Summary
Surgery is an important treatment modality for the majority of solid organ cancers. Unfortunately, cancer recurrence following surgery of curative intent is common, and typically results in refractory disease and patient death. Surgery and other perioperative interventions induce a biological state conducive to the survival and growth of residual cancer cells released from the primary tumour intraoperatively, which may influence the risk of a subsequent metastatic disease. Evidence is accumulating that anaesthetic and analgesic interventions could affect many of these pathophysiological processes, influencing risk of cancer recurrence in either a beneficial or detrimental way. Much of this evidence is from experimental in vitro and in vivo models, with clinical evidence largely limited to retrospective observational studies or post hoc analysis of RCTs originally designed to evaluate non-cancer outcomes. This narrative review summarises the current state of evidence regarding the potential effect of perioperative anaesthetic and analgesic interventions on cancer biology and clinical outcomes. Proving a causal link will require data from prospective RCTs with oncological outcomes as primary endpoints, a number of which will report in the coming years. Until then, there is insufficient evidence to recommend any particular anaesthetic or analgesic technique for patients undergoing tumour resection surgery on the basis that it might alter the risk of recurrence or metastasis.
Keywords: anaesthesia, general; anaesthesia, inhalational; anaesthesia, intravenous; analgesia; cancer, recurrence; inflammation; opioid; stress response; surgery
Cancer is the second leading cause of death worldwide, resulting in 9.6 million deaths in 2018, and the overall global incidence of new cancer cases is predicted to increase from 14 million in 2012 to 24 million by 2035.1, 2 Surgery is potentially curative for many solid organ cancers, and over 80% of patients with cancer will undergo at least one surgical procedure.3 Unsurprisingly then, cancer-related surgeries form a large and expanding workload for surgeons and anaesthetists worldwide.
Recurrence following surgery presents a huge morbidity and mortality burden for patients, as well as a wider societal and economic burden. A metastatic disease is typically refractory to treatment, and the majority of cancer-related deaths are due to metastasis.4 Recurrence following surgical resection varies dramatically and is influenced by many factors, including primary organ affected and tumour grade and stage. Undoubtedly, surgical technique affects recurrence risk given the requirement to achieve clear sample margins and minimise tumour handling to prevent the dispersal of tumour cells. What is less clear is whether anaesthetic, analgesic, or other perioperative interventions (e.g. the use of steroids, beta blockers, or systemic lidocaine) may also have an influence on cancer recurrence.5 Initial retrospective studies suggesting that certain techniques may have beneficial effects on reducing recurrence rates were followed by others with contradictory results. Laboratory studies, examining the effects of commonly used anaesthetic and analgesic drugs on cancer cells in vitro, have suggested a signal that some agents demonstrate potentially beneficial anticancer effects and others potentially detrimental cancer-promoting effects.6 Definitive evidence requires prospective, randomised clinical trials. To date, no trial has been published that examines the effect of an anaesthetic or analgesic technique during primary cancer surgery on long-term oncological outcomes, such as disease-free survival, although a number are in progress and should report in the coming years.
This review outlines the significant progress that has been made in understanding the pathophysiological mechanisms in the perioperative period that underlie cancer recurrence and metastasis, and how anaesthetic and analgesic agents may influence them.
Mechanisms of cancer recurrence following surgery
The mechanisms underlying post-surgical cancer recurrence are complex and incompletely understood. Following an intended curative surgical resection of a primary tumour, cancer may recur at a number of sites by a variety of mechanisms:7
-
(i)
Local recurrence at the tumour resection site due to proliferation of residual cells
-
(ii)
Lymph-node metastasis due to tumour cells released into the lymphatic system before or during the procedure
-
(iii)
Distant organ metastasis due to seeding by circulating tumour cells (CTCs) released before or during the procedure
-
(iv)
Seeding within a body cavity (e.g. peritoneal spread)
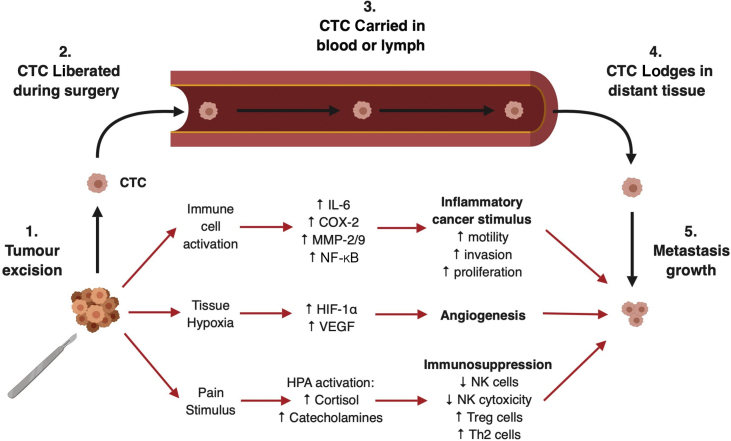
The likelihood that individual cancer cells will ‘seed’ in tissue and progress to a clinically significant metastatic disease is influenced by pathophysiological changes induced by surgery (see Fig. 1), and potentially by anaesthesia and perioperative events, including hypothermia and blood transfusion.8 Cancer cells exist in a complex tissue microenvironment involving the interplay of surrounding non-cancerous stromal cells, immune system cells, extracellular matrix, chemokines, cytokines, and myriad other factors.9 This delicate microenvironment is easily disrupted by tissue trauma, and the surgical intervention aiming to eliminate the disease may inadvertently create conditions that promote not only survival, but progression, proliferation, and spread of residual cancer cells. Such surgery-induced physiological changes are numerous and include inflammation, tissue hypoxia, angiogenesis, surgical stress response, and immunosuppression.7 These changes can drive the process known as ‘epithelial-to-mesenchymal transition’, whereby epithelial cancer cells develop a mesenchymal phenotype facilitating cellular motility, and thus, metastatic potential.10
Fig. 1.
Schematic representation of the pathophysiological mechanisms induced by surgery that promote survival and growth of metastatic deposits formed by circulating tumour cells (CTCs) released intraoperatively. COX-2, cyclo-oxygenase-2; HIF, hypoxia-inducible factor; HPA, hypothalamic–pituitary–adrenal axis; IL-6, interleukin 6; MMP, matrix metalloprotease; NF-κB, nuclear factor kappa B; NK, natural killer cell; Th2, Type 2 helper T cell; Treg, regulatory T cell; VEGF, vascular endothelial growth factor. Created with BioRender.
Stress response and immunosuppression
Identifying and eliminating cancer cells are a crucial function of human immunity, and prominent in the delivery of this ‘immune surveillance’ are the natural killer (NK) cells of the innate immune system and the cytotoxic CD8+ T cells of the adaptive immune system.11 After surgery, there is an initial pro-inflammatory phase, followed by a period of immunosuppression during which the immune system's ability to detect and eradicate cancer cells is diminished.12 This suppression may be related to a combination of mechanisms, including the surgical stress response activating the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenal (HPA) axis, increased circulating inflammatory mediators, hypothermia, allogeneic blood transfusion, and potentially anaesthetic or analgesic agents.7, 13, 14 Activation of the HPA axis stimulates the release of cortisol and catecholamines—these humoral factors not only inhibit the proliferation and antitumour activity of NK cells and CD8+ T cells, but also promote the proliferation of regulatory T cells (Tregs) and Type 2 helper T cells (Th2), which have pro-tumour effects.7 Tumour cells bear ß-adrenoceptors that, upon activation, trigger the increased expression of factors associated with metastasis, including pro-inflammatory interleukin-6 (IL-6), pro-angiogenic vascular endothelial growth factor (VEGF), and matrix metalloprotease enzymes (including MMP-2 and MMP-9, which degrade extracellular matrix and facilitate cancer cell mobility and invasion).8
Inflammation
Even an optimal surgical technique necessarily injures normal tissue in order to remove a tumour. Trauma to residual non-cancerous tissue induces inflammation required for the physiological wound-healing response to occur, but is potentially detrimental to other organs.15 The inflammatory process involves the release of humoral factors, including prostaglandins (PGE), cytokines (e.g. IL-6), tumour necrosis factor alpha, and chemokines, resulting in the recruitment and activation of macrophages, neutrophils, and fibroblasts.16 Recruited cells release further cytokines and growth factors, which promote desirable localised tissue healing. Unfortunately, these factors may also promote residual cancer cell viability at the site of resection by stimulating cancer cell proliferation and migration, as well as depressing the immune function by inhibiting NK cytotoxicity.17, 18 Moreover, distant sites of inflammation, with disrupted endothelial surfaces and bathed in growth factors, may provide favourable sites for seeding by CTCs liberated during surgery, a phenomenon termed ‘inflammatory oncotaxis’.19
The mechanisms underlying inflammation involve the increased expression of numerous components of inflammatory pathways implicated in tumorigenesis, including20
-
(i)
Enzymes (e.g. MMP and cyclo-oxygenase-2 [COX-2])
-
(ii)
Transcription factors (e.g. hypoxia-inducible factor 1-alpha [HIF-1], nuclear factor kappa-B [NF-κB], and signal transducer and activator of transcription 3)
-
(iii)
Chemokine receptors (e.g. CXC chemokine receptor 2 [CXCR2])
Individual differences of expression of certain factors between patients may influence disease progression (e.g. overexpression of COX-2 and CXCR2 is implicated in progression of several cancer types).21, 22 These cellular pathways have been examined as potential mechanisms to explain how anaesthetic and analgesic agents may influence disease progression (e.g. lidocaine reducing MMP release in lung cancer cells, or COX-2 inhibitors inhibiting angiogenesis).23, 24
Angiogenesis
The disrupted perfusion of injured tissue produces a hypoxic cellular microenvironment leading to the up-regulated expression of HIF-1. This transcription factor promotes the expression of many components of tissue repair pathways, including VEGF, extracellular matrix, and cell adhesion proteins.25 The resultant angiogenesis drives the creation of new healthy tissue, but also promotes the proliferation of residual cancer cells.26 VEGF also causes lymphatic dilation and remodelling in the tumour environment, providing cancer cells with alternative exit routes from the primary site.27 Overexpression of HIF-1 or VEGF is associated with poor prognosis in numerous cancer types.28, 29 Modulation of HIF and VEGF expression by anaesthetic and analgesic drugs, including volatile agents, local anaesthetics (LA), and opioids, has been postulated and is discussed further in this article.30, 31, 32
Anaesthetic agents
Propofol
Propofol is the most commonly used i.v. anaesthetic agent for induction and maintenance of anaesthesia. In vitro studies have shown propofol to possess anti-inflammatory properties as well as stimulatory effects on immune function, potentially resulting in beneficial effects on cancer recurrence, although definitive clinical evidence of this remains elusive.33
Laboratory studies
Considerable preclinical research has focused on the immune-modulating effects of propofol. Tumours excised from patients randomised to receive propofol–paravertebral anaesthesia for breast cancer surgery were shown to have increased infiltration by NK and T helper cells compared with an inhalational technique, suggestive of a beneficial anticancer effect on immune function.34 Researchers also sampled serum from patients undergoing cancer surgery under different anaesthetic techniques, and examined the effect of this serum on immune cells and cancer cells in vitro. Two studies found that serum sampled after operation from women randomised to propofol–paravertebral anaesthesia for breast cancer surgery preserved donor NK cell activity in vitro, compared to reduced NK activity with serum from women receiving a sevoflurane–opioid technique.35, 36 Caution must be exercised when interpreting the results of these studies, as the individual contributions of propofol and regional techniques to measured outcomes are difficult to separate and the mechanisms of the observations are not elucidated. Furthermore, not all in vitro studies support the hypothesis that propofol has beneficial immune-stimulating effects: breast cancer patients randomised to either propofol–remifentanil or sevoflurane anaesthesia were found to have no difference in postoperative serum cytokine concentrations or NK and cytotoxic T-lymphocyte numbers.37
Aside from any effect on immune function, propofol possibly affects malignant cells directly through a variety of putative mechanisms. One is inhibition of oncogenes, such as neuroepithelial cell-transforming gene 1 (NET1) and sex-determining region Y box 4 (SOX4), which are overexpressed in certain cancers and associated with poorer prognosis.38 Propofol has been shown to reduce the expression of these genes in vitro, leading to the inhibition of cancer cell activity.39, 40, 41 In prostate cancer cells, propofol inhibits androgen receptor expression in vitro, indicating a potential beneficial effect, as androgenic stimulation is implicated in prostate cancer progression.42 Additionally, propofol has been shown to down-regulate HIF-1 in cancer cells in vitro—with potential inhibitory effects on angiogenesis, and therefore, tumour growth.43 Contrary to the beneficial antitumour effects of propofol found in much laboratory research, isolated studies have suggested that propofol may possess pro-tumour effects: increased breast cancer cell migration following propofol exposure was observed in two studies.44, 45
Clinical studies
Several retrospective clinical studies have detected reduced cancer recurrence risk in patients who received propofol-based anaesthesia rather than volatile agents during surgery for various cancer types.46, 47 Improved overall survival following propofol-based techniques has been found in other studies.48, 49 In a large retrospective study examining anaesthesia type and patient survival post-cancer surgery, 2607 patients in the i.v. group were propensity matched with an equal number in the inhalational group—inhalational anaesthesia was associated with an increased risk of death (hazard ratio [HR]: 1.46; 95% confidence interval [CI]: 1.29–1.66) following a multivariable analysis of confounders in the matched group.50 These apparent beneficial effects of propofol have not universally been found: several retrospective studies examining a variety of cancer surgeries reported no difference in either survival or recurrence between TIVA and volatile-based groups.51, 52, 53 On balance, however, available evidence from retrospective studies currently appears to suggest that propofol may have beneficial effects in resisting metastasis and improving survival, which warrants a definitive evaluation in a prospective, randomised controlled clinical trial. No large RCT examining cancer recurrence following propofol vs volatile anaesthesia for cancer surgery has been completed to date. A number of such trials are underway and are listed in Table 1.
Table 1.
Selected ongoing prospective RCTs listed on clinicaltrials.gov and updated in 2018–9, which examine anaesthetic techniques and post-surgical cancer outcomes. GA, general anaesthesia; RA, regional anaesthesia.
| Study ID | Title | Design | Intervention | Cancer outcome measurements | Estimated completion |
|---|---|---|---|---|---|
| NCT00418457 | Regional Anesthesia and Breast Cancer Recurrence | Multicentre prospective RCT (n=1311) | Volatile+opioids vs propofol TIVA+RA | 1°: Cancer recurrence rate | March 2019 |
| NCT03034096 | General Anesthetics in Cancer Resection Surgery | Multicentre prospective RCT (n=2000) | Propofol TIVA vs volatile | 1°: Overall survival 2°: Recurrence-free survival |
December 2020 |
| NCT02786329 | Influence of Anesthesia on Postoperative Outcome and Complications in Colorectal Cancer Patients | Single-centre prospective 2×2 factorial RCT (n=450) | 2×2: Propofol vs volatile; lidocaine vs placebo | 1°: Overall survival and recurrence rate | December 2021 |
| NCT03109990 | Impact of Dexmedetomidine on Postoperative Tumor Recurrence in Patients with Breast Cancer | Multicentre prospective RCT (n=460) | Intraoperative dexmedetomidine infusion vs placebo | 1°: Overall survival and recurrence rate | April 2024 |
| NCT03172988 | Dexamethasone, Flurbiprofen Axetil and Long-Term Survival After Lung Cancer Surgery | Multicentre prospective 2×2 factorial RCT (n=844) | 2×2: Flurbiprofen, dexamethasone, saline, and lipid microspheres | 1°: Overall survival at 3 yr 2°: Recurrence-free survival |
August 2023 |
| NCT02840227 | Effect of Combined GA/RA on Cancer Recurrence in Patients Having Lung Cancer Resections | Multicentre prospective RCT (n=2000) | GA+epidural vs GA+opioids | 1°: Cancer-free survival | December 2021 |
Volatile anaesthesia
Volatile agents are the most commonly used method for the maintenance of general anaesthesia worldwide. It is established that volatile anaesthetic agents have effects on the immune system and the inflammatory response.54, 55 However, conflicting evidence exists as to whether volatiles activate or inhibit these pathways, and it remains unclear whether clinical outcomes are influenced.
Laboratory studies
Volatile agents modulate the immune response via a number of cellular targets, including gamma-aminobutyric acid, glycine, acetylcholine, and serotonin receptors present on immune cells, such as neutrophils, macrophages, and NK cells.56 Serum taken from patients who underwent volatile anaesthesia was observed to stimulate cancer cell activity and inhibit NK activity compared to serum from those receiving a propofol–regional technique.35, 36, 57 In contrast, a study examining immune cell and cytokine differentiation in breast cancer patients randomised to either propofol or sevoflurane anaesthesia found no significant differences between the two groups, with the authors concluding that any differential immune modulation related to the agents used may be minimal.58
Volatile agents potentially exert direct effects on cancer cells themselves. Sevoflurane has been shown to increase breast cancer cell proliferation and migration in vitro.59 Volatiles also appear to stimulate ovarian cancer cell migration and other pro-metastatic processes when tested in vitro, with increased expression of a variety of growth factors and matrix-degrading enzymes also noted.60, 61 Conversely, other studies have suggested that inhalational agents may actually directly inhibit cellular mechanisms that drive metastasis, and that any overall effect on recurrence may be related to cancer type: one study showed that sevoflurane exposure stimulated renal cancer cell viability and migration, whereas an opposite, inhibitory effect was seen with non-small-cell lung carcinoma (NSCLC) cells.62
Volatile anaesthetics are known to be protective against ischaemia–reperfusion injury in a variety of clinical contexts and organ systems.63 This protection is associated with induced expression of the angiogenesis-regulating factor HIF-1α, a mechanism that is perhaps protective in the setting of reperfusion injury, but in cancer surgery promotes malignant recurrence.64 Isoflurane has been found to increase the expression of HIF in prostate and renal cell carcinoma cells in separate studies, both findings associated with increased cancer cell migration and proliferation.43, 65
Clinical studies
Retrospective clinical studies comparing volatile vs propofol-based techniques have already been discussed. In summary, these studies have either shown that inhalational agents increase the risk of cancer recurrence and/or decrease the overall survival, or have shown no difference between the two groups in terms of these outcomes.46, 47, 48, 50, 51, 53 Ongoing prospective trials are listed in Table 1.
Local anaesthetics and regional anaesthesia
Local anaesthetics provide effective inhibition of pain signal transmission, with neuraxial or regional blockade providing both effective intraoperative anaesthesia and postoperative analgesia. In terms of cancer recurrence, several benefits may accrue from regional techniques:
-
(i)
Attenuation of the stress response may reduce the associated immunosuppression and preserve the innate immune system's ability to eliminate residual cancer cells.
-
(ii)
Reduced pain allows for opioid dose reduction, attenuating their possible detrimental effect on cancer recurrence.
-
(iii)
Volatile anaesthetic requirements may be reduced, also reducing any potential adverse effect on recurrence.
-
(iv)
More recent evidence suggests that amide LAs potentially possess direct antitumour effects on cancer cells (see below).
Laboratory studies
The effect of regional anaesthesia on serum markers of the surgical stress response has been examined, with conflicting results. In a study randomising prostatectomy patients to either epidural or opioid-based analgesia, serum cortisol and insulin were reduced in the epidural group post-procedure, but only one cytokine (IL-17) was significantly elevated in the opioid group after operation, with the authors concluding that epidural analgesia attenuates the stress response, but not the inflammatory response.66 In another trial, colorectal cancer (CRC) patients were randomised to epidural or patient-controlled analgesia after operation, and serum concentrations of cytokines, insulin, and cortisol were measured.67 Again, a minimal difference was found in serum inflammatory cytokines, suggesting that epidural anaesthesia does not effectively attenuate the inflammatory response.
Plausible experimental evidence supports the hypothesis that amide LAs may exert direct inhibitory effects on tumour cells, distinct from their effects on neurones. In vitro, bupivacaine directly inhibits prostate and ovarian cancer cell viability, proliferation, and migration at clinically relevant concentrations.68 Amide LAs reduce breast cancer cell viability and migration at high concentration, however, not at low plasma concentrations typically resulting from perioperative LA infusions.69 In vivo evidence supports a beneficial effect of i.v. lidocaine in reducing tumour size and metastatic burden in mouse models of cancer.70, 71, 72 It is unclear how amide LAs may exert such an anticancer effect—possible candidate mechanisms include their known sodium channel inhibitory effect or possibly other means, such as inhibition of the Src oncogene or DNA demethylation.73, 74, 75
Clinical studies
Much of the current interest in whether anaesthesia may influence outcomes following cancer surgery was spurred by the publication of several retrospective clinical studies over a decade ago, which suggested that regional techniques may be protective against cancer recurrence.76, 77 In recent years, a plethora of similar retrospective studies have been published (selected studies are summarised in Table 2). Results have been varied, with some suggestive of a beneficial effect of regional anaesthesia on overall survival, recurrence-free survival, or biochemical recurrence, whilst others have detected no difference and a small minority has even reported detrimental effects.
Table 2.
Selected retrospective studies of regional anaesthesia and clinical outcomes. BCR, biochemical recurrence; CSS, cancer-specific survival; GA, general anaesthesia; HR, hazard ratio; OR, odds ratio; OS, overall survival; PCA, patient-controlled analgesia; PFS, progression-free survival; PVB, paravertebral block; RCT-PHA, RCT-post hoc analysis; RFS, recurrence-free survival; TURBT, trans-urethral resection of bladder tumour; PCEA, patient-controlled epidural analgesia.
| Study authors | Year | Type | Surgery type | Techniques compared | Significant results |
|---|---|---|---|---|---|
| Biki and colleagues76 | 2008 | RC | Radical prostatectomy | GA+epidural (n=102); GA+opioid (n=123) | 57% reduction in BCR in epidural group |
| Tsui and colleagues78 | 2010 | RCT-PHA | Radical prostatectomy | GA+epidural (n=49); GA (n=50) | No difference in disease-free survival |
| Wuethrich and colleagues79 | 2010 | RC | Radical prostatectomy | GA+epidural (n=105); GA+opioid/NSAID (n=158) | Improved PFS in epidural group (HR: 0.45); no difference in BCR, CSS, or OS |
| Forget and colleagues80 | 2011 | RC | Radical prostatectomy | GA+epidural (n=578); GA (n=533) | No difference in BCR-free survival |
| Wuethrich and colleagues81 | 2013 | RC | Radical prostatectomy | GA+epidural (n=67); GA+opioid/NSAID (n=81) | No difference in BCR, RFS, or OS |
| Roiss and colleagues82 | 2014 | RC | Radical prostatectomy | GA+spinal (n=3047); GA alone (n=1725) | No difference in BCR, RFS, or OS |
| Sprung and colleagues83 | 2014 | RC | Radical prostatectomy | GA+epidural (n=486); GA+opioids (n=486) | No difference in RFS, CSS, or OS |
| Scavonetto and colleagues84 | 2014 | RC | Radical prostatectomy | GA+neuraxial (n=1642); GA alone (n=1642) | GA alone associated with increased systemic progression (HR: 2.81; P=0.008) and mortality (HR: 1.32; P=0.047) |
| Tseng and colleagues85 | 2014 | RC | Radical prostatectomy | Spinal+sedation (n=1166); GA (n=798) | No difference in BCR |
| Lee and colleagues86 | 2017 | RC | Thoracotomy for lung cancer | GA+epidural (n=619); GA+PVB (n=536); GA+PCA (n=574) | PVB associated with increased OS; no difference with epidural or PCA; no difference in recurrence between the three groups |
| Christopherson and colleagues87 | 2008 | RCT-PHA | Colectomy | GA+epidural (n=85); GA (n=92) | Improved OS with epidural in those without metastases at time of surgery, but only up to 1.46 yr postoperative |
| Gottschalk and colleagues88 | 2010 | RC | Colectomy | GA+epidural (n=256); GA (n=253) | No difference in RFS overall; improved RFS in patients aged >64 yr |
| Gupta and colleagues89 | 2011 | RC | Colectomy | GA+epidural (n=562); GA+PCA (n=93) | Epidural use for rectal (HR: 0.45) cancers associated with improved OS; no difference seen for colon cancers |
| Cummings and colleagues90 | 2012 | RC | Colectomy | GA+epidural (n=9670); GA (n=32 481) | Improved OS in epidural group (HR: 0.91), but no difference in cancer recurrence |
| Day and colleagues91 | 2012 | RC | Laparoscopic colectomy | GA+epidural (n=107); GA+spinal (n=144); GA+PCA (n=173) | No difference in OS or 5 yr disease-free survival |
| Holler and colleagues92 | 2013 | RC | Colectomy | GA+epidural (n=442); GA (n=307) | Improved OS in epidural group (HR: 0.73) |
| Vogelaar and colleagues93 | 2015 | RC | Colectomy | GA+epidural (n=399); GA (n=189) | Improved OS in GA group (HR: 0.77) |
| Hiller and colleagues94 | 2014 | RC | Gastrectomy or oesophagectomy | GA+epidural (n=97); GA (n=43) | Epidurals associated with improved OS and RFS at 2 yr in oesophagectomy group; no difference in gastrectomy group |
| Cummings and colleagues95 | 2014 | RC | Gastrectomy | GA+epidural (n=766); GA (n=1979) | No difference in OS or recurrence rate |
| Heinrich and colleagues96 | 2015 | RC | Oesophagectomy | GA+epidural (n=118); GA (n=35) | No difference in RFS or OS |
| Li and colleagues97 | 2016 | RC | Oesophagectomy | GA+epidural (n=178); GA (n=178) | No difference in OS or recurrence risk |
| Shin and colleagues98 | 2017 | RC | Gastrectomy | Epidural PCA (n=4325); i.v. PCA (n=374) | No difference in OS or RFS |
| Wang and colleagues99 | 2017 | RC | Gastrectomy | GA+epidural (n=1390); GA (n=2856) | Improved OS in epidural group (HR: 0.65) |
| Lacassie and colleagues100 | 2013 | RC | Advanced ovarian cancer | GA+epidural (n=37); GA (n=43) | No difference in OS or time to recurrence |
| Lin and colleagues101 | 2011 | RC | Ovarian serous adenocarcinoma | GA+epidural (n=106); GA+opioids (n=37) | Decreased OS in GA+opioids group (HR: 1.214) |
| de Oliveira and colleagues102 | 2011 | RC | Ovarian cancer debulking | GA+epidural (n=55); GA (n=127) | Intraoperative epidural use associated with reduced recurrence risk (HR: 0.37) |
| Capmas and colleagues103 | 2012 | RC | Ovarian cancer complete cytoreduction | GA+PCEA (n=47); GA (n=47) | No difference in OS or RFS |
| Tseng and colleagues104 | 2018 | RC | Ovarian cancer debulking | GA+epidural (n=435); GA (n=213) | Improved PFS and OS in epidural group |
| Doiron and colleagues105 | 2016 | RC | Radical cystectomy | GA+epidural (n=887); GA (n=741) | No difference in OS or CSS |
| Weingarten and colleagues106 | 2016 | RC | Radical cystectomy | GA+spinal (n=195); GA (n=195) | No difference in OS or RFS |
| Chipollini and colleagues107 | 2018 | RC | Radical cystectomy | GA+epidural (n=215); GA (n=215) | Worse RFS (HR: 1.67) and CSS (HR: 1.53) in epidural group |
| Choi and colleagues108 | 2017 | RC | TURBT | Spinal (n=718); GA (n=158) | Lower recurrence rate in spinal group (HR: 0.636) |
| Koumpan and colleagues109 | 2018 | RC | TURBT | Spinal (n=135); GA (n=96) | Increased recurrence rate (OR: 2.06) and earlier time to recurrence (HR: 1.57) in GA group |
| Zimmitti and colleagues110 | 2016 | RC | Colorectal liver metastectomy | GA+epidural (n=390); GA (n=120) | Epidural associated with improved RFS (HR: 0.74), but not OS |
| Exadaktylos and colleagues77 | 2006 | RC | Breast | GA+PVB (n=50); GA+opioid (n=79) | Increased recurrence-free survival in PVB group at 3 yr (88% vs 77%; P=0.012) |
| Myles and colleagues111 | 2011 | RCT-PHA | Abdominal cancer surgery | GA+epidural (n=230); GA (n=216) | No difference in OS or RFS |
Given the heterogeneity of the studies involved and their widely conflicting findings, it is difficult to draw an overall conclusion as to whether regional techniques affect survival or recurrence following cancer surgery. A meta-analysis of 10 studies published up to 2014 examining outcomes following prostatectomy found that regional techniques were not associated with longer biochemical recurrence-free survival, but were associated with improved overall survival (HR 0.81; 95% CI: 0.68–0.96; P=0.016).112 Another meta-analysis identified 21 studies (up to 2014) examining outcomes following neuraxial anaesthesia for a range of cancer surgeries,113 and observed an association between neuraxial anaesthesia and improved overall survival (HR: 0.853; 95% CI: 0.741–0.981; P=0.026) and improved recurrence-free survival (HR: 0.846; 95% CI: 0.718–0.998; P=0.047). However, a more recent meta-analysis of 28 retrospective studies published up to 2017 concluded that regional anaesthesia was not associated with improved survival, recurrence-free survival, or biochemical recurrence-free survival.114 The most recent Cochrane review of the topic concluded that evidence for a benefit of regional anaesthesia on tumour recurrence remains inadequate.115
A number of ongoing prospective randomised clinical trials are working to address the considerable knowledge gap (see Table 1). Of note, no significant clinical trial has yet been completed examining the effect of perioperative intravenous lidocaine on cancer recurrence, despite laboratory evidence suggesting benefit. NCT02786329 will examine lidocaine and recurrence following CRC surgery, but in a relatively small study population (n=450).
Analgesic agents
Opioids
As potent analgesic agents, opioids are widely used perioperatively for cancer surgery, and their effects on cancer have been examined in laboratory and (largely retrospective) clinical studies.
Laboratory studies
Opioids have long been associated with effects on immune function. Opioids are known to act indirectly on the nervous system, causing the release of biological amines that may attenuate innate immunity by inhibiting NK cytotoxicity.116 The HPA axis might also be stimulated, resulting in glucocorticoid release leading indirectly to immunosuppression.117 Direct effects on immune function may occur via opioid receptors, such as the mu-opioid receptor (MOR), or non-opioid receptors expressed by immune cells, including NK cells.116, 118
Different opioids produce different physiological effects on immune function. In rats, the MOR partial agonist, buprenorphine, prevented the depression of NK cell cytotoxicity and metastasis enhancement caused by surgery, contrary to the observed effects of fentanyl and morphine.117 Tramadol may actually possess immune-stimulating properties and enhances NK cytotoxicity in rats.119 Furthermore, not all laboratory studies are universally supportive of the hypothesis that morphine has detrimental effects on cancer biology, with a small number of both in vitro and in vivo studies finding that morphine exerts potentially beneficial antitumour effects.32, 120, 121
Cancer cells can express opioid receptors differently to non-cancerous tissue, enabling opioid-related stimulation of tumorigenic processes, including migration, angiogenesis, and metastasis.122 In vitro evidence suggests that MOR regulates opioid and growth-factor-induced receptor signalling, leading to the proliferation and migration of NSCLC cells.123, 124 The harmful influence of MOR has been detected clinically as well, with MOR overexpression associated in retrospective clinical studies with poorer outcomes in prostate cancer and oesophageal squamous cell cancer.125, 126
Mouse models of NSCLC and breast cancer have shown the opioid antagonists, methylnaltrexone and naloxone, to possess cancer-inhibiting effects.127, 128 Additionally, a follow-up analysis of RCTs designed to evaluate the effect of methylnaltrexone (on constipation in cancer patients receiving daily opioids) found improved survival in methylnaltrexone recipients, raising the hypothesis that opioid-related cancer growth may be inhibited by MOR antagonism.129
Despite the hypothesised cancer-stimulating effects of some opioids, it must be borne in mind that evidence exists that poorly controlled pain may drive malignant processes.130 The mechanism is unclear: it may be due to increased activity of both the SNS and the HPA axis, with the subsequent increase in circulating catecholamines and glucocorticoids attenuating the activity of immune cells.131 Clinically, poorly controlled pain or increased opioid requirements have retrospectively been associated with poorer survival in patients with advanced NSCLC.132 Therefore, the balance between the immunosuppressive effects of pain, on one hand, and opioids, on the other, may be the key to whether opioid treatment results in greater risk of cancer recurrence.
Retrospective clinical studies
Numerous retrospective studies have examined cancer surgery outcomes specifically in relation to the type and quantity of opioid administered perioperatively. In over 900 patients undergoing surgery for NSCLC, patients with Stage 1 disease had a significant association between high intraoperative fentanyl dose and decreased overall survival, and showed a trend towards reduced recurrence-free survival (HR: 1.12; 95% CI: 0.99–1.27; P=0.053); notably, Stages 2 and 3 patients did not share similar associations.133 A similar, albeit much smaller (n=99), retrospective study of NSCLC patients also found an association between opioid administration perioperatively and cancer recurrence.134 Conversely, in another study, no significant association was found between perioperative opioid dose in NSCLC patients and overall survival or recurrence.135 Similar negative findings were detected by a large (>1600 patients) retrospective study that found no association between intraoperative fentanyl dose and recurrence-free survival or overall survival in CRC patients.136 Conflicting results were also reported in two recent retrospective studies of US and Korean patients undergoing surgery for oesophageal squamous cell carcinoma: high-dose opioid treatment was strongly associated with disease recurrence in the Korean study, whereas the US study found improved recurrence-free survival and overall survival.137, 138
Summarising the totality of evidence regarding the influence of opioids on outcomes following cancer surgery is predictably difficult given the widely varying nature and results of studies to date. A meta-analysis of 147 publications examining analgesic treatment in animal cancer models concluded that, on the basis of limited evidence (opioids were examined in only 32 studies, most not involving surgical models), opioids do not influence metastasis.139 The most recent attempt at quantitative meta-analysis of perioperative opioids and CRC recurrence identified 13 publications of interest, but deemed them too heterogeneous to quantify an effect.140 In our opinion, the balance of evidence suggests a signal that opioids may facilitate metastasis in certain conditions, but, as with every anaesthetic intervention discussed in this review, the hypothesis must be tested in a prospective RCT before any major change in clinical practice is justified.
Non-steroidal anti-inflammatory drugs
Surgery causes an inflammatory response, which is implicated in cancer recurrence, so it follows that inhibition of inflammation may reduce postoperative recurrence.141 Evidence is growing that NSAIDs have beneficial anticancer effects, although many studies have been epidemiological in nature and have examined NSAID effects on cancer outcomes outside a surgical context.142
Laboratory studies
NSAID effects on cancer recurrence may be related to reduced prostaglandin synthesis due to COX inhibition or possibly other mechanisms affecting tumorigenesis, most of which remain poorly understood.143 Possible NSAID-related effects on factors determining cancer progression may include opioid-sparing analgesic effects, altered expression of transcription factors (such as NF-κB), or signalling proteins (such as epidermal growth factor receptor).144 In vitro studies have detected beneficial NSAID-related effects, including impaired cancer cell viability, proliferation, and migration, with both COX-dependent and COX-independent mechanisms implicated.145, 146 NSAIDs and COX-2 inhibitors have displayed a range of cancer-inhibiting effects in animal models, associated with potential mechanisms, such as reduced VEGF expression and down-regulation of the SOX2 oncogene.27, 147, 148
Clinical studies
Much effort has been spent studying outcomes related to long-term NSAID treatment (prior to or after diagnosis) in cancer patients. Regular NSAID use is associated with reduced CRC incidence in observational and randomised controlled studies.149 Regular NSAID use has also been associated with improved recurrence-free survival following surgery in observational studies examining a broad spectrum of cancers, including CRC and breast cancer.150, 151 Not all studies have produced clear evidence of benefit—a prospective analysis of over 34 000 breast cancer patients detected no association between post-diagnosis NSAID or COX-2 inhibitor use and breast cancer recurrence, although pre-diagnosis use appeared to reduce recurrence.152
Aside from long-term NSAID use, the perioperative administration of NSAIDs has been examined in retrospective studies, with variable results seen. One group examining breast cancer patients detected an association between perioperative NSAID treatment and improved cancer outcomes.153, 154 No beneficial effects on cancer outcomes were attributed to perioperative NSAID administration alone in retrospective studies examining prostate cancer and NSCLC surgery.80, 155, 156 However, enhanced survival has been reported with perioperative combined NSAID–dexamethasone treatment in retrospective studies of NSCLC patients.157 Prospective trials of COX-2 inhibitor treatment and cancer outcomes have been even more limited—one study examining postoperative rofecoxib use in CRC patients was terminated early due to concerns over potential cardiovascular side-effects, with no difference in overall survival or recurrence detected in recruited patients.158
In a common refrain, summarising the benefits or otherwise of NSAIDs in a cancer surgery context has proven difficult. A review of 16 studies examining cancer recurrence and perioperative NSAID use published up to 2017 found the studies either too heterogeneous or poor quality to attempt a meta-analysis, and the authors concluded that evidence supporting a beneficial effect of perioperative NSAIDs is equivocal.159 An RCT currently underway, which hopefully will add some clarity to the effect of perioperative NSAIDs on cancer outcomes, is NCT03172988 (see Table 1).
α-2-Adrenoceptor agonists
Despite their frequent use as sedative and analgesic agents, the effects of α-2-adrenoceptor agonists on cancer have rarely been studied. Given the generally pro-tumour effects of catecholamines, it may be postulated that agents that similarly activate adrenoceptors should also exhibit cancer-promoting effects. This has been shown in laboratory studies of animal and human cancer cells where exposure to α-2 agonists resulted in pro-tumoral effects, as well as detrimental effects on innate immune system cells.160, 161, 162, 163 Conversely, a small trial randomising patients undergoing radical gastrectomy to dexmedetomidine infusion or saline placebo found that dexmedetomidine resulted in reduced levels of catecholamines and pro-inflammatory cytokines, suggesting a potentially beneficial antitumour effect.164 Whilst most laboratory evidence points to a detrimental effect of -2 agonists in cancer surgery, it must also remembered that the use of such agents may allow for reduced patient exposure to opioids and volatile anaesthetics, both of which may be implicated in cancer recurrence. Where the balance of these factors lies in terms of clinical outcomes is unclear, however, to date, no beneficial effects on recurrence risk have been detected in clinical (albeit retrospective) studies examining NSCLC and breast cancer patients.153, 165
Other perioperative interventions
β-adrenoceptor antagonists
Although not typically regarded as analgesic agents per se, ß-adrenoceptor antagonist (ß-blocker) treatment may ameliorate the effects of surgery-induced SNS activation, and thus, limit the resulting cancer-promoting effects of catecholamines. Indeed, studies examining ß-blocker effects on various cancer cell types in vitro have largely pointed to beneficial anti-metastatic effects.166, 167 Benefits have also been detected in clinical trials, in which perioperative ß-blocker use in cancer patients reduced pro-inflammatory cytokines and prevented the catecholamine-induced elevation of pro-tumour Tregs.168, 169 Initial meta-analyses of retrospective clinical studies examining clinical outcomes in cancer patients suggested that ß-blocker use improves survival.170, 171 However, a more recent meta-analysis, including 27 studies published up to 2018, concluded that ß-blockers have no evident effect on cancer recurrence, and effects on disease-free survival and overall survival vary with tumour type from beneficial to harmful; the authors do note that the evidence base is variable and limited, especially in terms of tumour type and dosage and selectivity of ß-blocker used.172
Dexamethasone
Corticosteroids may reduce the cancer-stimulating effects of the surgical inflammatory response and appear, notionally at least, to hold promise as a perioperative therapy. However, at higher doses, corticosteroids also induce immunosuppression, potentially increasing the recurrence risk.173 Where the balance of effects lies is unknown and has not been widely studied for outcomes post-cancer surgery. Dexamethasone is commonly used intraoperatively as an anti-emetic, and thus, is an ideal candidate to examine; however, results, to date, have been inconsistent. A retrospective study of 309 patients undergoing surgery for endometrial cancer found no difference in recurrence risk, overall survival, or progression-free survival between those that received dexamethasone and those that did not.174 Recent retrospective cohort studies of NSCLC and pancreatic cancer patients suggested that perioperative dexamethasone may be associated with improved patient survival.157, 175 Conversely, reduced overall survival was detected in a retrospective analysis of intraoperative dexamethasone in rectal cancer patients.176 Prospective data are limited—a post hoc analysis of a small study of CRC patients (n=60) randomised to dexamethasone or placebo pre-surgery found that dexamethasone increased the metastasis risk.177 Given the largely retrospective and variable nature of clinical data available, higher-quality studies are required before a judgement on the benefits or otherwise of corticosteroids can be made. One RCT underway is NCT03172988, examining the dexamethasone effect on outcomes post-NSCLC surgery.
Blood transfusion
Transfusion of red blood cells (RBCs) and other blood products is frequently required during cancer surgery and in vitro this causes immunosuppression and inflammation—factors known to adversely affect cancer recurrence risk.178 Transfusion-related immune modulation is recognised as a pathophysiological phenomenon with various mechanisms suspected.14 Macrophages consuming iron released from damaged RBCs demonstrate compromised phagocytic activity and shift towards pro-tumour Th2 effector responses, while cytokines contained in transfused blood products contribute to inflammation.179 In vivo studies suggest that the cancer-stimulating effects of transfusion increase with increasing product storage duration.180
Clinical evidence summarised in meta-analyses of retrospective studies has suggested a harmful effect of perioperative allogeneic blood transfusion on outcomes (including recurrence) in a variety of cancer types, such as bladder, gastric, and prostate cancer.181, 182, 183 To date, the best-quality evidence supporting a detrimental effect of blood transfusion on cancer recurrence has come from several prospective randomised studies examining CRC patients—a 2006 Cochrane meta-analysis reported an overall odds ratio for recurrence of 1.42 (95% CI: 1.20–1.67), although study heterogeneity and insufficient data on surgical technique prevented the definitive establishment of a causal relationship.184 In summary, blood transfusion is associated in preclinical studies with inflammation and immunosuppression. It is also associated with increased risk of cancer recurrence in CRC, but whether this is attributable to patients with more aggressive cancer needing more transfusion or a true effect of transfusion increasing recurrence remains inconclusive.
Conclusions
Some evidence from in vitro and in vivo laboratory studies, as well as a number of observational clinical studies, suggests a signal that certain anaesthetic and analgesic agents are beneficial, and others detrimental, in preventing cancer progression or recurrence. Retrospective studies are inherently limited by their significant risk of bias, and only a prospective RCT can truly determine a causal link between an anaesthetic intervention and altered cancer outcomes. Several such RCTs are currently ongoing, and the results are expected within the next few years (Table 1). However, these trials largely study one cancer type, and given the widely heterogeneous biological and clinical behaviour of different cancers, trial results may not be reliably applicable to all malignancies. Therefore, considerable work will remain in the future to establish the effects of anaesthesia and analgesia on outcomes for cancer types not addressed in these studies.
When considering priorities for future research, it must be noted that systemic lidocaine remains largely unstudied in terms of clinical outcomes despite significant laboratory evidence of benefit pointing to an anti-metastatic effect of amide LAs. Perhaps then, the most promising techniques supported by the most scientifically plausible data for a future clinical trial are propofol TIVA vs sevoflurane inhalational anaesthesia, with and without a perioperative systemic lidocaine infusion. Evaluating these techniques in an adequately powered 2×2 factorial design trial, with oncological primary and secondary endpoints,185 would require approximately 6000 patients and a considerable period of follow-up. Meanwhile, in the absence of clinical evidence of harm associated with any agent, and whilst we await good-quality randomised trial data to support agent-related benefit, there still remain insufficient data to change the current clinical practice.186
Authors' contributions
Structure/outline/overall direction: DJB.
Literature review: TW.
Current evidence synthesis: TW.
Writing first draft: TW.
Critical revision: AS, DM, DJB.
All authors read and approved the final manuscript.
Declaration of interest
DJB and DM are board members of the British Journal of Anaesthesia.
Handling editor: J.G. Hardman
Editorial decision: 23 April 2019
References
- 1.Pilleron S., Sarfati D., Janssen-Heijnen M. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer. 2019;144:49–58. doi: 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan R., Alatise O.I., Anderson B.O. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16:1193–1224. doi: 10.1016/S1470-2045(15)00223-5. [DOI] [PubMed] [Google Scholar]
- 4.Mehlen P., Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 5.Byrne K., Levins K.J., Buggy D.J. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63:184–192. doi: 10.1007/s12630-015-0523-8. [DOI] [PubMed] [Google Scholar]
- 6.Duff S., Connolly C., Buggy D.J. Adrenergic, inflammatory, and immune function in the setting of oncological surgery: their effects on cancer progression and the role of the anesthetic technique in their modulation. Int Anesthesiol Clin. 2016;54:48–57. doi: 10.1097/AIA.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 7.Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 8.Neeman E., Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30:S32–S40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey S.C., Amedei A., Aquilano K. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35:S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alazawi W., Pirmadjid N., Lahiri R., Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264:73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 13.Du G., Liu Y., Li J., Liu W., Wang Y., Li H. Hypothermic microenvironment plays a key role in tumor immune subversion. Int Immunopharmacol. 2013;17:245–253. doi: 10.1016/j.intimp.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Youssef L.A., Spitalnik S.L. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol. 2017;24:551–557. doi: 10.1097/MOH.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam A., Hana Z., Jin Z., Suen K.C., Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 17.Szalayova G., Ogrodnik A., Spencer B. Human breast cancer biopsies induce eosinophil recruitment and enhance adjacent cancer cell proliferation. Breast Cancer Res Treat. 2016;157:461–474. doi: 10.1007/s10549-016-3839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angka L., Khan S.T., Kilgour M.K., Xu R., Kennedy M.A., Auer R.C. Dysfunctional natural killer cells in the aftermath of cancer surgery. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081787. 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerHagopian R.P., Sugarbaker E.V., Ketcham A. Inflammatory oncotaxis. JAMA. 1978;240:374–375. [PubMed] [Google Scholar]
- 20.Sethi G., Shanmugam M.K., Ramachandran L., Kumar A.P., Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1–15. doi: 10.1042/BSR20100136. [DOI] [PubMed] [Google Scholar]
- 21.Sobolewski C., Cerella C., Dicato M., Ghibelli L., Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffer T., Daqing M. The emerging role of chemokine receptor CXCR2 in cancer progression. Transl Cancer Res. 2016;5(Suppl 4):S616–S628. [Google Scholar]
- 23.Farooqui M., Li Y., Rogers T. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–1531. doi: 10.1038/sj.bjc.6604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piegeler T., Schlapfer M., Dull R.O. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br J Anaesth. 2015;115:784–791. doi: 10.1093/bja/aev341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokmic Z., Musyoka J., Hewitson T.D., Darby I.A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol. 2012;296:139–185. doi: 10.1016/B978-0-12-394307-1.00003-5. [DOI] [PubMed] [Google Scholar]
- 26.Schito L., Semenza G.L. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Le C.P., Nowell C.J., Kim-Fuchs C. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye L.Y., Zhang Q., Bai X.L., Pankaj P., Hu Q.D., Liang T.B. Hypoxia-inducible factor 1α expression and its clinical significance in pancreatic cancer: a meta-analysis. Pancreatology. 2014;14:391–397. doi: 10.1016/j.pan.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Shen W., Li H.L., Liu L., Cheng J.X. Expression levels of PTEN, HIF-1α, and VEGF as prognostic factors in ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21:2596–2603. [PubMed] [Google Scholar]
- 30.Zhao H., Iwasaki M., Yang J., Savage S., Ma D. Hypoxia-inducible factor-1: a possible link between inhalational anesthetics and tumor progression? Acta Anaesthesiol Taiwan. 2014;52:70–76. doi: 10.1016/j.aat.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Yang B., Qian F., Li W., Li Y., Han Y. Effects of general anesthesia with or without epidural block on tumor metastasis and mechanisms. Oncol Lett. 2018;15:4662–4668. doi: 10.3892/ol.2018.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khabbazi S., Nassar Z.D., Goumon Y., Parat M.O. Morphine decreases the pro-angiogenic interaction between breast cancer cells and macrophages in vitro. Sci Rep. 2016;6:31572. doi: 10.1038/srep31572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang S., Liu Y., Huang L., Zhang F., Kang R. Effects of propofol on cancer development and chemotherapy: potential mechanisms. Eur J Pharmacol. 2018;831:46–51. doi: 10.1016/j.ejphar.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Desmond F., McCormack J., Mulligan N., Stokes M., Buggy D.J. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311–1319. [PubMed] [Google Scholar]
- 35.Buckley A., McQuaid S., Johnson P., Buggy D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113:i56–i62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 36.Jaura A.I., Flood G., Gallagher H.C., Buggy D.J. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014;113:i63–i67. doi: 10.1093/bja/aet581. [DOI] [PubMed] [Google Scholar]
- 37.Lim J.A., Oh C.S., Yoon T.G. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. 2018;18:159. doi: 10.1186/s12885-018-4064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Ju H.L., Yuan X.Y., Wang T.J., Lai B.Q. SOX4 is a potential prognostic factor in human cancers: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18:65–72. doi: 10.1007/s12094-015-1337-4. [DOI] [PubMed] [Google Scholar]
- 39.Ecimovic P., Murray D., Doran P., Buggy D.J. Propofol and bupivacaine in breast cancer cell function in vitro—role of the NET1 gene. Anticancer Res. 2014;34:1321–1331. [PubMed] [Google Scholar]
- 40.Du Q., Liu J., Zhang X., Zhu H., Wei M., Wang S. Propofol inhibits proliferation, migration, and invasion but promotes apoptosis by regulation of Sox4 in endometrial cancer cells. Braz J Med Biol Res. 2018;51 doi: 10.1590/1414-431X20176803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou C.L., Li J.J., Ji P. Propofol suppresses esophageal squamous cell carcinoma cell migration and invasion by down-regulation of sex-determining region Y-box 4 (SOX4) Med Sci Monit. 2017;23:419–427. doi: 10.12659/MSM.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatsumi K., Hirotsu A., Daijo H., Matsuyama T., Terada N., Tanaka T. Effect of propofol on androgen receptor activity in prostate cancer cells. Eur J Pharmacol. 2017;809:242–252. doi: 10.1016/j.ejphar.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 43.Huang H., Benzonana L.L., Zhao H. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–1349. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garib V., Niggemann B., Zanker K.S., Brandt L., Kubens B.S. Influence of non-volatile anesthetics on the migration behavior of the human breast cancer cell line MDA-MB-468. Acta Anaesthesiol Scand. 2002;46:836–844. doi: 10.1034/j.1399-6576.2002.460714.x. [DOI] [PubMed] [Google Scholar]
- 45.Meng C., Song L., Wang J., Li D., Liu Y., Cui X. Propofol induces proliferation partially via downregulation of p53 protein and promotes migration via activation of the Nrf2 pathway in human breast cancer cell line MDA-MB-231. Oncol Rep. 2017;37:841–848. doi: 10.3892/or.2016.5332. [DOI] [PubMed] [Google Scholar]
- 46.Jun I.J., Jo J.Y., Kim J.I. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020. doi: 10.1038/s41598-017-14147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.H., Kang S.H., Kim Y., Kim H.A., Kim B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69:126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Z.F., Lee M.S., Wong C.S. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129:932–941. doi: 10.1097/ALN.0000000000002357. [DOI] [PubMed] [Google Scholar]
- 49.Zheng X., Wang Y., Dong L. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther. 2018;11:1141–1148. doi: 10.2147/OTT.S156792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wigmore T.J., Mohammed K., Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 51.Kim M.H., Kim D.W., Kim J.H., Lee K.Y., Park S., Yoo Y.C. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget. 2017;8:90477–90487. doi: 10.18632/oncotarget.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh T.K., Kim K., Jheon S. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control. 2018;25 doi: 10.1177/1073274818775360. 1073274818775360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enlund M., Berglund A., Andreasson K., Cicek C., Enlund A., Bergkvist L. The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119:251–261. doi: 10.3109/03009734.2014.922649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stollings L.M., Jia L.J., Tang P., Dou H., Lu B., Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. doi: 10.1097/ALN.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y.M., Song B.C., Yeum K.J. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int. 2015;2015:242709. doi: 10.1155/2015/242709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuki K., Eckenhoff R.G. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. 2016;123:326–335. doi: 10.1213/ANE.0000000000001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Y.J., Li S.Y., Cheng Q. Effects of anaesthesia on proliferation, invasion and apoptosis of LoVo colon cancer cells in vitro. Anaesthesia. 2016;71:147–154. doi: 10.1111/anae.13331. [DOI] [PubMed] [Google Scholar]
- 58.Oh C.S., Lee J., Yoon T.G. Effect of equipotent doses of propofol versus sevoflurane anesthesia on regulatory T cells after breast cancer surgery. Anesthesiology. 2018;129:921–931. doi: 10.1097/ALN.0000000000002382. [DOI] [PubMed] [Google Scholar]
- 59.Ecimovic P., McHugh B., Murray D., Doran P., Buggy D.J. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33:4255–4260. [PubMed] [Google Scholar]
- 60.Luo X., Zhao H., Hennah L. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831–839. doi: 10.1093/bja/aeu408. [DOI] [PubMed] [Google Scholar]
- 61.Iwasaki M., Zhao H., Jaffer T. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. 2016;7:26042–26056. doi: 10.18632/oncotarget.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciechanowicz S., Zhao H., Chen Q. Differential effects of sevoflurane on the metastatic potential and chemosensitivity of non-small-cell lung adenocarcinoma and renal cell carcinoma in vitro. Br J Anaesth. 2018;120:368–375. doi: 10.1016/j.bja.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 63.Wu L., Zhao H., Wang T., Pac-Soo C., Ma D. Cellular signaling pathways and molecular mechanisms involving inhalational anesthetics-induced organoprotection. J Anesth. 2014;28:740–758. doi: 10.1007/s00540-014-1805-y. [DOI] [PubMed] [Google Scholar]
- 64.Ma D., Lim T., Xu J. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol. 2009;20:713–720. doi: 10.1681/ASN.2008070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benzonana L.L., Perry N.J., Watts H.R. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 66.Fant F., Tina E., Sandblom D. Thoracic epidural analgesia inhibits the neuro-hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. Br J Anaesth. 2013;110:747–757. doi: 10.1093/bja/aes491. [DOI] [PubMed] [Google Scholar]
- 67.Siekmann W., Eintrei C., Magnuson A. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal Dis. 2017;19:O186–O195. doi: 10.1111/codi.13643. [DOI] [PubMed] [Google Scholar]
- 68.Xuan W., Zhao H., Hankin J., Chen L., Yao S., Ma D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci Rep. 2016;6:26277. doi: 10.1038/srep26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li R., Xiao C., Liu H., Huang Y., Dilger J.P., Lin J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer. 2018;18:666. doi: 10.1186/s12885-018-4576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson M.Z., Crowley P.D., Foley A.G. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br J Anaesth. 2018;121:76–85. doi: 10.1016/j.bja.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 71.Chamaraux-Tran T.N., Mathelin C., Aprahamian M. Antitumor effects of lidocaine on human breast cancer cells: an in vitro and in vivo experimental trial. Anticancer Res. 2018;38:95–105. doi: 10.21873/anticanres.12196. [DOI] [PubMed] [Google Scholar]
- 72.Xing W., Chen D.T., Pan J.H. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology. 2017;126:868–881. doi: 10.1097/ALN.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 73.Dan J., Gong X., Li D., Zhu G., Wang L., Li F. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomed Pharmacother. 2018;103:823–828. doi: 10.1016/j.biopha.2018.04.106. [DOI] [PubMed] [Google Scholar]
- 74.Chamaraux-Tran T.N., Piegeler T. The amide local anesthetic lidocaine in cancer surgery—potential antimetastatic effects and preservation of immune cell function? a narrative review. Front Med (Lausanne) 2017;4:235. doi: 10.3389/fmed.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lirk P., Hollmann M.W., Fleischer M., Weber N.C., Fiegl H. Lidocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in breast cancer cells in vitro. Br J Anaesth. 2014;113:i32–i38. doi: 10.1093/bja/aeu201. [DOI] [PubMed] [Google Scholar]
- 76.Biki B., Mascha E., Moriarty D.C., Fitzpatrick J.M., Sessler D.I., Buggy D.J. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 77.Exadaktylos A.K., Buggy D.J., Moriarty D.C., Mascha E., Sessler D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsui B.C., Rashiq S., Schopflocher D. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth. 2010;57:107–112. doi: 10.1007/s12630-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 79.Wuethrich P.Y., Hsu Schmitz S.F., Kessler T.M. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology. 2010;113:570–576. doi: 10.1097/ALN.0b013e3181e4f6ec. [DOI] [PubMed] [Google Scholar]
- 80.Forget P., Tombal B., Scholtes J.L. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur J Anaesthesiol. 2011;28:830–835. doi: 10.1097/EJA.0b013e32834b7d9a. [DOI] [PubMed] [Google Scholar]
- 81.Wuethrich P.Y., Thalmann G.N., Studer U.E., Burkhard F.C. Epidural analgesia during open radical prostatectomy does not improve long-term cancer-related outcome: a retrospective study in patients with advanced prostate cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roiss M., Schiffmann J., Tennstedt P. Oncological long-term outcome of 4772 patients with prostate cancer undergoing radical prostatectomy: does the anaesthetic technique matter? Eur J Surg Oncol. 2014;40:1686–1692. doi: 10.1016/j.ejso.2014.02.223. [DOI] [PubMed] [Google Scholar]
- 83.Sprung J., Scavonetto F., Yeoh T.Y. Outcomes after radical prostatectomy for cancer: a comparison between general anesthesia and epidural anesthesia with fentanyl analgesia: a matched cohort study. Anesth Analg. 2014;119:859–866. doi: 10.1213/ANE.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 84.Scavonetto F., Yeoh T.Y., Umbreit E.C. Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: a large, retrospective matched cohort study. Br J Anaesth. 2014;113:i95–i102. doi: 10.1093/bja/aet467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tseng K.S., Kulkarni S., Humphreys E.B. Spinal anesthesia does not impact prostate cancer recurrence in a cohort of men undergoing radical prostatectomy: an observational study. Reg Anesth Pain Med. 2014;39:284–288. doi: 10.1097/AAP.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee E.K., Ahn H.J., Zo J.I., Kim K., Jung D.M., Park J.H. Paravertebral block does not reduce cancer recurrence, but is related to higher overall survival in lung cancer surgery: a retrospective cohort study. Anesth Analg. 2017;125:1322–1328. doi: 10.1213/ANE.0000000000002342. [DOI] [PubMed] [Google Scholar]
- 87.Christopherson R., James K.E., Tableman M., Marshall P., Johnson F.E. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 88.Gottschalk A., Ford J.G., Regelin C.C. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology. 2010;113:27–34. doi: 10.1097/ALN.0b013e3181de6d0d. [DOI] [PubMed] [Google Scholar]
- 89.Gupta A., Bjornsson A., Fredriksson M., Hallbook O., Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107:164–170. doi: 10.1093/bja/aer100. [DOI] [PubMed] [Google Scholar]
- 90.Cummings K.C., 3rd, Xu F., Cummings L.C., Cooper G.S. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population-based study. Anesthesiology. 2012;116:797–806. doi: 10.1097/ALN.0b013e31824674f6. [DOI] [PubMed] [Google Scholar]
- 91.Day A., Smith R., Jourdan I., Fawcett W., Scott M., Rockall T. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. Br J Anaesth. 2012;109:185–190. doi: 10.1093/bja/aes106. [DOI] [PubMed] [Google Scholar]
- 92.Holler J.P., Ahlbrandt J., Burkhardt E. Peridural analgesia may affect long-term survival in patients with colorectal cancer after surgery (PACO-RAS-Study): an analysis of a cancer registry. Ann Surg. 2013;258:989–993. doi: 10.1097/SLA.0b013e3182915f61. [DOI] [PubMed] [Google Scholar]
- 93.Vogelaar F.J., Abegg R., van der Linden J.C. Epidural analgesia associated with better survival in colon cancer. Int J Colorectal Dis. 2015;30:1103–1107. doi: 10.1007/s00384-015-2224-8. [DOI] [PubMed] [Google Scholar]
- 94.Hiller J.G., Hacking M.B., Link E.K., Wessels K.L., Riedel B.J. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand. 2014;58:281–290. doi: 10.1111/aas.12255. [DOI] [PubMed] [Google Scholar]
- 95.Cummings K.C., 3rd, Patel M., Htoo P.T., Bakaki P.M., Cummings L.C., Koroukian S. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: a population-based study. Reg Anesth Pain Med. 2014;39:200–207. doi: 10.1097/AAP.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heinrich S., Janitz K., Merkel S., Klein P., Schmidt J. Short- and long term effects of epidural analgesia on morbidity and mortality of esophageal cancer surgery. Langenbecks Arch Surg. 2015;400:19–26. doi: 10.1007/s00423-014-1248-9. [DOI] [PubMed] [Google Scholar]
- 97.Li W., Li Y., Huang Q., Ye S., Rong T. Short and Long-term outcomes of epidural or intravenous analgesia after esophagectomy: a propensity-matched cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shin S., Kim H.I., Kim N.Y., Lee K.Y., Kim D.W., Yoo Y.C. Effect of postoperative analgesia technique on the prognosis of gastric cancer: a retrospective analysis. Oncotarget. 2017;8:104594–104604. doi: 10.18632/oncotarget.21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Wang L., Chen H., Xu Y., Zheng X., Wang G. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: a retrospective study. Oncotarget. 2017;8:62658–62665. doi: 10.18632/oncotarget.16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lacassie H.J., Cartagena J., Branes J., Assel M., Echevarria G.C. The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population. Anesth Analg. 2013;117:653–660. doi: 10.1213/ANE.0b013e3182a07046. [DOI] [PubMed] [Google Scholar]
- 101.Lin L., Liu C., Tan H., Ouyang H., Zhang Y., Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106:814–822. doi: 10.1093/bja/aer055. [DOI] [PubMed] [Google Scholar]
- 102.de Oliveira G.S., Jr., Ahmad S., Schink J.C., Singh D.K., Fitzgerald P.C., McCarthy R.J. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg Anesth Pain Med. 2011;36:271–277. doi: 10.1097/AAP.0b013e318217aada. [DOI] [PubMed] [Google Scholar]
- 103.Capmas P., Billard V., Gouy S. Impact of epidural analgesia on survival in patients undergoing complete cytoreductive surgery for ovarian cancer. Anticancer Res. 2012;32:1537–1542. [PubMed] [Google Scholar]
- 104.Tseng J.H., Cowan R.A., Afonso A.M. Perioperative epidural use and survival outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer. Gynecol Oncol. 2018;151:287–293. doi: 10.1016/j.ygyno.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doiron C.R., Jaeger M., Booth C.M., Wei X., Siemens R.D. Is there a measurable association of epidural use at cystectomy and postoperative outcomes? A population-based study. Can Urol Assoc J. 2016;10:321–327. doi: 10.5489/cuaj.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weingarten T.N., Taccolini A.M., Ahle S.T. Perioperative management and oncological outcomes following radical cystectomy for bladder cancer: a matched retrospective cohort study. Can J Anaesth. 2016;63:584–595. doi: 10.1007/s12630-016-0599-9. [DOI] [PubMed] [Google Scholar]
- 107.Chipollini J., Alford B., Boulware D.C. Epidural anesthesia and cancer outcomes in bladder cancer patients: is it the technique or the medication? A matched-cohort analysis from a tertiary referral center. BMC Anesthesiol. 2018;18:157. doi: 10.1186/s12871-018-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi W.J., Baek S., Joo E.Y. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget. 2017;8:87667–87674. doi: 10.18632/oncotarget.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koumpan Y., Jaeger M., Mizubuti G.B. Spinal anesthesia is associated with lower recurrence rates after resection of nonmuscle invasive bladder cancer. J Urol. 2018;199:940–946. doi: 10.1016/j.juro.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 110.Zimmitti G., Soliz J., Aloia T.A. Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23:1003–1011. doi: 10.1245/s10434-015-4933-1. [DOI] [PubMed] [Google Scholar]
- 111.Myles P.S., Peyton P., Silbert B., Hunt J., Rigg J.R., Sessler D.I. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]
- 112.Lee B.M., Singh Ghotra V., Karam J.A., Hernandez M., Pratt G., Cata J.P. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5:387–395. doi: 10.2217/pmt.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weng M., Chen W., Hou W., Li L., Ding M., Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016;7:15262–15273. doi: 10.18632/oncotarget.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grandhi R.K., Lee S., Abd-Elsayed A. The relationship between regional anesthesia and cancer: a metaanalysis. Ochsner J. 2017;17:345–361. [PMC free article] [PubMed] [Google Scholar]
- 115.Cakmakkaya O.S., Kolodzie K., Apfel C.C., Pace N.L. Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database Syst Rev. 2014:Cd008877. doi: 10.1002/14651858.CD008877.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boland J.W., Pockley A.G. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175:2726–2736. doi: 10.1111/bph.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Franchi S., Panerai A.E., Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun. 2007;21:767–774. doi: 10.1016/j.bbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Xie N., Gomes F.P., Deora V. Activation of mu-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain Behav Immun. 2017;61:244–258. doi: 10.1016/j.bbi.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 119.Gaspani L., Bianchi M., Limiroli E., Panerai A.E., Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129:18–24. doi: 10.1016/s0165-5728(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 120.Koodie L., Yuan H., Pumper J.A. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. 2014;184:1073–1084. doi: 10.1016/j.ajpath.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Afsharimani B., Baran J., Watanabe S., Lindner D., Cabot P.J., Parat M.O. Morphine and breast tumor metastasis: the role of matrix-degrading enzymes. Clin Exp Metastasis. 2014;31:149–158. doi: 10.1007/s10585-013-9616-3. [DOI] [PubMed] [Google Scholar]
- 122.Wigmore T., Farquhar-Smith P. Opioids and cancer: friend or foe? Curr Opin Support Palliat Care. 2016;10:109–118. doi: 10.1097/SPC.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 123.Singleton P.A., Mirzapoiazova T., Hasina R., Salgia R., Moss J. Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth. 2014;113:i103–i108. doi: 10.1093/bja/aeu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lennon F.E., Mirzapoiazova T., Mambetsariev B. The mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zylla D., Gourley B.L., Vang D. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y.F., Xu Q.X., Liao L.D. Association of mu-opioid receptor expression with lymph node metastasis in esophageal squamous cell carcinoma. Dis Esophagus. 2015;28:196–203. doi: 10.1111/dote.12165. [DOI] [PubMed] [Google Scholar]
- 127.Mathew B., Lennon F.E., Siegler J. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bimonte S., Barbieri A., Cascella M. The effects of naloxone on human breast cancer progression: in vitro and in vivo studies on MDA.MB231 cells. Onco Targets Ther. 2018;11:185–191. doi: 10.2147/OTT.S145780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janku F., Johnson L.K., Karp D.D., Atkins J.T., Singleton P.A., Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27:2032–2038. doi: 10.1093/annonc/mdw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Page G.G., Blakely W.P., Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. doi: 10.1016/s0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 131.Cole S.W., Nagaraja A.S., Lutgendorf S.K., Green P.A., Sood A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zylla D., Kuskowski M.A., Gupta K., Gupta P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth. 2014;113:i109–i116. doi: 10.1093/bja/aeu351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cata J.P., Keerty V., Keerty D. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–908. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maher D.P., Wong W., White P.F. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113:i88–i94. doi: 10.1093/bja/aeu192. [DOI] [PubMed] [Google Scholar]
- 135.Oh T.K., Jeon J.H., Lee J.M. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tai Y.H., Wu H.L., Chang W.K., Tsou M.Y., Chen H.H., Chang K.Y. Intraoperative fentanyl consumption does not impact cancer recurrence or overall survival after curative colorectal cancer resection. Sci Rep. 2017;7:10816. doi: 10.1038/s41598-017-11460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oh T.K., Jeon J.H., Lee J.M. Association of high-dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: reinterpreting ERAS protocols for long-term oncologic surgery outcomes. Dis Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox074. [DOI] [PubMed] [Google Scholar]
- 138.Du K.N., Feng L., Newhouse A. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg. 2018;127:210–216. doi: 10.1213/ANE.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 139.Hooijmans C.R., Geessink F.J., Ritskes-Hoitinga M., Scheffer G.J. A systematic review and meta-analysis of the ability of analgesic drugs to reduce metastasis in experimental cancer models. Pain. 2015;156:1835–1844. doi: 10.1097/j.pain.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diaz-Cambronero O., Mazzinari G., Cata J.P. Perioperative opioids and colorectal cancer recurrence: a systematic review of the literature. Pain Manag. 2018;8:353–361. doi: 10.2217/pmt-2018-0029. [DOI] [PubMed] [Google Scholar]
- 141.Wang D., Dubois R.N. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Basha R., Baker C.H., Sankpal U.T. Therapeutic applications of NSAIDS in cancer: special emphasis on tolfenamic acid. Front Biosci (Schol Ed. 2011;3:797–805. doi: 10.2741/s188. [DOI] [PubMed] [Google Scholar]
- 143.Alfonso L., Ai G., Spitale R.C., Bhat G.J. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014;111:61–67. doi: 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liggett J.L., Zhang X., Eling T.E., Baek S.J. Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets. Cancer Lett. 2014;346:217–224. doi: 10.1016/j.canlet.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ceponyte U., Paskeviciute M., Petrikaite V. Comparison of NSAIDs activity in COX-2 expressing and non-expressing 2D and 3D pancreatic cancer cell cultures. Cancer Manag Res. 2018;10:1543–1551. doi: 10.2147/CMAR.S163747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kashfi K., Rayyan Y., Qiao L.L. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J Pharmacol Exp Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 147.Shi C. High COX-2 expression contributes to a poor prognosis through the inhibition of chemotherapy-induced senescence in nasopharyngeal carcinoma. Int J Oncol. 2018;53:1138–1148. doi: 10.3892/ijo.2018.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thyagarajan A., Saylae J., Sahu R.P. Acetylsalicylic acid inhibits the growth of melanoma tumors via SOX2-dependent-PAF-R-independent signaling pathway. Oncotarget. 2017;8:49959–49972. doi: 10.18632/oncotarget.18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rigas B., Tsioulias G.J. The evolving role of nonsteroidal anti-inflammatory drugs in colon cancer prevention: a cause for optimism. J Pharmacol Exp Ther. 2015;353:2–8. doi: 10.1124/jpet.114.220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ng K., Meyerhardt J.A., Chan A.T. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2015;107:345. doi: 10.1093/jnci/dju345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang X.Z., Gao P., Sun J.X. Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta-analysis. Cancer Causes Control. 2015;26:589–600. doi: 10.1007/s10552-015-0539-y. [DOI] [PubMed] [Google Scholar]
- 152.Cronin-Fenton D.P., Heide-Jorgensen U., Ahern T.P. Low-dose aspirin, nonsteroidal anti-inflammatory drugs, selective COX-2 inhibitors and breast cancer recurrence. Epidemiology. 2016;27:586–593. doi: 10.1097/EDE.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Forget P., Vandenhende J., Berliere M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 154.Forget P., Bentin C., Machiels J.P., Berliere M., Coulie P.G., De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113:i82–i87. doi: 10.1093/bja/aet464. [DOI] [PubMed] [Google Scholar]
- 155.Choi J.E., Villarreal J., Lasala J. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med. 2015;4:825–833. doi: 10.1002/cam4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee B.M., Rodriguez A., Mena G. Platelet-to-lymphocyte ratio and use of NSAIDs during the perioperative period as prognostic indicators in patients with NSCLC undergoing surgery. Cancer Control. 2016;23:284–294. doi: 10.1177/107327481602300312. [DOI] [PubMed] [Google Scholar]
- 157.Huang W.W., Zhu W.Z., Mu D.L. Perioperative management may improve long-term survival in patients after lung cancer surgery: a retrospective cohort study. Anesth Analg. 2018;126:1666–1674. doi: 10.1213/ANE.0000000000002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Midgley R.S., McConkey C.C., Johnstone E.C. Phase III randomized trial assessing rofecoxib in the adjuvant setting of colorectal cancer: final results of the VICTOR trial. J Clin Oncol. 2010;28:4575–4580. doi: 10.1200/JCO.2010.29.6244. [DOI] [PubMed] [Google Scholar]
- 159.Cata J.P., Guerra C.E., Chang G.J., Gottumukkala V., Joshi G.P. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. Br J Anaesth. 2017;119:750–764. doi: 10.1093/bja/aex225. [DOI] [PubMed] [Google Scholar]
- 160.Bruzzone A., Pinero C.P., Castillo L.F. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155:494–504. doi: 10.1038/bjp.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Szpunar M.J., Burke K.A., Dawes R.P., Brown E.B., Madden K.S. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013;6:1262–1272. doi: 10.1158/1940-6207.CAPR-13-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang C., Datoo T., Zhao H. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology. 2018;129:1000–1014. doi: 10.1097/ALN.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 163.Chen G., Le Y., Zhou L. Dexmedetomidine inhibits maturation and function of human cord blood-derived dendritic cells by interfering with synthesis and secretion of IL-12 and IL-23. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Y., Xu X., Liu H., Ji F. Effects of dexmedetomidine on patients undergoing radical gastrectomy. J Surg Res. 2015;194:147–153. doi: 10.1016/j.jss.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 165.Cata J.P., Singh V., Lee B.M. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33:317–323. doi: 10.4103/joacp.JOACP_299_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Glasner A., Avraham R., Rosenne E. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 167.Wang F., Liu H., Xu R. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol Med Rep. 2018;17:5213–5221. doi: 10.3892/mmr.2018.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haldar R., Shaashua L., Lavon H. Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun. 2018;73:294–309. doi: 10.1016/j.bbi.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 169.Zhou L., Li Y., Li X. Propranolol attenuates surgical stress-induced elevation of the regulatory T cell response in patients undergoing radical mastectomy. J Immunol. 2016;196:3460–3469. doi: 10.4049/jimmunol.1501677. [DOI] [PubMed] [Google Scholar]
- 170.Choi C.H., Song T., Kim T.H. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol. 2014;140:1179–1188. doi: 10.1007/s00432-014-1658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhong S., Yu D., Zhang X. Beta-blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cancer Prev. 2016;25:440–448. doi: 10.1097/CEJ.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 172.Yap A., Lopez-Olivo M.A., Dubowitz J. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth. 2018;121:45–57. doi: 10.1016/j.bja.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 173.Kunicka J.E., Talle M.A., Denhardt G.H., Brown M., Prince L.A., Goldstein G. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol. 1993;149:39–49. doi: 10.1006/cimm.1993.1134. [DOI] [PubMed] [Google Scholar]
- 174.Merk B.A., Havrilesky L.J., Ehrisman J.A., Broadwater G., Habib A.S. Impact of postoperative nausea and vomiting prophylaxis with dexamethasone on the risk of recurrence of endometrial cancer. Curr Med Res Opin. 2016;32:453–458. doi: 10.1185/03007995.2015.1123146. [DOI] [PubMed] [Google Scholar]
- 175.Sandini M., Ruscic K.J., Ferrone C.R. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long-term survival in pancreatic cancer. Ann Surg Oncol. 2018;25:4020–4026. doi: 10.1245/s10434-018-6827-5. [DOI] [PubMed] [Google Scholar]
- 176.Yu H.C., Luo Y.X., Peng H., Kang L., Huang M.J., Wang J.P. Avoiding perioperative dexamethasone may improve the outcome of patients with rectal cancer. Eur J Surg Oncol. 2015;41:667–673. doi: 10.1016/j.ejso.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 177.Singh P.P., Lemanu D.P., Taylor M.H., Hill A.G. Association between preoperative glucocorticoids and long-term survival and cancer recurrence after colectomy: follow-up analysis of a previous randomized controlled trial. Br J Anaesth. 2014;113:i68–i73. doi: 10.1093/bja/aet577. [DOI] [PubMed] [Google Scholar]
- 178.Cata J.P., Wang H., Gottumukkala V., Reuben J., Sessler D.I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hod E.A., Zhang N., Sokol S.A. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Atzil S., Arad M., Glasner A. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109:989–997. doi: 10.1097/ALN.0b013e31818ddb72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cata J.P., Lasala J., Pratt G., Feng L., Shah J.B. Association between perioperative blood transfusions and clinical outcomes in patients undergoing bladder cancer surgery: a systematic review and meta-analysis study. J Blood Transfus. 2016;2016:9876394. doi: 10.1155/2016/9876394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Agnes A., Lirosi M.C., Panunzi S., Santocchi P., Persiani R., D’Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: a systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol. 2018;44:404–419. doi: 10.1016/j.ejso.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 183.Li S.L., Ye Y., Yuan X.H. Association between allogeneic or autologous blood transfusion and survival in patients after radical prostatectomy: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Amato A., Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006:Cd005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Buggy D.J., Freeman J., Johnson M.Z. Systematic review and consensus definitions for standardised endpoints in perioperative medicine: postoperative cancer outcomes. Br J Anaesth. 2018;121:38–44. doi: 10.1016/j.bja.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 186.Buggy D.J., Borgeat A., Cata J. Consensus statement from the BJA workshop on cancer and anaesthesia. Br J Anaesth. 2015;114:2–3. doi: 10.1093/bja/aeu262. [DOI] [PubMed] [Google Scholar]