Abstract
Aims
The aims of this study were to evaluate health‐related quality of life (HRQL) among patients treated with extracorporeal membrane oxygenation (ECMO) in northern Norway over a period of 27 years (1988–2015) and to identify variables related to HRQL.
Methods and results
A retrospective inquiry of the ECMO registry at the University Hospital of North Norway identified 74 ECMO‐treated patients (mean age 49 years, 65% males). Acute cardiac failure was the dominant indication (58%), and venoarterial ECMO was the dominant mode of treatment (87%). Mortality for discharged patients was recorded on 20 September 2016. Thirty (41%) survivors were identified. Twenty‐three survivors were eligible for the survey and received a set of questionnaires at home. The main outcome measure was HRQL as measured with the 36‐item Short‐Form health survey (SF‐36) (RAND Short Form‐36 v1.2). Other questionnaires covered demographic information, problems with functioning in usual daily activities (such as hobbies, household chores, family, or work), employment status, and psychological distress. The survey was completed by 20 (87%) survivors (mean age = 49 years, 12 men). Indications for ECMO treatment (VA = 90%) had been respiratory failure (25%), cardiac failure (60%), and extracorporeal cardiopulmonary resuscitation (15%). The average time since ECMO treatment was 6.5 years. Seventy‐five percent reported mental HRQL (SF‐36 Mental Component Summary, mean = 43, SD = 5) or physical HRQL (SF‐36 Physical Component Summary, mean = 43, SD = 4.5) within the normal range (T = 50 ± 10) in comparison with age‐matched population data from national norms. Half of the responders reported problems on the SF‐36 subscales general health and role physical. Seventy percent reported problems on the SF‐36 subscale role emotional. All but one responder lived independently without any organized care, and 90% reported no problems related to basic self‐care. Half of those in working age had returned to work after ECMO treatment. Forty percent of the responders reported some degree of restrictions in usual daily activities, problems with mobility (35%), anxiety/depression (35%), or pain/discomfort (55%). Significant univariate associations were observed for poorer HRQL and higher reports of psychological distress, pain, and experiencing restrictions in usual everyday activities. Improved HRQL was significantly related to an extended time since ECMO treatment.
Conclusions
Our survey indicates an overall positive long‐term HRQL outcome for our ECMO survivors. A subset reported problems with functioning and HRQL. Future research should focus on identification of the survivors at risk for poor recovery who may benefit from rehabilitation interventions.
Keywords: ECMO, ELSO, Quality of life, Health status
Introduction
Patient‐reported health status is acknowledged as an important measure of health in clinical practice and research trials.1 There remains much scope for research to provide information about the long‐term symptom burden, functional status, and quality of life after treatment with extracorporeal membrane oxygenation (ECMO), particularly for adults.2, 3, 4, 5
Previous outcome studies show poorer health‐related quality of life (HRQL) in adult survivors of ECMO treatment than in healthy age‐matched and gender‐matched controls but show less difference when ECMO survivors are compared with survivors of cardiac surgery procedures or with survivors of general intensive care treatment without ECMO support.2, 4, 5, 6, 7
In order to improve health status and quality of life in ECMO survivors with long‐term problems, it is important to characterize which factors do influence their mental HRQL and physical HRQL.1, 7 From previous research, it seems probable that physical impairments with mobility restrictions,3 neurological injuries,8 mental health problems,2, 9, 10 and cognitive impairments11, 12 are potential obstacles for return to an optimal life situation after ECMO treatment. However, research on influential factors for HRQL in ECMO survivors has received limited attention.7
In Norway, the first ECMO treatment was performed at the University Hospital of North Norway (UNN) in 1988.13 From this initial experience, ECMO was used sporadically at our institution as an attempted life‐saving intervention in critically ill patients. From January 2013, an institutional ECMO protocol was implemented with three principal predefined indications for treatments such as refractory cardiogenic shock and severe respiratory failure and as an extracorporeal cardiopulmonary resuscitation (E‐CPR) effort.
The aims of this retrospective, single‐institution survey were to identify the long‐term survivors from the entire treatment era (1988–2015), to clarify their HRQL, and to characterize influential factors for HRQL.
Methods
Study population and hospital setting
All patients treated with ECMO in the intensive care unit between 1988 and 2015 at the UNN were identified by searching the hospital's electronic databases. The hospital is located at 69°N and is the primary referral centre for a large area (112 000 km2), covering the northern part of Norway and Svalbard with ~500 000 inhabitants. In this region, adult cardiac surgery and ECMO treatment are localized to one centre, serving 11 smaller emergency hospitals.
Data collection
Patient medical characteristics were collected by reviewing hospital records (clinical, laboratory, and operative notes) from the first registered time of critical illness requiring ECMO treatment. The standardized data‐reporting sheet from the Extracorporeal Life Support Organization (https://www.elso.org/Portals/0/Files/ELSOECLSRegistryForm.pdf) was used to guide the documentation of patient characteristics, pre‐ECMO and on‐ECMO clinical status, laboratory information, complicating events requiring intervention during ECMO treatment, and the causes of death.14 Owing to the long time‐span of inclusion and the heterogeneity of the indications and patient treatments over this time period, we have presented our patients' medical characteristics in three temporal cohorts: 1988–2000, 2001–12, and 2013–15. The last period represents the time after introduction of a predefined inclusion protocol (date 1 January 2013).
Mortality after discharge was recorded in 20 September 2016. Written consent for study participation was obtained by mail from long‐term survivors. The survey was sent only to survivors aged ≥18 years and without documentation of extensive cognitive problems (two patients excluded). Inclusion and exclusion of survivors for the survey are shown in Figure 1.
Figure 1.
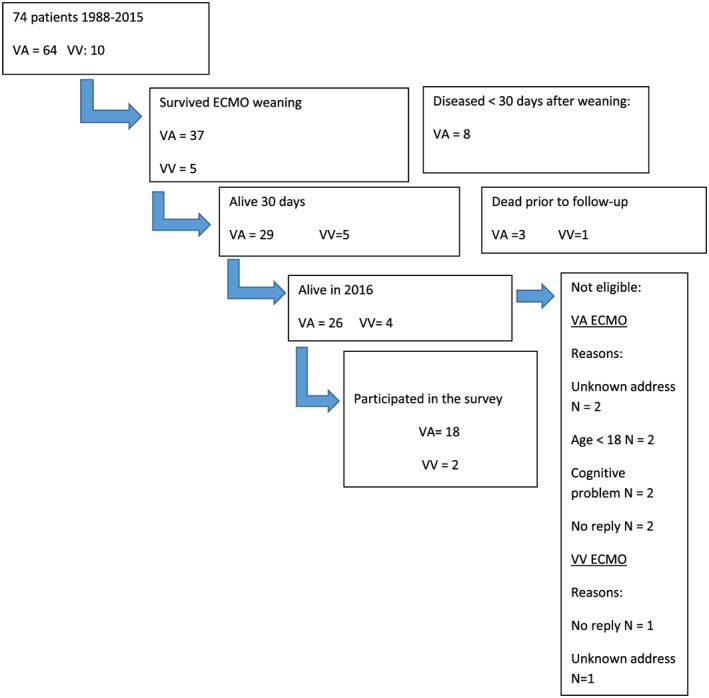
Flow chart shows the inclusion and exclusion of survivors for the survey. ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
The study complies with the Declaration of Helsinki 15 and was approved by the Regional Committee for Research Ethics in Medicine and Health Sciences in North Norway (Institutional Protocol Number 2015/1068).
Measurement methods
HRQL was measured with the Norwegian standard Version 1.2 of the 36‐item Short‐Form health survey (SF‐36).16 SF‐36 is a generic, profile‐based HRQL measure designed to capture a person's perception of how his or her health status has interfered with his or her psychological, social, and physical functioning for the past 4 weeks. It provides a measure of the relative burden of disease along eight different dimensions of health within a Physical Component Summary (PCS) scale and a Mental Component Summary (MCS) scale. Each participant's data were scored according to the recommended procedure and then compared with age‐corrected normative data from the general Norwegian population.16, 17 Age‐corrected scores were then transformed into T‐scores (mean = 50, SD = 10) such that scores between 40 and 60 represent the statistical normal range for all subscales and summary component scales.
Information about problems related to mobility (walking), self‐care (washing/dressing), usual activities (work, study, housework, family, leisure activities), pain/discomfort, and anxiety/depression were assessed with the descriptive system of the EuroQoL five‐dimension five‐level (EQ‐5D‐5L) health questionnaire.18 Each of the five dimensions is rated on a 5‐point scale, ranging from 1 ‘no problems’ to 5 ‘extreme problems’.
Psychological distress was assessed using the Hospital Anxiety and Depression Scale (HADS).19 The HADS is a 14‐item questionnaire designed to assess symptoms of mood disorders in non‐psychiatric hospital outpatients. Seven questions assess anxiety symptoms, and seven assess symptoms of depression (scored 0–3 for each question), with each scale having a maximum score of 21. Scores at or above eight points on either the depression or anxiety scale are suggested to be clinically relevant.20
Cognitive problems were assessed with the Everyday Memory Questionnaire 13‐item version (EMQ‐R).21 The EMQ‐R consists of 13 items, where each item is rated on a 5‐point scale ranging from A, scored as zero (‘Once or less in the last month’), to E, scored as four (‘Once or more in a day’). The items were summed and given a scale in the range of 0–52. The summary score was evaluated for its correlation with mental HRQL and physical HRQL.
Statistical analyses
We used descriptive statistics to present demographic and medical characteristics of the participants and their scores on the questionnaires. Continuous variables were reported as mean (standard deviation and/or range), while categorical variables were reported as counts (percent). Group comparisons of variables between the three time cohorts were analysed by one‐way ANOVA or binary logistic regression. The SF‐36 PCS and MCS scores were not significantly different from a normal distribution, as shown by the Shapiro–Wilk tests and by inspection of Q–Q plots of the residuals. Hence, parametric testing was used to assess group differences and correlations with the PCS and MCS SF‐36 variables. A significance level of P < 0.05 was used. All statistical analyses were performed using SPSS 25.0 (IBM, SPSS Inc., Chicago, IL, USA).
Results
Patients treated with extracorporeal membrane oxygenation, 1988–2015
As shown in Table 1, the total number of ECMO‐treated patients was 74, with a marked increase after implementation of the institutional protocol in 2013. Most patients treated with ECMO at our hospital were adult men (65%), mean age 49 years (0–76), and the main indication for support was acute cardiac failure (58%). In the large majority (>90%) of these patients, the cardiac failure was new onset without any known cardiac diseases prior to ECMO treatment. Venoarterial (VA) ECMO treatment was the dominant configuration (87%). Venovenous (VV) ECMO was used only sporadically (14%). The average duration of ECMO support was 6 days (8 h to 21 days), with an average hospital stay of 19 (1–126) days.
Table 1.
Characteristics of patients treated with extracorporeal membrane oxygenation in the three time cohorts and the total sample
| Characteristics of ECMO‐treated patients in the three time cohorts (n = 74) |
1988–2000 n = 10 (14%) |
2001–12 n = 16 (22%) |
2013–15 n = 48 (64%) |
1988–2015 n=74 (100%) |
|---|---|---|---|---|
| Mean (SD) (range) or no (%) | Mean (SD) (range) or no (%) | Mean (SD) (range) or no (%) | Mean (SD) (range) or no (%) | |
| Age (years) | 31 (21) (0–58) | 48 (21) (0–73) | 52 (16) (2–76) | 48.6 (0–76) |
| Age < 18 | 2 (20) | 1 (6) | 2 (4) | 5 (7) |
| Male | 5 (50) | 12 (75) | 31 (65) | 48 (65) |
| ECMO mode | ||||
| VA | 7 (70) | 15 (94) | 42 (87.5) | 64 (86.5) |
| VV | 3 (30) | 1 (6) | 6 (12.5) | 10 (13.5) |
| Reason for support | ||||
| Cardiac; all indications | 2 (20) | 12 (75) | 29 (60) | 43 (58) |
| Cardiogenic shock | 0 | 8 | 18 | 26 |
| Post‐cardiotomy | 2 | 4 | 11 | 17 |
| Pulmonary | 5 (50) | 1 (6) | 7 (15) | 13 (18) |
| E‐CPR | 3 (30) | 3 (19) | 12 (25) | 18 (24) |
| Bridge to transplant | 0 | 3 (19) | 3 (6) | 6 (8) |
| Days ventilated pre‐ECMO | 4 (10) (0–26) | 0.25 (0.4) (0–1) | 0.6 (1.5) (0–9) | 0.9 (0–26) |
| Pre‐pH < 7.2 | 4 (40) | 8 (50) | 24 (50) | 36 (49) |
| Cardiac arrest pre‐ECMO | 3 (30) | 6 (38) | 27 (56) | 36 (49) |
| Cannulation | ||||
| Central/peripheral | 2/4 (20/40) | 1/11 (6/69) | 4/39 (8/81) | 7/54 (10/73) |
| Surgical/percutaneous | 4/2 (40/20) | 11/1 (69/6) | 41/2 (86/4) | 56/5 (76/7) |
| Not registered | 4 (40) | 4 (25) | 5 (10) | 13 (17) |
| Days on ECMO | 6 (6) (1–21) | 4.5 (3) (0.3–14) | 6.6 (6) (0.3–21) | 6 (0.3–21) |
| Days of hospital stay | 15 (10) (5–29) | 13 (10) (1–35) | 21 (26) (1–125) | 19 (1–125) |
| Discharged ongoing ECMO | 0 | 2 (13) | 6 (13) | 8 (11) |
| Survived ECMO | 6 (60) | 8 (50) | 28 (58) | 42 (57) |
| Survived 30 days | 5 (50) | 7 (44) | 22 (46) | 34 (46) |
| Cardiac; all indications | 0 | 5 (42) | 13 (45) | 18 (42) |
| Cardiogenic shock | 0 | 3 (38) | 11 (61) | 14 (54) |
| Post‐cardiotomy | 0 | 2 (50) | 2 (19) | 4 (24) |
| Pulmonary | 3 (60) | 1 (100) | 4 (57) | 8 (62) |
| E‐CPR | 2 (67) | 1 (33) | 5 (42) | 8 (44) |
| Survivors with neurological injury | ||||
| Central | 0 | 0 | 5 (23) | 5 (15) |
| Peripheral | 2 (40) | 1 (14) | 7 (32) | 10 (29) |
ECMO set‐up was defined by the last configuration used. The occurrence of neurological injuries in survivors is as described by discharge note information.
Survival rates for the different indications for ECMO treatment are shown in Table 1. Survival rates were not significantly different between the three time cohorts. The main effect of age was not significantly different between the cohorts [F(2, 73) = 5.7, P = 0.005]. However, adult patients treated in the first cohort (1988–2000) were significantly younger than adults treated between 2013 and 2015 (P = 0.001) and adults treated between 2001 and 2012 (P = 0.024).
Complications during ECMO support and the causes of early deaths are shown in Table 2. The most common causes of death were irreversible organ failure involving the brain or the heart in the VA group and multiorgan failure in the VV group. All but four deaths occurred within 30 days. One patient died after 90 days, one after 15 months, and two after 7 and 26 years.
Table 2.
Complications requiring intervention during extracorporeal membrane oxygenation support in the 30 day survivors and non‐survivors, and causes of death for venoarterial and venovenous extracorporeal membrane oxygenation shown separately
| Complications requiring intervention during ECMO support in the 30 day survivors and non‐survivors, and causes of death | Survived VA n = 29 18 men Age 42 (0–73) | Died n = 35 25 men Age 54 (2–76) | Survived VV n = 5, 4 men Age 50 (22–68) | Died n = 5 1 man Age 44 (18–57) |
|---|---|---|---|---|
| Mechanical complications | ||||
| Cannula problems | 24% (7) | 26% (9) | 20% (1) | 20% (1) |
| Other | 14% (4) | 6% (2) | 20% (1) | 40% (2) |
| Patient related complications | ||||
| Haemorrhagic | ||||
| GI haemorrhage | 24% (7) | 9% (3) | 20% (1) | 20% (1) |
| Cannulation site bleeding | 52% (15) | 49% (17) | 20% (1) | 40% (2) |
| Surgical site bleeding | 14% (4) | 29% (10) | 20% (1) | 20% (1) |
| Haemolysis (plasma Hgb > 50 mg/dL) | 3% (1) | 0 | 0 | 0 |
| DIC | 7% (2) | 3% (1) | 0 | 0 |
| Central nervous systema | ||||
| Infarction | 7% (2) | 11% (4) | 0 | 0 |
| Haemorrhage | 0 | 6% (2) | 0 | 0 |
| Brain death | 0 | 9% (3) | 0 | 0 |
| Renal | ||||
| 1.5 < creatinine < 3.0 mg/L | 45% (13) | 40% (14) | 20% (1) | 0 |
| Creatinine > 3 mg/L | 14% (4) | 17% (6) | 0 | 20% (1) |
| Haemodialysis | 28% (8) | 37% (13) | 0 | 40% (2) |
| Haemofiltration | 10% (3) | 29% (10) | 20% (1) | 20% (1) |
| CAVHD | 0 | 0 | 0 | 20% (1) |
| Pulmonary | ||||
| Pneumothorax | 7% (2) | 6% (2) | 0 | 0 |
| Pulmonary haemorrhage | 10% (3) | 17% (6) | 0 | 20% (1) |
| Cardiopulmonary | ||||
| Inotropes on ECLS | 52% (15) | 83% (29) | 80% (4) | 80% (4) |
| Myocardial stunning by echocardiography | 17% (5) | 26% (9) | 0 | 0 |
| Hypertension requiring vasodilator | 14% (4) | 9% (3) | 0 | 0 |
| Tamponade: blood | 17% (5) | 20% (7) | 0 | 0 |
| CPR required | 10% (3) | 11% (4) | 0 | 0 |
| Cardiac arrhythmia | 31% (9) | 57% (20) | 0 | 0 |
| Other | 3% (1) | 6% (2) | 0 | 0 |
| Infectious | ||||
| Culture proven new infection | 24% (7) | 20% (7) | 0 | 0 |
| WBC < 1500 | 0 | 0 | 0 | 20% (1) |
| Metabolic | ||||
| Glucose < 40 mg/L | 7% (2) | 20% (7) | 0 | 0 |
| Glucose > 240 mg/L | 3% (1) | 0 | 0 | 0 |
| pH < 7.20 | 24% (7) | 43% (15) | 20% (1) | 40% (2) |
| pH > 7.6 | 7% (2) | 0 | 0 | 0 |
| Hyperbilirubinaemia | 21% (6) | 31% (11) | 0 | 40% (2) |
| Lower extremity | ||||
| Limb ischaemia | 0 | 3% (1) | 0 | 0 |
| Compartment syndrome | 0 | 3% (1) | 0 | 0 |
| Fasciotomy | 0 | 6% (2) | 0 | 0 |
| Amputation | 3% (1) | 0 | 0 | 0 |
| Causes of death | ||||
| Irreversible organ failure | 46% (16) | 20% (1) | ||
| Cardiac | 38% (6) | |||
| CNS | 38% (6) | |||
| Pulmonary | 20% (1) | |||
| Other organ | 25% (4) | |||
| Multiorgan failure | 34% (12) | 80% (4) | ||
| Haemorrhage | 3% (1) | |||
| Not registered | 17% (6) |
All but one early death occurred within 30 days.
CAVHD, continuous arteriovenous haemodialysis; DIC, disseminated intravascular coagulation; ECLS, extracorporeal life support; GI, gastrointestinal; Hgb, haemoglobin; WBC, white blood cell.
As verified by computed tomography.
In survivors of VA ECMO treatment, cardiopulmonary complications were the most frequent, followed by haemorrhagic and renal complications. These complications occurred at a higher rate in the VA group that died, as did metabolic complications. Least frequent in the surviving VA group were complications related to the lower extremities and central nervous system (CNS) complications. These complications were more common among the patients who died than among those who survived VA ECMO. Complications involving the CNS or the lower extremities did not occur in the VV group for either survivors or non‐survivors. Cardiopulmonary and haemorrhagic complications were most frequent in the group of VV survivors, while additional renal and metabolic complications occurred at a high rate in the non‐survivors of VV ECMO treatment. No patients required long‐term renal replacement therapy.
Additional discharge note information of neurological injuries is shown in Table 1. Five survivors of VA ECMO suffered CNS injuries. All events occurred after 2013. The CNS injuries were verified by cerebral imaging during hospital stay and were described as cerebral infarction, cerebral bleeding, or hypoxic–ischaemic brain injury. All five patients had undergone VA ECMO treatment for either cardiogenic shock (40%) or E‐CPR (60%). One of them also suffered peripheral nerve injury. All five were resuscitated from cardiac arrest.
Peripheral neural injuries were registered in 10 survivors (29%), equally distributed over the three time cohorts, and occurred in all indications: pulmonary (P) (20%), E‐CPR (20%), and cardiac (C) (60%). Peripheral neuropathies were most often foot drop or critical illness polyneuropathies.
Survey responders
The survey responders were 12 men and 8 women, with an average age of 48 (SD = 16) years. The average time from ECMO to follow‐up was 6.5 (SD = 7) years, and 60% of the responders had been treated after implementation of the institutional ECMO protocol in 2013. One of the responders had been a child at the time of ECMO treatment. Seventy‐five percent were treated with VA ECMO, for C (60%), P (25%), or E‐CPR (15%) indications. The average time on ECMO was 8 (SD = 7) days, and the average time of hospital stay was 20 (SD = 14) days. Peripheral neurological sequelae were documented in six (30%) survey responders and CNS injury in one responder (5%). Information about current chronic disorders requiring outpatient treatment was collected from the survey responders' hospital journals and showed that 11 (55%) were receiving outpatient treatment for a chronic cardiac condition, 4 (20%) had diabetes, 2 (10%) had a chronic pulmonary condition, 1 had cancer, and 1 had renal disease. The demographic characteristics of the survey responders are shown in Table 3, and the results from the survey are displayed in Table 3 and Figures 2 and 3.
Table 3.
Demographic characteristics of the survey responders and their results from the questionnaires
| Result from the survey responders n = 20 | n (%) or mean (SD) |
|---|---|
| Male/female | 12 (60)/8 (40) |
| Current age | 48 (15) |
| Marital status | |
| Alone | 7 (35) |
| Married/with partner | 13 (65) |
| Living situation | |
| Home independent | 18 (90) |
| Home with help from others | 2 (10) |
| Sheltered housing/nursing home | 0 |
| Education | |
| Primary school | 5 (25) |
| High school | 13 (65) |
| University level | 2 (10) |
| Working situation | |
| Full time | 7 (35) |
| Part time | 1 (5) |
| Retired | 4 (20) |
| Disability pension | 8 (40) |
| Psychological distress | |
| HADS depression scale | 3 (2.6) |
| HADS anxiety scale | 4.5 (4) |
| HADS depression scale > 8 | 0 |
| HADS anxiety scale > 8 | 3 (15) |
| Cognitive complaints | |
| EMQ‐13 score | 10 (9) |
| SF‐36 | |
| Mental Component Summary (MCS) | 43 (5), n < 40 = 5 |
| Physical Component Summary (PCS) | 43 (4.5), n < 40 = 5 |
| Physical functioning | 50 (10), n < 40 = 3 |
| General health | 38 (11), n < 40 = 11 |
| Role physical | 38 (13), n < 40 = 10 |
| Role emotional | 35 (13), n < 40 = 14 |
| Social role | 45 (11), n < 40 = 6 |
| Emotional well‐being | 49 (9), n < 40 = 3 |
| Pain | 47 (9), n < 40 = 4 |
| Vitality | 43 (11), n < 40 = 5 |
SF‐36 scores shown as both mean (SD) and the number of individuals with T‐scores < 40 on each scale.
EMQ‐13, Everyday Memory Questionnaire—13‐item version; HADS, Hospital Anxiety and Depression Scale.
Figure 2.
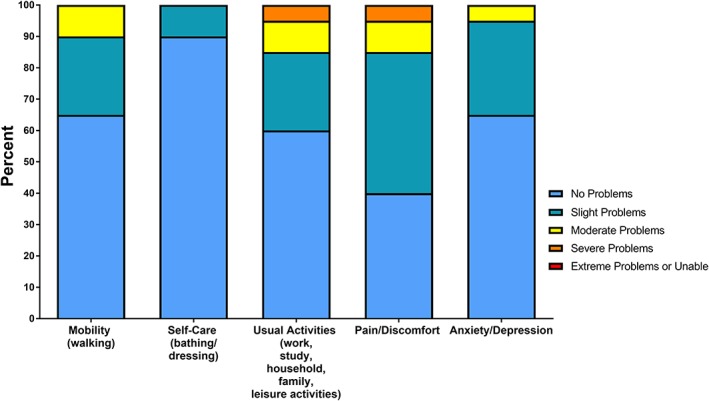
EQ‐5D‐5L. Frequency (percentage) of the severity of problems in daily functioning reported by the ECMO‐treated long‐term survivors on the EQ‐5D‐5L functional system (n = 20). ECMO, extracorporeal membrane oxygenation; EQ‐5D‐5L, EuroQoL five dimensions five levels.
Figure 3.
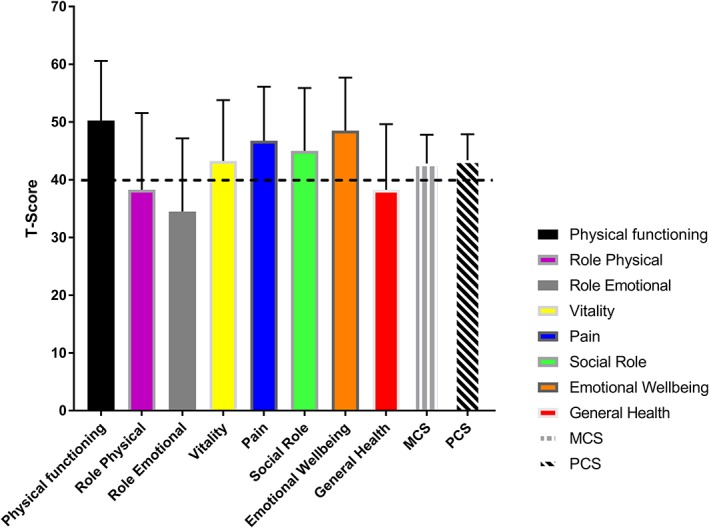
SF‐36 scores from all eight subscales and the two component summary scales presented in standardized T‐scores (mean = 50, SD = 10). Coloured bars show mean; error bars show SD. A T‐score within −40 to 60 represents the statistical normal range. Dashed horizontal line represents the lower limit of the normal range. Higher scores indicate better HRQL. HRQL, health‐related quality of life; MCS, Mental Component Summary; PCS, Physical Component Summary; SF‐36, 36‐item Short‐Form health survey.
EuroQoL five dimensions five levels
As shown in Figure 2, the majority of the responders reported no problems with mobility (65%), self‐care (90%), usual everyday activities (60%), with anxiety/depression (65%). None reported extreme problems, and severe problems were unusual (n = 1). However, slight or moderate problems were reported for self‐care (10%), mobility (35%), anxiety/depression (35%), usual activities (40%), and pain/discomfort (55%). As shown in Table 3, all were living in their private homes, and the large majority reported receiving no organized care. Fifty percent of those of working age had returned to work.
36‐Item Short‐Form health survey
The SF‐36 results are displayed in Figure 3 and Table 3. The average MCS and PCS scores were in the low normal range than were age‐matched national population norms. The subscales for general health, emotional role functioning, and physical role functioning were below the normal range.
Table 3 shows the number of individuals with HRQL scores below the normal range (T < 40) on each of the SF‐36 scales. Seven (35%) survivors scored below the normal range on the PCS and/or MCS scales. These individuals were all long‐term survivors of VA ECMO for cardiac (n = 6) or E‐CPR (n = 1) indications.
Hospital Anxiety and Depression Scale
No patients reported symptoms above the clinical cut‐off on the depression scale. Three (15%) responders reported symptoms above the clinical cut‐off on the anxiety scale. HADS scores are shown in Table 3.
Correlations with health‐related quality of life
Poorer MCS scores on the SF‐36 were significantly correlated with more cognitive problems as reported on the EMQ‐R (r = −0.64, P = 0.002) and more symptoms of anxiety (r = −0.50, P = 0.026) and depression (r = −0.63, P = 0.003) as reported on the HADS. Also, a significantly lower MCS score was correlated with a higher degree of difficulties with personal care (r = −0.45, P = 0.046) and pain/discomfort (r = −0.46, P = 0.042) as reported on the EQ‐5D‐5L.
Poorer PCS scores on the SF‐36 were significantly correlated with higher reports of depression symptoms as reported on the HADS (r = −0.63, P = 0.003) and with the EQ‐5D‐5L domains of pain/discomfort (r = −0.65, P = 0.002) and problems with handling daily activities (r = −0.45, P = 0.04).
Patients' demographic variables (age, sex, education, marital status, return to work) or ECMO characteristics (mode, indication, duration of ECMO support) were not significantly associated with the MCS or PCS scores. Survivors treated prior to 2013 had significantly better HRQL scores than had survivors treated after 2013, indicative of better HRQL with longer time since treatment [MCS: F(1, 19) = 4.5, P = 0.48; PCS: F(1, 19) = 4.5, P = 0.49].
Discussion
We identified all ECMO‐treated patients from the UNN during a 27 year period and evaluated HRQL in adult survivors. Despite increasing patient volume, wider indications, and older patients accepted in recent times, survival rates have been relatively stable and compare well with international reports.22
The main observation from our follow‐up demonstrated that our survivors had mental HRQL and physical HRQL in the statistical lower normal range compared with age‐matched national data. Psychological distress was low, and 50% of survivors had returned to work. Longer time since treatment was associated with improved HRQL. Psychological distress, memory problems, pain, and difficulties with usual activities were significantly correlated with a poorer HRQL.
It should be noted that our SF‐36 profile showed poor scores on the subscale for general health and on the subscales for emotional and physical role functioning. It is likely that the general health subscale mirrors not only the adverse effects of past critical illness and ECMO treatment but also the chronic diseases that were frequent in our long‐term survivors. Poor results on the two role‐functioning subscales are likely related to half of our sample not having returned to work after ECMO treatment.
Firm comparisons with previous HRQL studies are complicated by heterogeneity in patient and treatment characteristics, comparator groups, and follow‐up times. Nevertheless, earlier studies have also reported acceptable, although variable, HRQL when using the SF‐36.2, 4, 5, 23, 24, 25 High rates of return to independence in self‐care and daily activities, as found in the present study, have also been reported previously in other studies using the EQ‐5D descriptive system.3, 7, 23, 24, 26, 27
Our registered levels of psychological distress are low compared with those of several other studies using the HADS.2, 3, 5, 9, 10 In a prospective longitudinal study, Tramm et al. found that mental health improved during the first year after ECMO and that psychological distress was higher in survivors who received psychological care prior to ECMO treatment.3 Somewhat at odds with this finding, Risnes et al. found high rates of psychiatric morbidity several years after ECMO treatment, even in the survivors with no history of psychiatric disorder prior to ECMO treatment.9 Our 50% return‐to‐work rate is higher than that found by Hodgson et al.28 and Camboni et al.26 but lower than that reported by Tramm et al.,3 where two‐thirds of the sample had returned to work by 1 year after ECMO treatment.
In accordance with a number of previous studies, we showed that HRQL improves with longer time since ECMO treatment.2, 3, 5, 7, 10, 25 Although this result is encouraging, health workers should prioritize to identify survivors at risk for a poor HRQL, as well as factors that may hamper and facilitate HRQL after survival at the various time points in the recovery process.7, 23 In the present study, the subgroup of responders with a poor overall HRQL was survivors of VA ECMO for cardiogenic shock or E‐CPR indications. Prior studies suggest that these ECMO survivors are at higher risks for neurological injuries and cognitive impairments.8, 11 In our survivors, we detected mainly peripheral nerve injuries and, to a much lesser extent, CNS injuries. Without recent neuroimaging and neuropsychological testing, it remains uncertain if some of the survivors in our study had neurocognitive impairments that affected their functioning or HRQL.3, 5, 8, 11, 12
In the present study, more symptoms of psychological distress, memory problems, and pain, as well as difficulties with usual activities in everyday life, were significantly correlated with a poorer HRQL. If replicated in future studies, preferably with larger samples, our presented correlations may suggest that these issues need consideration in follow‐up care after survival and in upcoming research on HRQL after successful ECMO treatment.5, 6, 29, 30
Our study is limited by retrospective data retrieval prone to lack of precision and bias. Data were collected from a single centre over a long period of time, which limits generalizability. The small sample sizes prohibited multivariable modelling and statistical analyses regarding the subgroups of patients and long‐term survivors. Confounders may influence our observed correlations. HRQL prior to ECMO treatment was not known. HRQL was assessed at only one occasion and the responders varied greatly in time since ECMO treatment. Loss to follow‐up and exclusion of two long‐term survivors with brain injury may have skewed the outcome measures in a positive direction.
The strengths of our survey are the long follow‐up times and the fact that HRQL and functional outcome were evaluated with generic questionnaires commonly used in the ECMO literature and generally well validated in several populations. Nevertheless, psychometric validation of HRQL instruments in the ECMO population has yet to be performed. Additionally, it is currently unclear which instruments best capture the outcomes that are most important to the survivors at various time points after ECMO treatment.7, 29 Studies of HRQL in adult ECMO survivors with neurological injuries are currently lacking and should receive future attention to inform rehabilitation services. For the subgroup of ECMO patients with CNS injury, alternatives to generic HRQL questionnaires should be considered, as should the inclusion of measures of neuropsychological functioning.1, 3, 5, 11 Influential factors for HRQL after successful ECMO survival warrants further evaluation to inform researchers and practitioners regarding potential targets for rehabilitation interventions.1, 5, 7
Conflict of interest
None declared.
Funding
This work was supported by the Norwegian Health Authorities Northern Region [grant number 2015/558‐67/153] and the University Hospital of North Norway, Tromsø, Norway.
Acknowledgements
The investigators thank the survey responders who volunteered their time for this research project.
Ørbo M. C., Karlsen S. F., Pedersen E. P., Hermansen S. E., Rønning P. B., Nergaard K. A., Næsheim T., and Myrmel T. (2019) Health‐related quality of life after extracorporeal membrane oxygenation: a single centre's experience, ESC Heart Failure, 6, 701–710. 10.1002/ehf2.12433.
References
- 1. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation 2013; 127: 2233–2249. [DOI] [PubMed] [Google Scholar]
- 2. Muller G, Flecher E, Lebreton G, Luyt C‐E, Trouillet J‐L, Bréchot N, Schmidt M, Mastroianni C, Chastre J, Leprince P. The ENCOURAGE mortality risk score and analysis of long‐term outcomes after VA‐ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016; 42: 370–378. [DOI] [PubMed] [Google Scholar]
- 3. Tramm R, Ilic D, Sheldrake J, Pellegrino V, Hodgson C. Recovery, risks, and adverse health outcomes in year 1 after extracorporeal membrane oxygenation. Am J Crit Care 2017; 26: 311–319. [DOI] [PubMed] [Google Scholar]
- 4. Spangenberg T, Schewel J, Dreher A, Meincke F, Bahlmann E, van der Schalk H, Kreidel F, Frerker C, Stoeck M, Bein B. Health related quality of life after extracorporeal cardiopulmonary resuscitation in refractory cardiac arrest. Resuscitation 2018; 127: 73–78. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox ME, Jaramillo‐Rocha V, Hodgson C, Taglione MS, Ferguson ND, Fan E. Long‐term quality of life after extracorporeal membrane oxygenation in ARDS survivors: systematic review and meta‐analysis. J Intensive Care Med 2017: 0885066617737035. [DOI] [PubMed] [Google Scholar]
- 6. Elliott D, Davidson JE, Harvey MA, Bemis‐Dougherty A, Hopkins RO, Iwashyna TJ, Wagner J, Weinert C, Wunsch H, Bienvenu OJ. Exploring the scope of post‐intensive care syndrome therapy and care: engagement of non‐critical care providers and survivors in a second stakeholders meeting. Crit Care Med 2014; 42: 2518–2526. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh FT, Huang GS, Ko WJ, Lou MF. Health status and quality of life of survivors of extra corporeal membrane oxygenation: a cross‐sectional study. J Adv Nurs 2016; 72: 1626–1637. [DOI] [PubMed] [Google Scholar]
- 8. Xie A, Lo P, Yan TD, Forrest P. Neurologic complications of extracorporeal membrane oxygenation: a review. J Cardiothorac Vasc Anesth 2017; 31: 1836–1846. [DOI] [PubMed] [Google Scholar]
- 9. Risnes I, Heldal A, Wagner K, Boye B, Haraldsen I, Leganger S, Møkleby K, Svennevig JL, Malt UF. Psychiatric outcome after severe cardio‐respiratory failure treated with extracorporeal membrane oxygenation: a case‐series. Psychosomatics 2013; 54: 418–427. [DOI] [PubMed] [Google Scholar]
- 10. Mirabel M, Luyt C‐E, Leprince P, Trouillet J‐L, Léger P, Pavie A, Chastre J, Combes A. Outcomes, long‐term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med 2011; 39: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 11. Risnes I, Wagner K, Nome T, Sundet K, Jensen J, Hynås IA, Ueland T, Pedersen T, Svennevig JL. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg 2006; 81: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 12. von Bahr V, Kalzén H, Hultman J, Frenckner B, Andersson C, Mosskin M, Eksborg S, Holzgraefe B. Long‐term cognitive outcome and brain imaging in adults after extracorporeal membrane oxygenation. Crit Care Med 2018; 46: e351–e358. [DOI] [PubMed] [Google Scholar]
- 13. Bjertnaes L, Vaage J, Almdahl S, Lil M, Nilsen P, Hansen K, Solbø J, Jolin Å, Hotvedt R, Olafsen K. Extracorporeal membrane oxygenation (ECMO) as lung or heart assist. Acta Anaesthesiol Scand 1996; 40: 293–301. [DOI] [PubMed] [Google Scholar]
- 14. Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal life support organization registry report 2012. ASAIO J 2013; 59: 202–210. [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–1294. [DOI] [PubMed] [Google Scholar]
- 16. Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form health survey (SF‐36): I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 17. HÅvard Loge J, Kaasa S. Short Form 36 (SF‐36) health survey: normative data from the general Norwegian population. Scand J Soc Med 1998; 26: 250–258. [PubMed] [Google Scholar]
- 18. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy (Amsterdam, Netherlands) 1990; 16: 199. [DOI] [PubMed] [Google Scholar]
- 19. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 20. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 21. Royle J, Lincoln NB. The Everyday Memory Questionnaire—revised: development of a 13‐item scale. Disabil Rehabil 2008; 30: 114–121. [DOI] [PubMed] [Google Scholar]
- 22. Burrell AJ, Pellegrino VA, Wolfe R, Wong WK, Cooper DJ, Kaye DM, Pilcher DV. Long‐term survival of adults with cardiogenic shock after venoarterial extracorporeal membrane oxygenation. J Crit Care 2015; 30: 949–956. [DOI] [PubMed] [Google Scholar]
- 23. Schoenrath F, Hoch D, Maisano F, Starck CT, Seifert B, Wenger U, Ruschitzka F, Wilhelm MJ. Survival, quality of life and impact of right heart failure in patients with acute cardiogenic shock treated with ECMO. Heart Lung: J Acute Crit Care 2016; 45: 409–415. [DOI] [PubMed] [Google Scholar]
- 24. Anselmi A, Flécher E, Corbineau H, Langanay T, Le Bouquin V, Bedossa M, Leguerrier A, Verhoye J‐P, Ruggieri VG. Survival and quality of life after extracorporeal life support for refractory cardiac arrest: a case series. J Thorac Cardiovasc Surg 2015; 150: 947–954. [DOI] [PubMed] [Google Scholar]
- 25. Combes A, Leprince P, Luyt C‐E, Bonnet N, Trouillet J‐L, Léger P, Pavie A, Chastre J. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008; 36: 1404–1411. [DOI] [PubMed] [Google Scholar]
- 26. Camboni D, Philipp A, Rottenkolber V, Zerdzitzki M, Holzamer A, Floerchinger B, Lunz D, Mueller T, Schmid C, Diez C. Long‐term survival and quality of life after extracorporeal life support: a 10‐year report. Eur J Cardiothorac Surg 2017; 52: 241–247. [DOI] [PubMed] [Google Scholar]
- 27. Guihaire J, Dang Van S, Rouze S, Rosier S, Roisne A, Langanay T, Corbineau H, Verhoye J‐P, Flécher E. Clinical outcomes in patients after extracorporeal membrane oxygenation support for post‐cardiotomy cardiogenic shock: a single‐centre experience of 92 cases. Interact Cardiovasc Thorac Surg 2017; 25: 363–369. [DOI] [PubMed] [Google Scholar]
- 28. Hodgson CL, Hayes K, Everard T, Nichol A, Davies AR, Bailey MJ, Tuxen DV, Cooper DJ, Pellegrino V. Long‐term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit Care 2012; 16: R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K. The importance of patient‐reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014; 35: 2001–2009. [DOI] [PubMed] [Google Scholar]
- 30. Abrams D, Garan AR, Abdelbary A, Bacchetta M, Bartlett RH, Beck J, Belohlavek J, Chen Y‐S, Fan E, Ferguson ND. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 2018: 1–13. [DOI] [PubMed] [Google Scholar]


