Short abstract
Opioids are common analgesics for pain relief in HIV patients. Ironically, emerging clinical data indicate that repeated use of opioid analgesics in fact leads to a heightened chronic pain state. To understand the underlying pathogenic mechanism, we generated a mouse model to study the interactive effect of morphine and HIV-1 gp120 on pain pathogenesis. We simulated chronic pain in the model by showing that repeated morphine administrations potentiated HIV-1 intrathecal gp120-induced pain. Several spinal cellular and molecular pathologies that are implicated in the development of HIV-associated pain are exacerbated by morphine, including astroglial activation, pro-inflammatory cytokine expression and Wnt5a signaling. We further demonstrated that inhibition of Wnt5a not only reversed the glial activation and cytokine upregulation but also the exacerbation of gp120-induced pain. These studies establish a mouse model for the opioid exacerbation of HIV-associated pain and reveal potential cellular and molecular mechanisms by which morphine enhances the pain.
Keywords: Pain, HIV-1, opioid, morphine, Wnt, neuroinflammation, inflammasome, astrocyte
Introduction
Chronic pain deteriorates the quality of life of over 55% of HIV-1/AIDS patients.1,2 Current clinical practice frequently relies on long-term use of prescription opioid analgesics to control severe pain in these patients.3,4 Unfortunately, besides the powerfully acute analgesic effect, emerging clinical data indicate that repeated use of opioid analgesics promotes peripheral neuropathy and chronic pain in HIV patients.4–6 This side effect is of great clinical importance, as it suggests that HIV patients receiving opioids to relieve pain may paradoxically experience more pain as a result of treatment. Currently, little is known about how opioid analgesics can exacerbate HIV-related pain.
Analysis of postmortem tissues of HIV patients shows that gp120, rather than other HIV proteins, is specifically associated with the development of the pain disorder.7 The analysis also reveals an array of cellular and molecular pathologies in the pain transmission pathway that specifically manifest in the HIV patients who develop pain disorders but not in the patients without pain problems. These pathologies include the degeneration of peripheral sensory fibers and synapses in the spinal cord, the activation of astrocytes, and the upregulation of pro-inflammatory cytokines.7,8 In addition, the spinal upregulation of a neuroinflammatory regulator Wnt5a9,10 is also specifically associated with the expression of pain in HIV patients.11 The HIV pain model generated by intrathecal (i.t.) injection of mice with gp120 (hereafter the gp120 model) simulates these human neuropathologies that are associated with HIV pain.7,12 The collective findings indicate that these cellular and molecular aberrations likely contribute to the pathogenesis of HIV-associated pain. However, whether or not these pathogenic processes are exacerbated by chronic morphine is not clear.
Emerging clinical data suggest that chronic use of opioid analgesics paradoxically enhances pain sensitivity, which is known as opioid-induced hyperalgesia (OIH).13 The expression of OIH may contribute to the common practice of dose escalation in order to effectively control pain, which can eventually lead to the problems of overdosing.14,15 The proposed pathogenic processes of OIH also include neuroinflammation, manifested by glial reaction and upregulation of pro-inflammatory cytokines founded in HIV-1-associated pain.6 Hence, there is a potential pathogenic interaction between OIH- and HIV-1-associated pain, via shared signaling pathways. For instance, the HIV-1 gp120 coreceptor CCR5 or CXCR4 also regulate morphine tolerance, dependence, and reward.16–18 On the other hand, Toll-like receptors, the innate immune receptors that function as the nonclassical opioid receptors in glial cells,19–22 are also modulated by gp120.23–25 In addition, both morphine and gp120 can activate N-methyl-D-aspartate receptors in the spinal cord, which is implicated in the development of pain by triggering downstream cascades such as cytokine and chemokine signaling.26–31 The identification of shared pathways indicates that HIV and morphine may cooperatively dysregulate pain neural circuits to promote pain pathogenesis. However, this hypothesis has not been directly tested.
In this study, we used the gp120 model to determine the effect of repeated administration of morphine on pain behaviors. In addition, we also determined the effect of specific cellular and molecular pathologies that are implicated in the development of gp120-induced pain. Our results show that chronic morphine treatment exacerbates not only the expression of pain in the gp120 model but also the pain pathogenic processes. These findings provide evidence for the opioid exacerbation of HIV-associated pain in animals and reveal the pathogenic process that involves Wnt5a-regulated neuroinflammation.
Methods
Animals
Young adult C57BL6 mice (2–3 months old, 20–25 g) were purchased from Harlan Labs. Preliminary experiments with male and female mice did not reveal evident differences in molecular, cellular, and behavioral analyses. Hence, we only included male animals to avoid the potential compound effect of menstruation. Animal procedures were approved by the University of Texas Medical Branch Animal Care and Use Committee. Mice were housed in cages with soft bedding and free access to food and water under a 12-h reverse light–dark cycle and 20°C temperature control.
Materials
Recombinant HIV-1Bal envelope glycoprotein gp120 (Cat # 4961) was obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH. Antibodies used for immunoblotting and/or immunostaining included glial fibrillary acidic protein (GFAP; 1:5000, 04–1062 for immunoblotting; 1:500, MAB360 for immunostaining; Millipore), Iba1 (1:1000, ab178847, Abcam), CD11b (1:1000, ab13357, Abcam), Wnt5a (1:1000, 2530, Cell Signaling Technology), Wnt3a (1:1000, MAB1324, R&D), phosphorylated c-Jun N-terminal kinase (p-JNK; 1:1000, 9251, Cell Signaling Technology), caspase 1 (1:1000 for immunoblotting, 1:200 for immunostaining, 06–503, Millipore), interleukin (IL)-1β (1:500, ab1413-I, Millipore), tumor necrosis factor (TNF)-α (1:500, ab2148P, Millipore), and β-actin (1:1000, sc-1616-R, Santa Cruz Biotechnology).
I.t. injection
I.t. injection was performed as described before.7 Gp120 and Box5 (Storkbio Ltd.) were stored at −80°C and dissolved in phosphate-buffered saline (PBS) immediately before i.t. injection. Briefly, mice were anesthetized with isoflurane (3% during the first 1–2 min induction phase and 2% for the following maintenance phase) with a flow of oxygen. For i.t. injection, a 30.5-gauge stainless steel needle was attached to a 10 µl Luer tip syringe. The operator used the left thumb and middle finger to hold the mouse caudal paralumbar region and then used the index finger to locate the intervertebral space between L5 and L6. After location orientation, the operator used the right hand to insert the needle into the intervertebral space at a 45° angle. A sudden tail movement or twitch was identified as a correct i.t. placement of needle tip. After getting the positive tail signal, drug solution (5 µl) was injected slowly (1 µl/s). To avoid any spillage of solution, the needle was held at the position for at least 30 s before removal.
Mechanical pain behavioral test
Paw withdrawal thresholds (PWTs) were measured by von Frey testing on the plantar surface of the hind paw. Three days before testing, mice were habituated to the testing surroundings for 2 h per day. On the testing day, a series of calibrated von Frey filaments (0.1 to 2.0 g) were applied perpendicularly to the central area of the hind paw. The PWT values were calculated using the Dixon “up and down paradigm” method. To minimize the subjective effect and bias, behavioral tests were performed under double-blind conditions. The experimenter did not know the particular treatment of individual animals.
Western blotting analysis
The lumbar spinal dorsal horn (SDH) tissues were dissected out and homogenized in 300 µl radioimmunoprecipitation assay lysis buffer (1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, pH 7.4), supplemented with a protease inhibitor cocktail (Sigma). After homogenization, the lysates were put on ice for 30 min and centrifuged at 12,000×g for 10 min. The supernatants were collected and transferred to new tubes for protein concentration assays using a bicinchoninic acid protein assay kit (product 23227; Pierce). Equal amounts of protein (50 µg) were prepared and heated in SDS-polyacrylamide gel electrophoresis sample buffer (95°C; 5 min) and loaded on a 10% to 12% SDS-polyacrylamide gel. After gel separation and electrophoresis, the proteins were electrophoretically transferred to 0.2 µm polyvinylidene fluoride membranes. For hybridization, the membranes were washed once in Tris-buffered saline with Tween (TBST) buffer (50 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.6) and incubated sequentially with primary antibodies and horseradish peroxidase-conjugated secondary antibodies diluted in blocking buffer (TBST buffer with 5% nonfat milk powder or 5% bovine serum albumin (BSA)). Immunoblotting signals were visualized using an Enhanced Chemiluminescence kit (Thermo Scientific). The band intensity was quantified using NIH ImageJ software, and β-actin was included as a loading control.
Fluorescent immunostaining
Mice were anesthetized and transcardially perfused with ice-cold PBS. The segments of lumbar spinal cord were quickly dissected out and cut into two parts: one for immunostaining and the other for immunoblotting. For immunostaining, the tissues were immediately perfused with ice-cold fixative solution containing 4% paraformaldehyde in PBS for 12 h at 4°C, followed by cryoprotection in 30% sucrose for 24 h at 4°C. After embedding in optimal cutting temperature compound (Sakura Finetek), the tissues were mounted on a microtome (Leica), and 10 µm frozen sections were prepared for indirect fluorescent immunostaining. The sections were incubated in blocking buffers (5% BSA, 0.3% Triton X-100 in PBS) for 1 h at room temperature, followed by overnight incubation with primary antibodies diluted in blocking buffer. The sections were subsequently stained with fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch) for 1 h at room temperature. Stained sections were mounted with mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories). All images were captured using a confocal microscope system (Nikon). NIH ImageJ software was used for quantitative analysis.
Statistical analysis
Statistical analysis was performed using Prism 7 (GraphPad) software. Information of specific statistical test methods and animal numbers is provided in the figure legends. Quantitative data were presented as means ± standard error of the mean.
Results
Chronic morphine administration exacerbated gp120-induced pain
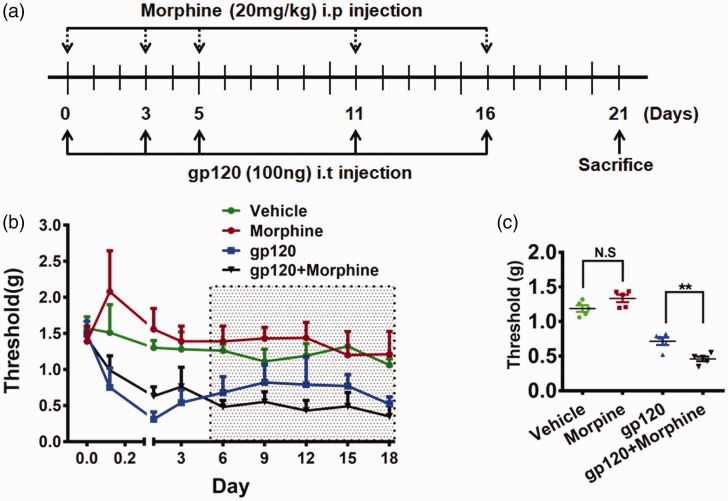
Our previous studies found that gp120 levels in the SDH of pain-positive HIV patients were around 10-fold higher than that in pain-negative patients, while Tat (Trans-Activator of Transcription of HIV-1) and Vpr (Viral Protein R of HIV-1) inversely correlated with HIV-associated pain, suggesting that gp120 is a critical contributor to the pathogenesis of HIV-associated pain.7 As “pain-positive” HIV patients were inclined to use opioids to alleviate pain, we chose a gp120 mouse model to study the potential interaction of chronic morphine and HIV-associated pain. To better imitate the effect of opioids on HIV-associated pain, we used a paradigm of repeated drug administration (Figure 1(a)). As shown in Figure 1(a), gp120 was intrathecally injected into mice on days 0, 3, 5, 11, and 16. Morphine was repeatedly injected into gp120 mice at a dose equivalent to the high end of clinical application (intraperitoneal (i.p.), 20 mg/kg) on the same day. Mechanical sensitivity was measured 5 h postmorphine injection. Von Frey tests showed that the mice developed hyperalgesia after a single gp120 injection, and multiple injections induced mechanical hypersensitivity that maintained at least for 18 days (Figure 1(b)). The results showed that before day 5 the average withdrawal threshold of the gp120 group was 0.53 ± 0.22 g (n = 6), while that of the gp120+morphine group was 0.79 ± 0.18 g (n = 6; p < 0.05, gp120 vs. gp120+morphine). This observation indicated that morphine caused an analgesic effect on gp120-induced pain during this period of treatment (Figure 1(b)). However, after day 5, the average threshold of the gp120 group was 0.73 ± 0.25 g (n = 6), while that of the gp120+morphine group was 0.43 ± 0.24 g (n = 6, p < 0.01) (Figure 1(c)). This observation indicated that long-term morphine administration potentiated the gp120-induced hyperalgesia. In contrast, for animals without gp120 administration, although morphine treatment also induced an analgesic effect during the early phase, it did not cause detectable hyperalgesia during the later phase (Figure 1(c)). This result suggested that under these experimental conditions repeated morphine treatment by itself did not cause hyperalgesia.
Figure 1.
Chronic morphine administration potentiated gp120-induced hyperalgesia. (a) Temporal diagram of drug administration. Gp120 and morphine were administrated by intrathecal and intraperitoneal injection, respectively, to simulate chronic presence. At day 21, the mice were euthanized for molecular and cellular analyses. (b) Threshold of mechanical pain (hyperalgesia) in the hind paw was measured by von Frey tests. Pain behavioral test was performed 5 h postmorphine injection. (c) Statistical comparison of the data points among groups in the shaded area in panel (b). Morphine exacerbation of gp120-induced pain started to express after day 6. With this administration paradigm, morphine alone did not cause significant hyperalgesia (p > 0.05). (**p < 0.01; ***p < 0.001; one-way analysis of variance, n = 6).
Chronic morphine exacerbated glial reaction in the spinal cord
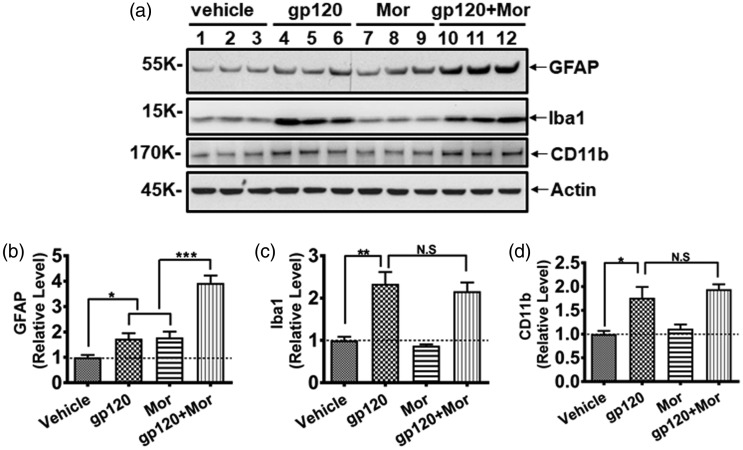
Next, we investigated the potential mechanism by which chronic morphine use caused heightened chronic pain. Because both HIV-1 proteins (e.g., gp120) and opioids can activate glial cells in the SDH,7,31–33 we wondered whether morphine potentiates gp120-induced pain by enhancing the glial reaction in the SDH. To investigate the potential interaction of chronic gp120 and morphine on glial activation, we performed immunoblotting to measure glial marker levels in the SDH at day 21 (Figure 2(a)), a time point that was chosen to assess the chronic interaction between gp120 and morphine on glia. The results showed that although repeated i.t. gp120 or i.p. morphine injection separately upregulated GFAP (astrocyte marker) by ∼70% (73 ± 38% and 78 ± 40% vs. vehicle, respectively, p < 0.05), their combination increased GFAP expression by ∼300% (293 ± 51% vs. vehicle; 127 ± 30% vs. gp120; 121 ± 29% vs. morphine; all with p < 0.001) (Figure 2(b)), suggesting a supra-additive effect (as opposed to a simple additive). On the other hand, although chronic gp120 treatment alone significantly increased the microglia/macrophage marker Iba1 (2.3 ± 0.5-fold vs. vehicle, p < 0.01) or CD11b (1.8 ± 0.4-fold vs. vehicle, p < 0.05), coadministration of gp120 and morphine did not significantly enhance its upregulation (Figure 2(c) and (d)). It is worth noting that microglia was not drastically activated by morphine in this experiment, although we did observe microglial activation in different paradigm of morphine administration (unpublished results). Generally, these data revealed an interaction of gp120 and morphine in activating astrocytes but not microglia under the conditions used in this study.
Figure 2.
gp120 and morphine cooperatively induced astrocyte activation in the SDH. (a) Immunoblots of GFAP (astrocyte marker), Iba1, and CD11b (microglia/macrophage marker). (b to d) Quantitative summary of panel (a). The same animals used for Figure 1 were analyzed (*p < 0.05; **p < 0.01; ***p < 0.001; N.S., not significant; one-way analysis of variance with Tukey post hoc tests, n = 6). GFAP: glial fibrillary acidic protein.
Morphine and gp120 cooperatively enhanced spinal Wnt5a upregulation
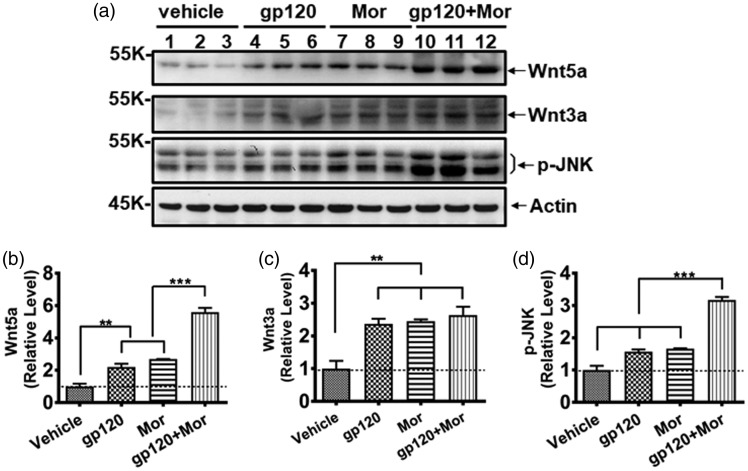
Given the critical role of active astrocytes in HIV-associated pain,7 we sought to reveal the cellular basis of the pro-hyperalgesic effect of chronic morphine on astrocyte activation. Our recent studies with primary neuron/glia cocultures showed that Wnt5a stimulated TNF-α and IL-1β expression, indicating that Wnt5a activity is involved in activating glial cells.9 Additionally, we found Wnt ligands (including Wnt5a and Wnt3a) and downstream effector proteins that were specifically increased in the SDH of HIV patient with chronic pain but not in the “pain-negative” HIV patients.11 Based on these and other data, we wondered whether chronic morphine use promoted astrocyte activation in the gp120 model through Wnt signaling. To test this, we firstly determined the expression levels of Wnt ligands. Immunoblotting results showed repeated i.t. gp120 or i.p. morphine injection individually increased Wnt5a protein (2.2 ± 0.4-fold and 2.3 ± 0.1-fold vs. vehicle, respectively, p < 0.01). However, cotreatment of gp120 and morphine caused a potentiation of upregulation of Wnt5a (5.7 ± 0.5-fold vs. vehicle; 2.5 ± 0.2-fold vs. gp120; 2.4 ± 0.2-fold vs. morphine; all with p < 0.001) (Figure 3(a) and (b)). In contrast, although gp120 or morphine treatment upregulated Wn3a protein (2.4 ± 0.3-fold and 2.5 ± 0.1-fold vs. vehicle, respectively, p < 0.01), their combination did not drastically enhance further Wnt3a upregulation (Figure 3(a) and (c)). We also measured the protein level of p-JNK because our previous studies revealed that Wnt5a regulated gp120-induced expression of cytokines through the JNK signaling pathway.9 Similar to Wnt5a, gp120 and morphine cotreatment also cooperatively facilitated upregulation of p-JNK (3.7 ± 0.2-fold vs. vehicle; 1.8 ± 0.1-fold vs. gp120; 1.7 ± 0.1-fold vs. morphine; all with p < 0.001), indicating that there is a potential role of Wnt5a/JNK signaling in the interaction of morphine and gp120 on astrocyte activation.
Figure 3.
Enhancement of Wnt5a upregulation by gp120 and morphine in the SDH. (a) Immunoblots of Wnt5a, Wnt3a, and p-JNK. (b to d) Quantitative summary of panel (a). Potentiation of gp120 and morphine on Wnt5a and p-JNK but not Wnt3a (**p < 0.01; ***p < 0.001; one-way analysis of variance with Tukey post hoc tests, n = 6). The same animals used for Figure 1 were analyzed. JNK: c-Jun N-terminal kinase.
Inhibition of Wnt5a signaling suppressed spinal astrocyte activation induced by gp120 and morphine
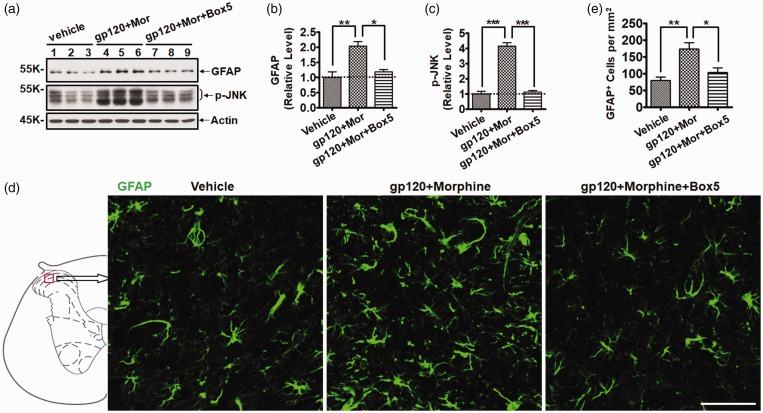
To determine whether Wnt5a signaling is critical for the astrocytic activation driven by chronic gp120 and morphine administration, we inhibited Wnt5a signaling with Box5, which is a Wnt5a-derived hexapeptide that specifically antagonizes Wnt5a activity.34 To block Wnt5a signaling, Box5 (16 µg) was i.t. administrated 30 min prior to gp120/morphine injection. Immunoblotting results showed that gp120+morphine injection significantly increased the expression of GFAP (2.0 ± 0.3-fold vs. vehicle, p < 0.01) at day 21 and that Box5 markedly attenuated the increase of GFAP by 85% (p < 0.05) (Figure 4(a) and (b)). Box5 alone had no effect on basal GFAP levels (data not shown). Similarly, the increase of p-JNK, a downstream effector protein of Wnt5a signaling, was also diminished by Box5 administration (p < 0.001) (Figure 4(a) and (c)). To further confirm that Box5 not only inhibited the increase of GFAP protein but also the activation of astrocyte cells, the spinal cord tissues were collected for immunofluorescent staining of GFAP. The staining results indicated that the number of GFAP+ astrocyte cells in the SDH increased after gp120 and morphine cotreatment (2.2 ± 0.3-fold vs. vehicle, p < 0.01). Importantly, Box5 blocked the increase of astrocyte cells by 92% (p < 0.05) (Figure 4(d) and (e)), which is consistent with the immunoblotting results. These results suggest that Wnt5a/JNK signaling might lead to the collaborative effect of gp120 and morphine on astrocytic activation.
Figure 4.
Box5 abolished the activation of astrocytes induced by gp120 and morphine. (a) Immunoblots of GFAP and p-JNK. (b and c) Quantitative summary of panel (a). (d) Immunostaining of GFAP in the SDH. Mice were administrated with gp120 and morphine as described in Figure 1(a), except that Box5 (16 µg, Wnt5a antagonist) was i.t. injected 30 min before gp120/morphine administration. The regions shown in the representative micrograph were indicated by the box in the scheme on the left. (e) Quantification of GFAP+ cells in the SDH of panel (d) (*p < 0.05; **p < 0.01; ***p < 0.001; one-way analysis of variance with Tukey post hoc tests, n = 3). GFAP: glial fibrillary acidic protein; JNK: c-Jun N-terminal kinase.
Wnt5a signaling was critical for spinal neuroinflammation induced by gp120 and morphine
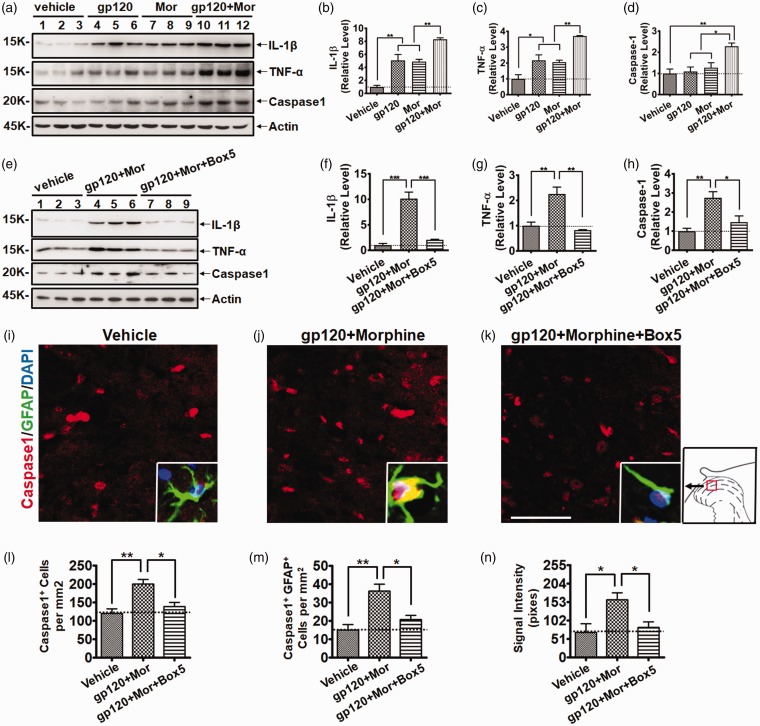
A major biological phenotype of activated astrocytes is the release of pro-inflammatory cytokines, which plays critical roles in chronic pain pathogenesis.35 Because astrocyte activation in HIV patients with chronic pain is associated with increases of the pro-inflammatory cytokines IL-1β and TNF-α, we determined whether chronic gp120 and morphine administration acted cooperatively on cytokine release. Immunoblotting results showed that gp120 or morphine treatment alone upregulated mature IL-1β protein (5.1 ± 1.6-fold and 4.9 ± 0.7-fold vs. vehicle, respectively, p < 0.01). Notably, the combination of gp120 and morphine cooperatively enhanced IL-1β expression (8.3 ± 0.4-fold vs. vehicle; 1.6 ± 0.1-fold vs. gp120; and 1.7 ± 0.1-fold vs. morphine; all with p < 0.01) (Figure 5(a) and (b)). Similar to IL-1β, an enhancement of mature TNF-α increase was also observed (3.7 ± 0.1-fold vs. vehicle, p < 0.001; 1.7 ± 0.1-fold vs. gp120, p < 0.01; 1.8 ± 0.1-fold vs. morphine, p < 0.01) (Figure 5(a) and (c)). To study the potential role of the inflammasome in regulating cytokine processing in activated astrocytes in the SDH, we measured the protein level of caspase 1, which converts pro-cytokines to mature cytokines (e.g., IL-1β and IL-18) after being activated by the inflammasome.36 Interestingly, neither gp120 nor morphine upregulated the active form of caspase 1 (P20) in the SDH (Figure 5(a) and (c)). Only the combination of gp120 and morphine significantly increased the active caspase 1 (2.3 ± 0.3-fold vs. vehicle, p < 0.01; 2.1 ± 0.2-fold vs. gp120, p < 0.05; 1.8 ± 0.2-fold vs. morphine, p < 0.05) (Figure 5(a) and (c)), suggesting that chronic treatment of gp120 and morphine can act cooperatively on cytokine production through activation of the inflammasome.
Figure 5.
Wnt5a antagonist blocked pro-inflammatory pathways activated by gp120 and morphine. (a) Immunoblots of IL-1β, TNF-α, and caspase 1 after gp120/morphine treatment. The same animals were analyzed as in Figure 1. (b to d) Quantitative summary of panel (a). Gp120 and morphine promoted the upregulation of pro-inflammatory regulators. (e) Effect of Box5 on IL-1β, TNF-α, and caspase 1 upregulation induced by gp120 and morphine. (f to h) Quantitative summary of panel (e). The same animals were analyzed as in Figure 4. Box5 attenuated the upregulation of pro-inflammatory regulators in the SDH. (i to k) Double staining of GFAP and caspase 1 in the SDH. The regions shown in the representative micrograph were indicated by the box in the scheme on the right. (l to n) Quantification of caspase 1+, caspase 1+/GFAP+, and caspase 1 staining intensity in the GFAP+ cells in the SDH. Box5 blocked caspase 1 activation in astrocytes in the SDH (*p < 0.05; **p < 0.01; ***p < 0.001; one-way analysis of variance with Tukey post hoc tests; for experiment (a) to (d), n = 6; for experiment (e) to (n), n = 3). GFAP: glial fibrillary acidic protein; IL: interleukin; TNF: tumor necrosis factor; DAPI: 4′,6-diamidino-2-phenylindole.
Next, to explore the significance of Wnt5a signaling in regulating the neuroinflammation induced by gp120 and morphine, we used Box5 to antagonize Wnt5a activity. The immunoblotting results showed that after Box5 was applied to the spinal cord, the combination of gp120 and morphine failed to drive IL-1β and TNF-α upregulation (Figure 5(e) to (g)). Importantly, Box5 also prevented the gp120+morphine-induced increase of caspase 1 (Figure 5(e) and (h)), indicating a crucial role of Wnt5a signaling in controlling inflammasome activation. To further confirm that gp120+morphine-induced inflammasome activation occurred in reactive astrocytes in the SDH, double staining of caspase 1 and GFAP in the spinal cord was performed. Fluorescent staining results revealed that the combination of gp120 and morphine markedly increased the number of caspase 1+ cells in the SDH (1.7 ± 0.2-fold vs. vehicle, p < 0.01) (Figure 5(i) and (l)). Notably, both the quantity (2.4 ± 0.4-fold vs. vehicle, p < 0.01) (Figure 5(i) and (m)) and signal intensity (2.3 ± 0.3-fold vs. vehicle, p < 0.05) (Figure 5(i) and (n)) of caspase 1+/GFAP+ cells significantly increased upon gp120+morphine stimulation, demonstrating that inflammasome-mediated cytokine processing occurred in reactive astrocytes in the SDH. Similar to the immunoblotting results, Wnt5a inhibition by Box5 also abolished the caspase 1 upregulation in astrocytes (Figure 5(i) to (n)). These data reveal a critical role of Wnt5a signaling in the inflammasome-mediated neuroinflammation induced by gp120 and morphine.
Wnt5a signaling is essential for morphine to exacerbate gp120-induced pain
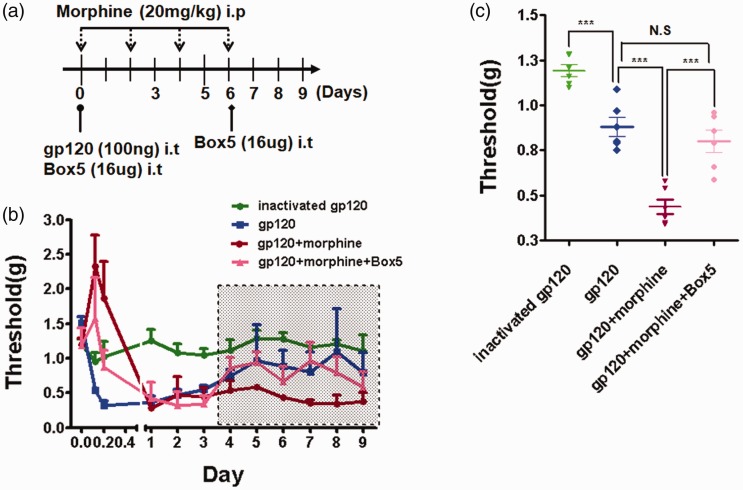
The above results revealed that Wnt5a signaling is crucial to regulate gp120+morphine-induced neuroinflammation and astrocytic activation at the molecular and cellular levels. We next evaluated the effect of Wnt5a signaling on morphine enhancement of gp120-induced hyperalgesia at the behavioral level. Considering that the exacerbated effect of morphine on gp120-induced hyperalgesia was clearly detectable at day 6 (Figure 1), we designed a short-term injection program (Figure 6(a)). Similar to the results of Figure 1, gp120 injection induced long-lasting hyperalgesia, and morphine markedly potentiated the pain (p < 0.001) (Figure 6(b) and (c)). Importantly, after injection of the Wnt5a antagonist Box5, the average PWT (between days 4 and 9) significantly rose from (0.45 ± 0.11 g) to (0.88 ± 0.13 g) (p < 0.001) (Figure 6(b) and (c)). These data show that Wn5a signaling is critical for morphine to enhance gp120-induced hyperalgesia.
Figure 6.
Box5 blocked the morphine potentiation of gp120-induced pain. (a) Drug administration paradigm. In the simplified procedure, gp120 was i.t. injected on day 0 and morphine was i.p. administrated on days 0, 2, 4, and 6. Box 5 was i.t. delivered 30 min before gp120/morphine injection on days 0 and 6. (b) Hyperalgesia measured by von Frey tests. Identical to Figure 1(a), mechanical pain test was performed 5 h postmorphine injection. (c) Statistical comparison of the data points among groups in the shaded area in panel (b). After day 4, Box5 attenuated the potentiation effect of morphine on gp120-induced hyperalgesia (***p < 0.001; N.S., not significant; one-way analysis of variance with Tukey post hoc tests, n = 6).
Discussion
In this study, we show that repeated administration of morphine in the gp120 pain model leads to increased hypersensitivity to mechanical stimulation. This observation is consistent with the clinical findings that chronic use of morphine and other opioid analgesics exacerbates pain in HIV patients.4,5 Therefore, this animal model may provide a relevant system to elucidate the spinal pathogenic mechanism of opioid analgesic-induced exacerbation of HIV-associated pain. Indeed, the model not only develops morphine-exacerbated pain behaviors but also manifests various cellular and molecular pathologies observed in the pain-positive patients,7,8,11 including neuroinflammation, that are implicated in pain pathogenesis.7,8,11 The effect of opioids in potentiating the preexisting pain conditions was reported by previous clinical or preclinical studies.37–39 For example, in a rat neuropathic pain model, morphine administration markedly prolonged pain condition.40
Our data reveal multiple cellular and molecular changes in the gp120 model that are exacerbated by morphine treatment. For instance, astrocytes are known to specifically activate in the spinal cords of HIV patients who develop pain but not from patients who do not develop pain, indicating their critical roles in the pathogenesis of HIV-associated pain.8 Significantly, repeated morphine treatments enhanced the activation of astrocytes in the gp120 model, supporting the idea that morphine promotes the astroglial reaction to facilitate the development of gp120-induced pain. The expression of neuroinflammation, as suggested by the upregulation of pro-inflammatory cytokines usually together with glial activation, is a prominent molecular hallmark that develops specifically in the spinal cord of the “pain-positive” HIV patients, indicating its potentially important contribution to the pathogenesis of HIV pain.7 Again, this HIV pain-associated process is exacerbated by morphine administration. It is possible that morphine exacerbates the gp120-induced pain via a mechanism involving these cellular and molecular processes.
Interestingly, we did not observe evident microglial activation in this study (Figure 2). Previous studies identified microglial activation as a crucial modulator of OIH and tolerance.41–43 The discrepancy is probably due to the differences in experimental conditions, including morphine administration paradigms (e.g., temporal patterns of administration, doses, and delivery routes) and animal species. Consistent with this notation, we also observed morphine-induced microglial activation in other studies with different paradigm of drug administration (in preparation for submission).
Our previous studies elucidated that Wnt5a plays a key role in regulating the pathogenesis of HIV-associated pain in the gp120 model.11,12 Significantly, Wnt5a, which is specifically upregulated in the “pain-positive” patients,11 increases in the gp120 model, and this upregulation is enhanced by morphine (Figure 3). Furthermore, we show here that a Wnt5a-specific antagonist abolishes the exacerbation of astrocyte activation as well as cytokine upregulation in the gp120 model. These findings suggest that Wnt5a is a master regulator through which morphine causes the exacerbation of gp120-induced pain. In support of this idea, we demonstrated that inhibition of Wnt5a by the antagonist abolished the exacerbation of mechanical allodynia induced by morphine (Figure 6). Based on these collective findings, we suggest that morphine functionally interacts with gp120 to exacerbate the pathogenesis of HIV-associated pain via Wnt5a-regulated neuroinflammation.
Authors’ Contribution
YS and SY performed the experiments; YS and SJT wrote the manuscript; and SJT designed the study.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health Grants R01NS079166, R01DA036165, and R01NS095747 (to SJT) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Aouizerat BE, Miaskowski CA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Lee KA. Risk factors and symptoms associated with pain in HIV-infected adults. J Assoc Nurses AIDS Care 2010; 21: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobalian A, Tsao JCI, Duncan RP. Pain and the use of outpatient services among persons with HIV: results from a nationally representative survey. Medical Care 2004; 42: 129–138. [DOI] [PubMed] [Google Scholar]
- 3.Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician 2012; 15: ES157–ES168. [PubMed] [Google Scholar]
- 4.Smith HS. Treatment considerations in painful HIV-related neuropathy. Pain Physician 2011; 14: E505–E524. [PubMed] [Google Scholar]
- 5.Önen NF, Barrette E-P, Shacham E, Taniguchi T, Donovan M, Overton ET. A review of opioid prescribing practices and associations with repeat opioid prescriptions in a contemporary outpatient HIV clinic. Pain Pract 2012; 12: 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Liu X, Tang S-J. Interactions of opioids and HIV infection in the pathogenesis of chronic pain. Front Microbiol 2016; 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan S-B, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A, Sarna SK, Tang S-J. Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol 2014; 75: 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci 2012; 32: 10833–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Shi Y, Shu J, Gao J, Wu P, Tang SJ. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem 2013; 288: 13610–13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Zhong L, Yang X, Andersson T, Huang M, Tang S-J. Wnt5a signaling contributes to Aβ-induced neuroinflammation and neurotoxicity. PLoS One 2011; 6: e22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Shu J, Gelman BB, Lisinicchia JG, Tang SJ. Wnt signaling in the pathogenesis of human HIV-associated pain syndromes. J Neuroimmune Pharmacol 2013; 8: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan S, Ji G, Li B, Andersson T, Neugebauer V, Tang S-J. A Wnt5a signaling pathway in the pathogenesis of HIV-1 gp120-induced pain. Pain 2015; 156: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008; 24: 479–496. [DOI] [PubMed] [Google Scholar]
- 14.Angst MD, Martin S, Clark MD. Opioid-induced hyperalgesiaA qualitative systematic review. Anesthesiology 2006; 104: 570–587. [DOI] [PubMed] [Google Scholar]
- 15.Collett BJ. Opioid tolerance: the clinical perspective. Br J Anaesth 1998; 81: 58–68. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend 2007; 88: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonek M, McLane VD, Stevens DL, Lippold K, Akbarali HI, Knapp PE, Dewey WL, Hauser KF, Paris JJ. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav Immun 2018; 69: 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res 2002; 280: 192–200. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 2010; 167: 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Li H, Zhang Y, Sun X, Hanley GA, LeSage G, Zhang Y, Sun S, Peng Y, Yin D. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem Biophys Res Commun 2010; 391: 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, Lesage G, Yin D. Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett 2011; 489: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 2012; 109: 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, Tremblay MJ, Mossman KL, Ashkar AA, Kovacs C, Bowdish DME, Snider DP, Kaul R, Kaushic C. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 2013; 191: 4246–4258. [DOI] [PubMed] [Google Scholar]
- 24.El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest 2011; 40: 498–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 2007; 104: 3396–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res 1999; 847: 18–25. [DOI] [PubMed] [Google Scholar]
- 27.Ye L, Xiao L, Bai X, Yang S-Y, Li Y, Chen Y, Cui Y, Chen Y. Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats. Neurosci Lett 2016; 634: 79–86. [DOI] [PubMed] [Google Scholar]
- 28.Ru W, Tang SJ. HIV-1 gp120Bal down-regulates phosphorylated NMDA receptor subunit 1 in cortical neurons via activation of glutamate and chemokine receptors. J Neuroimmune Pharmacol 2016; 11: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology 2006; 27: 217–228. [DOI] [PubMed] [Google Scholar]
- 30.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001; 410: 988–994. [DOI] [PubMed] [Google Scholar]
- 31.Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338: 160–182. [DOI] [PubMed] [Google Scholar]
- 32.Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci 2001; 21: 2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YN, Tsai RY, Lin SL, Chien CC, Cherng CH, Wu CT, Yeh CC, Wong CS. Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKCgamma signaling pathway. Behav Brain Res 2012; 229: 401–411. [DOI] [PubMed] [Google Scholar]
- 34.Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, Axelsson L, Andersson T. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci U S A 2009; 106: 19473–19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009; 10: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzan E, Eisenberg E, Treister R, Haddad M, Pud D. A negative correlation between hyperalgesia and analgesia in patients with chronic radicular pain: is hydromorphone therapy a double-edged sword? Pain Physician 2013; 16: 65–76. [PubMed] [Google Scholar]
- 38.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 2008; 139: 431–438. [DOI] [PubMed] [Google Scholar]
- 39.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain 2006; 7: 43–48. [DOI] [PubMed] [Google Scholar]
- 40.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 2016; 113: E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leduc-Pessah H, Weilinger NL, Fan CY, Burma NE, Thompson RJ, Trang T. Site-specific regulation of P2X7 receptor function in microglia gates morphine analgesic tolerance. J Neurosci 2017; 37: 10154–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrini F, Trang T, Mattioli T-AM, Laffray S, Del’Guidice T, Lorenzo L-E, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu J-M, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013; 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, Chen ML, Zhang YQ, Zhao ZQ. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci. 2010; 30: 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]