spinal neuronal sensitisation, we evaluated spinal Fos expression as a marker of neuronal sensitisation after ROR2 siRNA or scrambled control siRNA injection. Intrathecal injection of ROR2 siRNA, but not of scrambled siRNA, significantly inhibited the CCI-induced increase in Fos expression [126 (12) vs 81 (8) cells, P<0.05; 120 (11) vs 70 (7) cells, P<0.05; 120 (11) vs 70 (7), P<0.05, Fig. 2f and g].
Fig 2.
Manipulation of receptor tyrosine kinase-like orphan receptor ROR2 in the spinal cord regulates pain behaviours and neuronal sensitisation. (a) Validation of ROR2 small interfering RNA (siRNA) transfection efficiency in vivo. ***P<0.001, n=6. (b and c) effect of ROR2 siRNA (10 μg in 0.5 μl) on development of CCI-induced thermal hyperalgesia (b) and mechanical allodynia (c). **P<0.01, ***P<0.001, Scramble siRNA-Sham vs Scramble siRNA-CCI; #P<0.05, ##P<0.01, ###P<0.001, Scramble siRNA-CCI vs ROR2 siRNA-CCI; n=8. (d and e) Effect of ROR2 siRNA (10 μg in 0.5 μl) on maintenance of CCI-induced thermal hyperalgesia (d) and mechanical allodynia (e). **P<0.01, ***P<0.001, Sham-Scramble siRNA vs CCI-Scramble siRNA; #P<0.05, ##P<0.01, ###P<0.001, CCI-Scramble siRNA vs CCI-ROR2 siRNA; n=8. (f and g) Fos expression after intrathecal injection of ROR2 siRNA (10 μg in 0.5 μl) or scramble siRNA in CCI mice; representative immunocytochemical staining is shown in (f) and quantitative data are shown in (g). Dotted lines in the above behaviour test figures indicate Fos evaluation times. *P<0.05, ***P<0.001, n=6. (h) Tyrosine phosphorylation of ROR2 after spinal administration of Wnt5a (0.5 μg in 0.5 μl). *P<0.05, ***P<0.001, n=6. (i and j) Effect of Wnt5a (0.5 μg in 0.5 μl) on the thermal hyperalgesia (i) and mechanical allodynia (j) in naïve mice. **P<0.01, ***P<0.001, Scramble siRNA-Saline vs Scramble siRNA-Wnt5a; #P<0.05, ##P<0.01, ###P<0.001, Scramble siRNA-Wnt5a vs ROR2 siRNA-Wnt5a; n=8. (k) Fos expression after intrathecal injection of Wnt5a (0.5 μg in 0.5 μl) or saline in naïve mice. Fos was assessed 2 h after Wnt5a injection. ***P<0.001, n=6.
Activation of ROR2 produced pain behaviours and spinal neuronal sensitisation
Because knockdown of spinal ROR2 attenuated pain behaviours and spinal neuronal sensitisation induced by CCI, we questioned whether activation of ROR2 could reproduce this. Wnt5a, a non-canonical Wnt ligand, is capable of activating ROR225 and is upregulated in the spinal cord after peripheral nerve injury.16, 17 Activation of ROR2 by Wnt5a was validated by immunoprecipitation [1.9 (0.2)-fold of control, P<0.01, Fig 2h]. Activation of ROR2 by intrathecal injection of Wnt5a, but not saline, produced a significant decrease in thermal pain latency and mechanical pain threshold (P<0.01, scrambled siRNA-saline vs scrambled siRNA-Wnt5a, Fig. 2i and j) and increased spinal Fos expression [11 (1) vs 10 (12) cells, P<0.001, Fig 2k] in naive mice. Furthermore, Wnt5a-induced thermal hyperalgesia, mechanical allodynia (P<0.05, scrambled siRNA-Wnt5a vs ROR2 siRNA-Wnt5a, Fig. 2i and j) and Fos expression [89 (11) vs 9 (1) cells, P<0.001, Fig 2k] were prevented by pretreatment with ROR2 siRNA, suggesting that Wnt5a-induced pain behaviours are mediated by the ROR2 receptor. Together, these findings indicate that ROR2 in the spinal cord contributes to the modulation of neuropathic pain processing.
ROR2 modulated neuropathic pain via phosphorylation of the GluN2B subunit
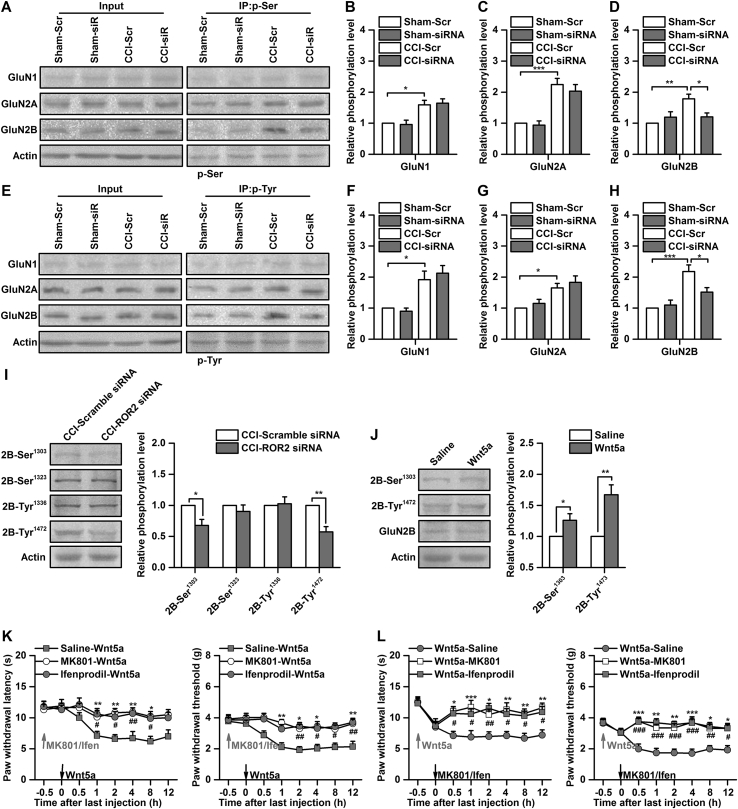
ROR2, as a non-canonical Wnt receptor, colocalises with the NMDA receptor and upregulates synaptic NMDA receptor currents.13, 14 Phosphorylation of the NMDA receptor at serine and tyrosine residues regulates the electrophysiological properties of the NMDA receptor.26 To determine whether spinal ROR2 modulates neuropathic pain via phosphorylation of the NMDA receptor, we treated the mice with ROR2 siRNA or scrambled siRNA after CCI and examined alterations of NMDA receptor phosphorylation. Serine phosphorylation of the GluN1, GluN2A, and GluN2B subunits was significantly increased on Day 7 after CCI [by 1.6 (0.1)-, 2.2 (0.2)-, and 1.8 (0.2)-fold of sham-Scr, P<0.05, Figs 3a–d]; however, only increased serine phosphorylation of the GluN2B subunit was reversed by ROR2 siRNA [1.8 (0.2) vs 1.2 (0.1), P<0.05, Fig 3b]. Similarly, CCI increased tyrosine phosphorylation of the GluN1, GluN2A, and GluN2B subunits in the spinal cord on Day 7 after CCI [by 1.9 (0.3)-, 1.6 (0.1)-, 2.2 (0.2)-fold of sham-Scr, P<0.05, Figs 3e–h], while the increase in tyrosine phosphorylation of the GluN2B subunit was reversed by knockdown of ROR2 [2.2 (0.2) vs 1.5 (0.1), P<0.05, Fig 3g].
Fig 3.
Phosphorylation of GluN2B is required for modulation of neuropathic pain related to receptor tyrosine kinase-like orphan receptor ROR2. (a) Representative western blots showing the effect of ROR2 small interfering RNA (siRNA) on serine phosphorylation of N-methyl-D-aspartic acid (NMDA) receptors induced by chronic constriction injury (CCI). P-Ser, serine phosphorylation. (b) Quantitative data of serine phosphorylation of GluN1 subunit. *P<0.05, n=6. Scr, scramble. (c) Quantitative data of serine phosphorylation of GluN2A subunit. ***P<0.001, n=6. (d) Quantitative data of serine phosphorylation of GluN2B subunit. *P<0.05, **P<0.01, n=6. (e) Representative western blots showing the effect of ROR2 siRNA (10 μg in 0.5 μl) on tyrosine phosphorylation of NMDA receptors induced by CCI. (f) Quantitative data of tyrosine phosphorylation of GluN1 subunit. *P<0.05, n=6. (g) Quantitative data of tyrosine phosphorylation of GluN2A subunit. *P<0.05, n=6. (h) Quantitative data of tyrosine phosphorylation of GluN2B subunit. *P<0.05, ***P<0.001, n=6. Phosphorylation of NMDA receptor was assessed on Day 11, the same as in Figure 2d. (i) Representative western blots and quantitative data for phosphorylation of GluN2B subunit at Ser 1303, Ser 1323, Tyr1336, and Tyr1472 sites after ROR2 siRNA (10 μg in 0.5 μl) or scrambled siRNA injection. *P<0.05, **P<0.01, n=6. (j) Representative western blots and quantitative data for phosphorylation of GluN2B subunit at Ser1303 and Tyr1472 sites after Wnt5a (0.5 μg in 0.5 μl) or saline injection. *P<0.05, **P<0.01, n=6. (k) Effect of pretreatment with MK801(2.5 μg in 0.5 μl) and ifenprodil (0.5 μg in 0.5 μl) on thermal hyperalgesia and mechanical allodynia induced by Wnt5a. *P<0.05, **P<0.01, Saline-Wnt5a vs MK801-Wnt5a; #P<0.05, ##P<0.01, Saline-Wnt5a vs Ifenprodil-Wnt5a; n=8. Ifen, ifenprodil. (l) Effect of posttreatment with MK801 (2.5 μg in 0.5 μl) and ifenprodil (0.5 μg in 0.5 μl) on thermal hyperalgesia and mechanical allodynia induced by Wnt5a. *P<0.05, **P<0.01, ***P<0.001, Wnt5a-Saline vs Wnt5a-MK801; #P<0.05, ##P<0.01, ###P<0.01, Wnt5a-Saline vs Wnt5a-Ifenprodil; n=8.
The GluN2B subunit has several serine and tyrosine phosphorylation sites, of which Ser 1303, Ser 1323, Tyr1472, and Tyr1336 are involved in pathologic pain and contribute to modulation of NMDA receptor function.27 To identify the phosphorylation sites involved in GluN2B subunit phosphorylation, we assessed phosphorylation of the GluN2B subunit at these four sites using phospho-site-specific antibodies. CCI-induced phosphorylation at Ser1303 and Tyr1472 were markedly decreased by intrathecal injection of ROR2 siRNA. In contrast, no significant alterations were found in phosphorylation at Ser1323 and Tyr1336 [0.7 (0.1)-, 0.6 (0.1)-fold of CCI-scrambled siRNA, P<0.05, Fig 3i]. Phosphorylation at Ser1303 and Tyr1472 was increased after activation of ROR2 by intrathecal injection of Wnt5a in naive mice [1.3 (0.1)-, 1.7 (0.2)-fold of saline, P<0.05, Fig 3j]. Finally, behavioural testing showed that thermal hyperalgesia and mechanical allodynia induced by Wnt5a were inhibited and reversed by pre- or posttreatment with the NMDA receptor antagonist MK-801 or the GluN2B subunit selective inhibitor ifenprodil (P<0.05, MK801-Wnt5a vs saline-Wnt5a, Wnt5a-MK801 vs Wnt5a-saline, Fig 1, Fig 3k and l). Together, these results suggest that phosphorylation of the GluN2B subunit at Ser1303 and Tyr1472 is required for modulation of neuropathic pain by ROR2 in the spinal cord.
Involvement of PKC and Src family kinases in phosphorylation of GluN2B
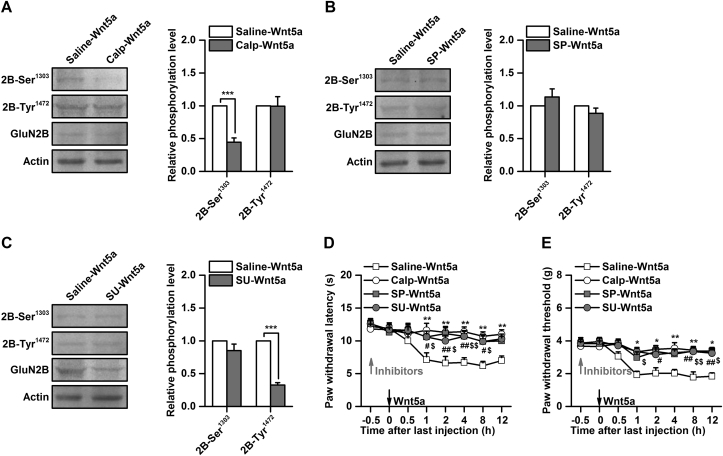
ROR2 exerts its effects by activating several downstream signalling kinases, such as Src family kinases (SFKs)28 or non-canonical Wnt signalling components, including PKC29 and JNK.30 To investigate the signalling pathways that are involved in phosphorylation of the GluN2B subunit by ROR2, we pretreated mice with various protein kinase inhibitors. Phosphorylation at Ser 1303, but not at Tyr1472, was significantly decreased by inhibition of PKC with calphostin C [0.4 (0.1)-fold of saline-Wnt5a, P<0.001, Fig 4a]. In contrast, pretreatment with the SFK inhibitor SU6656 significantly decreased phosphorylation at Tyr1472, but not at Ser1303 [0.3 (0.1)]-fold of saline-Wnt5a, P<0.001, Fig 4c]. No significant alteration in phosphorylation of Ser1303 or Tyr1472 was observed by inhibition of JNK with SP600125 [0.9 (0.1)-fold of saline-Wnt5a, P>0.05, Fig. 4b]. Consistent with inhibition of PKC and SFKs, behavioural testing showed that thermal hyperalgesia and mechanical allodynia induced by intrathecal injection of Wnt5a were reversed by calphostin C or SU6656 (P<0.05, vs saline-Wnt5a, Fig 4d and e). However, intrathecal injection of Wnt5a-induced thermal hyperalgesia and mechanical allodynia were also reversed by inhibition of JNK with SP600125 (P<0.05, vs saline-Wnt5a, Fig. 4d and e). Collectively, these results suggest that phosphorylation of the GluN2B subunit related to ROR2 in the spinal cord is dependent on PKC and SFKs.
Fig 4.
Involvement of protein kinase C (PKC) and Src in regulation of GluN2B phosphorylation related to receptor tyrosine kinase-like orphan receptor ROR2. (a) Effect of the PKC inhibitor calphostin C (0.2 μg in 0.5 μl) on phosphorylation of GluN2B-Ser 1303 and GluN2B-Tyr1472 induced by Wnt5a. ***P<0.001, n=6. (b) Effect of JNK inhibitor SP600125 (0.5 μg in 0.5 μl) on phosphorylation of GluN2B-Ser1303 and GluN2B-Tyr1472 induced by Wnt5a. n=6. (c) Effect of Src family kinase (SFK) inhibitor SU6656 (0.5 μg in 0.5 μl) on phosphorylation of GluN2B-Ser1303 and GluN2B-Tyr1472 induced by Wnt5a. ***P<0.001, n=6. (d and e) effect of calphostin C (0.2 μg in 0.5 μl), SP600125 (0.5 μg in 0.5 μl), and SU6656 (0.5 μg in 0.5 μl) on thermal hyperalgesia (d) and mechanical allodynia (e) induced by Wnt5a. *P<0.05, **P<0.01, Saline-Wnt5a vs calphostin C-Wnt5a; #P<0.05, ##P<0.01, Saline-Wnt5a vs SP600125-Wnt5a; $P<0.05, $$P<0.01, Saline-Wnt5a vs SU6656-Wnt5a; n=8. Calp, calphostin C; SP, SP600125; SU, SU6656.
Discussion
We show that demethylated ROR2 in the spinal cord is implicated in the regulation of neuropathic pain and neuronal sensitisation via phosphorylation of GluN2B. The major findings are: 1) long-term activation and increased expression of ROR2 participates in the development and maintenance of neuropathic pain and spinal neuronal sensitisation; 2) significant demethylation of a CpG island in the ROR2 gene promoter is induced by CCI; 3) an increase in phosphorylation of GluN2B mediates regulation of neuropathic pain by ROR2 in the spinal cord; and 4) PKC and SFKs are critical to ROR2-mediated increased phosphorylation of GluN2B. These findings demonstrate a novel mechanism for the modulation of neuropathic pain by ROR2 in the spinal cord.
ROR2, a member of the receptor tyrosine kinase-like orphan receptor family, plays an important role in neuronal development and neuronal plasticity.15 In cultured hippocampal neurons, ROR2 is essential for regulation of neurite outgrowth and axonal branching, possibly via microtubule-associated protein-mediated microtubule remodeling.31 Activation of ROR2 by Wnt5a enhances Disheveled 2 (DVL2) phosphorylation and polymerisation, neuronal progenitor cell self-renewal and differentiation, and peripheral target innervation of sympathetic neurones.32, 33, 34 Moreover, overexpression of ROR2 in hippocampal neurones increases NMDA receptor-mediated synaptic currents, and its knockdown decreases NMDA-mediated synaptic transmission.13 Using a CCI-induced neuropathic pain model, we found that activation and expression of ROR2 were upregulated in the spinal cord in neuropathic pain models. Moreover, knockdown of ROR2 could prevent and reverse CCI-induced increase in spinal Fos protein expression and pain behaviour. Activation of ROR2 by intrathecal injection of Wnt5a induced pain behaviour and spinal Fos protein expression in naive mice. These findings demonstrate that ROR2 expressed in the spinal cord contributes to modulation of neuropathic pain, which expands knowledge about the functional role of ROR2 in the CNS.
Central sensitisation, which is characterised by an increase in neuronal excitability, is considered a major mechanism that contributes to neuropathic pain.35 Induction and maintenance of neuronal sensitisation are largely dependent on activation of the NMDA receptor and its downstream signalling pathway,36 which are critical to development of neuronal sensitisation in the spinal cord during neuropathic pain.37 However, the regulatory mechanisms of the NMDA receptor in the spinal cord underlying neuropathic pain remain unclear. We found that activation of ROR2 increased phosphorylation of the GluN2B subunit, but not the GluN1 subunit, in the spinal cord. Knockdown of ROR2 inhibited CCI-induced increase in phosphorylation of GluN2B. We found that ROR2 mediates phosphorylation of the GluN2B subunit at Ser 1303 and Tyr1472. These findings suggested that ROR2-mediated phosphorylation of the GluN2B subunit could be an important mechanism for central sensitisation in the spinal cord during neuropathic pain.
Phosphorylation of GluN2B is a major regulatory mechanism for plastic changes in the NMDA receptor.27 Various protein kinases can phosphorylate GluN2B, such as PKA, PKC, and SFKs.27 Activation of ROR2 increases the activity of PKC and JNK in hippocampal neurones of juvenile mice,13, 14 and activation of ROR2 plays an important role in recruiting and activating SFKs.28 We found that PKC and SFKs, but not JNK, contribute to increased ROR2-mediated phosphorylation of the GluN2B subunit. Phosphorylation of GluN2B at Ser 1303 by PKC enhances NMDA receptor currents38; however, phosphorylation at Tyr1472 by SFKs increases surface expression of GluN2B.39, 40 Interestingly, inhibition of JNK did not change phosphorylation of GluN2B, but significantly reversed Wnt5a-induced thermal hyperalgesia and mechanical allodynia. Phosphorylation of synaptic scaffolding proteins by JNK is essential for anchoring the NMDA receptor at synaptic sites.41 We propose that JNK mediates modulation of neuropathic pain by ROR2 in an NMDA receptor trafficking-dependent manner.
Our findings revealed upregulation of ROR2 activation and expression after CCI, leading to increased activation of downstream SFK and PKC, which results in increased phosphorylation of GluN2B at Ser 1303 and Tyr1472 in the spinal cord. Upon phosphorylation of GluN2B, the electrophysiological properties of the NMDA receptor are changed to induce and maintain spinal neuronal sensitisation, accompanied by decreased withdrawal responses to mechanical and thermal stimuli after surgery for CCI in mice. Knockdown of ROR2 or intrathecal injection of GluN2B, SFK, and PKC-specific inhibitors attenuated pain behaviours in mice.
Activation of microglial cells in response to nerve injury has been implicated in the development of neuropathic pain.42 Co-culture of spinal neurones with microglia significantly upregulated ROR2 signaling.43 Therefore, it is possible that there is a cross-talk between the ROR2 signalling pathway and microglia under neuropathic pain conditions. Neuroinflammation may contribute to modulation of spinal nociceptive information related to ROR2. The underlying mechanisms need to be explored further.
Our study is not without limitations. The effect of ROR2 siRNA on pain behaviours was only investigated 15 days after CCI, and remains to be studied at the later time points of increased ROR2 expression (up to 21 days). Second, our data only showed demethylation of the CpG island in the ROR2 gene promoter induced by CCI. It is difficult to conclude whether the observed increase of ROR2 expression was because of direct effects of the demethylation of ROR2 gene promoter based on our data.
In conclusion, we identified an important role for a member of the receptor tyrosine kinase-like orphan receptor family, ROR2, in the development and persistence of neuropathic pain. Our findings may lead to novel targeted drug discovery for the treatment and relief of neuropathic pain.
Authors' contributions
Study design: XLZ, CML.
Study conduct: XLZ, CJZ, YNP, YW, HJX.
Data analysis: XLZ, CJZ.
Writing paper: XLZ, CJZ.
Revising paper: all authors.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Young Scholarship Program of the National Natural Science Foundation of China (81701101).
Handling editor: H.C. Hemmings Jr
Editorial decision: 20 August 2018
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.08.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Purcell A.L., Carew T.J. Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci. 2003;26:625–630. doi: 10.1016/j.tins.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol. 2014;6:a008912. doi: 10.1101/cshperspect.a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obata K., Tsujino H., Yamanaka H. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. 2002;99:121–132. doi: 10.1016/s0304-3959(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 4.Coull J.A., Beggs S., Boudreau D. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 5.Boucher T.J., Okuse K., Bennett D.L., Munson J.B., Wood J.N., McMahon S.B. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 6.Ro L.S., Chen S.T., Tang L.M., Jacobs J.M. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79:265–274. doi: 10.1016/s0304-3959(98)00164-x. [DOI] [PubMed] [Google Scholar]
- 7.Ugolini G., Marinelli S., Covaceuszach S., Cattaneo A., Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:2985–2990. doi: 10.1073/pnas.0611253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song X.J., Zheng J.H., Cao J.L., Liu W.T., Song X.S., Huang Z.J. EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain. 2008;139:168–180. doi: 10.1016/j.pain.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Liu S., Liu Y.P., Song W.B., Song X.J. EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. Pain. 2013;154:2823–2835. doi: 10.1016/j.pain.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Debebe Z., Rathmell W.K. Ror2 as a therapeutic target in cancer. Pharmacol Ther. 2015;150:143–148. doi: 10.1016/j.pharmthera.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford C.E., Qian Ma S.S., Quadir A., Ward R.L. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer. 2013;133:779–787. doi: 10.1002/ijc.27984. [DOI] [PubMed] [Google Scholar]
- 12.Green J.L., Kuntz S.G., Sternberg P.W. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerpa W., Latorre-Esteves E., Barria A. RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc Natl Acad Sci U S A. 2015;112:4797–4802. doi: 10.1073/pnas.1417053112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerpa W., Gambrill A., Inestrosa N.C., Barria A. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci. 2011;31:9466–9471. doi: 10.1523/JNEUROSCI.6311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrova I.M., Malessy M.J., Verhaagen J., Fradkin L.G., Noordermeer J.N. Wnt signaling through the Ror receptor in the nervous system. Mol Neurobiol. 2014;49:303–315. doi: 10.1007/s12035-013-8520-9. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Yuan S., Li B. Regulation of Wnt signaling by nociceptive input in animal models. Mol Pain. 2012;8:47. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan S., Shi Y., Tang S.J. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J Neuroimmune Pharm. 2012;7:904–913. doi: 10.1007/s11481-012-9370-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X., Zhang C., Zhang C., Peng Y., Wang Y., Xu H. MicroRNA-182-5p regulates nerve injury-induced nociceptive hypersensitivity by targeting ephrin type-b receptor 1. Anesthesiology. 2017;126:967–977. doi: 10.1097/ALN.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X.L., Wang Y., Zhang C.J., Yu L.N., Cao J.L., Yan M. PKA is required for the modulation of spinal nociceptive information related to ephrinB-EphB signaling in mice. Neuroscience. 2015;284:546–554. doi: 10.1016/j.neuroscience.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 21.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Yu L.N., Zhou X.L., Yu J. PI3K contributed to modulation of spinal nociceptive information related to ephrinBs/EphBs. PLoS One. 2012;7:e40930. doi: 10.1371/journal.pone.0040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., Ying J., Tong X. Epigenetic identification of receptor tyrosine kinase-like orphan receptor 2 as a functional tumor suppressor inhibiting beta-catenin and AKT signaling but frequently methylated in common carcinomas. Cell Mol Life Sci. 2014;71:2179–2192. doi: 10.1007/s00018-013-1485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma S.S., Srivastava S., Llamosas E. ROR2 is epigenetically inactivated in the early stages of colorectal neoplasia and is associated with proliferation and migration. BMC Cancer. 2016;16:508. doi: 10.1186/s12885-016-2576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green J., Nusse R., van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6:a009175. doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche K.W., Tingley W.G., Huganir R.L. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 27.Qiu S., Li X.Y., Zhuo M. Post-translational modification of NMDA receptor GluN2B subunit and its roles in chronic pain and memory. Semin Cell Dev Biol. 2011;22:521–529. doi: 10.1016/j.semcdb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Akbarzadeh S., Wheldon L.M., Sweet S.M., Talma S., Mardakheh F.K., Heath J.K. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn A.D., Moon R.T. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Yamanaka H., Moriguchi T., Masuyama N. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paganoni S., Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118:433–446. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho H.Y., Susman M.W., Bikoff J.B. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishita M., Itsukushima S., Nomachi A. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endo M., Doi R., Nishita M., Minami Y. Ror family receptor tyrosine kinases regulate the maintenance of neural progenitor cells in the developing neocortex. J Cell Sci. 2012;125:2017–2029. doi: 10.1242/jcs.097782. [DOI] [PubMed] [Google Scholar]
- 35.Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji R.R., Woolf C.J. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 37.Woolf C.J., Thompson S.W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 38.Liao G.Y., Wagner D.A., Hsu M.H., Leonard J.P. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- 39.Lavezzari G., McCallum J., Lee R., Roche K.W. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- 40.Roche K.W., Huganir R.L. Detection of protein phosphorylation in tissues and cells. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0514s11. [Chapter 5]: Unit 5 14. [DOI] [PubMed] [Google Scholar]
- 41.Farias G.G., Alfaro I.E., Cerpa W. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H., Alam A., Chen Q. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 2017;118:504–516. doi: 10.1093/bja/aex006. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T., Smits R., Ikenaka K. Microglia-induced activation of non-canonical Wnt signaling aggravates neurodegeneration in demyelinating disorders. Mol Cell Biol. 2016;22 doi: 10.1128/MCB.00139-16. pii: MCB.00139-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.