Abstract
Aims
The study evaluated exercise left ventricular global longitudinal strain (LVGLS) and invasive haemodynamics for major adverse cardiac events (MACE) prediction in heart‐transplanted (HTx) patients.
Methods and results
The study comprised 74 stable consecutive HTx patients who were followed at the Department of Cardiology, Aarhus University Hospital, Denmark, from August 2013 to January 2017. All patients were transplanted a minimum of 12 months before study entry and were included at the time of their routine annual coronary angiography. A symptom‐limited haemodynamic exercise test with simultaneous echocardiographic study was performed. The primary endpoint was MACE during follow‐up defined as heart failure hospitalization, treated rejection episodes, coronary event, or cardiac death.
The median time since transplant was 5 years [1:12] and the median follow‐up was 1095 days [391;1506]. Thirty patients (41%) experienced MACE during follow‐up. Patients who suffered MACE had an impaired resting and peak exercise systolic function in form of a lower LV ejection fraction (Rest: 56 ± 12% vs. 65 ± 7%, P < 0.001; Peak 64 ± 13% vs. 72 ± 6%, P < 0.01) and LVGLS (Rest: 13 ± 4% vs. 16 ± 2%, P < 0.01; Peak: 15 ± 6% vs. 20 ± 4%, P = 0.0001) than patients without MACE episodes. In contrast, resting diastolic filling patterns were comparable between patients suffering from MACE and patients without MACE.
At rest, pulmonary capillary wedge pressure (mPCWP) and cardiac index did not predict MACE, whereas increased right atrial pressure (mRAP) was associated with increased MACE risk. Patients with peak exercise mPCWP >23 mmHg [hazard ratio (HR) 2.5, 95% confidence interval (CI): 1.2–5.4], cardiac index <5.9 L/min/m2 (HR 2.7, 95% CI: 1.0–6.3), or mRAP >13 mmHg (HR 2.7, 95% CI: 1.1–6.3) had increased MACE risk.
Patients with exercise‐induced LVGLS increase <3.5% and peak exercise cardiac index <5.9 L/min/m2 [HR 6.1 (95% CI: 2.2–17.1)] or mPCWP >23 mmHg [HR 6.1 (95% CI: 2.1–17.5)] or mRAP >13 mmHg [HR 7.5 (95% CI: 2.3–23.9)] had the highest MACE risk.
Conclusions
Resting haemodynamic parameters were poor MACE predictors in long‐term HTx patients. In contrast, peak exercise mPCWP, mRAP, and CI were significant MACE predictors. LVGLS both at rest and during exercise were significant MACE predictors, and the combined model with peak exercise LVGLS and peak exercise mPCWP, mRAP, and CI clearly identified high‐risk HTx patients in relation cardiovascular endpoints independently of time since HTx.
Keywords: Cardiac allograft vasculopathy, Exercise haemodynamics, Heart transplant, Myocardial deformation imaging, Outcome, Strain imaging
Introduction
Heart transplantation (HTx) remains today the optimal treatment for symptomatic end‐stage heart failure patients. Along with immunosuppressive therapy, preoperative and post‐operative management and surveillance have improved during recent decades. Yet these efforts have only translated into an improved outcome during the first year after HTx whereas survival beyond the first year remains unchanged.1 Analysis of International Society for Heart and Lung Transplantation (ISHLT) registry data shows that cardiac allograft vasculopathy (CAV) and graft failure are among the major long‐term heart‐related mortality causes.1 While mild‐to‐moderate acute cellular rejection (ACR) is commonly seen after HTx, ACR rarely directly leads to death.1 Yet its appearance is strongly associated with long‐term myocardial dysfunction2 and CAV development.3 Therefore, it seems of paramount importance to identify HTx patients with early signs of graft dysfunction in order to avoid that symptomatic graft failure develops.
Longitudinal myocardial deformation assessment by left ventricular global longitudinal strain (LVGLS) has emerged as a robust method for assessment of subclinical myocardial dysfunction induced by ACR4, 5 or CAV.6 Furthermore, resting LVGLS has proven beneficial for risk stratification both early7, 8 and late following HTx,9 whereas the prognostic value of exercise myocardial deformation analysis remains unknown.
Right heart catheterization (RHC) remains the gold standard for evaluating loading conditions, pulmonary vascular resistance, and cardiac output. In the post‐transplant setting, RHC is often performed in symptomatic patients and can be used for classification of CAV severity with assessment of left ventricular (LV) and right ventricular (RV) filling pressure.10 Previous studies have demonstrated that loading conditions in patients with CAV are often within the normal range during resting conditions.11, 12 With exercise, LV and RV filling pressure increases are more pronounced in HTx patients than should be expected from studies in healthy individuals.13 Exercise‐induced elevated LV filling pressure has been associated with CAV and previous rejection burden.12 However, little is known about the prognostic value of invasive haemodynamic parameters in long‐term follow‐up HTx patients.
Thus, the present study aimed to evaluate the prognostic value of exercise myocardial deformation and invasive haemodynamics in long‐term HTx patients.
Methods
Patients
We enrolled 74 stable consecutive HTx patients who were followed at the Department of Cardiology, Aarhus University Hospital, Denmark, from August 2013 to January 2017. All patients were transplanted a minimum of 12 months before study entry and were included at the time of their routine annual coronary angiography. A comprehensive echocardiographic assessment of myocardial function was performed at rest and simultaneously with exercise RHC. The haemodynamic and echocardiographic data from a subgroup of the patients formed part of previous publications.12, 14, 15
The patients were followed prospectively from the time of exercise test until death or censoring on the 10 October 2018, whichever came first. The primary endpoint was major adverse cardiac events (MACE) during follow‐up. MACE included (i) coronary event, defined as (a) severe new coronary stenosis (≥70%) or (b) new percutaneous coronary intervention (PCI); (ii) hospitalization due to heart failure, defined as need of intravenous diuretics or inotropes; (iii) treated antibody‐mediated rejection or ACR episode (≥2R); and (iv) cardiovascular death. The secondary endpoint was coronary events during follow‐up. Patients who experienced MACE were stratified at the time of their first MACE episode. We included severe stenosis without PCI in the definition of coronary event. CAV is often clinically silent and the vessels diffusely affected. Therefore, the lack of PCI does not rule out severe CAV progression. In patients with established CAV at baseline, CAV progression was defined as new coronary branch stenosis (≥70%) or new occluded vessels or PCI treatment. Event adjudication was performed by two independent reviewers (T. C. and S. H. P.) blinded to baseline echocardiographic results and RHC data. If there was doubt about the adjudication, a third reviewer (H. E.) was asked and decided on the type of event. Death was defined as cardiovascular death if no non‐cardiovascular reason was revealed.
Right heart catheterization and exercise protocol
Right heart catheterization was performed with a standard 7.5‐F triple‐lumen Swan‐Ganz thermistor using a balloon‐tipped catheter (Edwards Lifesciences, Irvine, California, USA). The catheter was introduced into the right jugular vein using an ultrasound‐guided technique and advanced pressure waveforms, and guided into the pulmonary artery using fluoroscopy. A comprehensive, direct, and indirect assessment of haemodynamic parameters was performed at rest and during exercise, as described in a previous publication.12
A multistage symptom‐limited, semi‐supine bicycle exercise test was performed using the Cardiac Stress Table (Lode B.V., Netherlands). Workload started at 0 W and was increased by 25 W every 3 min. The patients were explicitly encouraged to maintain a fixed pedalling speed of 60 rounds per minute and to exercise until exhaustion (Borg >18).16 At each step of exercise, we measured the mean arterial blood pressure, heart rate, mean right atrial pressure (RAP), pulmonary artery pressures, pulmonary capillary wedge pressure (PCWP), cardiac output by thermodilution, and arteriovenous oxygen content difference. We used mRAP and mPCWP as markers of RV and LV filling pressure.
Transthoracic echocardiography
We used a commercially available ultrasound system (Vivid 9, GE Healthcare Horten, Norway) with a 3.5‐MHz‐phased array transducer (M5S). The frame rate was adjusted to increase 60 frames per second.
At each stage of exercise, the LV ejection fraction (LVEF) was measured based on the biplane method of discs.17 Peak LV systolic mitral annular velocity (LVS′) was estimated from the colour‐coded tissue velocity image as an average of septal, lateral, anterior, and posterior velocities. The magnitude of systolic LVGLS18 was obtained from frame‐by‐frame tracking of speckle patterns throughout the left‐sided myocardium in standard two‐dimensional (2D) cine loops. The region of interest was manually adjusted for optimal tracking results. Segments with an unacceptably low visual tracking quality were excluded. LVGLS was calculated at the time in systole when the value peaked using a 17‐segment model.19 The systole was defined as the time from aorta valve opening to aorta valve closing. Our group has previously reported repeatability of LVGLS and found a very low intra‐observer and inter‐observer variation both at rest and during exercise.5, 20
Data were analysed offline using dedicated software (EchoPAC PC SW‐Only, 113, GE‐Healthcare, Milwaukee, Wisconsin, USA) by a single investigator (T. C.) who was blinded to clinical status and invasive measurements.
Coronary angiography
Coronary angiography was performed annually or biannually through a 6‐F sheath inserted into the femoral artery. We used the ISHLT guidelines to classify the CAV burden.10 Patients with previous percutaneous intervention were classified as CAV 2 despite no present diameter stenosis ≥70% due to the common conception that the diffuse nature of CAV leads to affected epicardial as well as microvascular vessels.
Rejection burden
Biopsies were obtained routinely during the first two post‐operative years. Subsequently, biopsies were drawn only if rejection was clinically suspected. ACRs were graded according to ISHLT guidelines (1R–3R).21 All rejections ≥2R were treated with intravenous methylprednisolone 1 g for 3 days, and basal oral immunosuppression was adjusted if necessary. Biopsy‐score was calculated as: Biopsy‐score = (number of 1R rejections * 1 + number of 2R rejections * 2 + number of 3R rejections * 3)/total number of biopsies taken.
Statistical methods
Normally distributed data are presented as mean ± standard deviation; non‐normally distributed data are presented as median and interquartile range [IQR]. Categorical data are presented as absolute values and percentages. Histograms and Q–Q plots were used to check continuous values for normality of the data distribution. Between‐group differences were assessed by Student's t‐test. Sensitivity and specificity were obtained by receiver‐operating characteristic curves. Optimal between‐group cut‐off points for echocardiographic and haemodynamic parameters were defined as the intersection points of sensitivity and specificity in the receiver‐operating characteristic curves. Time‐to‐event data were evaluated by Kaplan–Meier estimates and Cox proportional hazards methods. Hazard ratios (HRs), 95% confidence intervals (CIs), and two‐sided P values were calculated using the Cox models. A multivariable Cox regression model was used to correct for time since transplantation.
P values <0.05 were considered statistically significant. Analyses were performed using STATA (STATA/IC 13, StataCorp LP, Texas, College Station, USA).
Results
Patient demographics
The median time since transplant was 5 years [1:12]. The median follow‐up for all patients was 1095 days [391;1506]. The median follow‐up was 367 days [327;943] in the MACE group vs. 1390 days [952–1643] in the group without MACE. No patients were lost to follow‐up.
During follow‐up, 30 HTx patients experienced at least one MACE episode. A total of 7 (9%) patients suffered treatment‐demanding rejections, 11 (15%) patients were hospitalized due to heart failure, 23 (52%) patients suffered a coronary event, and 8 (11%) patients died from cardiovascular disease. A total of eight patients died due to non‐cardiovascular disease.
Table 1 presents the baseline demographics of the MACE group and the no‐MACE group. As expected, time since transplantation was significantly longer in the MACE group than in the no‐MACE group (P < 0.01). The former had more prevalent CAV (P < 0.0001) than the latter. We found no between‐group difference in rejection burden, creatinine levels, and haemoglobin. In contrast, patients in the MACE group had significantly higher Troponin‐T (P < 0.05) and Nt‐Pro‐BNP levels (P < 0.05) than patients in the no‐MACE group.
Table 1.
Patient characteristics at the time of right heart catheterization
| MACE (n = 30) | No MACE (n = 44) | P value | |
|---|---|---|---|
| Male n (%) | 23 (77) | 32 (73) | 0.70 |
| Donor age (years) | 46 ± 10 | 42 ± 13 | 0.22 |
| Age (years) | 52 ± 15 | 54 ± 10 | 0.44 |
| Time since transplantation (years) | 10 ± 7 | 5 ± 5 | <0.01 |
| NYHA functional class >1 n (%) | 13 (43) | 4 (9) | <0.001 |
| Body mass index (kg/m2) | 26 ± 4 | 25 ± 5 | 0.51 |
| CAV and previous rejections | |||
| Graft vasculopathy n (%) | 22 (73) | 10 (23) | <0.0001 |
| Previous percutaneous intervention | 8 (27) | 2 (5) | <0.01 |
| Number of EMBs showing 1R | 9 [6;11] | 7 [4;9] | 0.06 |
| Number of EMBs showing ≥2R | 1 [0;1] | 0 [0;1] | 0.14 |
| Biopsy‐score | 0.5 [0.3;0.6] | 0.5 [0.4–0.6] | 0.39 |
| Medication | |||
| Prednisolone n (%) | 15 (50) | 22 (50) | 1.00 |
| Cyclosporine n (%) | 12 (40) | 11 (25) | 0.17 |
| Tacrolimus n (%) | 18 (60) | 32 (73) | 0.25 |
| Mycophenolate n (%) | 21 (70) | 38 (86) | 0.09 |
| Everolimus n (%) | 9 (30) | 10 (23) | 0.48 |
| Statins n (%) | 27 (90) | 38 (86) | 0.64 |
| ACE/ATII inhibitor n (%) | 22 (73) | 29 (66) | 0.50 |
| Calcium blocker (%) | 10 (33) | 21 (48) | 0.22 |
| Aspirin (%) | 19 (63) | 16 (36) | <0.05 |
| Furosemid or bumetanide n (%) | 10 (33) | 7 (16) | 0.08 |
| Biochemistry | |||
| Creatinine (μmol/L) | 120 [80;152] | 100 [82;118] | 0.18 |
| Haemoglobin (mmol/L) | 8.2 ± 1.1 | 8.4 ± 1.0 | 0.47 |
| Troponin‐T (ng/L) | 18 [6;37] | 12 [6;19] | <0.05 |
| NT‐ProBNP (ng/L) | 501 [319;1746] | 364 [182;768] | <0.05 |
Data are presented as per cent or mean ± standard deviation or median and [IQR]. CAV, cardiac allograft vasculopathy; EMB, endomyocardial biopsy; MACE, major adverse cardiac events; NYHA, New York Heart Association.
Echocardiographic graft function in the major adverse cardiac events group versus the no‐major adverse cardiac events group
Patients who suffered MACE had an impaired resting systolic function in form of a lower LVEF (56 ± 12% vs. 65 ± 7%, P < 0.001), LVGLS (13 ± 4% vs. 16 ± 2%, P < 0.01), and LVS′ (5.4 ± 1.3 cm/s vs. 6.2 ± 1.1 cm/s, P < 0.01) than patients without MACE episodes. In contrast, resting diastolic filling patterns were comparable between patients suffering from MACE and patients without MACE (E/A ratio 2.1 ± 0.7 vs. 2.0 ± 0.7, P = 0.43; E‐deceleration time 162 ± 59 ms vs. 163 ± 37 ms, P = 0.95; isovolumetric relaxation time 72 ± 26 ms vs. 64 ± 17 ms, P = 0.11; E/e′ ratio 11.5 ± 5.5 vs. 9.3 ± 5.2, P = 0.09). RV function by tricuspid annular plan systolic excursion (14.0 ± 4.3 mm vs. 14.4 ± 2.7 mm, P = 0.59) and RV S′ (7.2 ± 2.4 cm/s vs. 7.7 ± 1.7 cm/s, P = 0.35) did not differ between MACE groups. However, patients suffering MACE during follow‐up had significantly lower RV free‐wall longitudinal strain (19 ± 6% vs. 22 ± 4%, P < 0.05) than patients without MACE.
At peak exercise, LVGLS (15 ± 6% vs. 20 ± 4%, P = 0.0001) and LVEF (64 ± 13% vs. 72 ± 6%, P < 0.01) were significantly lower in patients suffering from MACE than in patients without MACE, Figure 1 .
Figure 1.
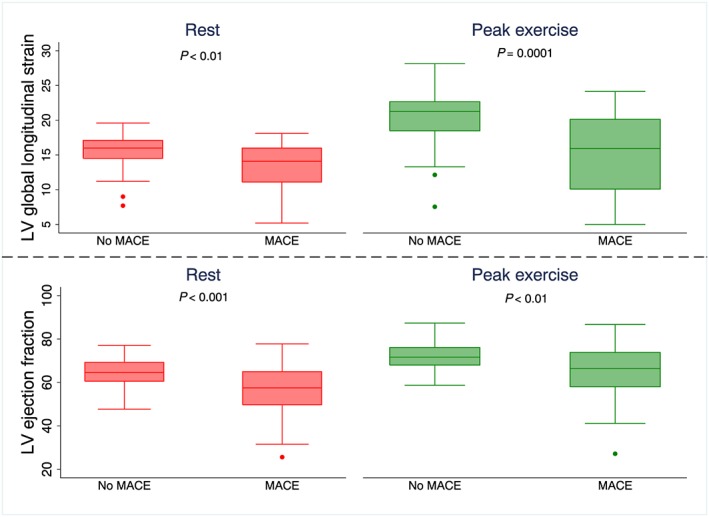
Boxplots with two‐sided P values showing left ventricular ejection fraction (LVEF) and left ventricular global longitudinal strain (LVGLS) at rest and at peak exercise in patients without major adverse cardiac events (no MACE) and in patients suffering major adverse cardiac events (MACE) during follow‐up.
In HTx patients, LVGLS both at rest and at peak exercise was weakly but significantly associated with PCWP (rest: r = −0.25, P < 0.05; peak: r = −0.31, P < 0.01), RAP (rest: r = −0.30, P < 0.01; peak: r = −0.38, P < 0.01) and cardiac index (rest: r = 0.35, P < 0.01; peak: r = 0.44, P < 0.001).
Invasive haemodynamics in the major adverse cardiac events group versus no‐major adverse cardiac events group
Table 2 shows invasive haemodynamics at resting and exercise conditions in HTx patients suffering from MACE and in HTx patients without MACE episodes during follow‐up.
Table 2.
Invasive haemodynamics at rest versus peak exercise in MACE versus no‐MACE HTx patients
| Rest | Peak exercise | |||||
|---|---|---|---|---|---|---|
| MACE (n = 26) | No MACE (n = 48) | P value | MACE (n = 25) | No MACE (n = 45) | P value | |
| Peak exercise METs (mL/kg/min) | 5.7 ± 1.5 | 6.0 ± 1.3 | 0.43 | |||
| Heart rate (beats/min) | 86 ± 16 | 84 ± 12 | 0.62 | 137 ± 17 | 129 ± 16 | <0.05 |
| MAP (mmHg) | 103 ± 12 | 99 ± 11 | 0.14 | 131 ± 18 | 127 ± 19 | 0.34 |
| AV diff (%) | 29 ± 6 | 27 ± 4 | 0.12 | 68 ± 9 | 64 ± 12 | 0.15 |
| SVRI (dynes/s/cm5/m2) | 2991 ± 565 | 2753 ± 558 | 0.08 | 1682 ± 485 | 1554 ± 474 | 0.34 |
| CI (L/min/m2) | 2.6 ± 0.4 | 2.8 ± 0.5 | 0.07 | 5.8 ± 1.6 | 6.4 ± 1.6 | 0.14 |
| SVI (mL/m2) | 32 ± 8 | 34 ± 7 | 0.23 | 42 ± 10 | 50 ± 12 | <0.01 |
| mRAP (mmHg) | 6 ± 4 | 4 ± 2 | <0.01 | 17 ± 10 | 11 ± 4 | <0.01 |
| mPAP (mmHg) | 21 ± 8 | 17 ± 4 | 0.08 | 38 ± 9 | 36 ± 7 | 0.28 |
| mPCWP (mmHg) | 12 ± 6 | 9 ± 3 | <0.05 | 28 ± 9 | 22 ± 9 | <0.01 |
| TPG (mmHg) | 9 ± 5 | 8 ± 3 | 0.43 | 10 ± 8 | 13 ± 7 | <0.05 |
| PVR (wood units) | 1.9 ± 1.4 | 1.5 ± 0.6 | 0.47 | 1.0 ± 1.0 | 1.2 ± 0.7 | 0.15 |
| PAC (mL/mmHg) | 4.9 ± 2.0 | 5.7 ± 2.2 | 0.09 | 3.9 ± 1.8 | 4.6 ± 1.8 | 0.17 |
Data are presented as mean ± standard deviation. AV diff, arterial‐venous saturation difference; CI, cardiac index; HTx, heart‐transplanted; MACE, major adverse cardiac events; mPAP, mean pulmonary arterial pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right atrial pressure; PAC, pulmonary arterial compliance; PVR, pulmonary vascular resistance; SVI, Stroke Volume Index; SVRI, Systemic Vascular Resistance Index; TPG, transpulmonary pressure gradient.
As depicted, the RV and LV filling pressures were higher in the MACE group than in the no‐MACE group during resting conditions. Pulmonary arterial compliance and cardiac index tended to be lower in the MACE group than in the no‐MACE group.
At peak exercise, we observed a significant difference in PCWP (P < 0.01), RAP (P < 0.01), and stroke volume (P < 0.01) between the MACE group and the no‐MACE group.
Primary endpoint: major adverse cardiac events prediction by echocardiographic graft function and invasive haemodynamics
Table 3 shows the optimal cut‐off points and the Cox regression analysis of the ability of echocardiographic parameters and invasive haemodynamics to predict MACE. At rest, RAP was the only parameter that predicted MACE. However, at peak exercise, RAP, PCWP, SVI, and cardiac index all predicted MACE, Figure 2 . We found that LVGLS was a strong MACE predictor both at rest and during exercise, Figure 3 A.
Table 3.
Univariable and multivariable cox regression analysis of the ability of haemodynamics to predict MACE
| Cut‐off value | Univariate HR (95% CI) | P value | Adjusteda HR (95% CI) | P value | |
|---|---|---|---|---|---|
| Rest | |||||
| Echocardiography | |||||
| LVEF (%) | 63 | 2.8 (1.3–6.0) | <0.01b | 2.5 (1.2–5.5) | <0.05 |
| LVGLS (%) | 15.2 | 2.9 (1.3–6.4) | <0.01 | 2.7 (1.2–6.0) | <0.05 |
| E/A ratio | 1.9 | 1.7 (0.8–3.5) | 0.16 | — | |
| E/e′ ratio | 8.7 | 1.6 (0.8–3.4) | 0.18 | — | |
| RV FW LS | 21.7 | 2.1 (1.0–4.5) | 0.06 | — | |
| Invasive haemodynamics | |||||
| CI rest (L/min/m2) | 2.8 | 1.3 (0.7–2.8) | 0.42 | — | |
| mRAP rest (mmHg) | 5 | 2.3 (1.1–4.8) | <0.05 | 2.0 (1.0–4.2) | 0.06 |
| mPAP rest (mmHg) | 18 | 1.9 (0.9–4.0) | 0.08 | — | |
| mPCWP rest (mmHg) | 9 | 1.5 (0.7–3.2) | 0.26 | — | |
| PAC rest (mL/mmHg) | 5.2 | 1.2 (0.6–2.5) | 0.63 | — | |
| Peak exercise | |||||
| Echocardiography | |||||
| LVEF (%) | 70 | 2.7 (1.2–6.0) | <0.05 | 2.7 (1.2–5.9) | <0.05 |
| LVGLS (%) | 19.7 | 3.2 (1.4–7.4) | <0.01 | 2.9 (1.3–6.6) | <0.05 |
| Delta LVGLS | 3.5 | 3.8 (1.7–8.7) | <0.01 | 2.9 (1.2–7.0) | <0.05 |
| Invasive haemodynamics | |||||
| CI peak (L/min/m2) | 5.9 | 2.7 (1.2–5.8) | <0.05 | 2.3 (1.1–5.1) | <0.05 |
| mRAP peak (mmHg) | 13 | 2.7 (1.1–6.3) | <0.05 | 2.4 (1.0–5.6) | <0.05 |
| mPAP peak (mmHg) | 38 | 1.5 (0.7–3.2) | 0.25 | — | |
| mPCWP peak (mmHg) | 23 | 2.5 (1.2–5.4) | <0.05 | 2.2 (1.0–4.8) | 0.05 |
| PAC peak (mL/mmHg) | 4 | 2.0 (1.0–4.3) | 0.07 | — | |
CI, cardiac index; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; MACE, major adverse cardiac events; mPAP, mean pulmonary arterial pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right atrial pressure; PAC, pulmonary arterial compliance; RV FW LS, right ventricular free wall longitudinal strain.
Adjusted for time since transplantation.
Significant after adjustment for presence of cardiac allograft vasculopathy.
Figure 2.
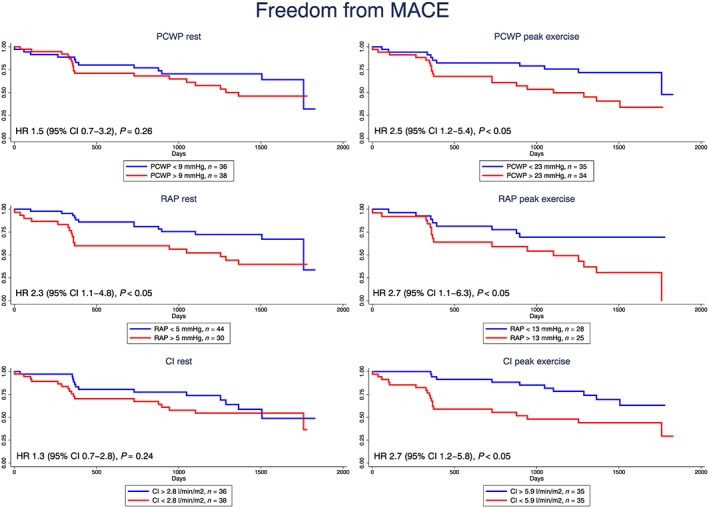
Kaplan–Meier survival curves and hazard ratios (HRs), 95% confidence intervals (CIs), and two‐sided P values from the Cox models for pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), and cardiac index (CI) at rest (left) and at peak exercise (right).
Figure 3.
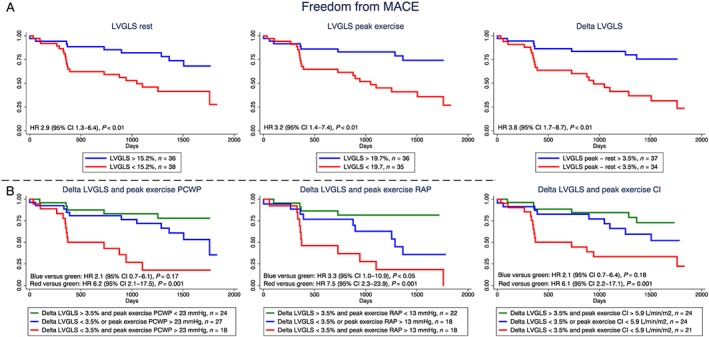
Kaplan–Meier survival curves and hazard ratios (HRs), 95% confidence intervals (CIs), and two‐sided P values from the Cox models for (A) left ventricular global longitudinal strain (LVGLS) at rest, at peak exercise, and the exercise‐induced increase in LVGLS; (B) the combined model of exercise‐induced left ventricular global longitudinal strain (LVGLS) increase and peak exercise pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), and cardiac index (CI). MACE, major adverse cardiac events.
The combined models of exercise LVGLS and haemodynamics in terms of peak exercise PCWP, RAP, and cardiac index were all strong predictors of MACE. The largest MACE risk was seen in patients with an exercise‐induced LVGLS increase <3.5% and either peak exercise cardiac index <5.9 L/min/m2 [HR 6.1 (95% CI: 2.2–17.1)] or peak exercise PCWP >23 mmHg [HR 6.1 (95% CI: 2.1–17.5)] or peak exercise RAP >13 mmHg [HR 7.5 (95% CI: 2.3–23.9)] had the highest MACE risk, Figure 3 B. Similarly, patients with both resting LVGLS <15.2% and 3D‐RVEF <51.7% had increased MACE risk compared with patients with LVGLS >15.2% and 3D‐RVEF >51.7% [HR 4.4 (95% CI: 1.8–10.7), P = 0.001].
Secondary endpoint: coronary event prediction by echocardiographic graft function and invasive haemodynamics
Table 4 shows the optimal cut‐off points and the Cox regression analysis of the ability of echocardiographic parameters and invasive haemodynamics to predict coronary events during follow‐up. Systolic function assessment by LVEF and LVGLS both at rest and at peak exercise predicted coronary events. The highest hazard was obtained by the deformation reserve in term of ΔLVGLS. RAP was a significant predictor of coronary events. The predictive ability increased during exercise. In contrast, PCWP did not predict coronary events and only CI at peak exercise predicted coronary events.
Table 4.
Univariable and multivariable cox regression analysis of the ability of haemodynamics to predict coronary events
| Cut‐off value | Univariate HR (95% CI) | P value | Adjusteda HR (95% CI) | P value | |
|---|---|---|---|---|---|
| Rest | |||||
| Echocardiography | |||||
| LVEF (%) | 62 | 3.4 (1.4–8.3) | <0.01b | 2.9 (1.2–7.3) | <0.05 |
| LVGLS (%) | 15.2 | 2.9 (1.2–7.2) | <0.05 | 2.6 (1.1–6.4) | <0.05 |
| E/A ratio | 1.9 | 1.9 (0.8–4.5) | 0.12 | — | |
| E/e′ ratio | 8.8 | 1.7 (0.8–4.0) | 0.18 | — | |
| RV FW LS | 21.7 | 2.0 (0.9–4.8) | 0.10 | ||
| Invasive haemodynamics | |||||
| CI rest (L/min/m2) | 2.8 | 1.0 (0.4–2.2) | 0.95 | — | |
| mRAP rest (mmHg) | 5 | 3.4 (1.5–8.1) | <0.01 | 2.9 (1.2–6.9) | <0.05 |
| mPAP rest (mmHg) | 18 | 2.5 (1.1–5.9) | <0.05 | 2.2 (0.9–5.2) | 0.07 |
| mPCWP rest (mmHg) | 10 | 2.3 (1.0–5.2) | 0.06 | — | |
| PAC rest (mL/mmHg) | 5.6 | 0.9 (0.4–2.2) | 0.90 | — | |
| Peak exercise | |||||
| Echocardiography | |||||
| LVEF (%) | 70 | 2.4 (1.0–5.7) | <0.05 | 2.3 (1.0–5.4) | 0.06 |
| LVGLS (%) | 19.3 | 3.0 (1.2–7.3) | <0.05 | 2.5 (1.0–6.3) | <0.05 |
| ΔLVGLS | 3.5 | 4.2 (1.6–10.7) | <0.01 | 3.0 (1.1–8.1) | <0.05 |
| Invasive haemodynamics | |||||
| CI peak (L/min/m2) | 5.8 | 2.2 (1.1–6.0) | <0.05 | 2.1 (0.9–4.9) | 0.10 |
| mRAP peak (mmHg) | 15 | 4.5 (1.6–12.7) | <0.01b | 3.8 (1.3–10.7) | <0.05b |
| mPAP peak (mmHg) | 39 | 1.8 (0.8–4.0) | 0.18 | — | |
| mPCWP peak (mmHg) | 26 | 2.0 (0.9–4.6) | 0.10 | — | |
| PAC peak (mL/mmHg) | 4 | 1.8 (0.8–4.2) | 0.18 | — | |
CI, cardiac index; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; mPAP, mean pulmonary arterial pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right atrial pressure; PAC, pulmonary arterial compliance; RV FW LS, right ventricular free wall longitudinal strain.
Adjusted for time since transplantation.
Significant after adjustment for presence of cardiac allograft vasculopathy.
Discussion
This is the first study to evaluate the prognostic value of echocardiography assessed myocardial deformation and invasive haemodynamics during exercise in long‐term HTx patients. The present study establishes normal values of invasive exercise haemodynamics in low‐risk and high‐risk HTx patients. We report the following main findings: (i) resting haemodynamic parameters were poor MACE predictors in long‐term HTx patients. (ii) In contrast, peak exercise PCWP, RAP, and CI were significant MACE predictors. (iii) LVGLS both at rest and during exercise were significant MACE predictors, and (iv) the combined model with peak exercise LVGLS and peak exercise PCWP, RAP, and CI clearly identified high‐risk HTx patients in relation cardiovascular end‐points independently of time since HTx.
Heart‐transplanted patients undergo routine graft function surveillance to detect graft dysfunction and deterioration. This traditionally involves echocardiographic assessment of systolic function by LVEF and diastolic function by trans‐mitral diastolic doppler flow and in some centres resting RHC. Even though this is performed routinely, the ability of these parameters to detect important cardiac hazards such as CAV and ACR is, at best, moderate.22 Resting LVGLS has emerged as a robust parameter for detection of subclinical myocardial dysfunction. Its clinical utility is most valuable in patients with preserved LVEF like often seen in HTx patients even if they have advanced CAV and ACR.22 Importantly, LVGLS is strongly associated with CAV6, 15 and is more likely to be affected by ACR5 than traditional echocardiographic parameters. Moreover, LVGLS had been demonstrated to possess great prognostic value after HTx, early7, 8 as well as late.9, 23 Recent guidelines therefore recommend routine LVGLS measuring during echocardiographic graft function surveillance.24 The present study extends the support of LVGLS; hence, we demonstrated a strong prognostic value of LVGLS during exercise.
Even though LVGLS may harbour great value in the surveillance of HTx patients, this parameter does not directly reflect LV and RV loading conditions. Theoretically, LV and RV loading conditions can be estimated by the trans‐mitral and trans‐tricuspid diastolic doppler filling pattern. However, several studies document a poor relation between the invasive filling pressure and the echocardiographic filling pattern in HTx patients.11, 25 Therefore, LV and RV filling pressure should be evaluated by RHC.
As graft failure remains an important late mortality cause after HTx, optimization of graft function surveillance has been a major issue for decades. Studies of invasive haemodynamics demonstrate that resting LV and RV filling pressures tend to normalize in most HTx patients during the first months after surgery, whereas pulmonary arterial compliance remains reduced.23, 26, 27 The results of the present study are in line with previous studies as we found resting LV‐filling and RV‐filling pressures within the normal range in most patients. However, the haemodynamic response to exercise is clearly abnormal in HTx patients. Thus, the CI increase is lower than expected from studies in healthy individuals.12 This documented inability to increase CI adequately during exercise might be attributable to several factors such as impaired ventricular contractile reserve,28, 29 an inadequate stroke volume reserve coupled with chronotropic incompetence,30, 31 increased afterload,32 and RV dysfunction.14 Another important characteristic of the haemodynamic response to exercise in HTx patients is pronounced PCWP increase.11, 12 The distinct differences in haemodynamics at peak exercise between HTx patients and healthy controls emphasize that assessment of invasive haemodynamics during resting conditions falls short as a measure of systolic and diastolic graft performance. Exercise testing should therefore be considered during haemodynamic graft function assessment in HTx patients as also recommended in heart failure patients with preserved LVEF.33 The majority of HTx patients with elevated LV filling pressure during exercise do not suffer severe CAV or high rejection burden.12 The prognostic result of these hazards may be reflected in the LVGLS magnitude. As a supplement, RHC with LV and RV filling pressure assessment may identify patients with restrictive physiology, that is, due to myocardial fibrosis, high impedance of the pulmonary vascular bed, pericardial restraint, or increased afterload. The combined model of echocardiographic assessed systolic performance and RHC assessed LV and RV filling pressure could therefore identify high‐risk patients independent of the underlying hazard.
Although RHC has been used in clinical practice post‐HTx, the prognostic value of haemodynamics is not well documented. A recent study by Kobayashi23 and colleagues showed that LVGLS and resting RAP at 1 year were complementary in assessing the 5‐year outcome after HTx. Our results support the findings in the previous study and suggest that exercise testing provides incremental value to the resting parameters. Notably, the time since HTx in our study and in the study by Kobayashi and colleagues are different. At 1 year after HTx, elevated RAP may be due to pulmonary stiffness induced by pre‐transplant pulmonary hypertension.27 However, in long‐term patients, as seen in the present study, the most likely causes of graft dysfunction are microvascular and macrovascular CAV and myocardial fibrosis. The effects of these hazards include perfusion and energy‐starved LV and RV myocardium during exercise characterized by a poor inotropic reserve and compensatory, elevated filling pressure. Exercise haemodynamics therefore had prognostic value adding to that of resting haemodynamics and myocardial deformation by identifying patients with compensated heart failure during resting conditions. Future studies should evaluate if risk stratification by exercise haemodynamic and LVGLS can be used for individualized medical therapy with a view to loading optimization, anti‐ischaemic medication, aggressive cholesterol‐lowering therapy, and optimized immunosuppression. In the interim, patients with an abnormal haemodynamic profile or LVGLS should be followed with frequent graft function and CAV surveillance.
Conclusions
Left ventricular and RV filling pressure at peak exercise predicted MACE in long‐term HTx patients. Furthermore, the study demonstrated that a combined model of exercise echocardiographic derived myocardial deformation and invasive haemodynamics was found to be the strongest model for MACE prediction.
Limitations
The present study reflects the experience of a single centre with a relatively small number of patients. Yet long‐term HTx patients are high‐event patients as also documented in the present study in which 41% (30/74) of the patients experienced MACE. Due to the relatively small cohort of patients, we were unable to simultaneously control for several variables. Furthermore, we were unable to determine if the hazard changed with increasing time since transplantation. However, we controlled the results for the influence of time since transplantation. In our study, we included patients with established CAV. The calculated cut‐off values cannot be directly transferred to de novo patients. Furthermore, we calculated optimal cut‐off points and tested these in the same cohort of patients. Therefore, the presented results reflect the best‐case scenario. The calculated cut‐off points should be tested in a larger independent cohort of HTx patients to evaluate if exercise testing provides incremental value to resting assessment in both patients with and without established CAV.
Conflict of interest
None declared.
Author contributions
T.S.C., H.E., B.B.L., and S.H. designed the research/study. T.S.C., H.E., S.M., and S.H. performed the research/study. T.S.C. and K.P.B.V. collected the data. T.S.C. analysed the data. T.S.C., H.E., B.B.L., K.P.B.V., S.M., and S.H. wrote the paper.
Funding
The study received funding from the Health Research Fund of the Central Denmark Region and the Danish Heart Association.
Acknowledgements
We would like to extend our gratitude to the nurses and physicians at the Cardiac Laboratory for their assistance with right heart catheterization. Furthermore, we would like to thank Lene Konrad and Lisbeth Lynge for their assistance during exercise testing.
Clemmensen, T. S. , Eiskjær, H. , Løgstrup, B. B. , Valen, K. P. B. , Mellemkjær, S. , and Poulsen, S. H. (2019) Prognostic value of exercise myocardial deformation and haemodynamics in long‐term heart‐transplanted patients. ESC Heart Failure, 6: 629–639. 10.1002/ehf2.12438.
Study type: Single centre, prospective cohort study.
ClinicalTrials.gov identifier: NCT02077764
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J. The registry of the International Society for Heart and Lung Transplantation: thirty‐second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34: 1244–1254. [DOI] [PubMed] [Google Scholar]
- 2. Clemmensen TS, Logstrup BB, Eiskjaer H, Hoyer S, Poulsen SH. The long‐term influence of repetitive cellular cardiac rejections on left ventricular longitudinal myocardial deformation in heart transplant recipients. Transpl Int 2015; 28: 475–484. [DOI] [PubMed] [Google Scholar]
- 3. Raichlin E, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Pereira NL, Daly RC, McGregor CG, Lerman A, Kushwaha SS. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant 2009; 28: 320–327. [DOI] [PubMed] [Google Scholar]
- 4. Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Serial changes in longitudinal graft function and implications of acute cellular graft rejections during the first year after heart transplantation. Eur Heart J Cardiovasc Imaging 2016; 17: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Changes in longitudinal myocardial deformation during acute cardiac rejection: the clinical role of two‐dimensional speckle‐tracking echocardiography. J Am Soc Echocardiogr 2015; 28: 330–339. [DOI] [PubMed] [Google Scholar]
- 6. Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Evaluation of longitudinal myocardial deformation by 2‐dimensional speckle‐tracking echocardiography in heart transplant recipients: relation to coronary allograft vasculopathy. J Heart Lung Transplant 2015; 34: 195–203. [DOI] [PubMed] [Google Scholar]
- 7. Sarvari SI, Gjesdal O, Gude E, Arora S, Andreassen AK, Gullestad L, Geiran O, Edvardsen T. Early postoperative left ventricular function by echocardiographic strain is a predictor of 1‐year mortality in heart transplant recipients. J Am Soc Echocardiogr 2012; 25: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 8. Eleid MF, Caracciolo G, Cho EJ, Scott RL, Steidley DE, Wilansky S, Arabia FA, Khandheria BK, Sengupta PP. Natural history of left ventricular mechanics in transplanted hearts: relationships with clinical variables and genetic expression profiles of allograft rejection. JACC Cardiovasc Imaging 2010; 3: 989–1000. [DOI] [PubMed] [Google Scholar]
- 9. Clemmensen TS, Eiskjaer H, Logstrup BB, Ilkjaer LB, Poulsen SH. Left ventricular global longitudinal strain predicts major adverse cardiac events and all‐cause mortality in heart transplant patients. J Heart Lung Transplant 2017; 36: 567–576. [DOI] [PubMed] [Google Scholar]
- 10. Mehra MR, Crespo‐Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy‐2010. J Heart Lung Transplant 2010; 29: 717–727. [DOI] [PubMed] [Google Scholar]
- 11. Meluzin J, Hude P, Krejci J, Spinarova L, Podrouzkova H, Leinveber P, Dusek L, Soska V, Tomandl J, Nemec P. Noninvasive prediction of the exercise‐induced elevation in left ventricular filling pressure in post‐heart transplant patients with normal left ventricular ejection fraction. Exp Clin Cardiol 2013; 18: 63–72. [PMC free article] [PubMed] [Google Scholar]
- 12. Clemmensen TS, Eiskjaer H, Logstrup BB, Mellemkjaer S, Andersen MJ, Tolbod LP, Harms HJ, Poulsen SH. Clinical features, exercise hemodynamics, and determinants of left ventricular elevated filling pressure in heart‐transplanted patients. Transpl Int 2016; 29: 196–206. [DOI] [PubMed] [Google Scholar]
- 13. Wolsk E, Bakkestrom R, Thomsen JH, Balling L, Andersen MJ, Dahl JS, Hassager C, Moller JE, Gustafsson F. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail 2017; 5: 337–346. [DOI] [PubMed] [Google Scholar]
- 14. Clemmensen TS, Eiskjaer H, Logstrup BB, Andersen MJ, Mellemkjaer S, Poulsen SH. Echocardiographic assessment of right heart function in heart transplant recipients and the relation to exercise hemodynamics. Transpl Int 2016; 29: 909–920. [DOI] [PubMed] [Google Scholar]
- 15. Clemmensen TS, Eiskjaer H, Logstrup BB, Tolbod LP, Harms HJ, Bouchelouche K, Hoff C, Frokiaer J, Poulsen SH. Noninvasive detection of cardiac allograft vasculopathy by stress exercise echocardiographic assessment of myocardial deformation. J Am Soc Echocardiogr 2016; 29: 480–490. [DOI] [PubMed] [Google Scholar]
- 16. Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category‐ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 1983; 15: 523–528. [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 18. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004; 17: 630–633. [DOI] [PubMed] [Google Scholar]
- 19. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539–542. [DOI] [PubMed] [Google Scholar]
- 20. Clemmensen TS, Eiskjaer H, Molgaard H, Larsen AH, Soerensen J, Andersen NF, Tolbod LP, Harms HJ, Poulsen SH. Abnormal coronary flow velocity reserve and decreased myocardial contractile reserve are main factors in relation to physical exercise capacity in cardiac amyloidosis. J Am Soc Echocardiogr 2018; 31: 71–78. [DOI] [PubMed] [Google Scholar]
- 21. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu‐Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24: 1710–1720. [DOI] [PubMed] [Google Scholar]
- 22. Dandel M, Hetzer R. Post‐transplant surveillance for acute rejection and allograft vasculopathy by echocardiography: usefulness of myocardial velocity and deformation imaging. J Heart Lung Transplant 2017; 36: 117–131. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi Y, Sudini NL, Rhee JW, Aymami M, Moneghetti KJ, Bouajila S, Kobayashi Y, Kim JB, Schnittger I, Teuteberg JJ, Khush KK, Fearon WF, Haddad F. Incremental value of deformation imaging and hemodynamics following heart transplantation: insights from graft function profiling. JACC Heart Fail 2017; 5: 930–939. [DOI] [PubMed] [Google Scholar]
- 24. Badano LP, Miglioranza MH, Edvardsen T, Colafranceschi AS, Muraru D, Bacal F, Nieman K, Zoppellaro G, Marcondes Braga FG, Binder T, Habib G, Lancellotti P. Document reviewers. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging 2015; 16: 919–948. [DOI] [PubMed] [Google Scholar]
- 25. Savage A, Hlavacek A, Ringewald J, Shirali G. Evaluation of the myocardial performance index and tissue doppler imaging by comparison to near‐simultaneous catheter measurements in pediatric cardiac transplant patients. J Heart Lung Transplant 2010; 29: 853–858. [DOI] [PubMed] [Google Scholar]
- 26. Lundgren J, Radegran G. Hemodynamic characteristics including pulmonary hypertension at rest and during exercise before and after heart transplantation. J Am Heart Assoc 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghio S, Crimi G, Pica S, Temporelli PL, Boffini M, Rinaldi M, Raineri C, Scelsi L, Pistono M, Totaro R, Guida S, Oltrona Visconti L. Persistent abnormalities in pulmonary arterial compliance after heart transplantation in patients with combined post‐capillary and pre‐capillary pulmonary hypertension. PLoS One 2017; 12: e0188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 29. Clemmensen TS, Molgaard H, Sorensen J, Eiskjaer H, Andersen NF, Mellemkjaer S, Andersen MJ, Tolbod LP, Harms HJ, Poulsen SH. Inotropic myocardial reserve deficiency is the predominant feature of exercise haemodynamics in cardiac amyloidosis. Eur J Heart Fail 2017; 19: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 30. Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013; 15: 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015; 8: 41–48. [DOI] [PubMed] [Google Scholar]
- 32. Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, Starling RC, Tang WH. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail 2012; 5: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]


