Short abstract
Patients with allergic rhinitis (AR) can suffer from mood disorders. The aim of this study was to investigate the clinical effect of a liposomal nasal spray (LN) containing vitamins A and E on the nasal mucosa in patients suffering from AR who had refused any type of anti-allergic treatment. For this purpose, the results of nasal cytology, Visual Analog Scale (VAS), Sino-Nasal Outcome Test-22 (SNOT-22), and Hospital Anxiety and Depression Scale (HADS) test were analyzed. Moreover, we evaluated the relationship between SNOT-22 and nasal cytology and between nasal symptoms and HADS scores. Statistical analysis revealed a significant decrease of scores at T1 in the LN treatment group as concerns VAS, SNOT-22, HADS-Anxiety test and a remarkable reduction of inflammatory cells detected with nasal cytology. Our study showed that higher levels of SNOT-22 corresponded to a higher level of HADS-Anxiety. The mechanisms underlying this relationship in AR patients are currently unknown, but we can suppose that improving mucosal trophism may contribute to the decrease of nasal symptoms and anxiety scores. The improvement of nasal symptoms, as measured by SNOT-22, was significantly correlated with the objective results of nasal cytology. These relationships between SNOT-22 and nasal cytology and between anxiety and cytology were investigated for the first time in our research.
Keywords: allergic rhinitis, nasal cytology, Sino-Nasal Outcome Test-22, HADS test, liposomes, mood disorder
Introduction
Allergic rhinitis (AR) is defined as when an immunoglobulin E (IgE)-mediated inflammatory response occurs in sensitized subjects challenging nasal symptoms such as rhinorrhea, blockage, sneezing, and itching. The most common tests for AR diagnosis are the percutaneous skin test and allergen-specific IgE antibody testing (radioallergosorbent testing).1–3
AR is a common problem with a negative impact on physical, social, and psychological well-being.4–9 AR is often part of a systemic inflammatory process associated with diseases such as allergic conjunctivitis, rhinosinusitis, and asthma.1,10
The impact of AR on the patient’s Quality of Life (QoL) (quality of sleep and psychological status) has received much more attention in recent years. Several studies have shown that a relationship exists between psychological factors and atopic disorders.6,8,11–16 The goal of this study was to investigate the clinical effect of a liposomal nasal spray (LN) containing vitamins A and E on the nasal mucosa in patients suffering from AR who had refused any type of anti-allergic treatment. For this purpose, the results of nasal cytology, Visual Analog Scale (VAS), Sino-Nasal Outcome Test-22 (SNOT-22), and Hospital Anxiety and Depression Scale (HADS) test were analyzed. Moreover, we evaluated the relationship between SNOT-22 and nasal cytology, HADS scores and nasal cytology, and between nasal symptoms and HADS scores.
Methods
This is a prospective, double-blind controlled study. Approval was received from the Ethical Committee (version 2, 10/23/2017). Written informed consent was obtained from all patients at the beginning of the study.
This study included 106 outpatients with perennial AR (56 females and 50 males), who had refused any type of anti-allergic treatment such as antihistamines, steroids, decongestants, anticholinergics, and leukotrienes.
They came from the outpatient Ear Nose Throat (ENT) Clinic of “San Salvatore” Hospital of L'Aquila (Italy). The patients were recruited between February 2017 and August 2018. Allergy was diagnosed by a positive prick test with common perennial allergens: Felis domesticus, Canis familiarus, Dermatophagoides sp., Alternaria alternata, Aspergillus fumigatus, and by nasal cytology.17 Only patients whose AR diagnosis was formulated at least 1 year before were included.
The exclusion criteria were tumor of sinuses, radiation therapy on head and neck, nose surgery, obstructive sleep apnoea syndrome surgery, turbinectomy, chronic sinusitis, nasal septum perforation, nasal fracture in the previous 3 months, untreated adenoidal hypertrophy, untreated asthma, sarcoidosis, Wegener’s granulomatosis, collapse of the nasal valve, smokers, and subjects younger than 18 years old.
The patients were randomly assigned to the study group (LN treatment group) of 53 patients treated with a liposomal (1000% w/w [100 g]) nasal spray with vitamin A (0.058% w/w [100 g]) and vitamin E (0.018% w/w [100 g]) and to the control group (rhynological saline solution [RH] control group) of 53 patients treated with 2.5 mL of sodium chloride 0.9% solution 2 times a day, in the morning and in the evening, in both the nasal cavities for 28 days.
The study lasted 30 days (1 day for the patient’s recruitment, 28 days of treatment, and 1 day for a follow-up visit). The first visit (T0) was performed on the day of the patient’s recruitment, and the follow-up visit (T1) was performed after 28 days of treatment with LN or saline solution.
At T0 and T1 a complete ENT examination, VAS, nasal cytology, SNOT-22 test, and HADS test were performed with the aim of evaluating all the variables taken into consideration.
The VAS was used in order to evaluate the severity of the nasal obstruction by scoring it on a continuous 10-cm horizontal line on which 0 cm and 10 cm represented no complaints and serious complaints associated with rhinitis, respectively.
Nasal cytology is a very useful diagnostic tool to detect cellular elements of the nasal epithelium due to allergen exposure, challenging stimuli, or inflammation. The diagnosis of nasal disorders by nasal cytology is based on the consideration that in healthy subjects the nasal mucosa is composed of ciliated, mucous-secreting, and basal cells, which are the common constituent of the pseudo-stratified epithelium. Besides small number of neutrophils, no other cells are detected in healthy individuals. In AR, the triggering event is allergen—IgE—mast cell interaction that leads to the early-phase response (mainly mediated by histamine). An intense infiltrate of eosinophils and mast cells (with lymphocytes and neutrophils) can be observed, strictly related to symptoms and exposure to allergens.18
The SNOT-22 test is used in order to evaluate the severity of nasal symptoms and their influence on the QoL. The questionnaire is composed by 22 chronic rhinosinusitis (CRS)-related questions, which evaluate the severity of symptoms and their influence on the QoL. The questionnaire is divided in 2 parts: 12 questions investigate physical symptoms (rhynologic symptoms, ear, and facial symptoms) and 10 the QoL (sleep function and psychological issues). All questions are based on a scale from 0 to 5, where 0 defines no problem and 5 defines maximal problems. The application of SNOT-22 in everyday clinical practice gives the opportunity to have more information for the clinician and facilitates the diagnosis and the treatment.19
The psychological status of each participant was assessed at T0 and T1 using the HADS test.20 The HADS test was proposed by Zigmoud and Snaith at the beginning of the 1980s in order to evaluate the psychological state of hospitalized patients. This test uses 2 sets of 7 questions each; the first one evaluates the anxious component (HADS-A), and the second one the depressive component (HADS-D). Questions about anxiety are number 1, 3, 5, 7, 9, 11, and 13; questions about depression are number 2, 4, 6, 8, 10, 12, and 14. The average time to fill the test is about 2 to 5 minutes. Each question is graded on a 3-point scale. For each question, the following answers may be given: 0—not at all, 1—infrequently, 2—lots of time, and 3—most of the time. Scores are obtained by adding the score of all questions for each set. The graduation is as follows: scores 0 to 7 are considered to be in the standard range (NON CASES); scores 7 to 11 are considered to be BORDERLINE; and scores > 11 are considered to be confirmed cases (CASES). The observer was trained in order to have fully consistent responses.21
Statistical Analysis
Data were analyzed and registered using the SAS 9.4 statistical software. The level of significance was set at P <.05.
The HADS-A variable has been re-encrypted by recording it in 3 dimensions with an ordinal qualitative character (normal <7; borderline 7–11; and pathological >11) as for the HADS-D variable.
Results
Ultimately, 106 patients were enrolled in the study: 53 patients were included in the LN treatment group and 53 patients in the RH control group. The treatment group consisted of 27 female and 26 male patients, while 24 female and 29 male patients were included in the RH group.
Patient ages ranged from 21 to 74 years, and the average age being 40.93 ± 13.84 years. Age distribution was similar in the 2 groups and averaged 39.66 ± 15.20 years in the LN group and 42.21 ± 12.35 years in the RH group.
All the discrete variables (VAS, SNOT-22, cytology, anxiety, and depression) were analyzed as frequencies and percentages in order to examine the differences between the 2 groups. Continuous variables were expressed as statistical average and standard deviation. At T0, similar average values for each variable were found in both groups (Table 1).
Table 1.
Statistical Results for Each Variable at T0–T1.
| RH Group |
LN Group |
|||
|---|---|---|---|---|
| Media ± SD T0 | Media ± SD T1 | Media ± SD T0 | Media ± DS T1 | |
| 42.21 ± 12.35 | 42.21 ± 12.35 | AGE | 39.66 ± 15.20 | 39.66 ± 15.20 |
| 6.47 ± 1.88 | 6.51 ± 2.00 | VAS | 6.57 ± 1.68 | 3.75 ± 1.41 |
| 8.11 ± 1.69 | 8.70 ± 3.45 | CYTOLOGY | 10.21 ± 7.02 | 5.57 ± 2.22 |
| 44.08 ± 17.49 | 43.81 ± 17.16 | SNOT-22 | 49.70 ± 12.88 | 34.32 ± 12.79 |
| 8.09 ± 3.12 | 8.28 ± 2.34 | HADS-A | 8.49 ± 2.79 | 6.40 ± 2.12 |
| 1.68 ± 2.08 | 1.32 ± 1.52 | HADS-D | 3.74 ± 3.50 | 3.28 ± 3.42 |
Abbreviations: SD, standard deviation; HADS, Hospital Anxiety and Depression Scale; LN, liposomal nasal spray; RH, rhynological saline solution; SNOT-22, Sino-Nasal Outcome Test-22; VAS, Visual Analog Scale.
Each Δvariable was compared and related to others using Pearson’s coefficient (Table 2) and Spearman’s coefficient (Table 3).
Table 2.
Correlations Between Variables Using Pearson’s Coefficient.
| ΔVAS | Δcyto | ΔSNOT-22 | Δanxiety | Δdepress | |
|---|---|---|---|---|---|
| ΔVAS | 1.00000 | .32294.0007 | .60036<.0001 | .42361<.0001 | −.00271.9780 |
| Δcyto | .32294.0007 | 1.00000 | .36916<.0001 | .18784.0538 | −.09529.3312 |
| ΔSNOT-22 | .60036<.0001 | .36916<.0001 | 1.00000 | .62392<.0001 | .07681.4339 |
| Δanxiety | .42361<.0001 | .18784.0538 | .62392<.0001 | 1.00000 | .11015.2610 |
| Δdepress | −.0027.9780 | −.0953.3312 | .07681.4229 | .11015.2610 | 1.00000 |
Abbreviations: SNOT-22, Sino-Nasal Outcome Test-22; VAS, Visual Analog Scale.
Table 3.
Correlations Between Variables Using Spearman’s Coefficient.
| ΔVAS | Δcyto | ΔSNOT-22 | Δanxiety | Δdepress | |
|---|---|---|---|---|---|
| ΔVAS | 1.00000 | .48777<.0001 | .65607<.0001 | .48619<.0001 | −.01542.8753 |
| Δcyto | .48777<.0001 | 1.00000 | .57042<.0001 | .38725<.0001 | −.07446.4481 |
| ΔSNOT-22 | .65607<.0001 | .57042<.0001 | 1.00000 | .59578<.0001 | .08957.3612 |
| Δanxiety | .48619<.0001 | .38725<.0001 | .59578<.0001 | 1.00000 | .05327.5876 |
| Δdepress | −.0154.8753 | −.0745.4481 | .08957.3612 | .05327.2610 | 1.00000 |
Abbreviations: SNOT-22, Sino-Nasal Outcome Test-22; VAS, Visual Analog Scale.
The statistical analysis, performed using Pearson’s coefficient (Table 2) and Spearman’s coefficient (Table 3), showed significant correlations between VAS, SNOT-22, cytology and HADS-A; conversely, no significant correlation was found between the HADS-D and any other variable.
At T0, the comparison between the 2 groups showed similar VAS scores. At T1, the LN group exhibited remarkable improvement of the nasal congestion (3.75 ± 1.14 vs 6.57 ± 1.68 at T0). Conversely, no significant differences were recorded at T1 in the group treated with saline solution (6.51 ± 2.00 vs 6.47 ± 1.88 at T0) (Figure 1)
Figure 1.
VAS in LN group versus RH group at T0/T1. LN, liposomal nasal spray; RH, rhynological saline solution; VAS, Visual Analog Scale.
As regards the inflammatory cell count, no significant differences were found comparing the 2 groups at T0. At T1, the LN group showed a significant decrease of cell counts (5.57 ± 2.22 vs 10.21 ± 7.02 at T0). By contrast, the RH group showed no significant variations (8.70 ± 3.45 vs 8.11 ± 1.69 at T0; Figure 2).
Figure 2.
Cyto in RH group versus LN group at T0/T1. cyto, cytology; LN, liposomal nasal spray; RH, rhynological saline solution.
From the scatter, which relates the cytology to the VAS results, it may be inferred that the decrease of nasal inflammatory cells at T1 corresponds to the decrease of VAS values (Figure 3). A lower number of inflammatory cells corresponded to lower values of the VAS, therefore to less severe nasal obstructive symptoms.
Figure 3.

Scatter Δcytology—ΔVAS. cyto, cytology; LN, liposomal nasal spray; RH, rhynological saline solution; VAS, Visual Analog Scale.
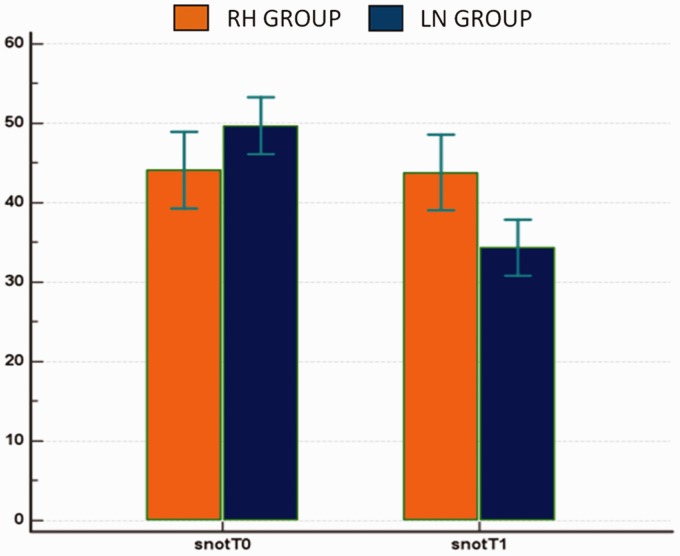
As far as delta SNOT-22 (T1–T0) is concerned, no significant results emerged in RH group because the SNOT-22 scores at T0 and at T1 were almost overlapping. Conversely, as regards the LN group, the SNOT-22 score at T0 was about 50 and decreased to 35 at T1 (Figure 4).
Figure 4.
SNOT-22 T0/T1 in LN group versus RH group. LN, liposomal nasal spray; RH, rhynological saline solution; SNOT, Sino-Nasal Outcome Test.
From the scatter, which relates the nasal cytology to the SNOT-22 results, it may be inferred that the decrease of nasal inflammatory cells at T1 corresponds to the decrease of SNOT-22 scores. A lower number of inflammatory cells corresponded to lower values of the SNOT-22 (Figure 5).
Figure 5.
Scatter cyto/SNOT-22. cyto, cytology; SNOT, Sino-Nasal Outcome Test.
Concerning the HADS test, a simple descriptive analysis showed different variations in the 2 groups. As regards the anxious component, the score decreased in the LN group in comparison with the RH group (6.40 ± 2.12 vs 8.49 ± 2.79 at T0). Conversely, as regards the depressive component, no significant differences were found comparing the 2 groups. (Table 1)
The comparison between the anxiety values and the number of inflammatory cells in the 2 groups showed that a direct relation between anxiety and cytology exists (Figure 6).
Figure 6.

Scatter correlation between anxiety and cytology.
The relationship between mood disorders and nasal symptoms was assessed by comparing the HADS test and the SNOT-22 test results. Our statistical analysis revealed a direct correlation between ΔSNOT-22 and ΔHADS-A (Figure 7).
Figure 7.

Scatter ΔSNOT-22—anxiety. LN, liposomal nasal spray; RH, rhynological saline solution; SNOT, Sino-Nasal Outcome Test.
Conversely, no direct correlation was detected between ΔSNOT-22 and Δdepressive component (Figure 8).
Figure 8.

Scatter ΔSNOT-22—Δdepressive component. SNOT, Sino-Nasal Outcome Test.
Discussion
According to Allergic Rhinitis and its Impact on Asthma guidelines,1 the standard care in the treatment of AR includes antihistamines, nasal decongestants or/and corticosteroids, cromones, antileucotrienes, and specific immunotherapy. We enrolled in the study 106 patients, who had refused the standard care to treat AR. We prescribed a liposomal spray solution containing vitamin A and vitamin E. Liposomes are phospholipid vesicles with dimensions varying between 25 nm and 1 µm, formed by a closed double lipid bilayer with an aqueous solution inside. This structure allows it to incorporate both hydrophilic and hydrophobic molecules, such as vitamins A and E, which are positioned within the lipid bilayer. In order to release the molecules transported to the action site, the lipid bilayer of the liposomes must merge with another lipid layer, such as the cell plasma membrane. The quality of the liposomes to be absorbed by each cell and to slowly release their content has made them an excellent tool for the administration of drugs, especially vitamins A and E which, being fat-soluble, are subject to rapid degradation by free radicals. Vitamins A and E stabilized and made more bioavailable by liposomes, play an antioxidant, epithelia-protective role, and contribute to improve the symptoms associated with allergic diseases.
Liposomes have been widely used in the pharmaceutical and food industries because of their biocompatibility, biodegradability, absence of toxicity, small size, and ability to carry a wide variety of bioactive compounds due to the amphiphilicity of the phospholipid encapsulating material.22 Several studies showed significantly higher absorption rates as well as greater pharmacological effects for drugs entrapped in liposomes,23–25 and the good mucosal targeting properties of liposomes have also been demonstrated.26
A liposomial nasal spray, containing vitamins A and E, was able to improve in vitro the survival and morphology of nasoseptal cartilage.27 Another LN containing vitamin E was better tolerated and improved the QoL more than dexpanthenol or isotonic NaCl spray in patients with rhinitis sicca.28 The prominent effects that vitamins A and E are well known for are their lubricating and moisturizing abilities.29 We assumed that vitamins A and E, associated with liposomes, could contribute to a better nasal trophism of the nasal mucosa and to a reduced response to allergic inflammation mediators in patients with AR.
The first goal of this study was to investigate the clinical effect of an LN containing vitamins A and E in patients with AR, by analyzing the results of VAS, nasal cytology, SNOT-22, and HADS test. The second goal was to evaluate the relationship between SNOT-22 and nasal cytology, HADS scores and nasal cytology, and between nasal symptoms and HADS scores.
At T0, no significant differences were found comparing the 2 groups as regards the VAS score. At T1, a remarkable reduction of the nasal obstruction was recorded in the LN treatment group. No improvements were recorded in the RH control group.
As regards the nasal cytology, the comparison between the 2 groups did not reveal noteworthy differences at T0. At T1, the LN-treated group showed a significant decrease in eosinophils, neutrophils, and basophils. Conversely, the RH control group showed no variations.
Analyzing the relationship between the nasal cytology and the VAS results, it may be inferred that the decrease of nasal inflammatory cells at T1 corresponded to the decrease of the VAS values, therefore to less severe nasal obstructive symptoms.
SNOT-22 was tested in AR patients and had been previously correlated with VAS for the seriousness of sino-nasal symptoms and mood disturbance. In Doulaptsi et al.’s study,30 VAS for sino-nasal symptoms in CRS showed a strong correlation with SNOT-22 scores. Farhoodet et al.31 assessed SNOT-22 scores in control population without CRS in order to define “a normal healthy score.” Other studies32,33 used the SNOT-22 test to assess the relationship between sinonasal symptoms in CRS and QoL. These studies revealed that depression and anxiety were significantly more common in patients with CRS compared to healthy controls. In our study, the SNOT-22 score decreased in the LN-treated group at the end of the study.
Our data showed a relationship between SNOT-22 and nasal cytology. No other studies can be found in the literature about this relationship.
Previous studies had proposed an LN as a nonpharmacological therapy concept for allergic rhinoconjunctivitis. In Bohm et al.’s study,34 the LN led to significant nasal symptoms relief, but QoL did not improved significantly. Weston and Mösges compared the LN to a steroid spray35 and revealed a similar significant reduction of symptoms and improvement in QoL in both treatments. Andersson et al. compared an intranasal lipidic microemulsion to isotonic saline solution and pointed out not only reduced allergen challenge-induced effects but also a reduction of plasma exudation in AR.36 Our study showed the effectiveness of a liposomal nasal solution containing vitamin A and vitamin E in reducing the inflammatory cells counts and in improving the nasal symptoms related to AR.
One possible hypothesis to explain these results is that this solution provides mechanical cleansing, and consequently, it removes antigens and improves the mucociliary clearance. Another possible theory takes into consideration the prominent effect of vitamins A and E, which are well known for their lubricating and moisturizing abilities. Furthermore, vitamins A and E, associated with liposomes, could contribute to a reduced response to allergic inflammation mediators in patients with AR.
Several scientific studies have shown that a relationship exists between psychological factors and atopic disorders.4–9 Patients with AR suffer from depression, sleep problems, and anxiety status more than people without allergic disease.6,11–16 Mental disorders affect about 1 in 4 adults annually.15 Depression is a common but serious mood disorder.37 It causes severe symptoms that affect how people feel, think, and handle daily activities, such as sleeping, eating, or working. To be diagnosed with depression, the symptoms must be present for at least 2 weeks.38 Anxiety is an unpleasant state of inner turmoil, often accompanied by nervous behaviors, such as pacing back and forth, somatic complaints, and rumination. It is often accompanied by restlessness, fatigue, problems in concentration, and muscular tension.37
At T0, the HADS test scores of the treated group were similar to the scores of the control group as regards both the anxious and the depressive components. In the treated group at T1, remarkable results were recorded referring to the anxious component but not to the depressive component. A direct correlation was detected between ΔSNOT-22 and ΔHADS-A—that is to say that severe nasal symptoms, which correspond to higher levels of SNOT-22, were closely correlated with higher scores of HADS-A: this suggests a greater predisposition to anxiety in patients with AR. Concerning the control group, no significant results emerged referring to each component. The results at T1 were comparable to those reported previously at T0; it may be inferred that RH group patients had no significant improvement of symptoms at the end of the study.
According with the literature, nasal obstruction may play a prominent role in increasing anxiety scores in patients suffering from AR,11 and our study showed a direct relationship between nasal symptoms and anxiety. Conversely, no direct correlation was detected between with ΔSNOT-22 and Δdepressive component.
The exact mechanisms underlying this effect in AR patients are currently unknown, but it is possible that increased Th2 cytokine and endocrine function may be involved, as stress-induced anxiety has been shown to be associated with significant interleukin 6, cortisol, and epinephrine production39–43 in AR patients.
Our statistical analysis revealed that higher levels of SNOT-22 correspond to higher level of HADS-A. Therefore, it may be inferred that a direct relation exists between nasal symptoms and anxiety.
Our study showed a significant relationship among the clinical improvement of nasal symptoms, the decrease of inflammatory cells counts, and the decrease of HADS-A score.
In order to better define the clinical situation of AR patient, we propose the use of the HADS questionnaire, which has proved to be a handy and reliable tool, useful in the mid-long term management of the patient with allergic nasal disease especially in aperiodic forms.
Conclusions
This study demonstrated the effectiveness of a liposomal nasal solution containing vitamin A and vitamin E in improving nasal symptoms and decreasing inflammatory cells counts in AR patients compared with a homogenous group of patients who used a saline solution.
The improvement of nasal symptoms, as measured by SNOT-22, was significantly correlated with the objective results of nasal cytology. This relationship between SNOT-22 and nasal cytology was investigated for the first time in our research.
A significant direct relationship emerged between nasal symptoms (ΔSNOT-22) and anxiety (HADS-A score) and between nasal cytology and anxiety (HADS-A score).
The exact mechanisms underlying this effect in AR patients are currently unknown, but we can suppose that a better condition of the nasal mucosa may decrease nasal symptoms and consequently the anxiety score.
These results suggest the use of HADS test for the evaluation of aperiodic AR patients.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by our institutional review board.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Lombardi C, Passalacqua G, Baiardini I, et al. , eds. Allergic Rhinitis and its Impact on Asthma (Italian Section). http:/www.progetto-aria.it/materiale/2017/slide-kit-aria-2017.ppt. Accessed July 17, 2019.
- 2.Mims JW. Epidemiology of allergic rhinitis. Int Forum Allergy Rhinol. 2014; 4(Suppl 2):S18–S20. [DOI] [PubMed] [Google Scholar]
- 3.Passali D, Cingi C, Staffa P, Passali F, Bayar Muluk N, Bellussi ML. The International Study of the Allergic Rhinitis Survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018; 8(1):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010; 125:S103–S115. [DOI] [PubMed] [Google Scholar]
- 5.Lack G. Pediatric allergic rhinitis and comorbid disorders. J Allergy Clin Immunol. 2001; 108(1 Suppl):S9–S15. [DOI] [PubMed] [Google Scholar]
- 6.Sih T, Mion O. Allergic rhinitis in the child and associated comorbidities. Pediatr Allergy Immunol. 2010; 21(1 Pt 2):e107–e113. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Zhang X, Zheng J, et al. Co-morbid psychological dysfunction is associated with a higher risk of asthma exacerbations: a systematic review and meta-analysis. J Thorac Dis. 2016; 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-Díaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: clinical implications. World Allergy Organ J. 2017; 10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kew KM, Nashed M, Dulay V, Yorke J. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev. 2016; 9:CD011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braunstahl GJ, Hellings PW. Allergic rhinitis and asthma: the link further unraveled. Curr Opin Pulm Med. 2003; 9:46–51 [DOI] [PubMed] [Google Scholar]
- 11.Vichyanond P, Suratannon C, Lertbunnaphong P, Jirapongsananuruk O, Visitsunthorn N. Clinical characteristics of children with non-allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol. 2010; 28:270–274. [PubMed] [Google Scholar]
- 12.Segboer CL, van Drunen CM, Terreehorst I, Rondon C, Hellings PW, Fokkens WJ. New findings in nonallergic rhinitis and local allergic rhinitis. Curr Otorhinolaryngol Rep. 2013; 1(2):106–112. [Google Scholar]
- 13.Xi L, Cao F, Zhang Y, Zhang L. Severity of nasal obstruction can predict the anxiety status of patients with allergic rhinitis but not patients with vasomotor rhinitis. Int Forum Allergy Rhinol. 2016; 6(11):1196–1203. [DOI] [PubMed] [Google Scholar]
- 14.Bell IR, Jasnoski ML, Kagan J, King DS. Depression and allergies: survey of a nonclinical population. Psychother Psychosom. 1991; 55(1):24–31. [DOI] [PubMed] [Google Scholar]
- 15.Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999; 40(6):491–496. [DOI] [PubMed] [Google Scholar]
- 16.Timonen M, Jokelainen J, Hakko H, et al. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003; 8(8):738–744. [DOI] [PubMed] [Google Scholar]
- 17.Gelardi M, Incorvaia C, Passalacqua G, Quaranta N, Frati F. The classification of allergic rhinitis and its cytological correlate. Allergy. 2011; 66:1624–1625. [DOI] [PubMed] [Google Scholar]
- 18.Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. Nasal cytology: practical aspects and clinical relevance. Clin Exp Allergy. 2016; 46:785–792 [DOI] [PubMed] [Google Scholar]
- 19.Mozzanica F, Preti A, Gera R, et al. Cross-cultural adaptation and validation of the SNOT-22 into Italian. Eur Arch Otorhinolaryngol. 2017; 274:887–895. [DOI] [PubMed] [Google Scholar]
- 20.Salaffi F, Stancati A. Scale di valutazione e malattie reumatiche. Mattioli 2001:196–197 (printed paper—correspond to 214–215 on web reference).
- 21.Costantini M, Musso M, Viterbori P, et al. Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer. 1999; 7:121–127. [DOI] [PubMed] [Google Scholar]
- 22.Emami S, Azadmard-Damirchi S, Hadi Peighambardoust S, Valizadeh H, Hesari J. Liposomes as carrier vehicles for functional compounds in food sector. J Exp Nanosci. 2016; 11(9):737–759 [Google Scholar]
- 23.Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003; 258(1–2):141–151 [DOI] [PubMed] [Google Scholar]
- 24.Coderch L, De Pera M, Fonollosa J, De La Maza A, Parra J. Efficacy of stratum corneum lipid supplementation on human skin. Contact Dermatits. 2002; 47:139–146 [DOI] [PubMed] [Google Scholar]
- 25.Niu M, Lu Y, Hovgaard L, Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomedicine. 2011; 6:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jubeh TT, Antler S, Haupt S, Barenholz Y, Rubinstein A. Local prevention of oxidative stress in the intestinal epithelium of the rat by adhesive liposomes of superoxide dismutase and tempamine. Mol Pharm. 2005; 2(1):2–11 [DOI] [PubMed] [Google Scholar]
- 27.Stoelzel K, Kohl B, Hoyer M, et al. Effect of nasal sprays on an in vitro survival and morphology of nasoseptal cartilage. Eur Arch Otorhinolaryngol, 2015; 272(4):877–888 [DOI] [PubMed] [Google Scholar]
- 28.Hahn C, Böhm M, Allekotte S, Mösges R. Tolerability and effects on quality of life of liposomal nasal spray treatment compared to nasal ointment containing dexpanthenol or isotonic NaCl spray in patients with rhinitis sicca. Eur Arch Otorhinolaryngol. 2013; 270(9):2465–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J Appl Physiol. 2009; 107(4):1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doulaptsi M, Prokopakis E, Seys S, Pugin B, Steelant B, Helling P. Visual analogue scale for sino-nasal symptoms severity correlates with Sino-Nasal Outcome Test 22: paving the way for a simple outcome tool of CRS burden. Clin Transl Allergy. 2018; 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhood Z, Schlosser RJ, Pearse ME, Storck KA, Nguyen SA, Soler ZM. Twenty‐two–item Sino‐Nasal Outcome Test in a control population: a cross‐sectional study and systematic review. Int Forum Allergy Rhinol. 2015; 6:271–277. [DOI] [PubMed] [Google Scholar]
- 32.Erskine SE, Hopkins C, Clark A, et al. Chronic rhinosinusitis and mood disturbance. Rhinology. 2017; 55(2):113–119. [DOI] [PubMed] [Google Scholar]
- 33.Philpott C, Erskine S, Hopkins C, et al. A case-control study of medical, psychological and socio-economic factors influencing the severity of chronic rhinosinusitis. Rhinology. 2016; 54(2):134–140. [DOI] [PubMed] [Google Scholar]
- 34.Böhm M, Avgitidou G, El Hassan E, Mösges R. Liposomes: a new non-pharmacological therapy concept for seasonal-allergic-rhinoconjunctivitis. Eur Arch Otorhinolaryngol. 2012; 269:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weston LA, Mösges R. Treating seasonal allergic rhinoconjunctivitis with a liposomal nasal spray. Allergologie. 2010; 33(5):196–204. [Google Scholar]
- 36.Andersson M, Greiff L, Wollmer P. Nasal treatment with a microemulsion reduces allergen challenge-induced symptoms and signs of allergic rhinitis. Acta Otolaryngol. 2008; 128(6):666–669. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 38.National Institute of Mental Health. https://www.nimh.nih.gov/health/topics/depression/index.shtml. Published 2018. Accessed July 17, 2019.
- 39.Assaf AM, Al-Abbassi R, Al-Binni M. Academic stress-induced changes in Th1- and Th2-cytokine response. Saudi Pharm J. 2017; 25(8):1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen E. Psychological stress and its relationship to cytokines and inflammatory diseases. In: Cytokines: Stress and Immunity 2nd ed. Abingdon, England: Taylor & Francis Group; 2007.
- 41.Chrousos GP. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J Allergy Clin Immunol. 2000; 106(5):S275–S291. [DOI] [PubMed] [Google Scholar]
- 42.Connor TJ, Brewer C, Kelly JP, Harkin A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 2005; 159(1–2):119–128. [DOI] [PubMed] [Google Scholar]
- 43.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-infl ammatory cytokines and a Th1-like response in stress-induced anxiety . Cytokine. 1998; 10:313–318. [DOI] [PubMed] [Google Scholar]