Abstract
Objective
Pain undertreatment, or oligoanalgesia, is frequent in the emergency department (ED), with major medical, ethical, and financial implications. Across different hospitals, healthcare providers have been reported to differ considerably in the ways in which they recognise and manage pain, with some prescribing analgesics far less frequently than others. However, factors that could explain this variability remain poorly understood. Here, we used neuroscience approaches for neural signal modelling to investigate whether individual decisions in the ED could be explained in terms of brain patterns related to empathy, risk-taking, and error monitoring.
Methods
For 15 months, we monitored the pain management behaviour of 70 ED nurses at triage, and subsequently invited 33 to a neuroimaging study involving three well-established tasks probing relevant cognitive and affective dimensions. Univariate and multivariate regressions were used to predict pain management decisions from neural activity during these tasks.
Results
We found that the brain signal recorded when empathising with others predicted the frequency with which nurses documented pain in their patients. In addition, neural activity sensitive to errors and negative outcomes predicted the frequency with which nurses denied analgesia by registering potential side-effects.
Conclusions
These results highlight the multiple processes underlying pain management, and suggest that the neural representations of others' states and one's errors play a key role in individual treatment decisions. Neuroscience models of social cognition and decision-making are a powerful tool to explain clinical behaviour and might be used to guide future educational programs to improve pain management in ED.
Keywords: analgesia, decision-making, empathy, neuroimaging, pain management
Editor's key points.
-
•
Many factors, including those related to clinicians, impact on acute pain management, resulting in significant inter-individual variability.
-
•
Neuroimaging techniques were used to explore how cognitive and emotional processes in emergency department nurses affected analgesia provision.
-
•
Neural networks associated with empathy or with risk assessment impacted on documentation of pain and analgesic contraindications.
-
•
Understanding factors contributing to individual variation in pain management is needed to develop targeted educational approaches.
The burden of unrelieved pain is a major unresolved public health problem, resulting in human suffering and economic costs. Unlike other medical conditions, pain is difficult to quantify objectively, and is mainly assessed using self-reports and indirect information about its intensity and aetiology, including medical history, previous experience, etc. As such, pain is frequently undertreated in hospitals (oligoanalgesia),1, 2 an issue which is exacerbated by the fact that healthcare providers vary widely in the willingness to prescribe analgesics, with only a fraction of this variability explainable by simple demographic characteristics (gender, age, or professional experience).3, 4, 5, 6, 7
In recent years, emergency departments (ED) worldwide have introduced computerised protocols to guide nurses in diagnosing and managing pain. Although these approaches improved the overall quality of pain management,8, 9, 10 they did not counteract oligoanalgesia, as ED nurses still underestimated and undertreated patients' pain to a variable degree.11, 12, 13, 14 This begs for the introduction of new approaches to better understand the processes underlying individual pain management decisions, which could lead to appropriate training procedures to reduce practice variation.
In the present study, we exploited recent advances in cognitive and affective neuroscience, which identified brain patterns related to personal affect and decision-making. In particular, a network involving the insula, cingulate cortex, and postcentral gyrus, was consistently implicated in empathising with other people's pain.15, 16 In addition, a partially-overlapping network in the anterior cingulate, anterior insula, and lateral prefrontal cortex was systematically associated with monitoring errors and negative outcomes from one's choices.17, 18 This growing knowledge about brain functions provided an opportunity to understand the processes underlying individual differences in pain management. In particular, we hypothesised that brain patterns related to empathy might explain individual differences in diagnosis, as healthcare providers who are less sensitive to others' suffering might report the pain of their patients less. Further, we predicted that brain patterns related to error-processing might also influence decisions at the bedside, as individuals most concerned about their performance might refrain from administering analgesics because of fear of side-effects.
Methods
Ethics approval
The study was approved by the Ethical Commission of Canton Vaud (CER-VD N°95/13) and conducted according to the declaration of Helsinki. Each participant signed an informed consent form.
Nurse-initiated analgesia protocol
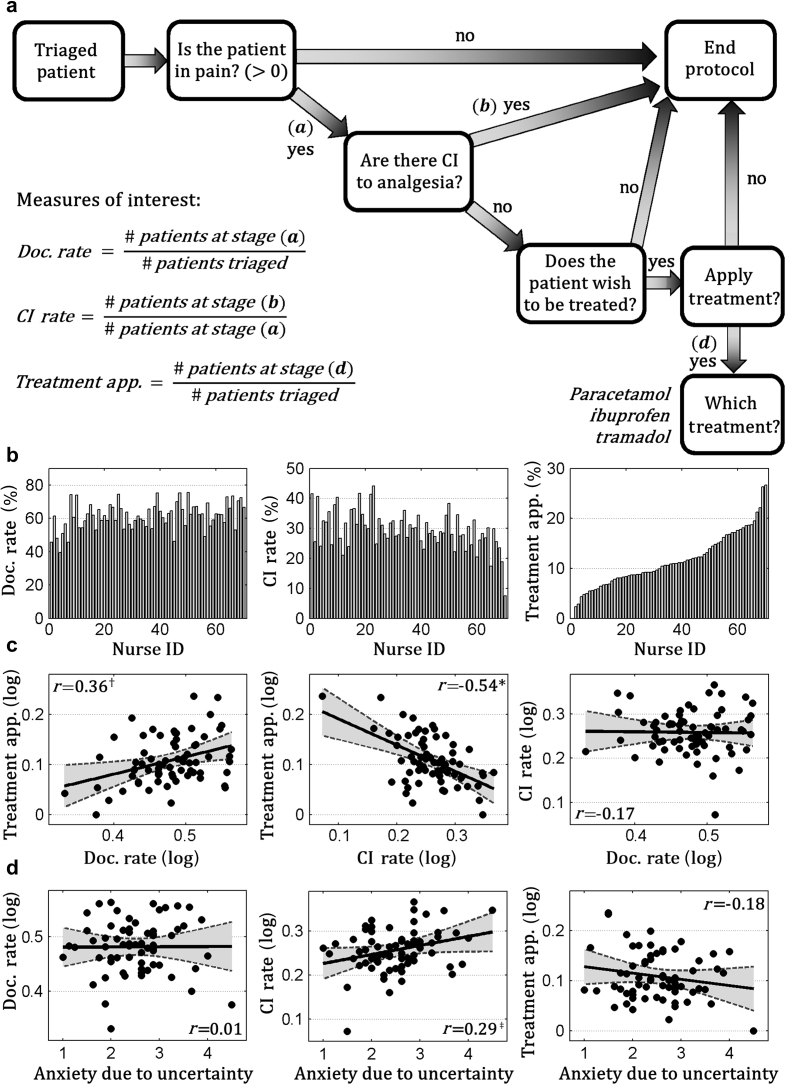
This study took advantage of a nurse-initiated analgesia protocol implemented in 2013 in the ED of the Lausanne University Hospital (Lausanne, Switzerland). The ED receives around 40 000 patients annually, each of which is initially triaged through the Swiss Emergency Triage Scale.19 Each nurse certified at using the protocol was prompted by an electronic health record to report: (1) whether the patient was in pain (> 0 using a numeric rating scale ranging from 0 [no pain] to 10 [the worst pain imaginable]); (2) whether there were contraindications (CIs) to analgesia; (3) whether the patient wished to receive analgesia; and (4) whether an appropriate treatment (paracetamol, ibuprofen, tramadol) should be selected (Fig. 1a). Importantly, as protocol data were recorded at triage, the assignment of patients to nurses was based exclusively on personnel availability, without any preselection in terms of acuity/aetiology. Hence, the nurses' identity was independent from the cases examined.
Fig 1.
(a) Flowchart including the key steps of the nurse-led protocol implemented in the emergency department. Nurses were expected to follow and document this procedure for each patient under their care. Data collected for each nurse over 15 months after the protocol implementation were used to estimate three different scalars indexing their pain management behaviour (pain documentation rate, contraindication rate, and treatment application). Each measure was computed as the percentage among patients who passed a specific protocol step, as noted in the flowchart. Full details are given in the Methods section. (b) Bar-graphs displaying between-nurse variability in pain management behaviour. Each subplot represents one of the three scalars of interest, whereas each bar represents one isolated nurse. Nurses' identity is coded with a number ranging from 1 to 70 according to their percentage of treatment application value. (c) Scatter plots describing the linear relation between the three measures. (d) Scatter plots describing the linear relation between the anxiety attributable to uncertainty score and each of the three behavioural measures of interest. Each plot shows a linear regression line (with a grey area describing the 95% confidence interval), plus the Pearson correlation coefficient. The significance of the correlation is highlighted as follows: *P<0.001, †P<0.01, ‡P<0.05. App., application; CI, contraindication; Doc, documentation.
Pain management measures
We used the electronic health record to retrieve information about the pain management decisions of each certified nurse for 15 months after the protocol implementation. Specifically, we focused on data from eligible patients (>16 yr old, in pain for less than 3 months, without history of drug/alcohol abuse, and no life-threatening condition) to estimate the following measures (see Fig. 1a for more details). Treatment application: proportion of decisions to deliver analgesia on triaged patients. This index was then broken down into two sub-indexes. (1) Documentation rate: the proportion of pain documentations on triaged patients. (2) CI rate: the proportion of CIs to analgesia documented in those patients who were in pain.
Participants
Nine months after the protocol implementation, all certified nurses were invited to take part in a survey probing for demographic information, work experience, and the anxiety from uncertainty scale.20 Subsequently, between 16 and 18 months after the protocol implementation, a subgroup was invited to take part in a study involving functional magnetic resonance imaging (fMRI). This subgroup included an equal proportion of individuals from each tertile of the Treatment Application distribution obtained from a preliminary analysis of protocol data (6 months from the implementation). This selection ensured that the tested individuals would represent a broad spectrum of protocol use.
Neuroimaging intervention
The neuroimaging study involved the following three experimental paradigms (see Supplementary material for more details).
1. Empathy for pain task.15, 21 Nurses saw pictures depicting hands in painful situations (wounded, pierced by a syringe, etc.), and control stimuli involving hands without any aversive feature. The task included 30 stimuli per condition, each presented for 2.5 s and followed by an inter-stimulus interval ranging between 2.5 and 4.1 s. This task lasted about 15 min.
2. Balloon analogue risk task.22, 23 Nurses had to adjust to risk in a gambling context, by pressing a key repeatedly to inflate a virtual balloon as much as possible and stop just before it exploded. If they stopped before the explosion, they received a virtual monetary gain proportional to the volume of air pumped (win condition); however, they received nothing if the balloon exploded (loss condition). The task involved 28 game iterations, each leading to a potential win/loss. Every game included up to 11 inflations, each remaining on the screen until a response was provided, and followed by an inter-inflation interval ranging between 1.5 and 2.5 s. Win/loss feedbacks lasted 2.5 s and were followed by an interval ranging between 2 and 4 s. The task never exceeded 15 min.
3. Social harm avoidance monitoring experiment.24 We implemented an error-monitoring task involving similar stakes to clinical decision making, where one's errors may cause harm to another person (the patient). The nurse inside the scanner took turns with a colleague outside (another nurse from the experimental group) in performing a dot-counting task. Overall, there were 98 trials, organised in 14 blocks (seven per player) of seven trials each. Every erroneous response had a 50% probability to cause a painful stimulation to the arm of the nurse outside the scanner, and was signalled with an ad hoc feedback for 5 s, followed by an interval ranging between 2 and 9 s. The overall amount of correct/erroneous trials depended on participants' proficiency in the counting task, whose difficulty was adjusted on-line to avoid ceiling/floor effects. The critical condition was when the nurse in the scanner caused pain to the one outside (one's painful errors). This was compared with a condition in which the same harmful outcome was caused by the nurse outside to him/herself (others' painful errors). The task lasted 12 min.
Data analysis
In the behavioural survey, we first assessed the dependency between the three pain management measures through Pearson's correlation coefficient. Subsequently, we assessed how each of these three measures was related with age, gender, years of experience, and anxiety for uncertainty. Results are reported as significant under an α=0.003 (Bonferroni-corrected for 15 tests).
Uncorrected effects (α=0.05) associated with anxiety for uncertainty scores are also reported, as one of the aims of the study was to investigate specifically how error/uncertainty processing might affect different stages of pain management.
For the neuroimaging investigation, we first preprocessed functional data of each nurse using SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/) to account for head movements, geometric distortions by the magnetic field, and anatomical differences between subjects. The preprocessed images were then fed to first-level general linear models (GLMs) testing, in each task, for increased activity in the main condition of interest, and for the tailored control (see previous studies15, 21, 22, 23, 24 and Supplementary material for details). The activity maps estimated in each individual GLM were then used for group-level analyses testing whether the condition of interest in each task: (1) exhibited increased activity with respect to the control; and (2) was linearly modulated by nurses' professional behaviour. Activations were reported if surviving correction for multiple comparisons for the whole brain or for regions-of-interest masks. These masks were obtained by reanalysing, under the same parameters used here, previous datasets obtained by running the same three paradigms on lay individuals15, 23, 24 (see Supplementary Tables S1–S3 for more details).
In addition, we used least absolute shrinkage and selection operator (LASSO)25, 26, 27, 28 and random forest (RF) regression29 to identify distributed patterns of activity that could predict nurses' professional behaviour. In particular, this analysis involved: (1) extracting the activity associated with each event of interest from a priori masks (the same used for the univariate analysis); (2) feeding the extracted signal to the two algorithms for multivariate modelling; (3) testing the generalisability of the estimated models through cross-validation techniques [i.e. assessing whether a model tailored on a portion of subjects could predict the clinical behaviour of the remaining (independent) subjects]; and (4) obtaining an overall mean squared error as the measure of prediction proficiency, which was then validated statistically through permutation techniques (see Supplementary material). The code for the multivariate analysis is available at the Open Science Framework (see Appendix 1).
Results
70 ED nurses responded to the survey and 33 agreed to take part in a subsequent neuroimaging investigation (see Table 1 for details). Two nurses asked to discontinue the neuroimaging session prematurely: hence, the balloon analogue risk task (BART) was completed by 32 participants and the social harm avoidance monitoring experiment (SHAME) by 31.
Table 1.
Demographic information. Eligible emergency department (ED) nurses responding to the survey, and subsequently subdivided into those who took part in the neuroimaging investigation, and those who did not. Each of the three groups is described in terms of overall size, number of women (including percentage value to the overall size), and median age, experience in the ED, and number of triages per nurse in a time window of 15 months (bracket values refer to inter-quartile range). For each of the measures reported, the subgroup taking part to the neuroimaging investigation discloses similar values to the group who did not
| Survey | Neuroimaging participants | Other participants | |
|---|---|---|---|
| Population size | 70 | 33 | 37 |
| Females, n (%) | 51 (73) | 22 (67) | 29 (78) |
| Age (yr) | 33 (31, 38) | 34 (31, 39) | 33 (30, 37) |
| ED experience (yr) | 6 (4, 9) | 9 (4, 13) | 6 (4, 8) |
| Triages per nurse | 452 (273, 694) | 480 (405, 694) | 445 (210, 692) |
Behavioural survey
When assessing the nurse-led analgesia protocol data, we found a large inter-individual variability in treatment application (Fig. 1b). This variability was related to both individual documentation rate and CI rate: nurses who applied analgesia more frequently were more inclined to document patients' pain (r=0.36, P=0.002), and less likely to report CIs (r=−0.54, P<0.001) (Fig. 1c). None of these indexes were associated with nurses' age, years of experience (|r|≤0.17, not significant) or gender (|t|≤0.99; except for potentially larger documentation rate in male nurses t(30.31)=2.15, P=0.039, uncorrected). Interestingly, nurses with higher scores on the anxiety from uncertainty scale showed higher CI rates (r=0.29, P=0.017 uncorrected; for the other indexes |r|≤0.18, not significant).
Neural responses to others' pain
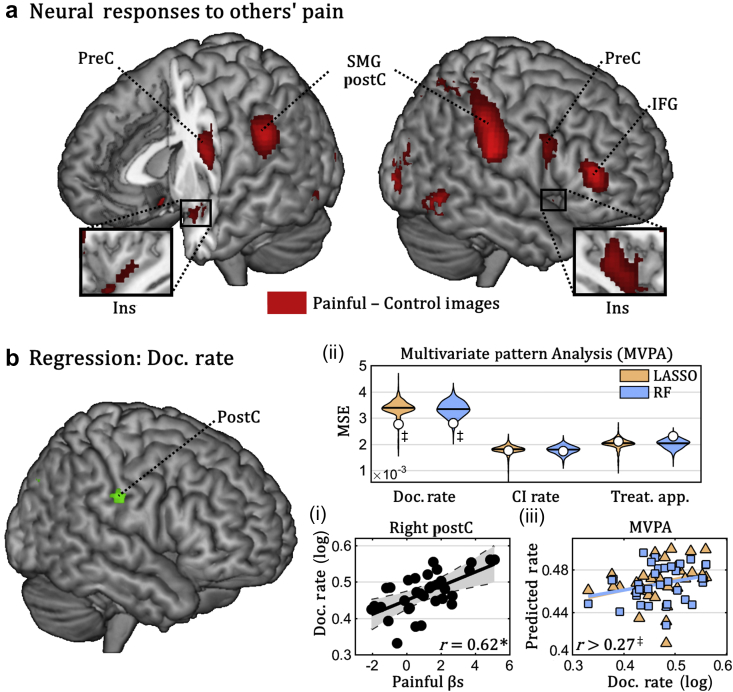
Subsequently, we engaged a subgroup of nurses in an fMRI task where they witnessed pictures of injured hands. This task recruited a brain network classically associated with pain-processing and empathy,15, 16, 21 involving the posterior insula, postcentral gyrus, and midline cortical areas (Fig. 2a). No activation was observed in the anterior insula and middle cingulate cortex, which are known to respond to others' pain in lay individuals, but not in professional healthcare providers.30, 31
Fig 2.
Empathy for pain. (a) Whole brain map depicting regions implicated in processing pictures of injured hands (painful–control images). (b) Linear regression of documentation rate. Surface rendering of a human brain highlighting suprathreshold coordinates in which neural responses to painful images explained nurses' documentation rate in univariate linear regression. Three subplots (i–iii) are also displayed. Subplot (i) describes the linear relation between documentation rate and the average parameter extracted by the right postcentral gyrus (grey area refers to the 95% confidence interval). Subplots (ii) and (iii) refer to data from multivariate pattern analysis (MVPA) (colour-coded according to the machine-learning algorithm used). On top, the overall proficiency of least absolute shrinkage and selection operator (LASSO) and random forest (RF) classifiers for prediction of the three clinical measures of interest is displayed. White circles refer to mean square error (MSE) associated with out-of-subject predictions, superimposed with violin-plots of the permutation-based null distribution of MSE. Subplot (iii) describes the linear regression between nurses' documentation rate and the value predicted by each of the two classifiers. CI, contraindication; Doc., documentation; IFG, inferior frontal gyrus; Ins, insula; PostC, postcentral gyrus; PreC, precentral gyrus; r, Pearson correlation coefficient; SMG, supramarginal gyrus; Treat. App., treatment application. *P<0.001, ‡P<0.05 associated with standard parametric analysis (for linear regressions) and permutation-based analysis (for MVPA).
We then tested whether these neural responses to others' pain could predict nurses' clinical behaviour. First, by using a univariate linear regression, we found a significant relationship between the activity in the right postcentral cortex and documentation rate, with stronger neural responses to injured hands in those who most frequently reported patients' pain in their daily work. We then tested whether clinical behaviour could be predicted from distributed patterns of brain activity (rather than isolated regions) during this task. For this purpose, we extracted the neural activity evoked by viewing injured hands from a predefined network (see Methods), and fed it to two machine learning algorithms (LASSO and RF) to predict clinical behaviour. Both algorithms revealed that empathy-related activity was a good predictor of the documentation rate of individual nurses (Fig. 2b). No significant effects (neither univariate nor multivariate) were associated with the other two measures.
Neural responses to negative outcomes
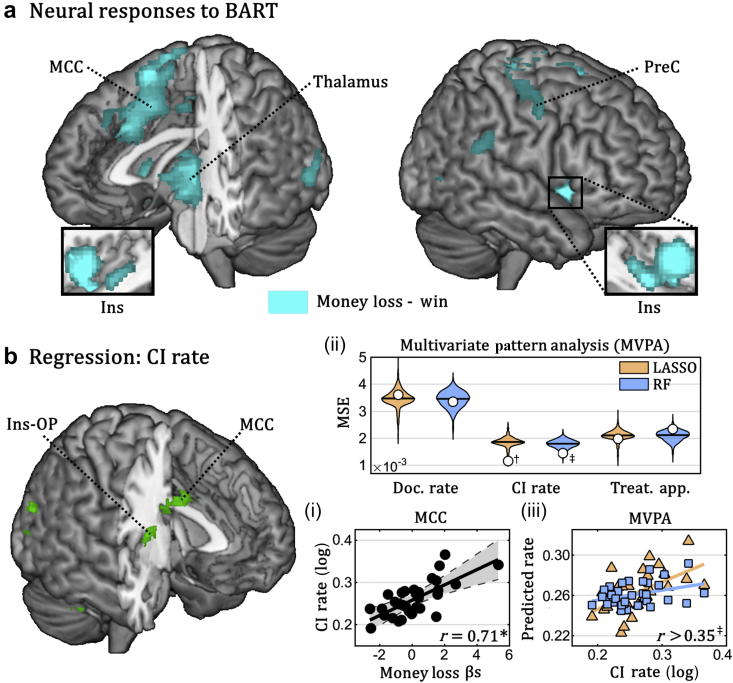
We performed similar analyses for brain activity evoked when observing self-caused errors and negative outcomes. When confronted with monetary losses (vs gains) in the BART,22, 23 nurses exhibited widespread activations in the middle cingulate cortex, anterior insula, and thalamus (Fig. 3a), a network often associated with the detection of errors,17, 18 and other salient outcomes.32, 33 Univariate linear regression showed that the activity of several regions within this network, including the insula and cingulate areas, were related to the documentation of CIs to analgesia. In addition, multivariate regression with LASSO and RF revealed that distributed patterns of activity related to money loss were a reliable predictor of nurses' CI rate (Fig. 3b).
Fig 3.
Balloon analogue risk task (BART). (a) Whole brain map depicting regions implicated in money loss (loss–win). (b) Linear regression of CI Rate. Surface rendering of a human brain highlighting suprathreshold coordinates in which neural responses to money loss explained nurses' CI rate in univariate linear regression. Three subplots (i–iii) are also displayed. Subplot (i) describes the linear relation between CI rate and the average parameter extracted by the middle cingulate cortex (grey area refers to the 95% confidence interval). Subplots (ii) and (iii) refer to data from multivariate pattern analysis (MVPA) (colour-coded according to the machine-learning algorithm used). On top, the overall proficiency of least absolute shrinkage and selection operator (LASSO) and random forest (RF) classifiers for prediction of the three clinical measures of interest. White circles refer to mean square error (MSE) associated with out-of-subject predictions, superimposed with violin-plots of the permutation-based null distribution of MSE. Subplot (iii) describes the linear regression between nurses' CI rate and the value predicted by each of the two classifiers. BART, balloon analogue risk task; CI, contraindication; Ins, insula; MCC, middle cingulate cortex; OP, parietal operculum; PreC, precentral gyrus; r, Pearson correlation coefficient; Treat. App., treatment application. *P<0.01, †P<0.01, ‡P<0.05 associated with standard parametric analysis (for linear regressions) and permutation-based analysis (for MVPA).
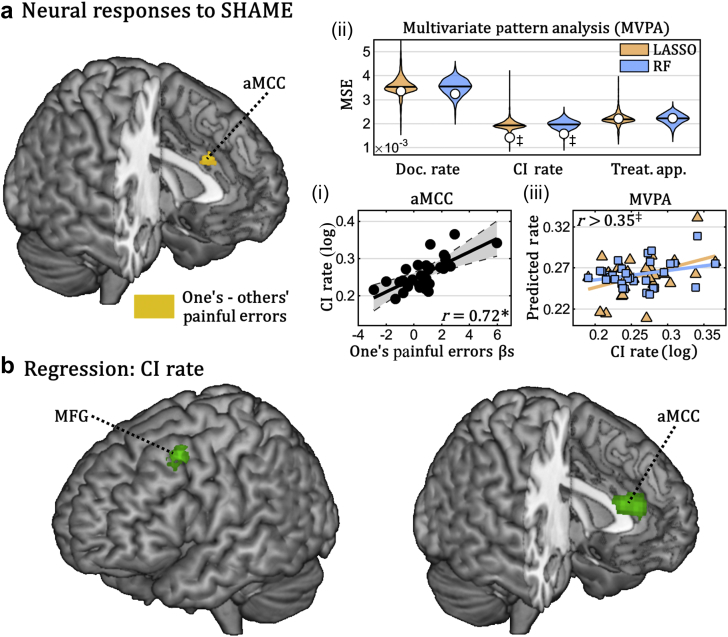
Similarly, when observing harmful consequences of their own (vs someone else's) errors in the SHAME,24 nurses activated the anterior portion of the middle cingulate cortex. Moreover, regression analysis showed that activity related to one's painful errors was linearly coupled with CI rate in both the middle cingulate cortex and the left middle frontal gyrus. Thus, as found for the BART, these areas were more strongly activated in those individuals who were more likely to spot CIs to analgesia. Finally, LASSO and RF regression confirmed that activity patterns in the network activated by harmful errors were a reliable predictor of CI rate (Fig. 4). Data from neither BART nor SHAME were significantly associated with the other two clinical measures.
Fig 4.
Social harm avoidance monitoring experiment (SHAME). (a) Whole brain map depicting regions implicated in painful outcomes of one's errors (one's–others’ painful errors). (b) Linear regression of CI rate. Surface rendering of a human brain highlighting suprathreshold coordinates in which neural responses to one's painful errors explained nurses' CI rate in univariate linear regression. Three subplots (i–iii) are also displayed. Subplot (i) describes the linear relation between CI rate and the average parameter extracted by the anterior middle cingulate cortex (grey area refers to the 95% confidence interval). Subplots (ii) and (iii) refer to data from multivariate pattern analysis (MVPA) (colour-coded according to the machine-learning algorithm used). On the top, the overall proficiency of least absolute shrinkage and selection operator (LASSO) and random forest (RF) classifiers for prediction of the three clinical measures of interest. White circles refer to mean square error (MSE) associated with out-of-subject predictions, superimposed with violin-plots of the permutation-based null distribution of MSE. Subplot (iii) describes the linear regression between nurses' CI rate and the value predicted by each of the two classifiers. aMCC, anterior middle cingulate cortex; CI, contraindication; Doc., documentation; MFG, middle frontal gyrus; Treat. App., treatment application. *P<0.001; ‡P<0.05 associated with standard parametric analysis (for linear regressions) and permutation-based analysis (for MVPA).
Discussion
Healthcare providers appraise and treat pain very differently from one another,3, 4, 5, 6, 7 resulting in patients being more or less likely to receive analgesia according to the person who is in charge of them. The demographic characteristics of healthcare providers only partially explain this variability,3 suggesting that other factors are at play. By using a battery of well-established questionnaires20 and experimental paradigms from neuroscience,15, 21, 22, 23, 24 we shed new light on the mechanisms underlying these inter-individual differences. First, the likelihood of reporting CIs to analgesia in clinical practice can be explained by personal anxiety towards uncertain outcomes (from the behavioural survey), and differences in brain responses to negative feedbacks (neuroimaging investigation). Second, the frequency of documenting patients' pain can be explained by differences in brain patterns evoked by witnessing others' injuries. Overall, our study underscores the role played by two main processes which exert opposite, but concurrent influences on the decision leading to the prescription of analgesics in clinical practice.
Ideally, choices such as documenting a symptom, reporting CIs, or prescribing treatment should be motivated exclusively by the clinical characteristics of patients. Hence, no variability should be observed between ED nurses, as long as they all handle a similar mix of cases, matched in aetiology and severity. Surprisingly however, nurses differ substantially from one another, ranging from those who prescribe analgesics to ∼5% up to 20% of patients (Fig. 1b; see also3, 4, 5, 6, 7). Considering that patients' assignment was independent of the nurses' identity, and that the clinical variables of interest were obtained by collapsing data from all cases handled by each operator in 15 months (see Methods), it is unlikely that the observed variability was influenced by the severity of patients examined. Instead, it is more plausible that each nurse is characterised by a personal disposition/attitude towards pain management. Previous studies have already categorised healthcare providers according to their attitudes (more vs less attentive to case severity,5 more vs less reliant on patients' self-reports11), without, however, shedding light on the processes that might contribute to this categorisation. Our study extends previous findings, not only by providing a working model according to which pain management is driven by two clear dimensions, but also by associating these processes with distinct brain networks.
Brain responses evoked by observing others' pain have been thoroughly investigated in neuroscience research, pointing to a major role of the insula, middle cingulate cortex, and postcentral gyrus.16 The most popular interpretation of these activations is that they reflect the engagement of circuits implicated in first-hand nociception, which are then re-enacted ‘empathetically’ when pain is not felt on oneself but observed in others.15, 16 Critically, however, these regions are not homogeneous in their function, but can be broadly classified into two functionally segregated networks, coding different aspects of the painful experience. In particular, brain patterns in the anterior insula and middle cingulate cortex might not be pain-specific, but generalise also to other aversive experiences such as arousing pictures,15 disgusting tastes, or monetary losses.34 Hence, these regions could serve a domain-general purpose involved in detecting events of high relevance for one's survival,32 including errors17, 18 and risky decisions,22, 23 with painful or financial consequences for oneself and others.33 In contrast, the posterior insula and postcentral somatosensory cortex appear to process pain in a more specific fashion, with little generalisation to other forms of affect.15, 35 This might underlie a sensory-specific component of the painful experience, which is re-enacted when also witnessing others' sufferance.15, 16 In our study, these functionally segregated networks were associated with independent components of pain management, with the postcentral gyrus predicting the frequency with which healthcare providers documented pain in patients, and the middle cingulate cortex predicting the frequency with which they noted potential CIs.
Overall, our study offers a comprehensive model of pain management decisions in which healthcare providers hold at least two distinct representations of their patient's state. First, there is the patient's current pain, which is estimated through evaluation of diagnostic signs and self-reports, but also influenced by doctors and nurses' empathic skills. Second, there is the patient's prospective state, which is estimated by predicting the potential consequences of analgesia and thus taps into one's ability to make decisions under uncertainty and to learn from previous errors. Critically, although healthcare providers are deontologically bound to relieve patients' current pain with analgesia, they are equally bound to prevent potential side-effects by withholding analgesia, a conflict which is resolved differently in each individual, based on specific characteristics of the case, but also personal traits of empathy, dispositions towards errors/uncertainty, etc. Training techniques already exist to modulate empathy and compassion,36 but also to help individuals reduce anxiety about potential errors.37 These could serve as a basis for future educational programs for doctors and nurses, to promote a more efficient pain treatment and a more coherent level of care.
In this study, we exploited the rare opportunity to monitor pain management behaviours of professional healthcare providers for 15 months, and relate them to brain activity patterns in well-known tasks. The drawback of this approach lies in the difficulty of obtaining independent cohorts (e.g. for assessing power or replicating effects), as other hospitals usually do not record the same behavioural indexes. The application of rigorous cross-validation techniques insured generalisability within the sample tested. However, only future implementations of the same pain management protocol in other EDs will allow us to extend our findings to different countries and healthcare systems.
Authors' contributions
Study design: CCD, MF, YF, PV, OH
Data collection: CCD, GS
Data analysis: CCD, LT
Interpretation of results: CCD, PV, OH
Manuscript drafting: CCD
Subjects recruitment: MF, EF
Manuscript critical revision: MF, GS, LT, EF, YF, PV, OH
Approved the final version of the manuscript and agree to be accountable for all aspects of the work (thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved): all authors.
Acknowledgements
We would like to thank Matthias Zunhammer for his advices in relation to the LASSO method and Franca Davenport and Kimberly C. Doell for overseeing the quality of the English text.
Handling editor: L. Colvin
Editorial decision: 04 January 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.01.039.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Swiss National Science Foundation grant numbers PP00O1_157424/1 (CCD) and 32003B_138413 (PV). UPSA pain foundation and the French Society for Emergency Medicine (SFMU; partially supported the salary of MF).
Appendix 1.
De-identified data files and scripts for the multivariate analyses are available at Open Science Framework: https://osf.io/2bved/.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Rupp T., Delaney K.A. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004;43:494–503. doi: 10.1016/j.annemergmed.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Wilson J.E., Pendleton J.M. Oligoanalgesia in the emergency department. Am J Emerg Med. 1989;7:620–623. doi: 10.1016/0735-6757(89)90286-6. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht E., Taffe P., Yersin B., Schoettker P., Decosterd I., Hugli O. Undertreatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective study. Br J Anaesth. 2013;110:96–106. doi: 10.1093/bja/aes355. [DOI] [PubMed] [Google Scholar]
- 4.Heins J.K., Heins A., Grammas M., Costello M., Huang K., Mishra S. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. J Emerg Nurs. 2006;32:219–224. doi: 10.1016/j.jen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Marquié L., Sorum P.C., Mullet E. Emergency physicians’ pain judgments: cluster analyses on scenarios of acute abdominal pain. Qual Life Res. 2007;16:1267–1273. doi: 10.1007/s11136-007-9228-y. [DOI] [PubMed] [Google Scholar]
- 6.Green C.R., Wheeler J.R.C., LaPorte F. Clinical decision making in pain management: contributions of physician and patient characteristics to variations in practice. J Pain. 2003;4:29–39. doi: 10.1054/jpai.2003.5. [DOI] [PubMed] [Google Scholar]
- 7.Barnett M.L., Olenski A.R., Jena A.B. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376:663–673. doi: 10.1056/NEJMsa1610524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly A.-M. Nurse-managed analgesia for renal colic pain in the emergency department. Aust Health Rev. 2000;23:185–189. doi: 10.1071/ah000185. [DOI] [PubMed] [Google Scholar]
- 9.Muntlin Å., Carlsson M., Säfwenberg U., Gunningberg L. Outcomes of a nurse-initiated intravenous analgesic protocol for abdominal pain in an emergency department: a quasi-experimental study. Int J Nurs Stud. 2011;48:13–23. doi: 10.1016/j.ijnurstu.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Cabilan C.J., Boyde M. A systematic review of the impact of nurse-initiated medications in the emergency department. Australas Emerg Nurs J. 2017;20:53–62. doi: 10.1016/j.aenj.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Vuille M., Foerster M., Foucault E., Hugli O. Pain assessment by emergency nurses at triage in the emergency department: a qualitative study. J Clin Nurs. 2018;27:669–676. doi: 10.1111/jocn.13992. [DOI] [PubMed] [Google Scholar]
- 12.Duignan M., Dunn V. Congruence of pain assessment between nurses and emergency department patients: a replication. Int Emerg Nurs. 2008;16:23–28. doi: 10.1016/j.ienj.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Modanloo M., Sayed Fatemi N., Bastani F., Peyrovi H., Behnampour N., Hesam M. Comparison of pain assessment by patients and triage nurses. Iran J Crit Care Nurs. 2010;4:23–28. [Google Scholar]
- 14.Puntillo K., Neighbor M., O’Neil N., Nixon R. Accuracy of emergency nurses in assessment of patients’ pain. Pain Manag Nurs. 2003;4:171–175. doi: 10.1016/s1524-9042(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 15.Corradi-Dell’Acqua C., Hofstetter C., Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamm C., Decety J., Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Klein T.A., Endrass T., Kathmann N., Neumann J., von Cramon D.Y., Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Taylor S.F., Stern E.R., Gehring W.J. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- 19.Rutschmann O.T., Hugli O.W., Marti C. Reliability of the revised Swiss Emergency Triage Scale: a computer simulation study. Eur J Emerg Med. 2018;25:264–269. doi: 10.1097/MEJ.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bovier P.A., Perneger T.V. Stress from uncertainty from graduation to retirement--a population-based study of Swiss physicians. J Gen Intern Med. 2007;22:632–638. doi: 10.1007/s11606-007-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao-Tasserit E., Corradi-Dell’Acqua C., Vuilleumier P. The good, the bad, and the suffering. Transient emotional episodes modulate the neural circuits of pain and empathy. Neuropsychologia. 2018;116:99–116. doi: 10.1016/j.neuropsychologia.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Rao H., Korczykowski M., Pluta J., Hoang A., Detre J.A. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schonberg T., Fox C.R., Mumford J.A., Congdon E., Trepel C., Poldrack R.A. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an FMRI investigation of the balloon analog risk task. Front Neurosci. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koban L., Corradi-Dell’Acqua C., Vuilleumier P. Integration of error agency and representation of others’ pain in the anterior insula. J Cogn Neurosci. 2013;25:258–272. doi: 10.1162/jocn_a_00324. [DOI] [PubMed] [Google Scholar]
- 25.Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.-W., Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L.J., Gianaros P.J., Manuck S.B., Krishnan A., Wager T.D. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan A., Woo C.-W., Chang L.J. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife. 2016;5:e15166. doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zunhammer M., Geis S., Busch V., Eichhammer P., Greenlee M.W. Pain modulation by intranasal oxytocin and emotional picture viewing—a randomized double-blind fMRI study. Sci Rep. 2016;6:31606. doi: 10.1038/srep31606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 30.Cheng Y., Lin C.-P., Liu H.-L. Expertise modulates the perception of pain in others. Curr Biol. 2007;17:1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y., Chen C., Decety J. How situational context impacts empathic responses and brain activation patterns. Front Behav Neurosci. 2017;11:165. doi: 10.3389/fnbeh.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 33.Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Corradi-Dell’Acqua C., Tusche A., Vuilleumier P., Singer T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat Commun. 2016;7:10904. doi: 10.1038/ncomms10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klimecki O.M., Leiberg S., Ricard M., Singer T. Differential pattern of functional brain plasticity after compassion and empathy training. Soc Cogn Affect Neurosci. 2014;9:873–879. doi: 10.1093/scan/nst060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keith N., Frese M. Effectiveness of error management training: a meta-analysis. J Appl Psychol. 2008;93:59–69. doi: 10.1037/0021-9010.93.1.59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.