Abstract
Aims
Conventional cytotoxic chemotherapy is still among the most effective treatment options for many types of cancer. However, cardiotoxicity, notably the decrease in left ventricular function under these regimens, can impair prognosis. Thus, prevention and treatment of cardiotoxicity are crucial. The present meta‐analysis aims to assess the efficacy of beta‐blockers or angiotensin‐converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs) for prevention of cardiotoxicity.
Methods and results
We systematically searched Pubmed, Cochrane, EMBASE, and Web of Science databases for randomized controlled trials published until February 2019. The analysis included randomized studies that reported on left ventricular ejection fraction (LVEF) after 6 months of chemotherapy in cancer patients who received beta‐blockers or ACE inhibitors/ARBs for prevention of cardiotoxicity compared with controls. Studies on combination cardioprotective therapies were excluded from the analysis. The primary endpoint was prevention of a decrease in LVEF as defined by the individual study and as assessed by either transthoracic echocardiography or magnetic resonance imaging. We here show that patients under anthracycline‐based chemotherapy have a moderate yet significant benefit in LVEF from beta‐blockers or ACEs/ARBs. The beta‐blocker analysis included 769 cancer patients, and the ACE inhibitors/ARBs analysis included a total of 581 cancer patients. The mean LVEF difference between the beta‐blocker group and the control group was 2.57% (95% confidence interval 0.63–4.51, P = 0.009). The mean difference for ACE inhibitors/ARBs was 4.71% (95% confidence interval 0.38–9.03, P = 0.03). However, the beneficial effects throughout the studies were variable as documented by significant heterogeneity between the studies.
Conclusions
Systematic evidence is needed to solidly found recommendations for cardioprotective prevention during chemotherapy. Likewise, trials on other neurohumoral drugs (spironolactone) and lipid‐lowering approaches are required to improve protection for cardio‐oncology patients.
Keywords: Cardio‐oncology, Cardiotoxicity, Meta‐analysis
Introduction
Cancer survivors often suffer from chemotherapy‐related heart failure, which limits their quality of life and increases mortality.1 Modern cancer therapy includes surgery, radiation, classical chemotherapy, targeted, and immune therapy. Heart failure represents the most common cardiovascular adverse event from cancer therapy.2 Both classical and novel cancer therapies contribute to the development of heart failure. Anthracyclines, the prototypic classical chemotherapy, continue to be a mainstay of anti‐cancer regimes but frequently induce heart failure.3 Cardiotoxic effects are dose dependent but may also occur at lower cumulative doses. In breast cancer patients, anthracycline administration is often followed by trastuzumab (HER2 receptor antagonist), which further increases the risk for heart failure.3 Several national and international cardio‐oncology organizations have forwarded recommendations for the surveillance of breast cancer patients before, during, and after therapy using imaging and biomarkers. For patients with signs of heart failure, the initiation of cardioprotective drug therapy has been recommended.4, 5, 6, 7 Evidence‐based guidelines are, however, missing.
The current American and European cardio‐oncology position papers refer to heart failure guidelines for treatment, which recommend first‐line use of beta‐blockers and angiotensin‐converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs). Several studies have tried to assess whether such therapy can prevent the development of heart failure when applied before the beginning of cancer therapy. There is currently little evidence, largely because of heterogeneity relating to the choice of drugs, study cohorts, and cancer entities. We therefore performed a meta‐analysis of randomized controlled trials comparing either beta‐blockers or ACE inhibitors/ARBs to control for heart failure prevention in patients receiving anthracycline chemotherapy.
Methods
The study was registered at PROSPERO (CRD42018082735). The analysis was performed as previously described,8, 9, 10 complying with the ‘Cochrane Handbook for Systematic Reviews of Interventions’,11 and the ‘Preferred Reporting of Items for Systematic Meta‐Analysis (PRISMA)’ reporting guidelines.12
A systematic search was conducted through Pubmed, Cochrane, EMBASE, and Web of Science databases, the major cardiology websites (www.tctmd.com, www.clinicaltrialresult.com, www.medscape.com, and www.cardiosource.com), and the abstracts or presentations from annual meetings of the major cardiovascular and cancer societies to identify relevant studies published until February 2019. The search was specific and sensitive using Medical Subject Headings terms and free text and considered studies published in English. The meta‐analysis included randomized studies that reported on left ventricular ejection fraction (LVEF) after 6 months of chemotherapy in cancer patients who received cardioprotective therapies compared with controls without such cardioprotective therapy. Trials with n < 15 patients were excluded. Studies on combination cardioprotective therapies, studies assessing cardioprotection in patients with established cardiotoxicity or 2 × 2 factorial design were excluded from the analysis. Only studies with assessment of LV function through transthoracic echocardiography and magnetic resonance imaging were included. The primary endpoint was prevention of a decrease in LVEF as defined by the individual study.
Two reviewers independently performed the search (M. T. and R. M.). A consensus was negotiated in case of disagreement (T. R.). The following data were collected: year of publication, type of prevention strategy, data on LVEF, and available reference values. For data synthesis, mean and standard deviation of LVEF were recorded. Heterogeneity between studies was tested using Q statistics, and inconsistencies were determined using the I 2 statistical test. We considered the presence of significant heterogeneity at 10% level of significance. A value of I 2 of 0–40% denotes that heterogeneity might not be important, 30–60% may represent moderate heterogeneity, 50–90% may represent substantial heterogeneity, and 75–100% may represent considerable heterogeneity.11
For statistical analysis, a random effects model was used. Data are expressed as relative risks and 95% confidence interval (CI) for dichotomous outcomes and mean difference for continuous variables. Descriptive statistics were performed using Revman 5.3 software (The Cochrane Collaboration). Funnel plot test (Egger's test) was used to evaluate publication bias.
Results
The systematic search as outlined in the Methods section revealed 183 potential trials. Review articles, experimental studies, and case reports were excluded. Thus, 14 studies were included into the final analysis. The majority of studies assessed patients receiving anthracycline therapy particularly using doxorubicin and epirubicin. The most frequent tumour disease was breast cancer followed by haematological malignancies. Eight eligible trials assessing beta‐blockers (carvedilol, nebivolol, bisoprolol, and metoprolol),13, 14, 15, 16, 17, 18, 19, 20 four studies assessing ACE inhibitors/ARBs (enalapril, telmisartan, candesartan, and perindopril),21, 22, 23, 24 and two studies assessing beta‐blockers and ACE inhibitors/ARBs in parallel arms25, 26 were identified (Table 1). The sample sizes in groups were rather low, ranging from n = 18 to n = 103. The beta‐blocker analysis included 769 cancer patients, and the ACE inhibitors/ARBs analysis included a total of 581 cancer patients. Two randomized controlled trials assessing spironolactone or combination strategies were excluded from the meta‐analysis but included in Table 1.27, 28 The LVEF difference was assessed after 6 months of chemotherapy.
Table 1.
Randomized controlled trials assessing cardioprotective regimes
| Study | Year of publication | Study design | Cancer type | Chemotherapy type | Study drug | Sample size | Median age | % women | Mean LVEF difference (%) |
|---|---|---|---|---|---|---|---|---|---|
| Cardinale21 | 2006 | RCT | Various | Various | Enalapril | 56 | 47 | 60 | 9.7 |
| Control | 58 | 44 | 67 | ||||||
| Kalay13 | 2006 | RCT | Various | Anthracycline | Carvedilol | 25 | 46 | 88 | 17.4 |
| Placebo | 25 | 49 | 84 | ||||||
| Georgakopoulos25 | 2010 | RCT | Lymphoma | Doxorubicin | Metoprolol | 42 | 51 | 48 | 3.3 |
| Enalapril | 43 | 47 | 49 | 2.8 | |||||
| Control | 40 | 49 | 47 | ||||||
| Kaya14 | 2012 | RCT | Breast cancer | Anthracycline | Nebivolol | 27 | 51 | 100 | 6.3 |
| Control | 18 | 50 | 100 | ||||||
| Dessi22 | 2013 | RCT | Various | Epirubicin | Telmisartan | 25 | 52 | 76 | 4 |
| Placebo | 24 | 53 | 75 | ||||||
| Bosch27 | 2013 | RCT | Haematological malignancies | Anthracycline and various | Enalapril and Carvedilol | 45 | 49 | 40 | 2.2 |
| Control | 45 | 50 | 47 | ||||||
| Elitok15 | 2014 | RCT | Breast cancer | Anthracycline | Carvedilol | 40 | 54 | 100 | 0.8 |
| Placebo | 40 | 52 | 100 | ||||||
| Akpek29 | 2015 | RCT | Breast cancer | Anthracycline and various | Spironolactone | 43 | 50 | 100 | 12.1 |
| Placebo | 40 | 50 | 100 | ||||||
| Boekhout23 | 2016 | RCT | Breast cancer | Anthracycline and Trastuzumab | Candesartan | 103 | 50 | 100 | 1 |
| Placebo | 103 | 50 | 100 | ||||||
| Tashakori Beheshti17 | 2016 | RCT | Breast cancer | Doxorubicin | Carvedilol | 30 | 42 | 100 | 1.76 |
| Placebo | 40 | 39 | 100 | ||||||
| Jhorawat16 | 2016 | RCT | Lymphoreticular malignancy | Adriamycin | Carvedilol | 27 | 43 | 14.8 | 3 |
| Placebo | 27 | 38 | 33.3 | ||||||
| Pituskin26 | 2017 | RCT | Breast cancer | Trastuzumab adjuvant | Perindopril | 33 | 53 | 100 | 3 |
| Bisoprolol | 31 | 50 | 100 | 5 | |||||
| Placebo | 30 | 51 | 100 | ||||||
| Janbabai24 | 2017 | RCT | Various | Anthracycline and various | Enalapril | 34 | 47 | 97 | 13.6 |
| Placebo | 35 | 47 | 88 | ||||||
| Abuosa18 | 2018 | RCT | Various | Doxorubicin | Carvedilol | 37 | 42 | 77 | 1 |
| Placebo | 38 | 40 | 76 | ||||||
| Avila19 | 2018 | RCT | Breast cancer | Anthracycline | Carvedilol | 96 | 50 | 100 | 0 |
| Placebo | 52 | 52 | 100 | ||||||
| Cochera20 | 2018 | RCT | Breast cancer | Doxorubicin | Nebivolol | 30 | 53 | 100 | 1 |
| Placebo | 30 | 52 | 100 |
LVEF, left ventricular ejection fraction; RCT, randomized controlled trial.
The results are very heterogeneous, and the improvement of LVEF by beta‐blockade and ACE inhibition/ARBs was rather small. The mean LVEF difference between the beta‐blocker group and the control group was 2.57%, 95% CI (0.63–4.51), P = 0.009. The analysis included 10 studies13, 14, 15, 16, 17, 18, 19, 20, 25, 26 with 385 patients in the beta‐blocker group and 384 control patients. The heterogeneity was considerable between the selected studies, as shown by an I 2 value of 86%, with P < 0.001 (Figure 1 ). The risk of bias was low, according to the funnel plot test (Supporting Information, Figure S1 ). Of note, the greatest beneficial effect was 17.4%,13 while several other studies including some of the larger ones reported no detectable effects, for example, the trial by Avila et al.19 in breast cancer patients.
Figure 1.
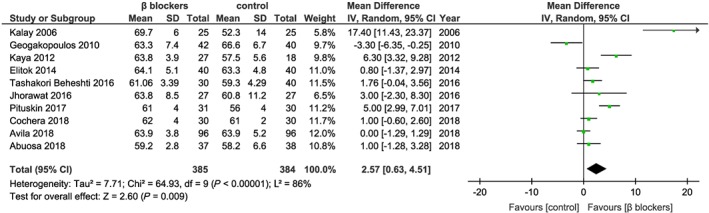
Studies for beta‐blockers in the prevention heart failure from anthracycline chemotherapy. The weight of each study is indicated as a percentage. Square boxes denote the risk ratio (RR), horizontal lines represent 95% confidence intervals (CIs), and the diamond plot represents the overall results of the included trials. SD, standard deviation.
The patients treated with ACE inhibitors/ARBs had a higher LVEF than controls after 6 months of chemotherapy, with mean difference = 4.71%, 95% CI (0.38–9.03), P = 0.03. Data are plotted from six studies21, 22, 23, 24, 25, 26 with 291 patients in the ACE inhibitors/ARBs group and 290 patients in the control group. The heterogeneity between the selected studies was considerable (I 2 = 94%, P < 0.001) (Figure 2 ). The risk of bias could not be assessed by the funnel plot test because the analysis included <10 studies. Of note, in the only study using spironolactone (n = 43 cancer patients with spironolactone vs. n = 40 cancer patients receiving control), a remarkable difference of 12.1% in favour of spironolactone in a cohort of breast cancer patients has been reported.29
Figure 2.
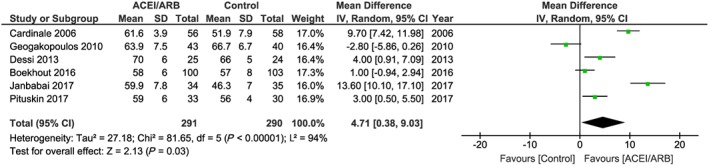
Studies for ACE inhibitors/ARBs in the prevention heart failure from anthracycline chemotherapy. The weight of each study is indicated as a percentage. Square boxes denote the risk ratio (RR), horizontal lines represent 95% confidence intervals (CIs), and the diamond plot represents the overall results of the included trials. ACEI, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers.
Discussion
Heart failure remains the most significant burden for patients receiving cancer therapy. Development of heart failure may occur during therapy or years after completion. Surveillance of patients at risk and feasible preventive therapies for younger patients (e.g. breast cancer patients or children and adolescents with haematological malignancies) remain incompletely studied. Evidence for guideline recommendations is currently not available. Based on the available data, heart failure therapy using beta‐blockers or ACE inhibitors/ABRs is associated with a moderate benefit in LVEF when applied before the beginning of cancer therapy in selected cohorts. Naturally, patients must be counselled regarding the adverse effects that come along with these pharmaceutical drugs.
This meta‐analysis has limitations. The patient cohorts were small across all studies, and individual patient data were not available. The studies only related to anthracycline‐induced heart failure and did not consider the novel, more powerful chemotherapies. Therefore, results cannot easily be extrapolated to novel targeted and immune therapies, which also largely contribute to cancer therapy‐associated heart failure development.1, 8, 30 The present studies differ with respect to the specific drug, doses, timing (notably with reference to the timing of chemotherapy), and duration of use. Protective effects seen, for example, with one specific beta‐blocker might not be readily detectable within the entire drug group (e.g. interaction of nebivolol with nitric oxide pathway). Interestingly, some studies included lower doses of anthracycline regimes, which may not have induced heart failure at significant rates.15 Heart failure is more likely with expected cumulative doses of doxorubicin (≥250 mg/m2 body surface area) or epirubicin (>600 mg/m2).1 Given the strong association with cardiac dysfunction in these patients, the efficacy of preventive therapies, the timing of drug application, and surveillance need to be evaluated in future trials. It remains furthermore unclear whether beneficial effects of preventive measures as seen in the analysed studies remain detectable once cancer therapy is terminated and beta‐blockers and/or ACE inhibitors/ARBs halted. This would clarify the question whether beta‐blockers and/or ACE inhibitors/ARBs per se improved cardiac function or prevented cardiotoxicity directly.
Left ventricular ejection fraction by echocardiography or magnetic resonance tomography is a surrogate endpoint, and endpoints referring to clinical symptoms, quality of life, and mortality were inconsistent. Recent evidence implicates that imaging by global longitudinal strain from speckle tracking or biomarker assessment may detect myocardial damage at much earlier stages than conventional assessment of ejection fraction by echocardiography or magnetic resonance imaging.31 These clinical parameters should be further evaluated with respect to the efficacy of preventive therapy. Combined approaches using imaging in conjunction with biomarkers may be superior to identify patients at risk or with subclinical cardiotoxicity.32
Data on combination therapy by both beta‐blockade and ACE inhibition/ARBs are scarce. Combination therapy using both agents was performed in the PRADA (Prevention of Cardiac Dysfunction during Adjuvant Breast Cancer Therapy) trial using a 2 × 2 factorial design to test the efficacy of the ARB candesartan and the beta‐blocker metoprolol. The LVEF reduction in all groups was moderate. Data could not be extracted for meta‐analysis, but overall, only in the candesartan arm was the LVEF reduction somewhat attenuated.28 The OVERCOME trial (Prevention of Left Ventricular Dysfunction with Enalapril and Carvedilol in Patients Submitted to Intensive Chemotherapy for Malignant Hemopathies) in patients with leukaemia also revealed a small benefit from combination therapy.27
In canonical heart failure, beta‐blockers are used to block sympathetic activity, reduce heart rate, and optimize excitation–contraction coupling. ACE inhibitors/ARBs are used to reduce blood pressure and attenuate cardiac remodelling. The pathophysiological mechanisms underlying anthracycline toxicity are complex and different from those in canonical heart failure5 including topoisomerase IIβ inhibition, formation of reactive oxygen species, impairment of endothelial nitric oxide synthase, and mitochondrial dysfunction.1 Future mechanistic studies must address these pathomechanisms and see whether or not beta‐blockers and ACE inhibitors/ARBs can target them. Such studies could also address the potential cardioprotection by dexrazoxane or blockade of mineralocorticoid receptors (e.g. spironolactone).
In conclusion, ACE inhibitors and beta‐blockers provide minimal preservation of LVEF. It is feasible to start these protective therapies in patients at risk scheduled for high dose classical chemotherapies particularly with pre‐existing cardiovascular risk factors. Development of adequate approaches for the prevention of chemotherapy‐related heart failure not only in terms of preservation of LVEF but even more in quality of life and survival is the eminent goal for cardio‐oncological research. This need is even greater with the advent of novel, more powerful chemotherapies.
Conflict of interest
The authors declare no conflict of interest.
Funding
No relevant funding.
Supporting information
Figure S1. Risk of bias in the beta‐blockers meta‐analysis. Studies with high precision are plotted near the average, and studies with low precision are spread evenly on both sides of the average, creating a roughly funnel‐shaped distribution, which indicates a non‐ significant publication bias (Egger's test).
Totzeck M., Mincu R. I., Heusch G., and Rassaf T. (2019) Heart failure from cancer therapy: can we prevent it?, ESC Heart Failure, 6, 856–862. 10.1002/ehf2.12493.
References
- 1. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio‐oncology—strategies for management of cancer‐therapy related cardiovascular disease. Int J Cardiol 2019; 280: 163–175. [DOI] [PubMed] [Google Scholar]
- 2. Snipelisky D, Park JY, Lerman A, Mulvagh S, Lin G, Pereira N, Rodriguez‐Porcel M, Villarraga HR, Herrmann J. How to develop a cardio‐oncology clinic. Heart Fail Clin 2017; 13: 347–359. [DOI] [PubMed] [Google Scholar]
- 3. Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline‐based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017; 18: e445–e456. [DOI] [PubMed] [Google Scholar]
- 4. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, Pina IL, Volgman AS, American Heart Association Cardiovascular Disease in W, Special Populations Committee of the Council on Clinical C, Council on C, Stroke N, Council on Quality of C, Outcomes R . Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 2018; 137: e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol 2017; 70: 2536–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Authors/Task Force M . ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016 2016; 37: 2768–2801. [DOI] [PubMed] [Google Scholar]
- 7. Barish R, Lynce F, Unger K, Barac A. Management of cardiovascular disease in women with breast cancer. Circulation 2019; 139: 1110–1120. [DOI] [PubMed] [Google Scholar]
- 8. Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti‐vascular endothelial growth factor activity: a meta‐analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol 2018; 25: 482–494. [DOI] [PubMed] [Google Scholar]
- 9. Mincu RI, Janosi RA, Vinereanu D, Rassaf T, Totzeck M. Preprocedural C‐reactive protein predicts outcomes after primary percutaneous coronary intervention in patients with ST‐elevation myocardial infarction a systematic meta‐analysis. Sci Rep 2017; 7: 41530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta‐analysis of more than 20 000 patients. J Am Heart Assoc 2017; 6: pii: e006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].http://handbook.cochrane.org (01 2018).
- 12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline‐induced cardiomyopathy. J Am Coll Cardiol 2006; 48: 2258–2262. [DOI] [PubMed] [Google Scholar]
- 14. Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, Berk V, Ardic I, Ergin A, Lam YY. Protective effects of nebivolol against anthracycline‐induced cardiomyopathy: a randomized control study. Int J Cardiol 2013; 167: 2306–2310. [DOI] [PubMed] [Google Scholar]
- 15. Elitok A, Oz F, Cizgici AY, Kilic L, Ciftci R, Sen F, Bugra Z, Mercanoglu F, Oncul A, Oflaz H. Effect of carvedilol on silent anthracycline‐induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six‐month follow‐up. Cardiol J 2014; 21: 509–515. [DOI] [PubMed] [Google Scholar]
- 16. Jhorawat R, Kumari S, Varma SC, Rohit MK, Narula N, Suri V, Malhotra P, Jain S. Preventive role of carvedilol in adriamycin‐induced cardiomyopathy. Indian J Med Res 2016; 144: 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tashakori Beheshti A, Mostafavi Toroghi H, Hosseini G, Zarifian A, Homaei Shandiz F, Fazlinezhad A. Carvedilol administration can prevent doxorubicin‐induced cardiotoxicity: a double‐blind randomized trial. Cardiology 2016; 134: 47–53. [DOI] [PubMed] [Google Scholar]
- 18. Abuosa AM, Elshiekh AH, Qureshi K, Abrar MB, Kholeif MA, Kinsara AJ, Andejani A, Ahmed AH, Cleland JGF. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J 2018; 70: S96–s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Goncalves Brandao SM, Rigaud VOC, Higuchi‐Dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for prevention of chemotherapy‐related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018; 71: 2281–2290. [DOI] [PubMed] [Google Scholar]
- 20. Cochera F, Dinca D, Bordejevic DA, Citu IM, Mavrea AM, Andor M, Trofenciuc M, Tomescu MC. Nebivolol effect on doxorubicin‐induced cardiotoxicity in breast cancer. Cancer Manag Res 2018; 10: 2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006; 114: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 22. Dessi M, Madeddu C, Piras A, Cadeddu C, Antoni G, Mercuro G, Mantovani G. Long‐term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin‐induced inflammation and oxidative stress assessed by serial strain rate. SpringerPlus 2013; 2: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, Los M, Smit WM, Nieboer P, Smorenburg CH, Mandigers CM, van der Wouw AJ, Kessels L, van der Velden AW, Ottevanger PB, Smilde T, de Boer J, van Veldhuisen DJ, Kema IP, de Vries EG, Schellens JH. Angiotensin II‐receptor inhibition with candesartan to prevent trastuzumab‐related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol 2016; 2: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 24. Janbabai G, Nabati M, Faghihinia M, Azizi S, Borhani S, Yazdani J. Effect of enalapril on preventing anthracycline‐induced cardiomyopathy. Cardiovasc Toxicol 2017; 17: 130–139. [DOI] [PubMed] [Google Scholar]
- 25. Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T, Galanopoulos A, Georgiakodis F, Zimeras S, Kyriakidis M, Ahimastos A. Cardioprotective effect of metoprolol and enalapril in doxorubicin‐treated lymphoma patients: a prospective, parallel‐group, randomized, controlled study with 36‐month follow‐up. Am J Hematol 2010; 85: 894–896. [DOI] [PubMed] [Google Scholar]
- 26. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary approach to novel therapies in cardio‐oncology research (MANTICORE 101‐Breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol 2017; 35: 870–877. [DOI] [PubMed] [Google Scholar]
- 27. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz‐Perez JT, de Caralt TM, Morales‐Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013; 61: 2355–2362. [DOI] [PubMed] [Google Scholar]
- 28. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storas TH, Hagve TA, Rosjo H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J 2016; 37: 1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akpek M, Ozdogru I, Sahin O, Inanc M, Dogan A, Yazici C, Berk V, Karaca H, Kalay N, Oguzhan A, Ergin A. Protective effects of spironolactone against anthracycline‐induced cardiomyopathy. Eur J Heart Fail 2015; 17: 81–89. [DOI] [PubMed] [Google Scholar]
- 30. Rassaf T, Totzeck M. Modern concepts in cardio‐oncology. J Thoracic Dis 2018; 10: S4386–S4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014; 63(25 Pt A): 2751–2768. [DOI] [PubMed] [Google Scholar]
- 32. Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM, Investigators I‐OS. Anthracycline‐induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society‐one trial. Eur J Cancer 2018; 94: 126–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Risk of bias in the beta‐blockers meta‐analysis. Studies with high precision are plotted near the average, and studies with low precision are spread evenly on both sides of the average, creating a roughly funnel‐shaped distribution, which indicates a non‐ significant publication bias (Egger's test).


