Abstract
Aims
Our aim was to investigate the glucose uptake in cancer patients suffering from different entities, using 18F‐FDG positron emission tomography–computed tomography scans. We further aimed at identifying potential variables altering cardiac and skeletal muscle glucose metabolism.
Methods and results
In a retrospective cohort study, we analysed cardiac and skeletal muscle 18F‐FDG uptake in onco‐positron emission tomography–computed tomography scans in adult patients suffering from Hodgkin's lymphoma, non‐Hodgkin's lymphoma, and non‐lymphatic cancer including patients suffering from thyroid cancer, bronchial carcinoma, and malignant melanoma. Univariate logistic regression models were created for increased cardiac and skeletal muscle 18F‐FDG uptake using cancer entity, sex, age, previous radiation, previous chemotherapy, diabetes, obesity, serum glucose levels, renal function, and thyroid function as parameters. Multivariate models were created by selecting variables according to Akaike's information criterion in a step‐down approach. Between 2014 and 2018, a total of 337 consecutive patients suffering from Hodgkin's lymphoma (n = 52), non‐Hodgkin's lymphoma (n = 57), and non‐lymphatic cancer (n = 228) were included in the analysis. Univariate logistic regression models showed high serum glucose levels to be associated with lower absorption rates in both cardiac and skeletal muscle (odds ratio [OR] 0.38 [0.23, 0.60, 95% confidence interval—CI], P < 0.0001, and 0.52 [0.33, 0.82, 95% CI], P < 0.005, respectively). Hodgkin's lymphoma was associated with an increase in cardiac uptake (OR 2.4 [1.3, 4.5, 95% CI], P < 0.005). Decreased skeletal muscle 18F‐FDG uptake was noted in elderly and obese patients. In our multivariate analysis, Hodgkin's lymphoma patients showed higher cardiac 18F‐FDG uptake, while non‐Hodgkin's lymphoma patients did not differ significantly from non‐lymphatic cancer patients (OR 1.6 [0.7, 3.3, 95% CI], P = 0.24). High serum glucose levels and prior chemotherapy were both associated with a significantly decreased cardiac 18F‐FDG uptake (OR 0.40 [0.24, 0.65, 95% CI], P < 0.0005, and 0.50 [0.27, 0.90, 95% CI], P < 0.05, respectively). Notably, prior chemotherapy did not influence FDG uptake in skeletal muscle to the same extent. Obesity and older age were both significantly associated with decreased gluteal 18F‐FDG uptake (OR 0.49 [0.27, 0.89, 95% CI], P < 0.05, and 0.47 [0.25, 0.87, 95% CI], P < 0.05).
Conclusions
Our data provide evidence for metabolic alterations in patients with Hodgkin's lymphoma related to cardiac glucose uptake in humans. This effect was independent from skeletal muscle metabolism.
Keywords: Glucose metabolism, Cardio‐oncology, 18F‐FDG, PET‐CT, Hodgkin's lymphoma, Cachexia
Introduction
Systemic metabolic effects of tumours have been linked to cachexia and cardiac wasting.1 Additionally, preclinical studies postulate a potential direct crosstalk between cancer cells and cardiomyocytes. Secreted ‘cachexokines’ can lead to detrimental cardiac metabolic changes, marked by an increased glucose metabolism, while the healthy heart metabolizes predominantly fatty acids.2, 3, 4 Furthermore, in vivo animal data on colon cancer and malignant melanoma linked systemic insulin depletion to a dysregulation of the cardiac glucose metabolism, visualized by 2‐deoxy‐2‐(18F)fluoro‐d‐glucose (FDG) positron emission tomography in combination with a low‐dose computed tomography (PET‐CT) scans.5 In humans, there are currently no data available for metabolic cardiac changes in cancer patients suffering from different tumour entitites.
Labelled metabolites used in PET‐CT are an established method to visualize and calculate metabolic activity in tissues (Figure 1). So far, limited data are available linking PET‐CT scans to human cardiac metabolism. FDG PET‐CT is commonly used to screen for metastases and assess neoplastic metabolic activity in distinct types of cancer. Recent studies were able to provide data for a potential cardio‐metabolic effect of radiotherapy using an I123‐labelled fatty acid analogue and FDG PET‐CT scans.6, 7, 8
Figure 1.
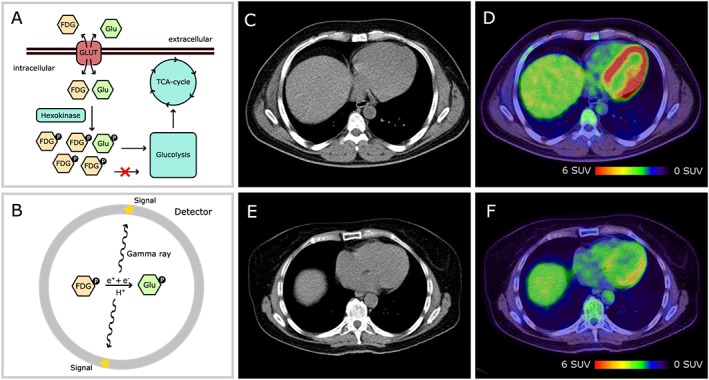
FDG positron emission tomography–computed tomography (PET‐CT) functionality and exemplary images. In the heart, FDG is absorbed like glucose and phosphorylated by hexokinase (A). Phosphorylated FDG does not enter glycolysis and cannot leave the cell via GLUT transporters (metabolic trap). Beta‐decay converts FDG to glucose (B). The subsequent annihilation reaction creates a gamma ray, which is detected by the PET scanner. Low‐dose CT scans are used for image fusion (C, E). Fused images including PET and CT (C, E) information are depicted in (D) and (F). High signal density is coloured in red, and low signal density is coloured in blue and green. (C) and (D) are taken from a Hodgkin's lymphoma patient, and (E) and (F) are taken from a non‐Hodgkin's lymphoma patient. Note the higher signal density in the left ventricle of a Hodgkin's lymphoma patient (D vs. F). SUV, standardized uptake value.
The goal of our analysis was to investigate possible alterations of the cardiac glucose uptake in cancer patients suffering from different tumour entities by using FDG PET‐CT scans. We further aimed at investigating differences in cardiac and skeletal muscle FDG uptake.
Methods
We retrospectively analysed PET‐CT scans from adult patients presenting in our nuclear medicine department between 2014 and 2018 suffering from Hodgkin's lymphoma, non‐Hodgkin's lymphoma, and non‐lymphatic cancer, namely, thyroid carcinoma, bronchial carcinoma, and malignant melanoma. The study was conducted according to the principles of the Declaration of Helsinki. Approval for this research was granted by the local research ethics committee. Patient‐related information was pseudonymized upon data extraction.
Positron emission tomography–computed tomography scans were performed according to a standard protocol. 350 to 370 mBq FDG was administered intravenously 60 min prior to the examination. A low‐dose whole‐body CT scan (Siemens Biograph, Siemens Healthcare Diagnostics, Eschborn, Germany) was used for attenuation correction and image fusion. Following the CT scan, a three‐dimensional emission scan was performed. Raw data were iteratively reconstructed and analysed using regions of interest as well as standardized uptake values (SUVs). Signals were measured in the free left ventricular wall, the aorta, and the gluteus muscle. The mean SUV of the region of interest was reported. The ROI was selected based on anatomic structures, not SUV intensity. Signals from the heart and gluteus muscle were normalized to the aorta. These ratios were subsequently logarithmized to achieve normal distribution.
Patient characteristics included sex, age, body mass index (BMI), patient history (prior chemotherapy, prior radiotherapy, and diabetes), serum creatinine levels, the estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) method), thyroid‐stimulating hormone levels, and fasting serum glucose levels. In addition, the cumulative doxorubicin dose was reported for all lymphoma patients. Data on left ventricular function were also collected, where available.
Statistical analyses were performed with R version 3.4.4 using the MASS and ggplot2 packages.9, 10, 11 Non‐normal distributed values are reported as median ± interquartile range (IQR) and compared using Wilcoxon rank‐sum tests. FDG absorption ratios were logarithmized to achieve normal distribution. An analysis of variance test was performed to test for differences within the groups. Tukey's honest significant difference method was applied for P‐level adjustment. Univariate logistic regression models were established, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for logarithmized signal ratios above the mean using each characteristic as variable. Multivariate models were created by selecting the variables according to Akaike's information criterion in a step‐down approach.12 Logistic regression results are reported with OR and a 95% CI.
Results
Between 2014 and 2018, a total of 337 patients suffering from Hodgkin's lymphoma (n = 52), non‐Hodgkin's lymphoma (n = 57), and non‐lymphatic cancer (n = 228) were analysed (Table 1). In this heterogeneous group, comprising patients with malignant melanoma (n = 81), thyroid cancer (n = 43), and bronchial carcinoma (n = 108), all non‐lymphatic cancer patients exhibited comparable cardiac FDG uptake (cardiac/aortic SUV: melanoma 2.3 ± 2.4, thyroid cancer 2.0 ± 1.9, and bronchial carcinoma 2.0 ± 2.4 [median ± IQR]). In contrast, patients with Hodgkin's lymphoma showed a significantly increased absorption when compared with non‐lymphatic cancer patients (3.5 ± 3.6 vs. 2.1 ± 2.3 [median ± IQR], P < 0.02), while those with non‐Hodgkin's lymphoma had equal absorption rates (2.1 ± 2.3 vs. 2.1 ± 2.1 [median ± IQR], P = 0.51). In a subset of lymphoma patients with available echocardiographic data (n = 76), patients exhibiting cardiac diastolic or systolic dysfunction (n = 22) showed similar FDG absorption levels as compared with their controls (n = 54, P = 0.69, Wilcoxon rank‐sum test, data not shown.) We further did not observe associated changes in cardiac hypertrophy (septal wall thickness) or left ventricular end‐diastolic diameter.
Table 1.
Study population
| HL (n = 52) | NHL (n = 57) | No lymphoma (n = 228) | ||||
|---|---|---|---|---|---|---|
| Male sex | 62% | 72% | 61% | |||
| Age | 40 ± 15 | 50 ± 17 | 64 ± 13 | *** | ### | +++ |
| BMI (kg/m2) | 25.11 ± 5.01 | 25.99 ± 7.67 | 25.88 ± 4.64 | |||
| Prior chemotherapy | 98% | 93% | 36% | ### | +++ | |
| Doxorubicin dose (mg/m2) | 179 ± 143 | 220 ± 113 | NA | |||
| Prior radiotherapy | 23% | 40% | 57% | ### | ||
| Serum glucose levels (mmol/L) | 5.6 ± 1.7 | 5.4 ± 1.1 | 6.0 ± 1.3 | ++ | ||
| Diabetes mellitus | 6% | 12% | 16% | |||
| Serum creatinine levels (μmol/L) | 72 ± 22 | 72 ± 18 | 79 ± 31 | |||
| Glomerular filtration rate (mL/min * 1.73 m2) | 111 ± 18 | 101 ± 20 | 85 ± 20 | ### | +++ | |
| Increased TSH (>4.0 mU/L) | 4.3% | 11.5% | 24.3% | # |
BMI, body mass index; HL, Hodgkin's lymphoma; NHL, non‐Hodgkin's lymphoma; TSH, thyroid‐stimulating hormone.
Continuous data are reported as mean ± standard deviation. Count data are reported as percentages. Statistics: Tukey's honest significance test was used for continuous data, and Yates's χ 2 test was used for count data applying Holm's method for P‐value adjustment.
P < 0.05 (HL vs. NHL).
P < 0.01 (HL vs. NHL).
P < 0.001 (HL vs. NHL).
P < 0.05 (HL vs. no lymphoma).
P < 0.01 (HL vs. no lymphoma).
P < 0.001 (HL vs. no lymphoma).
P < 0.05 (NHL vs. no lymphoma).
P < 0.01 (NHL vs. no lymphoma).
P < 0.001 (NHL vs. no lymphoma).
To investigate the influence of further variables on cardiac FDG uptake, we performed univariate logistic regression analyses. Hodgkin's lymphoma was associated with an increased cardiac FDG uptake (OR 2.4 [1.3, 4.5, 95% CI], P < 0.005), while serum glucose levels above 5.5 mmol/L (OR 0.38 [0.23, 0.60, 95% CI], P < 0.0001) and diabetes (OR 0.41 [0.19, 0.84, 95% CI], P < 0.05) were significantly associated with a decrease in cardiac FDG uptake. Notably, changes in gluteal FDG uptake were not significantly associated with a specific entity. Patients with advanced age (OR 0.62 [0.39, 0.99, 95% CI], P < 0.05), a BMI above 25 kg/m2 (OR 0.47 [0.30, 0.74, 95% CI], P < 0.005), or serum glucose levels above 5.5 mmol/L (OR 0.52 [0.33, 0.82, 95% CI], P < 0.005) were more likely to show low gluteal FDG uptake (Figure 2).
Figure 2.
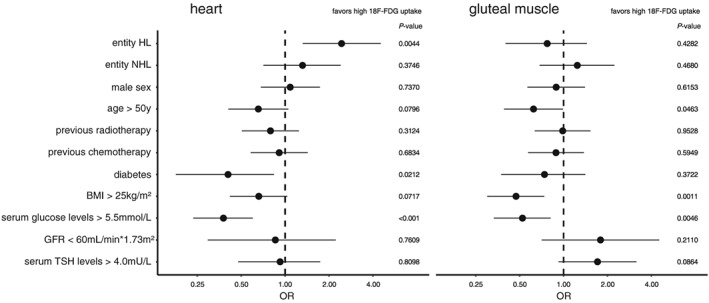
Univariate logistic regression analyses on cardiac and gluteal FDG uptake. Odds ratios (ORs) with 95% confidence intervals and P‐values are depicted for higher cardiac (left) or gluteal (right) 18F‐FDG absorption. Serum glucose levels above 5.5 mmol/L were significantly associated with lower absorption rates in both cardiac and skeletal muscle, while Hodgkin's lymphoma (HL) was only associated with an increase in cardiac absorption. BMI, body mass index; GFR, glomerular filtration rate; NHL, non‐Hodgkin's lymphoma; TSH, thyroid‐stimulating hormone.
We next calculated multivariate regression models. Variables were selected based on Akaike's information criterion in a step‐down approach.12 The models included the cancer entity, serum glucose levels above 5.5 mmol/L, previous chemotherapy for cardiac FDG uptake (Figure 3 A), glomerular filtration rate below 60 mL/1.73 m2, previous chemotherapy, BMI above 25 kg/m2, and age above 50 years for gluteal FDG uptake (Figure 3 B). In the multivariate model, Hodgkin's lymphoma was still significantly associated with an increase (OR 3.09 [1.46, 6.64, 95% CI], P < 0.005) and high serum glucose levels with a decrease (OR 0.40 [0.24, 0.65, 95% CI], P < 0.0005) in cardiac FDG uptake, which indicates an effect, independent from serum glucose concentration and previous chemotherapy (Figure 3 A). Notably, previous chemotherapy, which was not significantly changed in the univariate analysis, was accompanied by less FDG uptake in the multivariate model (OR 0.50 [0.27, 0.90, 95% CI], P < 0.05, compare Figure 2 and Figure 3 A). Gluteal FDG uptake was not significantly altered with previous chemotherapy. Overweight and advanced age, however, were associated with decreased FDG uptake (Figure 3 B). These findings indicate that different lymphoma entities exert distinct effects on cardiac glucose metabolism, which are not discernible in skeletal muscle (Figures 2 and 3).
Figure 3.
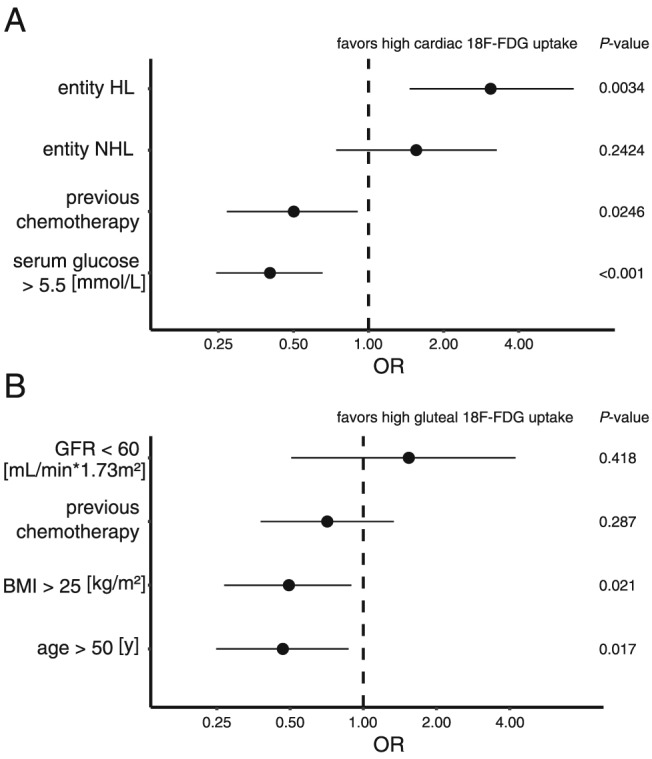
Multivariate logistic regression analyses on cardiac (A) and gluteal (B) FDG uptake. Hodgkin's lymphoma (HL) was significantly associated with high FDG absorption, while non‐Hodgkin's lymphoma (NHL) did not differ significantly from non‐lymphatic cancer. High serum glucose levels and prior chemotherapy were both associated with a significant decrease in cardiac FDG uptake. Interestingly, prior chemotherapy did not influence FDG uptake in skeletal muscle to the same extent. Obesity and older age were both significantly associated with decreased gluteal FDG uptake. Variables were selected in a step‐down approach using Akaike's information criterion. BMI, body mass index; GFR, glomerular filtration rate; OR, odds ratio.
Discussion
Based on an unbiased approach analysing 18F‐FDG uptake in patients with different cancer entities, we found an increased cardiac FDG uptake in patients with Hodgkin's lymphoma. Interestingly, these findings were not explained by differences in serum glucose levels or prior chemotherapy, which were in turn associated with a significant decrease in cardiac FDG uptake. This effect was not linked to systemic cachexia, and alterations in skeletal muscle glucose metabolism were mainly correlated with other factors, such as age and obesity. Of note, ‘metabolic remodelling’ and substrate utilization characterize the transition to heart failure.13, 14 Moreover, metabolic changes towards more glucose uptake were shown to be sufficient to induce cardiac dysfunction.3
In line with findings on heart failure, two studies analysing the effects of anthracyclines on FDG uptake in a total of 113 Hodgkin's lymphoma patients found a gradual increase in FDG uptake over time while on anthracycline treatment.15, 16 This increase was associated with a decrease in left ventricular function.15 Furthermore, a low left ventricular FDG uptake before doxorubicin treatment was associated with the development of chemotherapy‐induced cardiotoxicity.16 The reason why a low cardiac left ventricular FDG uptake at baseline is associated with the development of chemotherapy‐induced cardiotoxicity is yet not quite understood.
A study analysing sequential PET‐CTs of 20 lymphoma patients noted a spatially and temporally heterogeneous FDG uptake (SUVmax) not explained by fasting, age, gender, or cardiac risk factors.17 Another study investigating FDG uptake patterns in patients without previous cardiovascular disease described four different FDG uptake patterns, namely, none, diffuse, focal on diffuse, and focal enrichment. These patterns all occur in healthy hearts, while focal FDG uptake pattern was more prevalent in patients with an underlying cardiovascular disease.18
In our study, we found differences in the mean SUV of cardiac FDG uptake to be associated with the tumour entity itself. This might be indicative of an underlying crosstalk between the tumour and the heart as this effect was independent of previous chemotherapy and other factors. However, the retrospective design, the heterogenic study population, and the fact that our analysis is based on routine onco‐PET/CT scans without using an insulin clamp technique should be considered in interpreting the results.
Recent preclinical findings showed differences in cardiac glucose metabolism in mice inoculated with colon cancer and melanoma cells.5 However, in our patient cohort, we did not observe metabolic changes in patients with malignant melanoma. This could be possibly explained by different factors (tumour burden, melanoma subtype, patient characteristics, and cohort size).
Taken together, cardiac metabolism measured by nuclear imaging might open a new modality to assess patients at risk for cardiac complications from cancer. Additionally, it might serve as a novel tool to investigate previously unrecognized cardio‐oncological interactions with metabolic disturbances in animal models and patient populations.
Conflict of interest
M.S.A. reports receiving personal fees from Servier. The other authors declare no conflicts of interest.
Heckmann, M. B. , Totakhel, B. , Finke, D. , Anker, M. S. , Müller‐Tidow, C. , Haberkorn, U. , Katus, H. A. , and Lehmann, L. H. (2019) Evidence for a cardiac metabolic switch in patients with Hodgkin's lymphoma. ESC Heart Failure, 6: 824–829. 10.1002/ehf2.12475.
References
- 1. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJS, Beadle J, Argiles JM, Thum T, Földes G, Doehner W, Hilfiker‐Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014; 35: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schäfer M, Oeing CU, Rohm M, Baysal‐Temel E, Lehmann LH, Bauer R, Volz HC, Boutros M, Sohn D, Sticht C, Gretz N, Eichelbaum K, Werner T, Hirt MN, Eschenhagen T, Müller‐Decker K, Strobel O, Hackert T, Krijgsveld J, Katus HA, Diaz MB, Backs J, Herzig S. Ataxin‐10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol Metab Elsevier 2016; 5: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis E‐M, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Gröne H‐J, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018; 137: 2592–2608. [DOI] [PubMed] [Google Scholar]
- 4. Nickel A, Löffler J, Maack C. Myocardial energetics in heart failure. Basic Res Cardiol 2013; 108: 358. [DOI] [PubMed] [Google Scholar]
- 5. Thackeray JT, Pietzsch S, Stapel B, Ricke‐Hoch M, Lee C‐W, Bankstahl JP, Scherr M, Heineke J, Scharf G, Haghikia A, Bengel FM, Hilfiker‐Kleiner D. Insulin supplementation attenuates cancer‐induced cardiomyopathy and slows tumor disease progression. JCI Insight 2017; 2: pii: 93098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umezawa R, Takanami K, Kadoya N, Nakajima Y, Saito M, Ota H, Matsushita H, Sugawara T, Kubozono M, Yamamoto T, Ishikawa Y, Takeda K, Taki Y, Takase K, Jingu K. Assessment of myocardial metabolic disorder associated with mediastinal radiotherapy for esophageal cancer—a pilot study. Radiat Oncol 2015; 10: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takanami K, Arai A, Umezawa R, Takeuchi T, Kadoya N, Taki Y, Jingu K, Takase K. Association between radiation dose to the heart and myocardial fatty acid metabolic impairment due to chemoradiation‐therapy: prospective study using I‐123 BMIPP SPECT/CT. Radiother Oncol 2016; 119: 77–83. [DOI] [PubMed] [Google Scholar]
- 8. Jingu K, Kaneta T, Nemoto K, Ichinose A, Oikawa M, Takai Y, Ogawa Y, Nakata E, Sakayauchi T, Takai K, Sugawara T, Narazaki K, Fukuda H, Takahashi S, Yamada S. The utility of 18F‐fluorodeoxyglucose positron emission tomography for early diagnosis of radiation‐induced myocardial damage. Int J Radiat Oncol 2006; 66: 845–851. [DOI] [PubMed] [Google Scholar]
- 9. R Core Team . R: a language and environment for statistical computing. Vienna, Austria; 2018.
- 10. Venables WN, Ripley BD. Modern Applied Statistics with S, Fourth Edition. New York: Springer; 2002. [Google Scholar]
- 11. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag; 2009. [Google Scholar]
- 12. Wang H, Peng J, Wang B, Lu X, Zheng JZ, Wang K, Tu XM, Feng C. Inconsistency between univariate and multiple logistic regressions. Shanghai Arch Psychiatry Oxford University Press 2017; 29: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018; 15: 457–470. [DOI] [PubMed] [Google Scholar]
- 14. Müller OJ, Heckmann MB, Ding L, Rapti K, Rangrez AY, Gerken T, Christiansen N, Rennefahrt UEE, Witt H, Maldonado SG, Ternes P, Schwab DM, Ruf T, Hille S, Remes A, Jungmann A, Weis TM, Kreußer J, Gröne H‐J, Backs J, Schatz P, Katus HA, Frey N. Comprehensive plasma and tissue profiling reveals systemic metabolic alterations in cardiac hypertrophy and failure. Cardiovasc Res 2018. 10.1093/cvr/cvy274 [DOI] [PubMed] [Google Scholar]
- 15. Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S, Miglino M, Congiu AG, Ghigliotti G, Balbi M, Brunelli C, Sambuceti G, Ameri P, Spallarossa P. An increase in myocardial 18‐fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med 2018; 16: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauckneht M, Ferrarazzo G, Fiz F, Morbelli S, Sarocchi M, Pastorino F, Ghidella A, Pomposelli E, Miglino M, Ameri P, Emionite L, Ticconi F, Arboscello E, Buschiazzo A, Massimelli EA, Fiordoro S, Borra A, Cossu V, Bozzano A, Ibatici A, Ponzoni M, Spallarossa P, Gallamini A, Bruzzi P, Sambuceti G, Marini C. Doxorubicin effect on myocardial metabolism as a prerequisite for subsequent development of cardiac toxicity: a translational 18F‐FDG PET/CT observation. J Nucl Med 2017; 58: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 17. Thut DP, Ahmed R, Kane M, Djekidel M. Variability in myocardial metabolism on serial tumor 18F‐FDG PET/CT scans. Am J Nucl Med Mol Imaging e‐Century Publishing Corporation 2014; 4: 346–353. [PMC free article] [PubMed] [Google Scholar]
- 18. Nose H, Otsuka H, Otomi Y, Terazawa K, Takao S, Iwamoto S, Iwase T, Yamada H, Sata M, Harada M. The physiological uptake pattern of 18F‐FDG in the left ventricular myocardium of patients without heart disease. J Med Invest 2014; 61: 53–58. [DOI] [PubMed] [Google Scholar]


