Abstract
Aims
Left atrial (LA) remodelling is an important predictor of cardiovascular events of heart failure (HF) and atrial fibrillation. Data regarding diagnostic value of LA remodelling on diastolic dysfunction (DD) and preclinical HF remain largely unexplored.
Methods and results
We assessed LA dimension (LAD) in 8368 consecutive asymptomatic Asians (mean age: 49.7, 38.9% women) and related such measure to updated American Society of Echocardiography (ASE) DD criteria and newly revised N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) cut‐off (≥125 pg/mL) and HF with preserved ejection fraction criteria incorporating NT‐proBNP and echocardiography parameters by the European Society of Cardiology (ESC). LAD and indexed LAD (LADi) were both inversely correlated with myocardial relaxation e′ and positively associated with indexed LA volume, left ventricular E/e′, and tricuspid regurgitation velocity (all P < 0.001) and showed significantly graded increase across ASE‐defined ‘normal’, ‘inconclusive’, and ‘DD’ categories (30.9, 34.4, and 36.5 mm; 16.7, 19.1, and 20.6 mm/m2, for LAD/LADi, both P for trend: <0.001, respectively). Substantial differences of LAD/LADi (31.3 vs. 33.6 mm/16.7 vs. 19.2 mm/m2, both P < 0.001) between ESC low and high HF probability using NT‐proBNP cut‐off were also observed. Multivariate linear and logistic models demonstrated that LAD set at 34 mm was independently associated with ASE‐defined diastolic indices, DD existence, and elevated NT‐proBNP (all P < 0.05). The use of LAD further yielded high diagnostic accuracy in DD (area under receiving operative characteristic curve: 0.77, 95% confidence interval [0.73, 0.80]; negative predictive value: 97.9%) and in ESC‐recommended HF with preserved ejection fraction criteria (area under receiving operative characteristic curve: 0.70, 95% confidence interval [0.65, 0.75]; negative predictive value: 98.7%) with high predictive value in LA remodelling (>34 mL/m2; positive predictive value: 96%) and well‐discriminated ESC‐recommended NT‐proBNP (≥125 pg/mL, LAD: 37 mm) for HF.
Conclusions
Single utilization of atrial remodelling is highly useful for ruling out presence of DD and provides practical threshold for identifying preclinical HF based on most updated guidelines.
Keywords: Left atrium, ESC, Diastolic dysfunction, Heart failure, Atrial fibrillation, Echocardiography
Introduction
The left atrium (LA) serves as a sensitive marker of clinical and subclinical cardiovascular disease.1 Left atrial (LA) size is increased in a variety of cardiovascular disorders and is an indicator of chronically increased LA afterload and/or volume, especially in heart failure (HF).2, 3, 4, 5 Increased LA size has also been implicated in atrial fibrillation (AF)6, 7 and may be an indicator of early structural remodelling in senescence or in subjects with multiple clinical co‐morbid conditions including obesity8 or hypertension.9 LA size also appears to be a useful predictor of several cardiovascular outcomes.10, 11
Left atrium as a barometer may adapt to long‐standing diastolic dysfunction (DD) or HF owing to chronically elevated left ventricular (LV) filling pressure, even with preserved ventricular function.12, 13, 14 Therefore, accurate assessment of LA size or remodelling remains the cornerstone in recognizing DD as a clinical precursor for HF and forms the essential diagnostic key component for HF with preserved ejection fraction (HFpEF).15, 16 Because a standardized definition and index parameter for DD or clinical high‐risk population of HF remains obscure, a more recent update of DD scoring system issued by the American Society of Echocardiography (ASE) recommended the use of a cluster of quantitative diastolic measures for objective definition.17 Recently, the European Society of Cardiology (ESC)16 also provided relevant clinical N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) cut‐off, which predicts high probability for HF. However, the association of such measures with LA remodelling in a large asymptomatic population remains largely unexplored. This study therefore aimed to characterize LA remodelling and explore its association with DD or NT‐proBNP threshold for HF based on guideline‐recommended cut‐offs in an asymptomatic Asian population.
Methods
Study subjects
This cross‐sectional study analysed data of subjects who underwent annual cardiovascular evaluations at the MacKay Memorial Hospital (a tertiary medical centre in Northern Taiwan) between July 2003 and December 2012. For study participants who had multiple visits, only the visits for which echocardiogram data were available were included. Participants with missing key baseline variables and participants who did not have electrocardiography data for LA size were also excluded. Additional exclusion criteria were presence of an implanted pacemaker, pulmonary hypertension (defined as peak systolic pulmonary artery pressure ≥60 mmHg), impaired LV systolic function (LV ejection fraction <50%), hypertrophic cardiomyopathy, AF, thyroid dysfunction, significant primary valvular heart diseases (aortic or mitral valves) or history of valvular surgery, prior HF history or current symptoms of HF, or ongoing chest pain. Among totally 11 376 visits with echocardiography data available, we identified 8368 subjects with sufficient baseline information after exclusion criteria with part of this population published elsewhere.13 This study was approved by the local ethics committee in accordance with the Declaration of Helsinki in our hospital (MacKay Memorial Hospital), and patient information was de‐identified prior to analysis.
Conventional echocardiography and left atrial dimension determination
Echocardiography was performed as described previously,13 using the Philips Hewlett‐Packard (HP) Sonos 5500 ultrasound or the GE system (Vividi, Vingmed, Horten, Norway) equipped with the 2–4 MHz transducer. For both echocardiography systems, a standard imaging protocol was performed where M‐mode, linear‐based measures of LA dimension (LAD), LV end‐diastolic/systolic diameter, wall thickness, and LV mass (and indexed to body surface area) were all obtained and calculated using the ASE criteria.18 Further, LV volumes (both end‐diastolic and end‐systolic), LA volumes (LAVs) at maximum point, and derived LV ejection fraction were all determined by the modified biplane Simpson method using two‐dimensional (2D) echocardiography (from LV apical four‐chamber and two‐chamber views). Indexed LAV (LAVi) and LAD/LADi were calculated by dividing LAV and LAD by body surface area. All M‐mode images were acquired and recorded at a speed of 60–100 mm/s with the transducer placed at the third to the fifth inter‐costal space. All echocardiography loops were obtained for at least three beats for 2D‐based images and for at least 5–8 beats for M‐mode‐based measures. Image acquisition was performed with study participants in a left lateral decubitus position. Study participants were requested to hold their breath for 5–10 s if the image quality was suboptimal.
Assessment of diastolic functional indices and criteria for diastolic dysfunction
Diastolic assessment including transmitral inflow pulsed‐wave Doppler of LV early (E) and late diastolic (A) velocities were measured from the tip of the mitral leaflets of the LV apical four‐chamber view. Deceleration time (DT) and isovolumic relaxation time were also determined from same LV apical views, with minor adjustment and sliding of echo beam angle for continuous wave‐based Doppler isovolumic relaxation time acquisition from LV apical five‐chamber view. Peak tricuspid regurgitation (TR) systolic jet velocity was obtained during systole at the leading edge of spectral waveform from the four‐chamber view, with angle‐adjusted alignment of continuous wave Doppler echo beam. This also made it possible to assess the difference of pressure between the right ventricle and the right atrium. The simplified Bernoulli equation (P = 4 × [TR max]2) was used to quantify pressure differences using peak TR velocity, because this has been well validated with pulmonary artery systolic pressure utilizing the invasive right heart catheterization method.19
High frame rate myocardial velocity tissue Doppler imaging technique was used to determine early myocardial relaxation velocity (e′, both septal and lateral mitral annular areas were evaluated). LV filling pressure was estimated by dividing mitral inflow (E) by early myocardial relaxation velocity (e′). We further categorized diastolic function based on four variables recommended by the ASE17: (i) mitral annular velocity e′: septal e′ < 7 cm/s or lateral e′ < 10 cm/s; (ii) average E/e′ ratio > 14; (iii) LAV index >34 mL/m2; and (iv) peak TR velocity >2.8 m/s. Similarly, enlarged LAV was also part of HFpEF diagnostic components by more recent ESC criteria.16 We further developed ASE DD scores by calculating the summation of any available ‘positive’ diastolic index based on ASE in any individual (Figure 1 ), which reflected total numbers of abnormal diastolic parameters that met ASE cut‐off values. LV diastolic function was classified as ‘normal’ if more than half of the available diastolic indices did not meet the cut‐off values of ASE‐defined abnormal criteria, was defined as ‘DD’ if more than half of the variable parameters met the abnormal cut‐off values, and was classified as ‘inconclusive’ if half of the parameters did not meet the cut‐off values (Figure 1 ). Based on this, ASE DD scores were graded as 0, 1, 2, 3, or 4, respectively, for number of diastolic indices categorized as ‘abnormal’, with DD ratio defined as the ‘abnormal’ diastolic parameters divided by the total numbers of diastolic indices available. Finally, we further examined the diagnostic utilization of LAD/LADi measures for ESC‐proposed HFpEF criteria16 in current study, incorporating NT‐proBNP and three additional echocardiography parameters including indexed LAV (>34 mL/m2), sex‐specified LV mass index, and average E/e′ ratio and averaged e′ in study participants.
Figure 1.
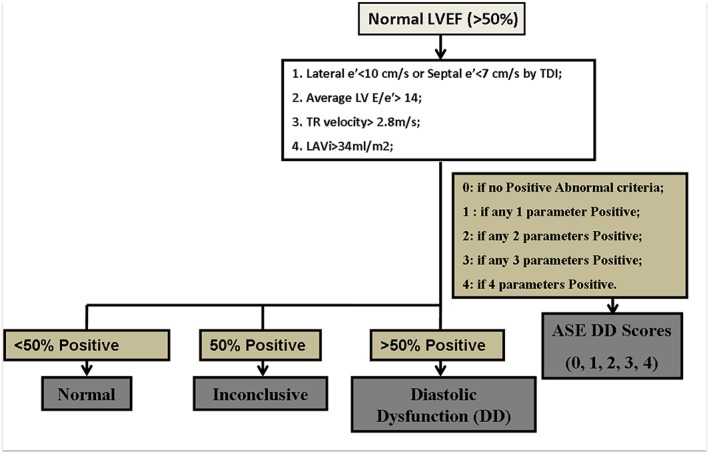
The classification and categorization of study participants and relevant diastolic dysfunction (DD) scoring developed based on American Society of Echocardiography (ASE)‐recommended criteria for diastolic functional measures. All participants had left ventricular ejection fraction (LVEF) > 50%. LAVi, left atrial volume index; LV, left ventricular; TDI, tissue Doppler imaging; TR, tricuspid regurgitation.
Determination of serum N‐terminal pro‐brain natriuretic peptide and renal function
N‐terminal pro‐brain natriuretic peptide (pg/mL) was determined using the electrochemiluminescence immunoassay (Roche E170, Roche Diagnostics) on samples collected by venipuncture. Renal function in terms of estimated glomerular filtration rate was assessed using the Modification of Diet in Renal Disease formula. Based on ESC clinical practice guideline criteria for high probability of HF, NT‐proBNP ≥ 125 pg/mL was categorized as high probability of clinical HF in our current study.
Statistical analysis
Continuous data were presented as mean and standard deviation. The independent two sample t‐test was performed to compare the continuous data between women and men (Supporting Information, Table S1 ). The one‐way ANOVA test with post hoc Bonferroni corrections was performed to compare the continuous data among any three categorization groups (e.g. as ‘normal’, ‘inconclusive’, and ‘DD’ in Table 1). Categorical data (e.g. gender, medical history, and co‐morbidity) were presented as numbers and percentages with the χ 2 test. The associations between LAD and LADi (which is clinically more practical without index) and all individual ASE‐defined four diastolic indices [including myocardial relaxation e′, LV E/e′, degree of LA enlargement (LAVi), and elevated TR velocity] were presented in univariate and multivariate linear regression models with age, gender, body size (body mass index), blood pressure, heart rate, fasting sugar level, lipid profiles including total cholesterol, high‐density lipoprotein, renal function in terms of estimated glomerular filtration rate, and baseline medical history of hypertension, diabetes mellitus, cardiovascular diseases, coronary artery diseases, and LV mass in a full model as confounders (Table 2). Paired comparisons of LAD/LADi in both genders were performed between the ‘normal’, ‘inconclusive’, and ‘DD’ groups by the ANOVA test (Supporting Information, Figure S2 ).
Table 1.
Baseline demographics, clinical characteristics, and diastolic indices of study participants stratified by ASE DD criteria
| ASE‐defined DD categorization | P (trend test/χ 2) | |||
|---|---|---|---|---|
| Normal (n = 7628) | Inconclusive (n = 521) | DD (n = 165) | ||
| Baseline characteristics | ||||
| Age, years | 48.8 (11.3) | 58.9 (11.8)* | 60.1 (10.9)* | <0.001 |
| Sex, female, % | 2886 (37.8) | 239 (45.9) | 96 (58.2) | <0.001 |
| BMI, kg/m2 | 24.2 (3.6) | 25.2 (3.7)* | 24.7 (3.8) | <0.001 |
| SBP, mmHg | 122.4 (17.3) | 131.7 (19.3)* | 133.7 (20.7)* | <0.001 |
| Heart rate, b.p.m. | 74.2 (10.3) | 73.6 (10.5) | 73.4 (10.6) | <0.001 |
| Medical history/co‐morbidity | ||||
| HTN, n (%) | 1360 (17.8) | 200 (38.4) | 62 (38.0) | <0.001 |
| Hyperlipidaemia, n (%) | 495 (6.5) | 59 (11.3) | 20 (12.1) | <0.001 |
| CVD, n (%) | 559 (7.3) | 94 (18.0) | 27 (16.4) | <0.001 |
| CAD, n (%) | 402 (5.3) | 70 (13.4) | 23 (13.9) | <0.001 |
| DM, n (%) | 473 (6.2) | 69 (13.2) | 24 (14.6) | <0.001 |
| Diastolic and left atrial indices | ||||
| PR interval, ms | 164.3 (20.6) | 169.9 (25.4)* | 171.6 (26.1)* | <0.001 |
| LAD, mm | 30.9 (4.6) | 34.4 (5.8)* | 36.5 (5.0)* | <0.001 |
| LADi, mm/m2 | 16.7 (2.5) | 19.1 (3.3)* | 20.6 (3.2)* | <0.001 |
| LAV (max), mL | 29.3 (10.4) | 36.1 (15.0)* | 40.9 (16.2)* , ** | <0.001 |
| LAVi, mL/m2 | 15.6 (5.1) | 20.1 (8.0)* | 22.7 (9.0)* , ** | <0.001 |
| TDI e′ (average), cm/s | 9.4 (2.3) | 6.7 (1.7)* | 6.4 (1.3)* | <0.001 |
| TDI E/e′ (mean) | 7.5 (2.0) | 11.7 (3.7)* | 13.8 (3.4)* , ** | <0.001 |
| TR velocity | 2.1 (0.3) | 2.4 (0.5)* | 2.8 (0.4)* , ** | <0.001 |
| No. of ASE DD scores | 0.34 (0.47) | 1.7 (0.5)* | 2.2 (1.1)* , ** | <0.001 |
| NT‐proBNP, pg/mL (IQR: 25th–75th) | 39.1 (12.5–50.3) | 108.9 (30.3–119)* | 160.5 (33.4–145.8)* , ** | <0.001 |
ASE, American Society of Echocardiography; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; DD, diastolic dysfunction; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; LAD, left atrial diameter; LADi, left atrial diameter index; LAV, left atrial volume; LAVi, left atrial volume index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SBP, systolic blood pressure; TDI, tissue Doppler imaging; TR, tricuspid regurgitation.
ANOVA P < 0.05 vs. normal group.
ANOVA P < 0.05 vs. inconclusive group.
Table 2.
The associations of LAD with ASE‐recommended diastolic indices or DD and ESC NT‐proBNP cut‐offs for HF
| All | Women | Men | ||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P‐value | Coefficient (95% CI) | P‐value | Coefficient (95% CI) | P‐value | |
| Linear regression models | ||||||
| Unadjusted univariate models (per 10 units + of LAD) | ||||||
| NT‐proBNP level (pg/mL) | 20.4 (15.6, 25.3) | <0.001 | 49.5 (40.0, 59.0) | <0.001 | 16.5 (11.0, 22.0) | <0.001 |
| LA volume (max) (mL) | 10.47 (9.95, 10.98) | <0.001 | 9.98 (9.20, 10.75) | <0.001 | 10.40 (9.70, 11.11) | <0.001 |
| LAVi (also ESC HFpEF16) (mL/m2) | 4.14 (3.86, 4.41) | <0.001 | 4.88 (4.43, 5.34) | <0.001 | 4.37 (4.01, 4.72) | <0.001 |
| Adjusted multivariate models (per 10 units + of LAD) | ||||||
| NT‐proBNP level (pg/mL) | 20.9 (14.6, 27.1) | <0.001 | 49.8 (37.3, 62.4) | <0.001 | 6.1 (−0.61, 12.8) | 0.075 |
| LAVi (also ESC HFpEF16) (mL/m2)a | 3.3 (3.0, 3.6) | <0.001 | 3.2 (2.6, 3.8) | <0.001 | 3.3 (2.9, 3.7) | <0.001 |
| TDI e′ (averaged) (cm/s) | −0.18 (−0.32, −0.03) | 0.016 | −0.4 (0.6, −0.1) | 0.004 | −0.1 (−0.3 0.08) | 0.28 |
| TDI E/e′ (mean) | 0.56 (0.39, 0.74) | <0.001 | 1.1 (0.7, 1.4) | <0.001 | 0.33 (0.13, 0.54) | 0.002 |
| TR velocity | 2.3 (2.0, 2.6) | <0.001 | 2.7 (2.1, 3.3) | <0.001 | 2.0 (1.6, 2.4) | <0.001 |
| OR | ||||||
| NT‐proBNP Abn (>125 pg/mL) | 2.0 (1.47, 2.77) | <0.001 | 2.10 (1.39, 3.17) | <0.001 | 2.10 (1.27, 3.42) | 0.004 |
| LAVi Abn (also ESC HFpEF16)a | 1.16 (1.11, 1.21) | <0.001 | 1.15 (1.08, 1.3) | <0.001 | 1.17 (1.10, 1.24) | <0.001 |
| TDI e′ (both) Abn | 1.02 (1.003, 1.04) | 0.025 | 1.05 (1.01, 1.09) | 0.02 | 1.02 (0.99, 1.04) | 0.21 |
| TDI E/e′ Abn | 1.11 (1.07, 1.16) | <0.001 | 1.10 (1.05, 1.17) | <0.001 | 1.12 (1.07, 1.19) | <0.001 |
| TR velocity Abn | 1.23 (1.19, 1.27) | <0.001 | 1.21 (1.16, 1.27) | <0.001 | 1.25 (1.20, 1.31) | <0.001 |
| NT‐proBNP | 2.08 (1.45, 2.71) | <0.001 | 4.98 (3.72, 6.23) | <0.001 | 0.61 (−0.06, 1.28) | 0.075 |
| DD Abn by ASE | 1.31 (1.25, 1.37) | <0.001 | 1.30 (1.22, 1.39) | <0.001 | 1.32 (1.23, 1.42) | <0.001 |
| NT‐proBNP ≥ 125 pg/mL | 1.07 (1.04, 1.11) | <0.001 | 1.08 (1.03, 1.12) | <0.001 | 1.08 (1.02, 1.13) | 0.004 |
Abn, abnormal; ASE, American Society of Echocardiography; CI, confidence interval; DD, diastolic dysfunction; ESC, European Society of Cardiology; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LAD, left atrial dimension; LA, left atrial; LAVi, left atrial volume index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; OR, odds ratio; TDI, tissue Doppler imaging; TR, tricuspid regurgitation.
For all study participants, models were adjusted for age, gender, body mass index, systolic blood pressure, heart rate, fasting sugar, total cholesterol, high‐density lipoprotein, estimated glomerular filtration rate, hypertension, diabetes mellitus, cardiovascular disease, and left ventricular mass; for men or women, models were adjusted for age, body mass index, systolic blood pressure, heart rate, fasting sugar, total cholesterol, high‐density lipoprotein, estimated glomerular filtration rate, hypertension, diabetes mellitus, cardiovascular disease, and left ventricular mass.
In which model BMI was not included.
We also examined the clinical odds ratio (OR) of individual abnormal ASE‐defined diastolic indices or presence of DD by logistic regression models, with (adjusted OR) and without (crude OR) adjusting for the same confounders. Area under receiving operative characteristic (AUROC) curves and the corresponding 95% confidence intervals (CIs) were calculated for prediction of individual abnormal diastolic indices and presence of DD using LAD/LADi measures separately. For LAD, optimal cut‐off was chosen from the largest summation of sensitivity and specificity, with positive (PPV) and negative predictive values (NPV) also reported.
All statistical analyses were evaluated at a two‐sided significance level of 0.05 and were performed using IBM spss software, version 22 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics and echocardiography measures of study subjects
Among totally 8368 participants (mean age of 49.7 ± 11.7 years) met the inclusion/exclusion criteria for this study (Supporting Information, Table S1 ), 8314 had any given (1‐4) echocardiography parameters for DD grading. The final study population comprised 3221 women and 5093 men, respectively. Echocardiography demonstrated that men had significantly greater LAD (32.1 vs. 29.9 mm), LV wall thickness, and volumes and larger total LV mass (indexed to height2) compared with women (all P < 0.05), although significantly lower mitral inflow E/A ratio, smaller LADi (16.4 vs. 17.8 mm/m2)/LAVi, lower myocardial relaxation e′ and LV E/e′, slightly smaller TR jet velocity (Supporting Information, Table S1 ), and significantly lower prevalence of individual ASE‐defined abnormal diastolic components of LAVi, LV E/e′, and TR velocity in men compared with women counterpart (all P < 0.05) (Supporting Information, Table S2 ).
Patients in the ‘inconclusive’ and ‘DD’ were accompanied by more prolonged PR intervals, greater LAD (30.9, 34.4, and 36.5 mm)/LADi (16.7, 19.1, and 20.6 mm/m2, respectively), larger LAVs, markedly lower myocardial relaxation e′, higher LV E/e′, and higher TR velocities compared with ‘normal’ group (Table 1, all P < 0.001). In addition, patients in the ‘DD’ group showed higher number of individual abnormal ASE‐defined diastolic indices (2.2 vs. 1.7 and 0.34, respectively) and higher NT‐proBNP levels compared with patients in the ‘inconclusive’ and ‘normal’ group (Table 1, all P < 0.001).
The associations among left atrial dimension, guideline‐recommended diastolic dysfunction, and N‐terminal pro‐brain natriuretic peptide cut‐off for heart failure
Among 8314 subjects eligible after exclusion, 7628 subjects (91.7%) were categorized as ‘normal’, with 521 (6.3%) categorized as ‘inconclusive’ and 165 (2.0%) categorized as ‘DD’ using the ASE DD criteria (Table 1). LAD correlated positively with PR interval (r = 0.18), LADi (r = 0.73), LAVs and LAV index (r = 0.45 and 0.35), and NT‐proBNP level (r = 0.11, all P < 0.001). Each 10 mm LAD increase was associated with 10.5 mL and 4.14 mL/m2 expansion of LAV (without and with indexing to body surface area, respectively) (Table 2). LAD and LAV indices were all inversely associated with myocardial relaxation e′ and were positively correlated to higher LV E/e′, TR velocity, numbers of abnormal DD indices, and higher NT‐proBNP (Supporting Information, Table S3 , all P < 0.001). Increasing age was associated with greater LAD/LADi (r = 0.26 and 0.40, both P < 0.001, respectively), lower myocardial relaxation e′, higher LV E/e′ (r = −0.62 and 0.44, both P < 0.001), more elevated NT‐proBNP (r = 0.23, P < 0.001), and higher TR velocity (r = 0.29, P < 0.001), with higher risk of DD increased with higher risk of DD (unadjusted OR: 2.31, 95% CI [2.02, 2.65], P < 0.001, for 10 year increment). In general, LAD/LADi showed better correlations with individual ASE‐based diastolic index than LAV parameters. The consistent associations among LAD and ASE‐based diastolic indices, DD, and NT‐proBNP were further confirmed by linear/logistic regression models (with and without adjustment) (Table 2, all P < 0.001).
Higher DD scores, ratios, or grading were all related to markedly ordered increase in LAD/LADi (30.9, 34.4, and 36.5 mm/16.7, 19.1, and 20.6 mm/m2, for LAD/LADi across ‘normal’, ‘inconclusive’, and ‘DD’ groups, both P for trend: <0.001) for both genders (Supporting Information, Figure S1 A and B). Men in general showed significantly larger LAD than women (32.1 vs. 29.9 mm) (Supporting Information, Figure S2 ), although LADi appeared to be higher in women (17.8 vs. 16.4 mm, P < 0.001), especially with larger LAD (r = 0.85 and 0.81 for women and men, P interaction for sex: <0.001; Figure 2 A). Both LAD and LADi seemed to be significantly larger using the NT‐proBNP cut‐off for high HF probability (≥125 pg/mL) when compared with the low probability (<125 pg/mL) group (33.6 vs. 31.3 mm/19.2 vs. 16.7 mm/m2, for LAD and LADi, respectively, P < 0.001) regardless of gender differences (Supporting Information, Figures S1 C and D and S2 ).
Figure 2.
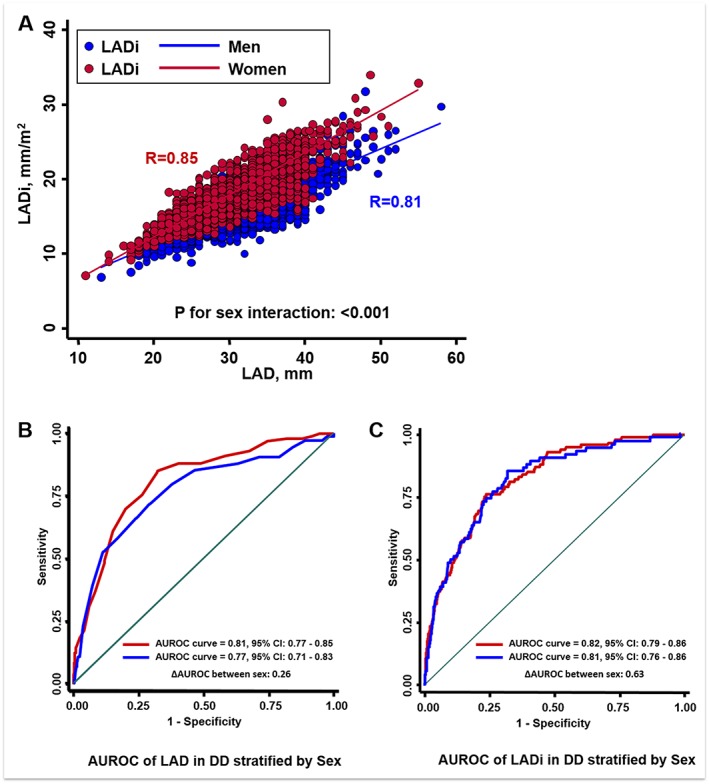
The linear relationships between left atrial dimension (LAD) (as X‐axis) and sex‐specified LAD indexed to body surface area (LADi) showed greater slope of LADi with larger LAD in women than men (A). The area under receiver operating characteristic (AUROC) curves of LAD/LADi for men and women, respectively, in identifying diastolic dysfunction (DD) using American Society of Echocardiography‐recommended guideline criteria. (B and C) Both LAD and LADi showed good diagnostic yield for such purpose. CI, confidence interval.
The diagnostic yield of left atrial dimension of guideline‐recommended diastolic dysfunction criteria and N‐terminal pro‐brain natriuretic peptide level
The optimal LAD cut‐off for identifying DD defined by ASE criteria was 34 mm (AUROC: 0.77, 95% CI [0.73, 0.80]) with high OR (OR: 6.27, 95% CI [4.47, 8.81]), 34 and 31.6 mm for men and women, respectively (AUROC: 0.77/0.81, 95% CI [0.71, 0.83]/[0.77, 0.85] for men and women) (Figure 2 B and Table 3). LAD set at 37 mm yielded a high PPV (96%) and high specificity (86.7%) for identifying abnormally high LAVi (>34 mL/m2) and showed high NPV values by using LAD cut‐offs in identifying DD in both sexes. Compared with LAD 37 mm as cut‐off in identifying abnormal LAVi (>34 mL/m2), which offered a relatively high specificity (86.7%) and low sensitivity (25.6%) (Table 3), LAD set at 34 mm provided better sensitivity (42%) at the cost of lower specificity (69%). By defining abnormally high NT‐proBNP for high probability of HF, the LAD cut‐off for all study participants again was 34 mm (AUROC: 0.63) (OR: 2.34, 95% CI [1.85, 2.94]) (34 [AUROC: 0.68] and 31.0 mm [AUROC: 0.67] for men and women, respectively) (Table 3). Similar AUROC by LADi for both gender groups was also observed (Figure 2 C). Finally, LAD set at 35 mm further yielded a sensitivity of 53.6% and acceptable specificity (69%) in identifying subjects who fulfilled ESC‐defined HFpEF criteria (AUROC: 0.70) (OR: 3.86, 95% CI [2.70, 5.52]), with relatively better NPV (98.7%) in total study subjects.
Table 3.
Diagnostic yield of LAD in relation to ASE‐recommended diastolic indices or DD and ESC NT‐proBNP cut‐offs for HF
| C‐statistics | LAD | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | AUROC | 95% CI | Cut‐off (mm) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| NT‐proBNP Abn (≥125 pg/mL) | 2.34 [1.85, 2.94] | 0.63 | [0.59, 0.66] | 34 | 50.5 | 69.6 | 50.49 | 69.62 |
| Echocardiography indices by ASE | ||||||||
| LAVi Abn (also ESC HFpEF16) | 2.24 [1.70, 2.96] | 0.57 | [0.54, 0.61] | 37 | 25.60 | 86.70 | 96 | 8 |
| TDI e′ (both) Abn | 2.45 [2.19, 2.73] | 0.65 | [0.64, 0.66] | 31 | 61.10 | 61.20 | 66.10 | 55.60 |
| TDI E/e′ Abn | 2.85 [2.17, 3.74] | 0.68 | [0.64, 0.71] | 32 | 67.30 | 58.40 | 7.50 | 95.20 |
| TR velocity Abn | 5.50 [4.41, 6.84] | 0.74 | [0.71, 0.77] | 35 | 62.10 | 77.20 | 11.60 | 95.40 |
| DD Abn by ASE | ||||||||
| All | 6.27 [4.47, 8.81] | 0.77 | [0.73, 0.80] | 34 | 74 | 68.80 | 5.30 | 97.90 |
| Women | 11.48 [6.70, 19.68] | 0.81 | [0.77, 0.85] | 31.6 | 86.50 | 70 | 8.70 | 96.80 |
| Men | 6.46 [3.65, 11.41] | 0.77 | [0.71, 0.83] | 34 | 82.60 | 63.70 | 3.20 | 98.60 |
| ESC criteria for HFpEF16 | 3.86 [2.70, 5.52] | 0.70 | [0.65, 0.75] | 35 | 53.6 | 77.0 | 4.9 | 98.7 |
Abn, abnormal; ASE, American Society of Echocardiography; AUROC, area under receiver operating characteristic curve; CI, confidence interval; DD, diastolic dysfunction; ESC, European Society of Cardiology; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LAD, left atrial dimension; LAVi, left atrial volume index; NPV, negative predictive value; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PPV, positive predictive value; TDI, tissue Doppler imaging; TR, tricuspid regurgitation.
Discussion
This study evaluated the impact of ASE‐recommended quantitative echo‐defined diastolic measures as well as ESC NT‐proBNP cut‐off for high probability of HF on LA remodelling in a large, asymptomatic Asians. Our results showed that increasing numbers of abnormal diastolic indices or presence of DD recommended by ASE as well as abnormal NT‐proBNP level (>125 pg/mL) were significantly associated with greater LA remodelling, defined by greater LAD. Our data suggested that LAD set at 34 mm yielded high negative predictive value for guideline‐based DD with high predictive accuracy for enlarged LAV (>34 mL/m2) and also discriminate high NT‐proBNP cut‐off (≥125 pg/mL) defined for HF well in a preclinical stage.
Our current findings were consistent with previous data showing that men had a significantly greater LAD compared with women (40 vs. 36 mm, respectively) and the correlation with DD existence,7 likely driven by increased myocardial stiffness, excessive extracellular matrix turnover/fibrosis from ageing, or associated co‐morbidities (such as hypertension and obesity).8, 20, 21, 22, 23, 24 Our findings further extended previous studies to a population with relatively low hypertension prevalence (<20%), indicating that subclinical LA remodelling might occur at a relatively early clinical stage. Because LA enlargement in adults may strongly predict cardiovascular diseases (e.g. HF, AF, and stroke25, 26), early recognition of LA remodelling in subjects with progressive pathological conditions plays a critical step for clinical risk stratification and preventive treatment. Maladaptive LA enlargement reflecting chronically increased afterload as a consequence of subclinical LV dysfunction or HF (American College of Cardiology Stage B)27 may indicate a stage beyond presence of DD, which could be load‐dependent and short‐term based.28 The thin‐walled structure makes LA particularly vulnerable to volume and pressure‐load conditions from long‐standing elevated LV filling pressure, resulting in chamber dilation or geometric alterations with greater susceptibility for AF.29 While atrial contraction may participate actively in LV filling during late diastolic phase, LA emptying function may deteriorate as LA remodelling continues with excessive interstitial fibrosis (or scar formation), causing retrograde elevation of pulmonary artery pressure and higher TR velocity.19 Owing to the complex geometry, it has been proposed that LAD may not enlarge equally as atrial remodelling progresses, which makes precise quantification of LA size clinically challenging.18
Our data showed a significant difference between guideline‐defined ‘normal’ and ‘DD’ groups (30.9 vs. 36.5 mm for all participants, P < 0.001) in both sexes, which challenges the traditional concept that LAD may be neither sensitive enough nor clinically feasible for assessment of LA remodelling in daily practice. Surprisingly, the differences in LAD between the ASE‐defined ‘normal’ and ‘DD’ groups remained significantly different (31.1 vs. 33.1 mm, P < 0.001) even after adjusting for several baseline covariates and LV mass (Supporting Information, Figure S2 ). Our data also suggested the potential use of LAD (34 mm) as useful threshold in identifying DD with relatively high accuracy (AUROC: 77%; sensitivity/specificity: 74/68.8%; and NPV: >95%) and relatively high specificity (86.7%) in identifying LA enlargement (>34 mL/m2; LAD: 37 mm) with good PPV (Table 3), indicating the potential feasibility in ‘labelling’ Stage B HF and help establish the clinical diagnosis of HFpEF recommended by most updated ESC HF guideline criteria.16 Taken together, these results suggested that lower LAD appears be particularly useful in excluding the possible existence of ‘DD’ as defined by ASE, whereas larger LAD actually implies true LAV expansion. Although LADi in general demonstrated similar accuracy, calculation of such information needs body surface area for indexation. Further, caution should be exercised during clinical interpretation of LADi, which possibly shows gender differences as in current study. Nonetheless, we are not claiming that LAD is superior to LADi for clinical use.
Our current findings are consistent with several recent large epidemiological reports showing that LAD may still be a useful marker for early stage hypertension,9 in identifying HFpEF,30, 31 and serve as a useful surrogate for AF prediction for regular drinkers.32 In the Strong Heart Study, LAD was used as a surrogate of target organ damage for cardiovascular events and diabetes incidence.33 In fact, the Cardiovascular Health Study reported that LA may actually remodel more severely from anteroposterior direction with HF.34 On the other hand, Armstrong et al.35 (CARDIA study) reported that LAD may be as useful as LAVs in predicting cardiovascular outcomes in a relatively healthy population. In a recent large meta‐analysis from population‐based cohort, arterial stiffness, a powerful clinical risk factor of development of DD was shown to independently predict LAD.36 Despite the traditional concept on the advantageous use of volume‐based LA measures over LAD, our data supported the clinical use of LAD for assessing DD and were concordant with Strong Heart Study and CARDIA studies as mentioned earlier.33, 35 This may be partly explained by the fact that LAD assessment is a relatively robust, simple measure with potentially higher reproducibility.30 Instead, LAVs are more prone to assessment errors or are less reproducible, with potentially higher technical dependency. Additionally, it is likely that in daily practice, more attention was paid to optimize LV endocardial delineation to avoid LV foreshortening during image acquisition37 and that true superior–inferior axial alignment of LA from apical views may be overlooked. Indeed, dimension‐based LAD/LADi showed better correlations with most diastolic indices than volume‐based LA measures (with and without index) in current work.
We had previously reported that NT‐proBNP, a clinical marker of LVH, DD, or HF,38 may rise in the asymptomatic stage of structural heart disease from ethnic Asians.39 However, data comparing LAD and NT‐proBNP levels from a large Asian population remain unexplored. So far, high NT‐proBNP value (≥125 pg/mL) based on current ESC guidelines accompanied by evident DD or structural anomalies has been proposed to be a recommended clinical cut‐off for high HF probability. Our current work showed that LAD discriminated NT‐proBNP levels with a cut‐off of 125 pg/mL, before (31.1 vs. 33.6 mm) and after (31.2 vs. 32.7 mm, both P < 0.001) adjustment of key clinical covariates and LV mass. Further, our data showed that LAD set at 30.6 and 34 mm, a value far below 40 mm, could efficiently provide abnormal cut‐off for subjects with high NT‐proBNP (≥125 pg/mL) in both women and men in a preclinical stage, respectively.
Limitations
An important limitation of this study was its retrospective nature and cross‐sectional design, and longitudinal follow‐up data were lacking. Additional prospective studies are required to further characterize the parameters that affect the LAD. Further, LA measures using volume‐based modality (such as Biplane Simpson's measures by 2D) can be more accurate in estimating true LA size, although LAD using M‐mode may be more suitable for large population screens. Our data suggested that LAD as a clinical surrogate of DD or abnormally high NT‐proBNP may start at a lower value, at which stage the concern about constrained LA remodelling from the anteroposterior dimension may not be relevant. Finally, it would be of interest to also assess or to integrate other cardiac function information, especially diastolic parameters,13 as part of the echo‐derived determinants in predicting LA remodelling, which may strengthen the mechanistic link between altered LV filling and LA adaptations.
Conclusions
Our data demonstrated the strong associations between guideline‐based cut‐offs for DD and high probability of clinical HF with LA structural remodelling by utilizing LAD in a large, preclinical stage of ethnic Asian population. The optimal LAD chosen for identifying guideline‐based functional abnormality cut‐offs was relatively low for both genders and yielded satisfactory discriminatory ability and high negative predictive accuracy. These data may have important implications for targeting specific populations at high risk of HF. Our data further provided useful threshold from a primary preventive standpoint by identifying modifiable clinical risks in subjects with high LAD in order to avoid progressive heart damage or incident AF in Asians.
Conflict of interest
None declared.
Funding
This work was partially funded by grants from the National Science Council (NSC‐101‐2314‐B‐195‐020, NSC‐103‐2314‐B‐010‐005‐MY3, 103‐2314‐B‐195‐001‐MY3, 101‐2314‐B‐195‐020‐MY1, MOST 103‐2314‐B‐195‐006‐MY3, and MOST 106‐2314‐B‐195‐008‐MY2), the MacKay Memorial Hospital (10271, 10248, 10220, 10253, 10375, 10358, and E‐102003), and the Taiwan Foundation for geriatric emergency and critical care.
Supporting information
Figure S1. Paired comparisons of LAD and LADi between groups of guideline‐based diastolic dysfunction and high probability for clinical HF.
Figure S2. Values and distribution of LAD across ASE‐recommended DD scoring (A) (0, 1, 2, 3, and 4), sex‐specific comparisons (B), together with sex‐specified LAD by ASE‐recommended DD categories (C, as “Normal”, “Inconclusive”, and “DD”) and ESC HF probability cutoff for abnormally high Nt‐ProBNP level (≥125 pg/mL, D).
Table S1. Baseline demographic, clinical characteristics and echocardiography indices of all study participants stratified by sex.
Table S2. Sex‐stratified prevalence and distribution of abnormal diastolic indices and diastolic dysfunction (DD) by ASE guideline.
Table S3. The associations of various LA measures, ASE recommended diastolic functional indices and Nt‐ProBNP level.
Acknowledgements
We would like to extend our gratitude to Bernard and Kuo‐Tzu Sung for their efforts and for their contribution to the graphs and illustrations in this work.
Tsai J.‐P., Sung K.‐T., Su C.‐H., Lai Y.‐H., Kuo J.‐Y., Yun C.‐H., Yen C.‐H., Hou C. J.‐Y., Wu T.‐H., Peng M.‐C., Hung T.‐C., Yeh H.‐I., and Hung C.‐L. (2019) Diagnostic accuracy of left atrial remodelling and natriuretic peptide levels for preclinical heart failure, ESC Heart Failure, 6, 723–732. 10.1002/ehf2.12430.
References
- 1. Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy ageing: the Cardiovascular Health Study. Circ Cardiovasc Imaging 2009; 2: 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol 1997; 29: 651–658. [DOI] [PubMed] [Google Scholar]
- 3. Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SB, Epstein SE. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation 1976; 53: 273–279. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA 1994; 271: 840–844. [PubMed] [Google Scholar]
- 5. Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 1999; 30: 2019–2024. [DOI] [PubMed] [Google Scholar]
- 6. Kosmala W, Saito M, Kaye G, Negishi K, Linker N, Gammage M, Marwick TH. Incremental value of left atrial structural and functional characteristics for prediction of atrial fibrillation in patients receiving cardiac pacing. Circ Cardiovasc Imaging 2015; 8: pii:e002942. [DOI] [PubMed] [Google Scholar]
- 7. Liu XK, Jahangir A, Terzic A, Gersh BJ, Hammill SC, Shen WK. Age‐ and sex‐related atrial electrophysiologic and structural changes. Am J Cardiol 2004; 94: 373–375. [DOI] [PubMed] [Google Scholar]
- 8. Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Döring A, Keil U, Hense HW, Schunkert H, MONICA/KORA Investigators . The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol 2009; 54: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 9. Su G, Cao H, Xu S, Lu Y, Shuai X, Sun Y, Liao Y, Li J. Left atrial enlargement in the early stage of hypertensive heart disease: a common but ignored condition. J Clin Hypertens 2014; 16: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tripepi G, Mattace‐Raso F, Mallamaci F, Benedetto FA, Witteman J, Malatino L, Zoccali C. Biomarkers of left atrial volume: a longitudinal study in participants with end stage renal disease. Hypertension 2009; 54: 818–824. [DOI] [PubMed] [Google Scholar]
- 11. Hassanin N, Alkemary A. Detection of left atrium myopathy using two‐dimensional speckle tracking echocardiography in participants with end‐stage renal disease on dialysis therapy. Echocardiography 2016; 33: 233–241. [DOI] [PubMed] [Google Scholar]
- 12. Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension 1995; 25: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 13. Chen C, Chen YT, Wang KT, Shih SC, Liu CC, Kuo JY, Hou CJY, Wu YJ, Hung CL, Yeh HI. The association among age, early mitral leaflet closure, cardiac structure, diastolic indices and NT‐proBNP in an asymptomaticTaiwanese population. IJC Heart Vasc 2015; 8: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur J Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39, e14. [DOI] [PubMed] [Google Scholar]
- 19. Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, Frenneaux MP. Assessment of pulmonary artery pressure by echocardiography—a comprehensive review. Int J Cardiol Heart Vasc 2016; 12: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Triposkiadis F, Tentolouris K, Androulakis A, Trikas A, Toutouzas K, Kyriakidis M, Gialafos J, Toutouzas P. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr 1995; 8: 801–809. [DOI] [PubMed] [Google Scholar]
- 21. Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr 2003; 4: 92–100. [DOI] [PubMed] [Google Scholar]
- 22. Casaclang‐Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol 2008; 51: 1–11. [DOI] [PubMed] [Google Scholar]
- 23. Kopeć G, Sobień B, Podolec M, Waligóra M, Brózda M, Zarzecka J, Loster B, Nessler J, Pająk A, Podolec P. The prevalence of abnormal echocardiographic findings in a sample of urban adult population. Kardiol Pol 2014; 72: 42–49. [DOI] [PubMed] [Google Scholar]
- 24. Gidding SS, Palermo RA, DeLoach SS, Keith SW, Falkner B. Associations of cardiac structure with obesity, blood pressure, inflammation, and insulin resistance in African–American adolescents. Pediatr Cardiol 2014; 35: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994; 89: 724–730. [DOI] [PubMed] [Google Scholar]
- 26. Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory participants with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol 2008; 102: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 28. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TSM. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006; 47: 2357–2363. [DOI] [PubMed] [Google Scholar]
- 29. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014; 63: 493–505. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida C, Nakao S, Goda A, Naito Y, Matsumoto M, Otsuka M, Shimoshikiryo M, Eguchi A, Lee‐Kawabata M, Tsujino T, Masuyama T. Value of assessment of left atrial volume and diameter in patients with heart failure but with normal left ventricular ejection fraction and mitral flow velocity pattern. Eur J Echocardiogr 2009; 10: 278–281. [DOI] [PubMed] [Google Scholar]
- 31. Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TSM. Usefulness of left atrial volume in predicting first congestive heart failure in patients ≥65 years of age with well‐preserved left ventricular systolic function. Am J Cardiol 2005; 96: 832–836. [DOI] [PubMed] [Google Scholar]
- 32. McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016; 5: e004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. Target organ damage and incident type 2 diabetes mellitus: the Strong Heart Study. Cardiovasc Diabetol 2017; 16: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons ≥65 years of age (the Cardiovascular Health Study). Am J Cardiol 2006; 97: 83–89. [DOI] [PubMed] [Google Scholar]
- 35. Armstrong AC, Liu K, Lewis CE, Sidney S, Colangelo LA, Kishi S, Ambale‐Venkatesh B, Arynchyn A, Jacobs DR, Correia LCL, Gidding SS, Lima JAC. Left atrial dimension and traditional cardiovascular risk factors predict 20‐year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging 2014; 15: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuznetsova T, Haddad F, Tikhonoff V, Kloch‐Badelek M, Ryabikov A, Knez J, Malyutina S, Stolarz‐Skrzypek K, Thijs L, Schnittger I, Wu JC. Impact and pitfalls of scaling of left ventricular and atrial structure in population‐based studies. J Hypertens 2016; 34: 1186–1194. [DOI] [PubMed] [Google Scholar]
- 37. Iwataki M, Takeuchi M, Otani K, Kuwaki H, Haruki N, Yoshitani H, Tamura M, Abe H, Otsuji Y. Measurement of left atrial volume from transthoracic three‐dimensional echocardiographic datasets using the biplane Simpson's technique. J Am Soc Echocardiogr 2012; 25: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 38. Dietl A, Stark K, Zimmermann ME, Meisinger C, Schunkert H, Birner C, Maier LS, Peters A, Heid IM, Luchner A. NT‐proBNP predicts cardiovascular death in the general population independent of left ventricular mass and function: insights from a large population‐based study with long‐term follow‐up. PLOS One 2016; 11: e0164060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hung TC, Wang KT, Yun CH, Kuo JY, Hou CJ, Liu CY, Wu TH, Bezerra HG, Cheng HY, Hung CL, Yeh HI. Value of serum N‐terminal B‐type natriuretic peptide in asymptomatic structural heart disease in Taiwanese population: comparisons with current ESC guidelines. Int J Cardiol 2017; 231: 195–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Paired comparisons of LAD and LADi between groups of guideline‐based diastolic dysfunction and high probability for clinical HF.
Figure S2. Values and distribution of LAD across ASE‐recommended DD scoring (A) (0, 1, 2, 3, and 4), sex‐specific comparisons (B), together with sex‐specified LAD by ASE‐recommended DD categories (C, as “Normal”, “Inconclusive”, and “DD”) and ESC HF probability cutoff for abnormally high Nt‐ProBNP level (≥125 pg/mL, D).
Table S1. Baseline demographic, clinical characteristics and echocardiography indices of all study participants stratified by sex.
Table S2. Sex‐stratified prevalence and distribution of abnormal diastolic indices and diastolic dysfunction (DD) by ASE guideline.
Table S3. The associations of various LA measures, ASE recommended diastolic functional indices and Nt‐ProBNP level.


