Abstract
Aims
The occurrence of hyperbilirubinaemia after heart surgery using cardiopulmonary bypass or post‐operative heart failure is fairly common. We investigated the incidence, predictive value, and post‐operative outcome of hyperbilirubinaemia after cardiac surgery in an effort to identify potential risk factors and significance on clinical outcome.
Methods and results
Between 2006 and 2016, 1272 (10.1%) out of 12 556 patients developed hyperbilirubinaemia, defined as bilirubin concentration >3 mg/dL, during post‐operative course at our institution. All patients who were operated using cardiopulmonary bypass were included. Hepatic dysfunction was diagnosed preoperatively in 200 patients (15.7%), whereas mean model of end‐stage liver disease score was 11.22 ± 4.99. Early mortality was 17.4% with age [hazard ratio (HR) 1.019, 95% confidence interval (CI) 1.008–1.029; P = 0.001], diabetes (HR 1.115, CI 1.020–1.220; P = 0.017), and emergent procedures (HR 1.315, CI 1.012–1.710) as multivariate predictors. Post‐operative predictors were low‐output syndrome (HR 3.193, 95% CI 2.495–4.086; P < 0.001), blood transfusion (HR 1.0, CI 1.0–1.0; P < 0.001), and time to peak bilirubin (HR 1.1, CI 1.0–1.1; P < 0.001). We found an increased correlation with mortality at 3.5 post‐operative day as well as an optimal cut‐off value for bilirubin of 5.35 mg/dL. A maximum bilirubin of 25.5 mg/dL was associated with 99% mortality. Survival analysis showed significantly decreased survival for patients who developed late, rather than early, hyperbilirubinaemia.
Conclusions
Post‐operative hyperbilirubinaemia is a prevalent threat after cardiopulmonary bypass, associated with high early mortality. The timing and amount of peak bilirubin concentration are linked to the underlying pathology and are predictors of post‐operative outcome. Patients with late development of steep hyperbilirubinaemia warrant meticulous post‐operative care optimizing cardiac and end organ functions before reaching the point of no return.
Keywords: Hyperbilirubinaemia, Liver failure, Low‐output syndrome, Cardiopulmonary bypass
Introduction
Several studies have reported the occurrence of early post‐operative hyperbilirubinaemia after cardiac surgery with varying incidence between 10% and 40%.1, 2, 3
Despite marked improvement in cardiopulmonary bypass (CPB) strategies, post‐operative hyperbilirubinaemia remains a serious concern, correlating with worse post‐operative outcomes.4, 5 Whether this form of liver dysfunction is an effect of CPB associated factors, that is, haemolysis, hypoperfusion, or systemic inflammatory response, remains to be asserted.1, 6, 7 Most studies were concerned with identifying factors predicting the incidence of post‐operative jaundice, following cardiac surgery. While transient moderate hyperbilirubinaemia is usually associated with a favourable outcome, late occurring ascending hyperbilirubinaemia was associated with higher mortality and morbidity.8
The aim of this study was to identify possible factors indicating the transition from transient jaundice to persistent marked hyperbilirubinaemia, raising cause for concern due to marked deterioration in liver function. A possible threshold marking the difference between these two entities may be useful for the clinician to identify patients at risk and reduce morbidity and mortality.
Methods
After approval of our institutional review board (S‐286/2010), data collected in a prospective manner from our cardiac surgery registry were analysed. During the period between October 2006 and September 2016, 1272 out of 12 556, who underwent routine or emergency surgery at our institution and developed post‐operative hyperbilirubinaemia, defined as total bilirubin concentration >3 mg/dL, were enclosed in our study. Only patients with the need for CPB installation, who were at least 18 years of age at the time of the procedure, were included. Operations were performed with the use of crystalloid or blood, antegrade cardioplegia, and on‐pump beating heart procedures.
Demographic data included age, gender, renal or hepatic dysfunction, history of myocardial infarction, presence of poor left ventricular function, and peripheral artery disease. Furthermore, cardiovascular risk factors such as arterial hypertension, diabetes mellitus, history of smoking, and obesity were collected. Mean laboratory values for total bilirubin and creatinine concentrations, international normalized ratio, platelets, haemoglobin, and leucocyte concentration, and liver function tests were measured. Risk stratification scores such as EuroSCORE and model of end‐stage liver disease (MELD) score were calculated. Preoperative status such as need for catecholamine support, invasive ventilation, and emergency operation was recorded.
Operative data such as mean operation time, CBP time, and cross‐clamping time, in addition to mean minimal temperature, were documented. Patient follow‐up was completed by routine questionnaire used for quality management at our clinic. Recorded outcome variables included early mortality (occurring up to 30 days after surgery). Post‐operative outcome analysis consisted of need and time for catecholamine support, need for blood/blood products and amount of transfusion, intubation time, and length of stay on intensive care unit (ICU). Adverse cardiovascular events such as low‐output syndrome (defined as inadequate cardiac output with or without the need for mechanical circulatory support and lactic acidosis), cerebrovascular events, post‐operative bleeding (>400 mL in 1 h), and need for surgical re‐exploration, new onset dialysis, re‐intubation, atrial flutter, and infections were included.
Statistical analysis
Statistical analysis was carried out using IBM SPSS statistics version 23 software (SPSS Chicago, IL). The data analysed are presented as continuous or categorical variables. Continuous data are expressed as mean ± SD when variables were assumed to be normally distributed. Categorical data are expressed as total numbers and percentages. Risk factors for early and midterm mortality were calculated using Cox regression analysis. A P‐value of less than 0.05 was considered statistically significant. Correlation between mortality and occurrence of maximum bilirubin concentration as well as value was calculated using receiver operator curve (ROC) analysis. Youden index was used for assessment of optimal cut‐off values. One‐year survival estimates were calculated using Kaplan–Meier method.
Results
The demographic data of our patients are depicted in Table 1. Mean age at the time of operation was 65.36 ± 12.8 years, and 355 (27.9%) of patients developing post‐operative hyperbilirubinaemia were female. The mean logistic EuroSCORE was 22.2 ± 19.5 and 997 (78.8%) patients presented with symptoms of congested heart failure, classified by the New York Heart Association as grades III and IV. Echocardiographic and/or catheterization data showed poor left ventricular function—defined as ejection fraction <35%—in 288 (21.8%) patients, while 388 (30.5%) had measured increase in pulmonary artery pressure. Previous myocardial infarction was found in 346 (27.2%) patients and previous percutaneous transluminal coronary angioplasty in 217 (17.1%), while 197 (15.7%) suffered from unstable angina. Renal impairment was present in 378 (29.7%) patients, whereas only 31 (2.4%) patients required haemodialysis therapy. The mean MELD score was calculated at 11.22 ± 4.99, and 200 (15.7%) patients had documented liver dysfunction on preoperative workup. Arterial hypertension was present in 988 (77.7%), diabetes mellitus in 317 (24.9%), obesity in 314 (24.7%), and peripheral artery occlusion disease (PAD) in 121 (9.5%) patients. History of smoking was found in 442 (34.8%) patients, while 278 (21.9%) suffered from chronic obstructive pulmonary disease. Emergent procedures were required in 328 (25.8%) patients, whereas 77 suffered from acute congestive heart failure, 76 were under catecholamine therapy, and 52 required invasive ventilation prior to operation (6.1%, 6%, and 4.1%, respectively). Preoperative bilirubin concentration was 1.56 ± 2.52 mg/dL, and further laboratory values are presented in Table 2.
Table 1.
Demographic characteristics in patients developing hyperbilirubinaemia
| Demographic variables | N = 1272 (%) |
|---|---|
| Age (years) | 65.36 ± 12.8 |
| EuroSCORE, logistic | 22.2 ± 19.5 |
| MELD score | 11.22 ± 4.99 |
| NYHA status III–IV | 997 (78.8) |
| Female | 355 (27.9) |
| LVEF ≤ 35% | 378 (29.7) |
| Pulmonary hypertension | 388 (30.5) |
| Diabetes mellitus | 317 (24.9) |
| History of smoking | 442 (34.8) |
| Arterial hypertension | 988 (77.7) |
| Atrial fibrillation | 190 (14.9) |
| PAD | 121 (9.5) |
| COPD | 278 (21.9) |
| Previous MI | 346 (27.2) |
| Previous PCI | 217 (21.8) |
| Unstable angina | 197 (15.7) |
| Renal impairment | 378 (29.7) |
| History of hepatic disease | 200 (15.7) |
| Preoperative dialysis | 31 (2.4) |
| Obesity | 314 (24.7) |
| Emergency procedure | 328 (25.8) |
| Previous cardiac surgery | 223 (17.5) |
| Acute CHF | 77 (6.1) |
| Preoperative intubation | 52 (4.1) |
| Need for catecholamine support | 76 (6) |
COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction, pulmonary hypertension defined as >35 mmHg; MELD, model for end‐stage liver disease; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery occlusion disease; PCI, percutaneous catheter intervention, renal impairment defined as creatinine concentration >2 mg/dL, obesity body mass index >30.
Table 2.
Preoperative laboratory values precardiac surgery
| Laboratory parameter | N = 1272 |
|---|---|
| Bilirubin (mg/dL) | 1.56 ± 2.52 |
| INR | 1.20 ± 0.37 |
| sGOT | 61.7 ± 60.1 |
| sGPT | 54.4 ± 57 |
| sCreatinine (mg/dL) | 1.22 ± 0.86 |
| Haemoglobin (g/L) | 12.3 ± 2.2 |
| Leucocytes × 103/L | 9.4 ± 5.2 |
| Platelets × 109/L | 221 ± 99 |
INR, international normalized ratio; sCreatinine, serum creatinine; sGOT, serum glutamic‐oxaloacetic transaminase; sGPT, serum glutamic‐pyruvic transaminase.
The type of surgical intervention and intraoperative data are outlined in Table 3. Leading cause for surgery was coronary artery bypass grafting (24.5%), followed by valve replacement or reconstruction surgery (20.4%) and combination of coronary artery bypass grafting and valvular replacement/reconstruction (17.5%). Further indications for surgery included aortic surgery including aneurysm repair and acute aortic dissection (17.2%), multiple valve interventions (10.8%), surgery for congenital/other heart defects (5.1%), and orthotropic heart transplantation (4.2%). CPB time was 169 ± 81 min, with a mean cross‐clamping time of 90 ± 52 min. Minimal temperature while on CPB reached 32.58 ± 3.34°C. Post‐operative findings are summarized in Table 4. The amount of transfusion was recorded with a mean of 1501 ± 1388 mL for packed red blood cells, 514 ± 834 mL for fresh frozen plasma, and 443 ± 424 mL for thrombocytes. Post‐operative inotropic support with administration of dobutamine was required in 782 (61.7%), while norepinephrine and epinephrine were used in 392 (31.0%) and 293 (23.2%), respectively.
Table 3.
Intraoperative parameters and specification of operation
| Intraoperative parameters | N = 1272 (%) |
|---|---|
| CPB time (min) | 169 ± 81 |
| Cross‐clamp time (min) | 90 ± 52 |
| Operation time (min) | 309 ± 123 |
| Minimal core temperature (°C) | 32.58 ± 3.34 |
| Isolated CABG | 312 (24.5) |
| Isolated valve surgery | 260 (20.4) |
| Combined CABG and valve surgery | 223 (17.5) |
| Aortic surgery | 219 (17.2) |
| Combined valve surgery | 138 (10.8) |
| Cardiac transplantation | 54 (4.2) |
| Other | 66 (5.1) |
| Valve surgery detailed | |
| Aortic valve | 441 |
| Mitral valve | 205 |
| Endocarditis | 162 |
Aortic surgery included repair for aneurysm and/or dissection; other surgeries included cardiac defect closures, reconstruction of congenital defects in the adult, and extirpation of neoplasms. CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass.
Table 4.
Post‐operative findings and adverse cardiac events
| Post‐operative finding | N = 1272 (%) |
|---|---|
| Low‐output syndrome | 360 (28.3) |
| Use of catecholamines | |
| Norepinephrine | 392 (31) |
| Dobutamine | 782 (61.7) |
| Epinephrine | 293 (23.2) |
| Time of inotropic support (days) | |
| Norepinephrine | 6.18 ± 9.9 |
| Epinephrine | 1.57 ± 4.5 |
| Use of blood/products (mL) | |
| PRBC | 1501 ± 1388 |
| FFP | 514 ± 834 |
| Thrombocytes | 443 ± 424 |
| ICU characteristics | |
| ICU stay (days) | 9.64 ± 16.61 |
| Intubation time (h) | 140 ± 267 |
| Post‐operative bleeding | 239 (18.8) |
| Need for surgical re‐exploration | 69 (5.4) |
| CPR | 104 (8.2) |
| Infection | 623 (49) |
| Cerebrovascular event | 37 (2.9) |
| Re‐intubation | 139 (10.9) |
| New onset dialysis | 425 (33.4) |
| AF | 640 (50.3) |
| Post‐operative laboratory findings | |
| INR | 1.2 ± 0.37 |
| Creatinine (mg/dL) | 1.84 ± 1.19 |
| Bilirubin (mg/L) | 6.51 ± 7.69 |
| Time to maximum bilirubin concentration (days) | 4.7 ± 10.2 |
| In‐hospital mortality | 221 (17.4) |
AF, atrial fibrillation; CPR, cardiopulmonary resuscitation; FFP, fresh frozen plasma; ICU, intensive care unit; INR, international normalized ratio; PRBC, packed red blood cell.
After surgery, the mean bilirubin concentration was 6.51 ± 7.69 mg/dL (range 3.1–89.0 mg/dL), and maximum bilirubin concentration was reached after 4.70 ± 10.2 days. The need for norepinephrine support was continued on ICU for a mean of 6.18 ± 9.9 days, while epinephrine was used for 1.57 ± 4.5 days. Invasive ventilation was continued for 140 ± 267 h, and 139 (10.9%) patients were re‐intubated due to respiratory failure. Post‐operative bleeding was encountered in 239 (18.8%) patients, while surgical re‐exploration was required in 69 (5.4%) patients. The mean creatinine concentration was 1.84 ± 1.19 mg/dL, and 425 (33.4%) patients required haemofiltration. Cerebrovascular events were developed in 37 (2.9%) patients. Further adverse events and post‐operative laboratory parameters are outlined in Table 4.
Early mortality in patients with documented post‐operative hyperbilirubinaemia occurred in 221 (17.4%) patients, while the overall mortality at the same time period was 4.2% (P < 0.001). The leading cause of death was multiple organ failure due to reduced cardiac output. Other causes included respiratory failure, septic shock, and haemorrhagic shock.
To further delineate possible risk factors, associated with this significant increase in mortality, univariate Cox regression analysis was performed using established predictors for the development of hyperbilirubinaemia after cardiac surgery. Moreover, we performed a multivariate Cox regression analysis for risk factors identified upon univariate regression. The results are presented in Table 5. Increased age and the presence of diabetes mellitus were preoperative independent predictors of early mortality, while blood transfusions and emergent procedures were identified as operative predictors of mortality. Post‐operative presence of low‐output syndrome and increasing time until the development of hyperbilirubinaemia were found to be independent predictors of early mortality in patients with hyperbilirubinaemia after cardiac surgery.
Table 5.
Cox regression analysis for in‐hospital mortality
| In‐hospital mortality | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| Characteristic | P | HR (95% CI) | P | HR (95% CI) |
| Age | 0.001 | 1.015 (1.006–1.025) | 0.001 | 1.019 (1.008–1.029) |
| EuroSCORE | <0.001 | 1.015 (1.010–1.019) | ||
| NYHA | <0.001 | 1.474 (1.320–1.646) | ||
| Diabetes mellitus | <0.001 | 1.220 (1.126–1.321) | 0.017 | 1.115 (1.020–1.220) |
| PHTN | 0.005 | 1.204 (1.057–1.371) | ||
| LVEF | <0.001 | 1.201 (1.126–1.281) | ||
| Bypass time | <0.001 | 1.003 (1.002–1.004) | ||
| Blood transfusion | <0.001 | 1.000 (1.000–1.000) | <0.001 | 1.000 (1.000–1.000) |
| Low output | <0.001 | 4.917 (3.971–6.089) | <0.001 | 3.193 (2.495–4.086) |
| Bilirubin max. | <0.001 | 1.027 (1.020–1.034) | ||
| T bilirubin max. | <0.001 | 1.030 (1.025–1.035) | <0.001 | 1.019 (1.011–1.026) |
| Emergency procedure | <0.001 | 1.657 (1.336–2.056) | 0.04 | 1.315 (1.012–1.710) |
Bilirubin max., maximum concentration of bilirubin; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association classification of heart failure; PHTN, pulmonary hypertension; T bilirubin max., time to maximum bilirubin concentration.
Hence, ROC analysis to identify timing of maximum hyperbilirubinaemia (as well as concentration increase) associated with increased mortality showed a correlation with mortality at 3.5 days after cardiac surgery (sensitivity: 67.9%, specificity: 85.8%) as well as an optimal cut‐off value for bilirubin concentration of 5.35 mg/dL (sensitivity: 65.2%, specificity: 77.5%). A maximum bilirubin concentration of 25.5 mg/dL was associated with 99% mortality (sensitivity: 100%, specificity: 85%) as depicted in Figure 1. Survival analysis (Figure 2) showed a significantly decreased 1 year survival for patients developing late (>3.5 days after surgery) rather than early hyperbilirubinaemia (log‐rank <0.0001). In the subgroup of patients with maximum bilirubin level <3.5 post‐operative day (POD), average bilirubin levels were significantly lower than in patients developing maximum values >3.5 POD as depicted in Figure 3. Figure 3 illustrates furthermore progression and depression of bilirubin values during post‐operative course.
Figure 1.
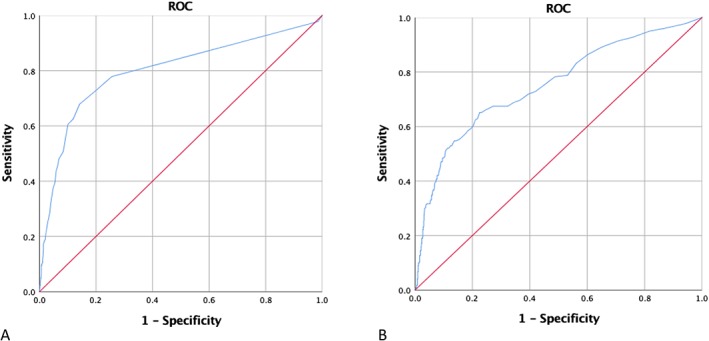
Receiver operator curve (ROC) analysis. (A) Correlation between in‐hospital mortality and increase in bilirubin at 3.5 days post‐operatively (sensitivity: 67.9%, specificity: 85.8%, area under the curve: 0.8). (B) Cut‐off value for bilirubin increase at 5.35 mg/dL and occurrence of in‐hospital mortality (sensitivity: 65.2%, specificity: 77.5%, area under the curve: 0.752).
Figure 2.
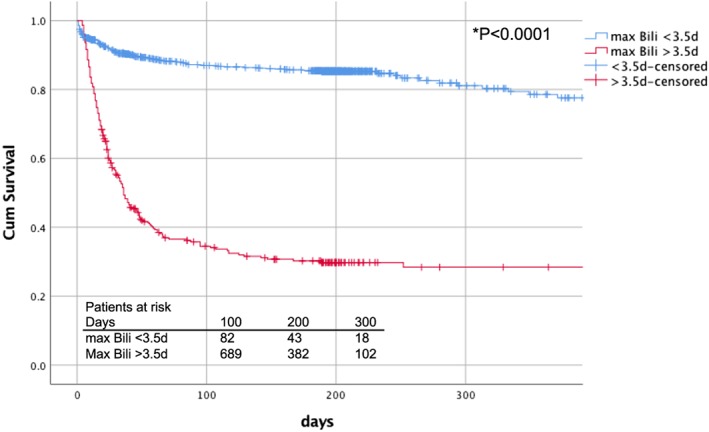
Kaplan–Meier analysis of 1 year survival. Depiction of survival in patients with maximum bilirubin increase before and after post‐operative day 3.5.
Figure 3.
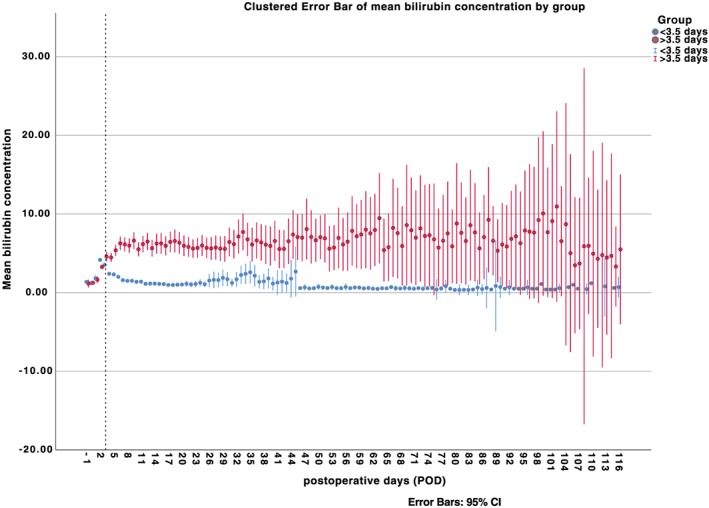
Overall bilirubin values. Depiction of overall bilirubin values divided into subgroups according to reached maximum bilirubin level less than and greater than 3.5 post‐operative day. The dashed line marks the end of post‐operative day 3. CI, confidence interval.
Discussion
It has been previously established that the use of extra corporeal circulation (ECC) is linked to early jaundice and transient liver dysfunction.5, 9 In case of progression to hepatic failure, the associated mortality rises drastically reaching up to 90%.3, 8, 10 Hence, several studies were concerned with the incidence of jaundice and liver dysfunction after the use of ECC and their predictive risk factors. However, little focus was aimed on the differentiation between early transient hyperbilirubinaemia and the development of hepatic failure. To our knowledge, this study represents the largest post‐operative analysis of hyperbilirubinaemia and its influence on early mortality in patients after cardiac surgery with use of CPB thus far.
The analysis of this contemporary cohort of cardiac surgery patients showed an overall incidence of post‐operative hyperbilirubinaemia of 10.1%, which is relatively low in comparison with recent literature, where incidence was reported between 3% and 35%, albeit it mostly exceeds 20%.1, 2, 3, 11, 12 In this study, all adult cardiac surgery patients with the installation of ECC were included. Preoperative evaluation showed the presence of any liver dysfunction in 15.7% of patients, and the calculated mean MELD score was 11.22 ± 4.99.
While controversies exist regarding whether the incidences differed according to performed operation, this study showed that once hyperbilirubinaemia was present, the mortality risk was the same, irrespective of the preceding procedure. The in‐hospital mortality increased from 4.2% in all patients to 17.4% in those with post‐operative hyperbilirubinaemia, which is comparable with reported early mortality between 19% and 25%.2, 12
Proportional hazards in Cox regression analysis identified increasing age and presence of diabetes mellitus as independent risk factors for early mortality with an odds ratio of 1.02 and 1.12 (95% confidence interval 1.008–1.029 and 1.02–1.22, respectively) in patients with hyperbilirubinaemia. While both factors are established risk factors for early mortality after cardiac surgery, their presence indicates diminished functional capacity of the liver and adds to the cumulative burden in the case of developed hyperbilirubinaemia.
Another factor associated with early mortality was the number of blood units transfused in patients with hyperbilirubinaemia. While increasing amount of blood transfusions contributes to haemolysis and thus the need for bile secretion, regression analysis showed an increase in early mortality with a hazard ratio of 1.0 and a narrow confidence interval, hence, suggesting that it is a surrogate parameter for the development of a critical perioperative state, rather than a direct cause of hyperbilirubinaemia and consecutive liver failure.
Hepatic dysfunction followed other serious perioperative complications such as low‐output syndrome and patients with emergent procedures. Therefore, multivariate regression analysis of early mortality identified both states as independent predictors of mortality with an odds ratio of 3.1 and 1.3. These states are correlated with perioperative and early post‐operative hypotension and hypoxia, which in turn are important factors in the development of post‐operative jaundice.3 While preoperative advanced New York Heart Association status, high logistic EuroSCORE, increased pulmonary pressure, and reduced left ventricular function all reflect forms of reduced cardiac output and therefore reduced perfusion, these factors were only correlated with mortality in univariate regression analysis. Although multifactorial in nature, reduced systemic blood flow during these conditions leads in turn to a reduced hepatic and biliary tract oxygen delivery and hypoperfusion.13 In addition, congestive heart failure with raised right atrial pressure is linked to liver congestion, further reducing its functional capacity.3, 13, 14 Prolonged CPB and cross‐clamp periods increase the need for inotropic drug support. Along with hypovolaemia, this can reduce systemic blood flow and cause subsequent hypoperfusion of the liver. These phenomena are most pronounced in the early post‐operative period. In addition, haemolysis, associated with CPB and its components, that is, cardiotomy suction, gaseous micro‐emboli, and necessity for transfusions, increases the burden of bilirubin disposal and bile transport.15 In most cases, this form of early jaundice is reversible, once cardiac output is sufficient and oxygen delivery is adequate.11 This was further confirmed in our findings, where the correlation between CPB time and mortality was not confirmed on multivariate regression. Accordingly, patients with restored adequate perfusion showed an initial temporary increase in bilirubin concentration. On the other hand, the later hyperbilirubinaemia develops with a steep slope; concern must be raised in regard to further systemic hypoperfusion. Hence, timing of bilirubin concentration increase was identified as an independent risk factor, as opposed to numeric maximum bilirubin concentration (hazard ratio 1.02, 95% confidence interval 1.01–1.03). This is further delineated by ROC analysis showing a correlation between increased mortality with increased bilirubin concentration at 3.5 days (POD) after initial CPB surgery (sensitivity: 65.2%, specificity: 85.8%). The optimal cut‐off bilirubin for increased bilirubin concentration was at 5.35 mg/dL (sensitivity: 65.2%, specificity: 77.5%). Survival analysis using Kaplan–Meier estimates comparing patients with developed hyperbilirubinaemia before and after POD 3.5 shows a significantly decreased 1 year survival for patients developing late hyperbilirubinaemia. These findings may point to the timing, where hyperbilirubinaemia transitions from being an immediate post‐operative sequel of CPB and its associated components to a secondary developed hepatic dysfunction. This form of hepatic dysfunction should be monitored as an indicator of congestive heart failure as observed in other studies.16 In this instance, focus should be directed on optimizing haemodynamics to prevent further deterioration, because the development of obstructive or ischaemic complications carries an especially dismal prognosis.4 This is moreover reflected in our findings concerning adverse clinical events showing prolonged ICU stay, need for invasive ventilation, high incidences of infections, or new onset dialysis when compared with uncomplicated patients. These observations in turn present surrogate markers reflecting the critical state of these patients.
Conclusions
The incidence of post‐operative hyperbilirubinaemia is a prevalent threat after CPB, associated with an increased early mortality. The timing and amount of peak bilirubin concentration may help differentiate between patients with reactive increase due to high demand in bile secretion and those with an underlying pathology of liver function due to systemic hypoperfusion. Hence, patients with late development of steep hyperbilirubinaemia warrant meticulous post‐operative care optimizing cardiac and end organ functions, before reaching the point of no return. Nevertheless, the provocative point of no return should not determine a patient's fatal fate. From our experience, patients are capable to recover from bilirubin levels exceeding 25.5 mg/dL, especially in patients of young age and restoration of adequate perfusion.
Conflict of interest
None declared.
Funding
None.
Farag M., Veres G., Szabó G., Ruhparwar A., Karck M., and Arif R. (2019) Hyperbilirubinaemia after cardiac surgery: the point of no return, ESC Heart Failure, 6, 694–700. 10.1002/ehf2.12447.
References
- 1. Wang MJ, Chao A, Huang CH, Tsai CH, Lin FY, Wang SS, Liu CC, Chu SH. Hyperbilirubinemia after cardiac operation. Incidence, risk factors, and clinical significance. J Thorac Cardiovasc Surg 1994; 108: 429–436. [PubMed] [Google Scholar]
- 2. Collins JD, Bassendine MF, Ferner R, Blesovsky A, Murray A, Pearson DT, James OF. Incidence and prognostic importance of jaundice after cardiopulmonary bypass surgery. Lancet 1983; 1: 1119–1123. [DOI] [PubMed] [Google Scholar]
- 3. Chu CM, Chang CH, Liaw YF, Hsieh MJ. Jaundice after open heart surgery: a prospective study. Thorax 1984; 39: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kraev AI, Torosoff MT, Fabian T, Clement CM, Perez‐Tamayo RA. Postoperative hyperbilirubinemia is an independent predictor of longterm outcomes after cardiopulmonary bypass. J Am Coll Surg 2008; 206: 645–653. [DOI] [PubMed] [Google Scholar]
- 5. An Y, Xiao YB, Zhong QJ. Hyperbilirubinemia after extracorporeal circulation surgery: a recent and prospective study. World J Gastroenterol 2006; 12: 6722–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumle B, Boldt J, Suttner SW, Piper SN, Lehmann A, Blome M. Influence of prolonged cardiopulmonary bypass times on splanchnic perfusion and markers of splanchnic organ function. Ann Thorac Surg 2003; 75: 1558–1564. [DOI] [PubMed] [Google Scholar]
- 7. Gardeback M, Settergren G, Brodin LA. Hepatic blood flow and right ventricular function during cardiac surgery assessed by transesophageal echocardiography. J Cardiothorac Vasc Anesth 1996; 10: 318–322. [DOI] [PubMed] [Google Scholar]
- 8. Michalopoulos A, Alivizatos P, Geroulanos S. Hepatic dysfunction following cardiac surgery: determinants and consequences. Hepatogastroenterology 1997; 44: 779–783. [PubMed] [Google Scholar]
- 9. Welbourn N, Melrose DG, Moss DW. Changes in serum enzyme levels accompanying cardiac surgery with extracorporeal circulation. J Clin Pathol 1966; 19: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arif R, Seppelt P, Schwill S, Kojic D, Ghodsizad A, Ruhparwar A, Karck M, Kallenbach K. Predictive risk factors for patients with cirrhosis undergoing heart surgery. Ann Thorac Surg 2012; 94: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 11. Mastoraki A, Karatzis E, Mastoraki S, Kriaras I, Sfirakis P, Geroulanos S. Postoperative jaundice after cardiac surgery. Hepatobiliary Pancreat Dis Int 2007; 6: 383–387. [PubMed] [Google Scholar]
- 12. Nishi H, Sakaguchi T, Miyagawa S, Yoshikawa Y, Fukushima S, Saito S, Ueno T, Kuratani T, Sawa Y. Frequency, risk factors and prognosis of postoperative hyperbilirubinemia after heart valve surgery. Cardiology 2012; 122: 12–19. [DOI] [PubMed] [Google Scholar]
- 13. Vazquez P, Lopez‐Herce J, Carrillo A, Sancho L, Bustinza A, Diaz A. Hepatic dysfunction after cardiac surgery in children. Pediatr Crit Care Med 2001; 2: 44–50. [DOI] [PubMed] [Google Scholar]
- 14. Hayashida N, Shoujima T, Teshima H, Yokokura Y, Takagi K, Tomoeda H, Aoyagi S. Clinical outcome after cardiac operations in patients with cirrhosis. Ann Thorac Surg 2004; 77: 500–505. [DOI] [PubMed] [Google Scholar]
- 15. Lockey E, McIntyre N, Ross DN, Brookes E, Sturridge MF. Early jaundice after open‐heart surgery. Thorax 1967; 22: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB, CHARM Investigators . Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009; 11: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]


