Abstract
This article comments on:
M. Bouzid, F. He, G. Schmitz, R. E. Häusler, A. P. M. Weber, T. Mettler-Altmann and J. de Meaux. 2019. Arabidopsis species deploy distinct strategies to cope with drought stress. Annals of Botany 124(1): 27–40.
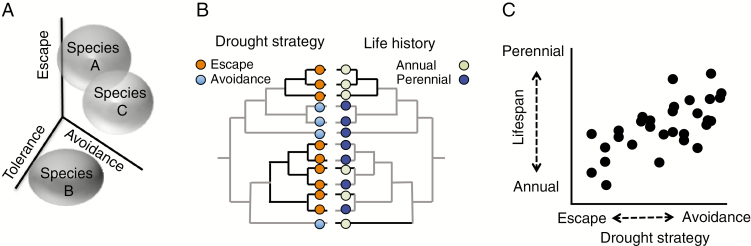
The idea that tradeoffs in resource allocation lead to the evolution of plant functional trait syndromes is widespread in plant ecology (Reich et al., 2003). The evolution of drought resistance is a prime example: a diverse set of physiological mechanisms underlie the ability to cope with limited water availability and these mechanisms are often classified into distinct strategies including drought escape, drought avoidance, and drought tolerance (Ludlow, 1989). Plants may escape drought by developing rapidly and reproducing before drought becomes severe while plants that avoid drought alter physiological conditions in order to reduce water loss and remain hydrated when water availability is low. Plants also may tolerate drought via altering cellular conditions to survive longer-term or more severe dehydration. While these strategies are not necessarily mutually exclusive, comparisons of drought strategies between closely-related species, such as done by Bouzid et al. (2019) in this issue of AoB, are rare. The drought strategy a species employs is hypothesized to be intimately linked to the life history and ecology of the species (Fig. 1; Kooyers, 2015). For instance, annual species that inhabit ephemeral environments would not experience selection for drought tolerance, and long-lived perennial trees would not employ an extensive drought escape strategy. Likewise, the drought strategy employed by a species should also be impacted by its biotic environment. For example, species that exist in uber-competitive environments may allocate relatively more resources toward rapid growth and reproduction to ensure fitness when encountering stress than a species in a competition-free environment.
Fig. 1.
Parameter space describing potential drought escape, drought avoidance, and drought tolerance phenotypes for three hypothetical species (A). Comparison of drought strategy (left tree) and life history strategy (right tree) phenotypes for multiple species in a diverse clade (B). Dark lines indicate where phenotypic shifts between drought or life history strategies have occurred. A phylogenetic generalized linear regression of the top clade in this tree could generate the hypothesized association between drought strategy and life history (C).
Although the connection between life history and drought avoidance strategy seems intuitive and has been routinely hypothesized within the plant ecology community, this association has rarely been investigated. In this issue, Bouzid et al. (2019) provide evidence that the drought strategy in the genus Arabidopsis is linked to the general life history strategy employed by each species. Although all species have relatively similar leaf-level phenotypes and water use efficiencies under well-watered conditions, responses to water limitation in a dry down experiment were quite divergent. The only annual species surveyed, Arabidopsis thaliana, employs a drought avoidance strategy where plants maintain homeostasis and do not wilt quickly, but are unable to survive wilting when it does occur. The perennial species A. lyrata is found in the driest habitats of the three species and employs a drought tolerance strategy where it wilts relatively rapidly, but is not badly damaged from wilting. Another perennial, A. halleri, is typically found in more competitive environments than the other species, and had an intermediate strategy between A. thaliana and A. lyrata where wilting occurs somewhat later than A. lyrata, but earlier than A. thaliana. A. halleri also demonstrates some ability to survive and recover from wilting, but not to the extent of A. lyrata.
The results here highlight that drought resistance strategies are not mutually exclusive and match the ecological niche of the species. A. halleri here employs an intermediate strategy that involves both drought avoidance and drought tolerance. This strategy appears well adapted to the ecological niche of the species as A. halleri occurs in more competitive environments than the other species and thus would presumably favour a strategy that allows maintenance of growth in the face of minor droughts, but switches to a drought tolerance strategy that limits wilting during more severe droughts. Notably, while A. halleri exhibits multiple strategies, both drought avoidance and drought tolerance strategies are weaker in A. thaliana or A. lyrata respectively (i.e., the jack-of-all-trades is a master of none). This suggests that there must be genetic or physiological trade-offs that limit the evolution of concurrent strategies. Such trade-offs have often been evaluated in intraspecific studies of other species between drought escape and drought avoidance (McKay et al., 2003 but see Kooyers et al., 2015), but are less often assessed between drought avoidance and tolerance mechanisms. This potential trade-off warrants future empirical and theoretical attention in order to develop physiological hypotheses for understanding drought strategy limitations and empirically assess multivariate trait complexes across Plantae.
The uniqueness of these results stresses the difficulty of research on drought resistance in natural populations. Rarely has a study compared the drought strategies of multiple closely related species in a controlled dry down experiment. More common experimental designs survey multiple drought resistance traits in a particular community under field conditions (Angert et al., 2007) or examine intraspecific variation from species that occur over dramatic mesic-xeric gradients in a common garden experiment (Kooyers et al., 2015). While all three approaches provide useful information on the evolution of drought resistance, a comparative approach (i.e. Fig. 1B, C) allows inclusion of species that occupy a wider range of ecological niches and employ a broader diversity of life history strategies as well as morphological and physiological adaptations to drought. Indeed, one comparable study examining drought resistance strategies in the Mimulus guttatus species complex finds that species from more water-limited environments have more pronounced drought escape strategies (Wu et al., 2010). In addition, a recent study of the genus Heliophila (Brassicaceae) finds a broad association between life history strategy and drought frequency where annual species are found in areas with more pronounced drought stress (Monroe et al., 2018). Utilizing diverse clades in a phylogenetic-controlled comparative approach is an intriguing direction that may provide new insights in the limits of drought strategy evolution.
One key to revealing associations between life history and drought strategies is defining morphological and physiological traits that can be used as reliable proxies for drought strategies. Surveying all the potential traits associated with drought resistance quickly becomes both expensive (e.g. δC13) or logistically challenging (e.g. root morphology). In practice, time to flowering is often used as a proxy for drought escape, water use efficiency via δC13 for drought avoidance, and lifetime fitness following a drought event for drought tolerance. Comparison of these metrics is challenging, especially measuring lifetime fitness in a biologically-realistic fashion. Bouzid et al. provide a useful proxy for quantifying drought avoidance and tolerance strategies by examining both how severe drought must become before wilting occurs as a measure of avoidance and how well plants recover from wilting events as a measure of tolerance. Thus, these phenotypes provide a method to quantify drought avoidance vs. tolerance reasonably quickly via automated methods on young plants in controlled drought treatments. Combining these wilting phenotypes with time to flowering and duration of flowering can provide a realistic way to assess tradeoffs between drought strategies and associations with other traits.
Assessing associations between drought resistance strategies and life history is important to understand the limitations of drought resistance evolution and determine how drought resistance fits into broader plant functional strategies. Despite drought resistance strategies having been hypothesized and debated for more than 30 years, there is still little consensus about the underlying constraints or even a quantification of relative strategies across Plantae. We hope that Bouzid et al.’s conclusions will stimulate comparative approaches to better examine whether or not trade-offs lead to distinct drought strategies that evolve in response to environmental differences in drought frequency, severity and timing.
Literature cited
- Angert AL, Huxman TE, Barron-Gafford GA, Gerst KL, Venable DL. 2007. Linking growth strategies to long-term population dynamics in a guild of desert annuals. Journal of Ecology 95: 321–331. [Google Scholar]
- Bouzid M, He F, Schmitz G, Häusler RE, Weber APM, Mettler-Altmann T, De M eaux J. 2019. Arabidopsis species deploy distinct strategies to cope with drought stress. Annals of Botany doi: 10.1093/aob/mcy237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyers NJ. 2015. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science 234: 155–162. [DOI] [PubMed] [Google Scholar]
- Kooyers NJ, Greenlee AB, Colicchio JM, Oh M, Blackman BK. 2015. Replicate altitudinal clines reveal that evolutionary flexibility underlies adaptation to drought stress in annual Mimulus guttatus. New Phytologist 206: 152–165. [DOI] [PubMed] [Google Scholar]
- Ludlow MM. 1989. Strategies of response to water stress. In: Structural and functional responses to environmental stresses. The Hague: SPB Academic Publishing, 269–281. [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. 2003. Genetics of drought adaptation in Arabidopsis thaliana I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology 12: 1137–1151. [DOI] [PubMed] [Google Scholar]
- Monroe JG, Gill B, Turner K, McKay JK. 2018. Drought frequency predicts life history strategies in Heliophila. bioRxiv doi: 10.1101/493270. [DOI] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Wu CA, Lowry DB, Nutter LI, Willis JH. 2010. Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia 162: 23–33. [DOI] [PubMed] [Google Scholar]