Abstract
Aims
This study aims to assess prognostic impact of Framingham criteria for heart failure (FC‐HF) in patients with stable heart failure (HF) with preserved ejection fraction (HFpEF).
Methods and results
In the prospective Karolinska‐Rennes (KaRen) study, we assessed stable HFpEF patients after an acute HF episode. We evaluated associations between the four descriptive models of HFpEF and the composite endpoint of all‐cause mortality and HF hospitalization. The descriptive models were FC‐HF alone, FC‐HF + natriuretic peptides (NPs) according to the PARAGON trial, FC‐HF + NPs + echocardiographic HFpEF criteria according to European Society of Cardiology HF guidelines, and FC‐HF + NPs + echocardiographic criteria according to the PARAGON trial. Out of the 539 patients enrolled in KaRen, 438 returned for the stable state revisit after 4–8 weeks, 13 (2.4%) patients died before the planned follow‐up, and 88 patients (16%) declined or were unable to return. Three hundred ninety‐nine patients have FC registered at follow‐up, and among these, the four descriptive models were met in 107 (27%), 82 (22%), 61 (21%), and 69 (22%) patients, and not met in 292 (73%). The 107 patients that had FC‐HF at stable state (descriptive model 1) could also be part of the other models because all patients in models 1–4 had to fulfil the FC‐HF. The patients in model 0 did not fulfil the criteria for FC‐HF but could have single FC. Of single FC, only pleural effusion predicted the endpoint [hazard ratio (HR) 3.38, 95% confidence interval (CI) 1.47–7.76, P = 0.004]. Patients without FC‐HF had better prognosis than patients meeting FC‐HF. The unadjusted associations between the four HFpEF descriptive models and the endpoint were HR 1.54, 95% CI 1.14–2.09, P = 0.005; HR 1.71, 95% CI 1.24–2.36, P = 0.002; HR 1.95, 95% CI 1.36–2.81, P = 0.001; and HR 2.05, 95% CI 1.45–2.91, P < 0.001, for descriptive models 1–4, respectively. No descriptive model independently predicted the endpoint.
Conclusions
In ambulatory HFpEF patients, a quarter met FC‐HF, while most met NP and echocardiography criteria for HF. Residual FC‐HF tended to be associated with increased risk for mortality and HF hospitalization, further strengthened by NPs and echocardiographic criteria, highlighting its role in clinical risk assessment.
Keywords: Heart failure with preserved ejection fraction, Prognosis, Echocardiography, Natriuretic peptides, Framingham criteria
Introduction
Heart failure (HF) is commonly categorized by left ventricular ejection fraction (LVEF) into HF with reduced ejection fraction (LVEF <40%), HF with midrange ejection fraction (LVEF 40–49%), or HF with preserved ejection fraction (HFpEF; LVEF ≥50%).1 HF, irrespective EF, has poor prognosis with high mortality and HF hospitalization rates,2, 3 in particular after a recent HF hospitalization.4
Regardless of LVEF, the diagnosis of HF requires signs and symptoms. The Framingham criteria for HF (FC‐HF),5 introduced in the 70s, is the most commonly used criterion for HF and is based solely on symptoms and clinical findings. Thereafter, the importance of natriuretic peptides (NPs) and refined echocardiographic measurements for the diagnosis of HF were recognized, as reflected in the European Society of Cardiology (ESC) HF guidelines 20161 and in the design of more recent HF trials including HFpEF studies such as the PARAGON trial.6
The HFpEF syndrome is associated with non‐cardiovascular (CV) co‐morbidities that may cause symptoms resembling those of HF, for example, chronic obstructive pulmonary disorder, which may complicate diagnosis.7 Therefore, there is increasing recognition of the need for suitable definitions of HFpEF and inclusion/exclusion criteria in HFpEF trials.
After an acute HF event, patients are often assigned a diagnosis of chronic HF. However, to what extent signs and symptoms persist chronically, whether definitions of HFpEF continue to be met, and the prognostic role of signs, symptom, and HFpEF criteria, over time, are unknown. Therefore, the aim was to assess the prognostic impact of FC‐HF and different descriptive models of HFpEF, based on echocardiography, NPs, and FC‐HF, in stable HFpEF patients after an acute HF event.
Methods
This analysis was based on the Karolinska‐Rennes (KaRen) study, a European multicentre prospective observational study designed to characterize HFpEF and to identify prognostic factors for long‐term mortality and morbidity.8 Patients presenting to hospital with acute HF were screened and included provided that they fulfilled signs and symptoms of HF according to FC,5 and LVEF ≥45% at echocardiography within 48 h, and N‐terminal pro brain natriuretic peptide (NT‐proBNP) >300 ng/L or BNP >100 ng/L. The LVEF cut‐off reflected the definition of HFpEF at the time of study design.8 Out of the 539 patients enrolled, 438 returned for the stable state revisit after 4–8 weeks, 13 (2.4%) patients died before the planned follow‐up, and 88 patients (16%) declined or were unable to return. The patients in this analysis had to have FC reassessed at the 4–8 week follow‐up. This visit was defined as baseline for assessing subsequent outcomes in the present analysis. At this visit, in addition to clinical assessment including the FC, patients underwent detailed echocardiography, medical history, and blood sampling including NPs. The echocardiographic assessment was performed on a ViVid 7 echo‐platform (GE VingMed, Horten, Norway) and analysed in a dedicated core centre in Hôpital Pontchaillou, Rennes, France. Each examination was interpreted once, and measurements were performed three times and averaged by an echocardiographer blinded to the specific clinical history of the patient.
Estimated glomerular filtration rate was calculated according to the Modification of Diet in Renal Disease equation, and NPs were analysed in the local hospital laboratory. Quality of life was assessed with the Minnesota Living with Heart Failure Questionnaire.9
Framingham criteria for heart failure
The FC‐HF consists of major (paroxysmal nocturnal dyspnoea, orthopnea, jugular venous distension, third heart sound, cardiothoracic ratio >0.5 on X‐ray, pulmonary oedema on X‐ray, and pulmonary crackling rales) and minor [peripheral oedema, nocturnal cough, dyspnoea at exercise, hepatomegaly, pleural effusion, and tachycardia (defined as ≥100 bpm)] criteria. To fulfil the FC‐HF, the presence of two major or one major + two minor criteria is required.5
Descriptive models of heart failure with preserved ejection fraction
The four descriptive models of HFpEF were used for which the FC‐HF was required for all (Figure 1 ). In addition, in descriptive models 2–4, HFpEF criteria from the ESC guidelines and the PARAGON trial6 were added. The descriptive models were (i) FC‐HF only; (ii) FC‐HF + NPs in sinus rhythm >300/100 and in atrial fibrillation >900/400 ng/L for NT‐proBNP or BNP, respectively; (iii) FC‐HF + NPs + ESC echocardiography HFpEF criteria1: relevant structural heart disease [increased left atrial volume index (LAVI) >34 mL/m2 and/or increased left ventricular mass index >95 g/m2 in women and >115 g/m2 in men] and/or diastolic dysfunction, reduced é (é average <9 cm/s), or increased E/é ratio (>13); and (iv) FC‐HF + NPs + echocardiographic HFpEF criteria from the ongoing PARAGON study6 that requires structural changes with ≥1 of the following: LAVI >28 mL/m2 and/or left atrial diameter >38 mm, or left ventricular hypertrophy, interventricular septal thickness >10 mm and/or posterior wall thickness in diastole >10 mm). Patients could fulfil more than one of the descriptive models 1–4. The patients not fulfilling the FC‐HF at the baseline outpatient visit were grouped as descriptive model 0 (Figure 1 ).
Figure 1.
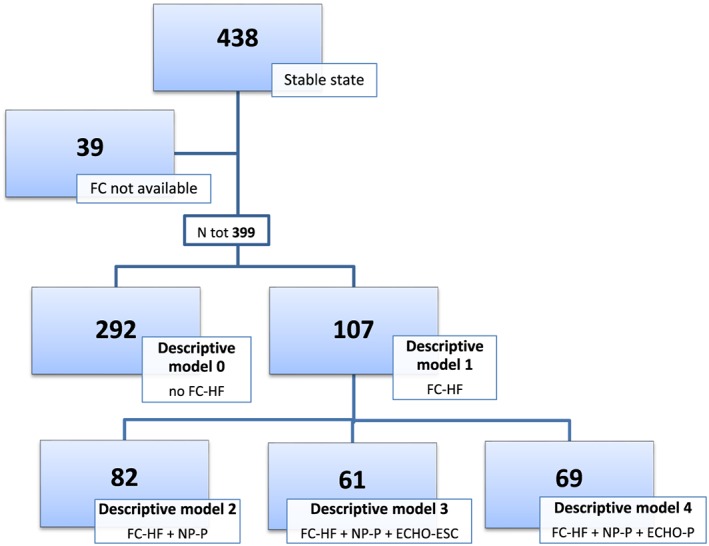
Flowchart of patients showing their distribution to descriptive models 1–4 of heart failure (HF) with preserved ejection fraction and those no longer fulfilling inclusion criteria for the study at stable state [absence of Framingham criteria for HF (FC‐HF), descriptive model 0]. For example, 69/107 patients with FC‐HF fulfilled criteria for descriptive model 4. FC‐HF: two major or one major + two minor. Natriuretic peptide (NP) criteria PARAGON (NP‐P): NT‐proBNP in sinus rhythm >300; in atrial fibrillation >900; BNP in sinus rhythm >100; and in atrial fibrillation >400. European Society of Cardiology ECHO HF with preserved ejection fraction criteria (ECHO‐ESC): relevant structural heart disease [left atrial (LA) enlargement/left ventricular (LV) hypertrophy] and/or diastolic dysfunction. Structural heart disease: increased LA volume index (>34 mL/m2) and/or increased LV mass index >95 g/m2 in women and >115 g/m2 in men. Diastolic dysfunction: reduced é (é average <9 cm/s) and/or increased E/é ratio (>13). PARAGON ECHO structural heart disease criteria (ECHO‐P): at least one of the following: LA volume index >28 mL/m2 and/or LA diameter >38 mm. LV hypertrophy: interventricular septal thickness >10 mm and/or posterior wall thickness in diastole >10 mm.
Primary endpoint
Patients were followed by telephone call every 6 months up to 18 months regarding potential hospitalizations and mortality until study closure in November 2012. This information was used for the primary composite endpoint of all‐cause mortality and HF hospitalization analysed according to our HFpEF descriptive models. All events were adjudicated by and defined according to clinical judgment by the local investigator. The study involved no intervention, and patients were managed routinely by their own physician.
Statistics
Descriptive data were presented as median [interquartile range (IQR)] or number (%) and compared with the Wilcoxon rank sum test and Pearson χ 2 test (Table 1). Kaplan–Meier analysis was used to assess the study composite endpoint consisting of survival time free from HF hospitalization.
Table 1.
Clinical characteristics of 399 patients according to the four descriptive models (DM) of heart failure with preserved ejection fraction
|
Descriptive model 0 No HF acc. to FC |
Descriptive model 1 HF acc. to FC |
P (DM 1 vs. DM 0) |
Descriptive model 2 FC + NP |
Descriptive model 3 FC + NP + ESC echo |
Descriptive model 4 FC + NP + PARAGON echo |
|
|---|---|---|---|---|---|---|
| N (399) | 292 (73%) | 107 (27%) | 82 (22%) | 61 (21%) | 69 (22%) | |
| Demographics and medical history | ||||||
| Age (years) | 77 (71–82) | 79 (72–84) | 0.039 | 81 (73–85) | 82 (74–85) | 81 (73–84) |
| Gender (female) | 166 (57%) | 57 (53%) | 0.520 | 41 (50%) | 32 (52%) | 35 (51%) |
| Smoking | 103 (35%) | 33 (31%) | 0.955 | 27 (33%) | 21 (34%) | 23 (33%) |
| Hypertension | 230 (79%) | 83 (78%) | 0.797 | 62 (77%) | 48 (80%) | 43 (78%) |
| AF | 178 (61%) | 72 (67%) | 0.444 | 63 (77%) | 49 (80%) | 54 (78%) |
| COPD | 37 (13%) | 16 (15%) | 0.552 | 12 (14%) | 6 (10%) | 9 (13%) |
| T2DM | 74 (25%) | 34 (32%) | 0.200 | 25 (30%) | 20 (33%) | 21 (30%) |
| CAD | 92 (32%) | 34 (32%) | 0.819 | 26 (32%) | 22 (36%) | 23 (33%) |
| Stroke | 28 (10%) | 13 (12%) | 0.456 | 11 (13%) | 7 (11%) | 9 (13%) |
| Renal disease | 85 (29%) | 33 (31%) | 0.487 | 27 (33%) | 19 (31%) | 21 (30%) |
| Cancer | 45 (15%) | 18 (17%) | 0.691 | 11 (14%) | 8 (14%) | 10 (15%) |
| Physical findings | ||||||
| SBP (mmHg) | 140 (120–151) | 139.5 (120–150) | 0.293 | 140 (120–150) | 140 (120–150) | 140 (120–150) |
| DBP (mmHg) | 75 (65–80) | 70 (60–80) | 0.437 | 70 (61–80) | 70 (60–80) | 70 (65–80) |
| Heart rate (bpm) | 68 (60–78) | 66 (57–72) | 0.176 | 65 (57–72) | 65 (57–71) | 65 (57–71) |
| BMI (kg/m2) | 27.6 (23.9–31.6) | 29.3 (25.2–33.5) | 0.026 | 29.2 (25.2–33.5) | 29.1 (25.3–33.5) | 29.3 (25.5–33.5) |
| Functional class, QoL | ||||||
| NYHA class I | 46 (16%) | 3 (2.8%9 | <0.001 | 2 (2.4%) | 1 (1.6%) | 1 (1.4%) |
| NYHA class II | 198 (69%) | 45 (42%) | 34 (41%) | 26 (43%) | 29 (42%) | |
| NYHA class III | 40 (14%) | 49 (46%) | 39 (48%) | 28 (46%) | 32 (46%) | |
| NYHA class IV | 1 (<0.1%) | 9 (8.5%) | 7 (8.5%) | 6 (10%) | 7 (10%) | |
| MLHFQ (0–105) | 26 (12–43) | 32 (22.5–50.5) | <0.001 | 31 (19–47) | 26 (14–44) | 27 (14–45) |
| Treatment | ||||||
| ACEi | 99 (34%) | 38 (36%) | 0.764 | 32 (39%) | 25 (41%) | 27 (39%) |
| ARB | 79 (27%) | 27 (25%) | 0.715 | 22 (27%) | 13 (21%) | 18 (26%) |
| Spironolactone | 34 (12%) | 11 (10%) | 0.703 | 7 (8.5%) | 4 (6.5%) | 5 (7.2%) |
| Loop diuretics | 123 (42%) | 72 (67%) | <0.001 | 60 (73%) | 45 (74%) | 53 (77%) |
| Thiazide diuretics | 47 (16%) | 16 (15%) | 0.782 | 11 (13%) | 7 (11%) | 8 (12%) |
| Ca channel blockers | 105 (36%) | 35 (33%) | 0.518 | 28 (34%) | 20 (33%) | 21 (30%) |
| Digoxin | 22 (7.5%) | 6 (5.6%) | 0.168 | 4 (4.9%) | 3 (4.9%) | 4 (5.8%) |
| Beta‐blocker | 195 (67%) | 67 (63%) | 0.256 | 53 (65%) | 42 (69%) | 46 (67%) |
| Anticoagulants | 118 (40%) | 48 (45%) | <0.001 | 37 (45%) | 30 (49%) | 35 (51%) |
| Antiplatelet therapy | 115 (39%) | 47 (44%) | 0.339 | 38 (46%) | 31 (51%) | 32 (46%) |
| Statins | 122 (42%) | 43 (40%) | 0.012 | 35 (43%) | 26 (43%) | 29 (42%) |
| Pacemaker | 38 (13%) | 17 (16%) | 0.461 | 12 (15%) | 8 (13%) | 9 (13%) |
| Echo parameters | ||||||
| LVEF (%) | 63 (57–68) | 62 (56–66) | 0.109 | 62 (56–65) | 62 (56–65) | 62 (55–65) |
| LVEDd (mm) | 47 (43–52) | 48 (43–52) | 0.538 | 48 (43–53) | 48 (43–53) | 48 (43–53) |
| LAVI (mL/m2) | 45 (38–56) | 50 (39–63) | 0.024 | 51 (37–62) | 50 (37–62) | 51 (37–62) |
| LVMI male (g/m2) | 128 (108–158) | 130 (104–160) | 0.524 | 131 (107–160) | 131 (115–160) | 131 (107–160) |
| LVMI female (g/m2) | 115 (94–128) | 121 (99–140) | 0.899 | 124 (99–140) | 124 (99–140) | 124 (99–140) |
| E/é | 11 (8–15) | 12 (10–18) | 0.004 | 12 (10–18) | 14 (10–19) | 12 (10–18) |
| Biochemistry | ||||||
| NT‐proBNP (ng/L) | 1196 (495–2359) | 1634 (695–3108) | 0.011 | 2353 (1301–3525) | 2369 (1429–3752) | 2727 (1429–3752) |
| BNP (ng/L) | 264 (113–523) | 366 (259–581) | 0.132 | |||
| p‐Glu (mmol/L) | 5.6 (5.1–7.2) | 6.1 (5.3–8.1) | 0.047 | 6.1 (5.4–8.2) | 6.1 (5.4–9.3) | 6.1 (5.4–8.5) |
| GFR (MDRD) | 59 (41–74) | 51 (38–67) | 0.071 | 48 (38–66) | 47 (37–65) | 47 (38–65) |
| GFR <30 | 34 (12.1%) | 12 (11.4%) | 0.856 | 9 (11.0%) | 7 (11.5%) | 8 (11.6%) |
| Sodium (mmol/L) | 140 (138–142) | 140 (138–141) | 0.699 | 140 (138–142) | 140 (138–141) | 139 (138–141) |
| Potassium (mmol/L) | 4.1 (3.8–4.5) | 4.1 (3.8–4.4) | 0.563 | 4.1 (3.8–4.4) | 4.1 (3.8–4.4) | 4.1 (3.8–4.4) |
| Hb (g/L) | 128 (112–135) | 120 (100–131) | 0.012 | 120 (100–130) | 117 (100–130) | 117 (100–130) |
| Anaemia | 106 (39%) | 58 (55%) | 0.003 | 47 (57%) | 38 (62%) | 43 62% |
ACEi, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation and/or atrial flutter; anaemia, Hb <120 in women and <130 in men; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; COPD, chronic pulmonary obstruction; DBP, diastolic blood pressure; GFR, glomerular filtration rate; Hb, haemoglobin; LVEDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MDRD, Modification of Diet in Renal Disease Study; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; p‐glu, plasma glucose; QoL, quality of life; renal disease, self‐reported, chart reported, or creatinine ≥100 mmol/L; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.
Data presented as median (interquartile range) or n (%).
The association between the individual FC as well as HF according to the four descriptive models of HFpEF and the composite endpoint was assessed in univariable and multivariable Cox regression models. In addition to the composite endpoint, CV death and non‐CV death were analysed as independent variables censoring for death of unknown cause. In the adjusted model, sex, age, glomerular filtration rate, haemoglobin, sodium, body mass index, systolic blood pressure, atrial fibrillation/flutter, coronary artery disease, chronic obstructive pulmonary disease, type 2 diabetes mellitus, and New York Heart Association (NYHA) class were included, all with known impact on morbidity and mortality. Due to non‐normal distribution, plasma and serum NPs were analysed in log‐transformed format. P‐values were two‐sided, and statistical significance was set at 0.05. Statistical analyses were performed using STATA software version 13.1.
Ethics
The French Ethics committees and the ethical review board at Karolinska Institutet approved the KaRen study, which was conducted according to International Conference on Harmonization and Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients.
Results
Patients
A total of 539 patients with acute HFpEF were enrolled in the KaRen study (Figure 1 , Table 1).10 Of these, 13 (2.4%) died during the hospitalization or after hospitalization but before the scheduled 4–8 week outpatient follow‐up, and 88 (16%) withdrew consent to return for detailed longitudinal assessment. Of the 438 stable outpatients (Figure 1 ), 399 patients have FC registered at the baseline visit and constituted the patient material in this study. For the entire study group, the mean age was 76 years, and 56% were women. Most patients were in NYHA II (62%), and the median BNP and NT‐proBNP were respectively 277 (IQR 136–570) and 1410 (IQR 515–2640) ng/L.
Patient characteristics according to heart failure with preserved ejection fraction descriptive models
Approximately one quarter (21–27%) of the patients fulfilled at least one of our four descriptive models of HFpEF: 1st descriptive model, 107 patients (27%); 2nd, 82 (22%); 3rd, 61 (21%); and 4th, 69 (22%) (Table 1). Thus, the same patients (of the 107) could be part of several descriptive models. The remaining 292 patients did not fulfil the FC‐HF (descriptive model 0) but could have single FC. The baseline variables, according to the presence or absence of the four descriptive models, are shown in Table 1. Patients in descriptive model 0 were younger and had lower NYHA class, NT‐proBNP, LAVI, and E/e´ compared with patients with FC‐HF (descriptive model 1). Minnesota Living with Heart Failure Questionnaire showed better quality of life in descriptive model 0 compared with descriptive model 1, median 26 and 32, respectively, P < 0.001, Table 1.
Presence of individual Framingham criteria according to heart failure with preserved ejection fraction descriptive models
The distribution of each individual FC according to our descriptive models is given in Figure 2 . Dyspnoea on exertion was the most prevalent criteria followed by peripheral oedema. In 292 patients, FC‐HF (at least two major or one major and two minor criteria) was not fulfilled at the baseline visit, but single FC could still have been present. The most commonly occurring single FC in descriptive model 0 was dyspnoea at exercise (63%) and peripheral oedema (22%). Among the FC, in particular, nocturnal cough, rales, and congestion on X‐ray were absent in this group.
Figure 2.
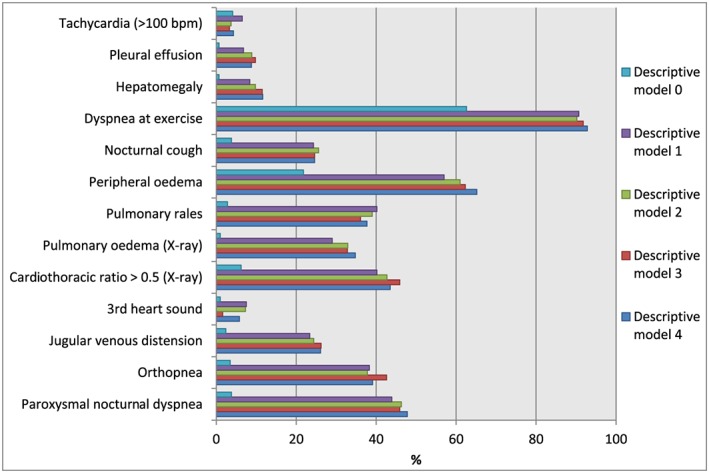
Prevalence of individual Framingham criteria for heart failure at stable state according to the four descriptive models of heart failure with preserved ejection fraction clarified in the methods section.
Follow‐up time and primary endpoint of freedom from all‐cause mortality and heart failure hospitalizations
The median follow‐up time was 19 months (IQR 9–34 months), and the composite endpoint occurred in 186 (47%) of the patients out of which 93 patients died (23%) (Figures 3 – 4 ). CV mortality constituted 38% (35 patients) and non‐CV 37% (34 patients). The cause of death was unknown in 24 patients (26%). The survival free from HF hospitalization by Kaplan–Meier analysis is shown in Figure 3 . Not surprisingly, patients without FC (descriptive model 0) had the best prognosis. For the rest, the probability for fulfilling the primary endpoint significantly increased as NPs, and especially, echocardiography criteria were added. After covariate adjustment, no descriptive model independently predicted the primary composite endpoint (Figure 4 ), but there was a trend towards significance when adding NPs and echocardiographic criteria to FC‐HF with the strongest trend seen when adding the PARAGON criteria (descriptive model 4). Among the single FC, only pleural effusion predicted the composite endpoint in adjusted model, hazard ratio (HR) 3.38 [95% confidence interval (CI)] (Table 2 ) with prevalence 7–10% in descriptive models 1–4 (Figure 2 ).
Figure 3.
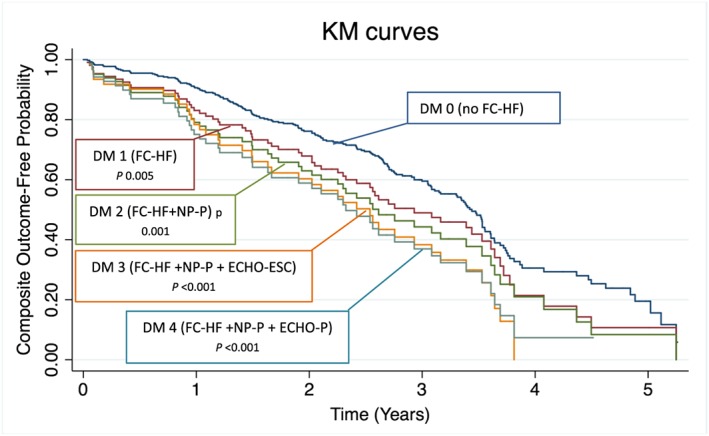
Kaplan–Meier (KM) curves showing the primary endpoint (survival free from heart failure hospitalization) for the four descriptive models (DMs) of heart failure with preserved ejection fraction clarified in the methods section. All definitions compared with descriptive model 0 (patients without heart failure according to Framingham criteria), P‐values from unadjusted cox regression analyses. ESC, European Society of Cardiology; FC‐HF, Framingham criteria for heart failure; NP, natriuretic peptide.
Figure 4.
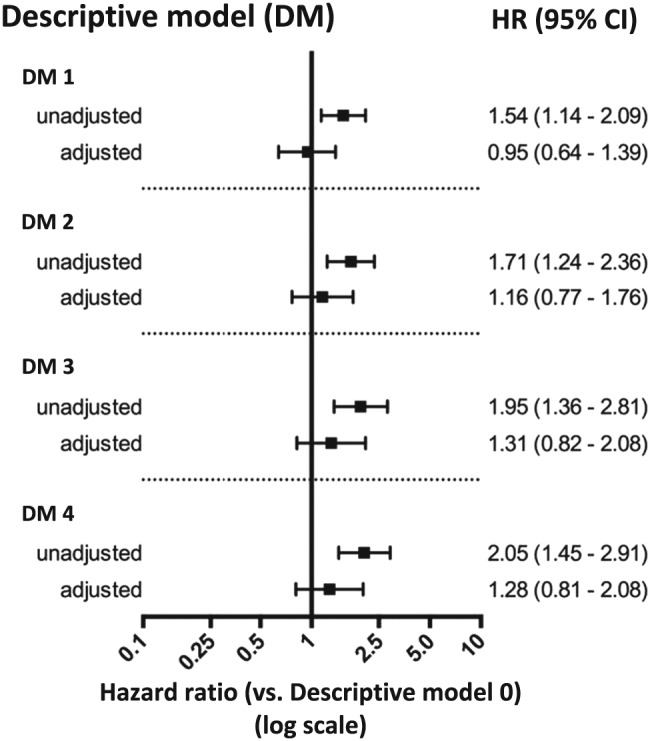
Heart failure with preserved ejection fraction according to the four descriptive models, clarified in the Methods section, as predictors of all‐cause mortality/heart failure hospitalization compared with descriptive model 0 (patients without heart failure according to Framingham criteria). Adjustments for gender, age, glomerular filtration rate, log haemoglobin, log sodium, systolic blood pressure, atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, type 2 diabetes mellitus, cancer, and New York Heart Association class. CI, confidence interval; HR, hazard ratio.
Table 2.
Cox regression analyses for the individual Framingham criteria of heart failure and the composite endpoint of heart failure hospitalization and death of any cause
| Framingham criteria | HR unadj. | 95% CI | P‐value | HR adj.a | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Major | ||||||
| Paroxysmal nocturnal dyspnoea | 1.65 | 1.16–2.35 | 0.005 | 0.87 | 0.55–1.38 | 0.556 |
| Orthopnea | 0.96 | 0.64–1.43 | 0.843 | 0.48 | 0.28–0.80 | 0.005 |
| Jugular venous distension | 1.54 | 0.97–2.45 | 0.070 | 1.02 | 0.55–1.90 | 0.951 |
| 3rd heart sound | 1.48 | 0.65–3.34 | 0.350 | 1.41 | 0.58–3.41 | 0.446 |
| Cardiothoracic ratio >0.5 on X‐ray | 1.00 | 0.99–1.00 | 0.194 | 1.12 | 0.71–1.78 | 0.613 |
| Pulmonary oedema on X‐ray | 1.00 | 0.99–1.00 | 0.075 | 1.53 | 0.89–2.65 | 0.124 |
| Pulmonary rales | 2.04 | 1.40–2.98 | <0.001 | 1.29 | 0.80–2.07 | 0.294 |
| Minor | ||||||
| Peripheral oedema | 1.61 | 1.18–2.17 | 0.002 | 1.33 | 0.93–1.90 | 0.118 |
| Nocturnal cough | 1.07 | 0.69–1.65 | 0.774 | 0.72 | 0.43–1.18 | 0.195 |
| Dyspnoea at exercise | 1.21 | 0.87–1.68 | 0.252 | 0.84 | 0.57–1.24 | 0.374 |
| Hepatomegaly | 1.98 | 1.01–3.88 | 0.046 | 0.81 | 0.37–1.78 | 0.605 |
| Pleural effusion | 5.04 | 2.45–10.4 | <0.001 | 3.38 | 1.47–7.76 | 0.004 |
| Tachycardia (>100 bpm) | 0.69 | 0.32–1.46 | 0.329 | 0.94 | 0.41–2.13 | 0.878 |
CI, confidence interval; HR, hazard ratio.
Adjustments for gender, age, glomerular filtration rate, log haemoglobin, log sodium, systolic blood pressure, atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, type 2 diabetes mellitus, cancer, and New York Heart Association class.
In addition to the primary endpoint, associations between the different HF descriptive model and CV death and non‐CV death were explored. In unadjusted analyses, both CV death and non‐CV death were significantly associated with all four descriptive models of HFpEF. In adjusted multivariable analyses, none of the descriptive models were significantly associated with CV death, but interestingly, descriptive models 2 and 4 were predictors of non‐CV death, HR 2.75 (95% CI 1.02–7.42, P‐value 0.045) and HR 2.97 (95% CI 1.04–8.47, P‐value 0.041), respectively.
Discussion
This is to our knowledge the first study to assess the prognostic importance of FC‐HF in ambulatory HFpEF patients following an acute HF hospitalization. Prevalence of HFpEF according to the FC‐HF was present in a quarter of patients, whereof 10% were in NYHA class IV. Most met NP and echocardiography criteria for HF. Residual FC‐HF at follow‐up tended to be associated with increased risk for mortality and HF hospitalization, further strengthened by NPs and echocardiographic criteria, highlighting its role in clinical risk assessment.
Heart failure with preserved ejection fraction characteristics
As expected in HFpEF patients,11, 12 the patients in KaRen were old [mean age 79 years for patients fulfilling FC‐HF (descriptive model 1)], were often female, and had a high prevalence of atrial fibrillation. Moreover, our HFpEF patients had several co‐morbidities, as expected in patients in their 80s, reflecting frailty. This could contribute to explain why in a previous analysis from KaRen, outcomes were greatly determined by non‐CV predictors, in particular age, non‐CV syncope, and anaemia, consistent with frailty.7 Conversely, it was notable in the present study that HF factors such as those in FC‐HF were associated with non‐CV outcomes, suggesting complex interactions between HF factors and co‐morbidities in determining CV and non‐CV outcomes.
Prognostic implications of the Framingham criteria
At enrolment in the KaRen study, FC‐HF was part of the inclusion criteria. However, in this study, we assessed their prevalence and prognostic implication post‐discharge at ambulatory follow‐up as part of the four different descriptive models. We did not aim to diagnose HFpEF at stable follow‐up. In the clinical setting, the clinician relies on patient history and clinical assessment, regardless of LVEF. At follow‐up after an acute HF admission, it would be valuable to know if signs and symptoms such as FC‐HF can predict prognosis and cause‐specific prognosis or if any single clinical signs carry greater prognostic importance than others.
We found that the absence of FC reflected better outcome and in univariate analysis, FC‐HF was associated with outcome. Analysing FC one by one, pleural effusion did predict the primary endpoint; however, it is an unspecific sign that can be related to other diagnoses.
Heart failure with preserved ejection fraction descriptive models 2 and 4, both including NP criterion, significantly predicted non‐CV death after adjustments possibly reflecting frailty of individuals fulfilling these descriptive models. However, this (CV death/non‐CV death) should be interpreted with caution because cause of death was unknown in a large proportion (26%).
Natriuretic peptides and echocardiography are increasingly available (although not always used) both in the acute setting and at follow‐up, enabling diagnosis establishment and use of combined measures for prognostic assessment. The echocardiographic HFpEF criteria have evolved to include the combination of high filling pressures with structural changes such as LAVI and LV hypertrophy or mass, and we have previously shown in KaRen that ESC echocardiography criteria were present in 76% of stable HFpEF13 and E/é in stable HFpEF predicted outcomes.14 In contrast, in the present study focusing on FC‐HF, the E/é criteria for HFpEF were part of descriptive model 31 but did not add prognostic information compared with structural changes alone as descriptive model 4 (derived from the PARAGON trial).6
We chose the NP and echo inclusion criteria from the PARAGON study as this study was the first to incorporate structural changes such as LAVI and hypertrophy in the inclusion criteria.
As in PARAGON, we used separate NP elevations for sinus rhythm and atrial fibrillation patients to potentially identify patients with worse outcome. In a registry‐based study from Sweden, we have previously showed that NPs were of particular prognostic value in HFpEF.15 Although NP criteria (descriptive model 2) in the present study added to predicting the primary outcome, the further addition with echocardiographic criteria was superior in identifying patients with poor outcome (Figure 3 ). Although our mortality analyses (CV death/non‐CV death) should be interpreted with caution due to a large proportion of deaths by unknown cause, we found that the addition of NPs and echocardiographic HFpEF criteria (descriptive models 2 and 4) significantly predicted non‐CV death after adjustments, which could reflect frailty.6
Implications for future studies of heart failure with preserved ejection fraction
Only a quarter of our patients would have been eligible for PARAGON reflecting the major difference between a registry cohort and an RCT. The PARAGON criteria as well as NP + ESC echocardiography criteria were fulfilled in a quarter of the patients and indeed provided enrichment for predicting the composite outcome. Taken together, this suggests that even in patients with recent acute HF hospitalization, verification of both HF signs and symptoms and reassessment of NPs and echocardiography criteria are helpful to ensure increased risk of HF events. However, these parameters also predicted non‐CV events. Thus, design of HFpEF trials remains difficult, because this needs to ensure the presence of HF, sufficient risk of CV events, not too high risk of non‐CV events (which presumably are not affected by HF specific interventions), and a stage of the syndrome where intervention may be the most effective.
Limitations
The four different HFpEF descriptive models applied in the present study were created post hoc, and no sample size calculation was made based on their presence or distribution yielding a small sample size. It is therefore possible that our study had a limited power to independently predict prognostic factors. Furthermore, the HFpEF descriptive model reflects the time when the KaRen study was designed, and hence, some of our study patients had HF with midrange ejection fraction according to current definitions.1 In analysing CV death and non‐CV death, there is uncertainty because cause of death was unknown in 26% of patients. Finally, patients who died prior to outpatient visit were excluded and may have been more likely to meet HF criteria, had they made it to the outpatient follow‐up, but even assuming all these patients would have met criteria, still only 30% met HF criteria at follow‐up.
Conclusions
In HFpEF patients, HF according to the FC‐HF was present in a quarter of patients, whereof 10% were in NYHA class IV. Most met NP and echocardiography criteria for HFpEF. Residual FC‐HF at follow‐up tended to be associated with increased risk for mortality and HF hospitalization, further strengthened by NPs and echocardiographic criteria, highlighting its role in clinical risk assessment and in identifying patients for future HFpEF research.
Conflict of interest
In this study, the authors had full access to all the data and take responsibility for its integrity and the data analysis. There are no conflicts of interest related to this study. However, to the extent of the findings in the KaRen trial that may affect the use of heart failure drugs or devices and future trials, we disclose the following: G.S.: research grants from Boehringer Ingelheim and Merck Sharp & Dohme; honoraria from Vifor, Servier, AstraZeneca, and Roche; C.H.: consulting fees from Novartis and speaker and honoraria from MSD; E.D.: speaker honoraria and consulting fees from Novartis, AstraZeneca; J.C.D.: research grants, speaker honoraria, and consulting fees from Medtronic and St Jude Medical; L.H.L.: research grants and speaker and honoraria from AstraZeneca, consulting honoraria from Novartis and St Jude Medical, and research grants from Boston Scientific; C.L.: principal investigator of REVERSE, a CRT study sponsored by Medtronic research grants, speaker honoraria, and consulting fees from Medtronic, speaker honoraria and consulting fees from St. Jude Medical.
Funding
The Prospective KaRen study was supported in part by grants from Fédération Française de Cardiologie/Société Française de Cardiologie, France, and Medtronic Bakken Research Center, Maastricht, The Netherlands. C.H. was supported by grants from Center for Gender Medicine Karolinska Institutet, Stockholm, Sweden. L.H.L. was supported by the Swedish Research Council (grant 2013‐23897‐104604‐23), Swedish Heart Lung Foundation (grants 20080409, 20100419), and the Stockholm County Council (grants 00556‐2009, 20110120). C.L. was supported by the Swedish Heart Lung Foundation (grants 20080498, 20110406) and the Stockholm County Council (grants 20090376, 20110610). No funding agency had any role in the design and conduct of the study, in the collection, management, analysis, or interpretation of the data.
Acknowledgements
The authors acknowledge Kambiz Shahgaldi and Maria Westerlind for echocardiogram assessments and the contribution from Gunilla Förstedt and Eva Wallgren at Karolinska University hospital for blood sampling, laboratory analyses, and patient care.
Löfström, U. , Hage, C. , Savarese, G. , Donal, E. , Daubert, J.‐C. , Lund, L. H. , and Linde, C. (2019) Prognostic impact of Framingham heart failure criteria in heart failure with preserved ejection fraction. ESC Heart Failure, 6: 830–839. 10.1002/ehf2.12458.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 3. Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J 2004; 25: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 4. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017; 70: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 5. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail 2017; 5: 471–482. [DOI] [PubMed] [Google Scholar]
- 7. Lund LH, Donal E, Oger E, Hage C, Persson H, Haugen‐Löfman I, Ennezat PV, Sportouch‐Dukhan C, Drouet E, Daubert JC, Linde C, on behalf of the KaRen Investigators . Association between cardiovascular vs. non‐cardiovascular co‐morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 992–1001. [DOI] [PubMed] [Google Scholar]
- 8. Donal E, Lund LH, Linde C, Edner M, Lafitte S, Persson H, Bauer F, Öhrvik J, Ennezat PV, Hage C, Löfman I, Juilliere Y, Logeart D, Derumeaux G, Gueret P, Daubert JC. Rationale and design of the Karolinska‐Rennes (KaRen) prospective study of dyssynchrony in heart failure with preserved ejection fraction. Eur J Heart Fail 2009; 11: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rector TS. Patient's self‐assessment of their congestive heart failure: II. Content, reli‐ability and validity of a new measure—The Minnesota Living with Heart Failure Questionnaire. Heart Fail 1987; 3: 198. [Google Scholar]
- 10. Donal E, Lund LH, Oger E, Hage C, Persson H, Reynaud A, Ennezat PV, Bauer F, Sportouch‐Dukhan C, Drouet E, Daubert JC, Linde C, KaRen Investigators . Baseline characteristics of patients with heart failure and preserved ejection fraction included in the Karolinska Rennes (KaRen) study. Arch Cardiovasc Dis 2014; 107: 112–121. [DOI] [PubMed] [Google Scholar]
- 11. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 12. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 13. Persson H, Donal E, Lund LH, Matan D, Oger E, Hage C, Daubert JC, Linde C, KaRen Investigators . Importance of structural heart disease and diastolic dysfunction in heart failure with preserved ejection fraction assessed according to the ESC guidelines—a substudy in the Ka (Karolinska) Ren (Rennes) study. Int J Cardiol 2018; 274: 202–207. [DOI] [PubMed] [Google Scholar]
- 14. Donal E, Lund LH, Oger E, Hage C, Persson H, Reynaud A, Ennezat PV, Bauer F, Drouet E, Linde C, Daubert C, on behalf of the KaRen investigators . New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: a subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur J Heart Fail 2015; 17: 680–688. [DOI] [PubMed] [Google Scholar]
- 15. Savarese G, Orsini N, Hage C, Dahlström U, Vedin O, Rosano GMC, Lund LH. Associations with and prognostic and discriminatory role of N‐terminal pro–B‐type natriuretic peptide in heart failure with preserved versus mid‐range versus reduced ejection fraction. J Card Fail 2018; 24: 365–374. [DOI] [PubMed] [Google Scholar]


