Abstract
Background and Aims
Water limitation is an important determinant of the distribution, abundance and diversity of plant species. Yet, little is known about how the response to limiting water supply changes among closely related plant species with distinct ecological preferences. Comparison of the model annual species Arabidopsis thaliana with its close perennial relatives A. lyrata and A. halleri, can help disentangle the molecular and physiological changes contributing to tolerance and avoidance mechanisms, because these species must maintain tolerance and avoidance mechanisms to increase long-term survival, but they are exposed to different levels of water stress and competition in their natural habitat.
Methods
A dry-down experiment was conducted to mimic a period of missing precipitation. The covariation of a progressive decrease in soil water content (SWC) with various physiological and morphological plant traits across a set of representative genotypes in A. thaliana, A. lyrata and A. halleri was quantified. Transcriptome changes to soil dry-down were further monitored.
Key Results
The analysis of trait covariation demonstrates that the three species differ in the strategies they deploy to respond to drought stress. Arabidopsis thaliana showed a drought avoidance reaction but failed to survive wilting. Arabidopsis lyrata efficiently combined avoidance and tolerance mechanisms. In contrast, A. halleri showed some degree of tolerance to wilting but it did not seem to protect itself from the stress imposed by drought. Transcriptome data collected just before plant wilting and after recovery corroborated the phenotypic analysis, with A. lyrata and A. halleri showing a stronger activation of recovery- and stress-related genes, respectively.
Conclusions
The response of the three Arabidopsis species to soil dry-down reveals that they have evolved distinct strategies to face drought stress. These strategic differences are in agreement with the distinct ecological priorities of the stress-tolerant A. lyrata, the competitive A. halleri and the ruderal A. thaliana.
Keywords: Arabidopsis halleri, Arabidopsis lyrata, Arabidopsis thaliana, avoidance strategy, drought stress, evolution, plant wilting, tolerance strategy, soil water content
INTRODUCTION
All physiological and cellular plant aspects depend on water, so limitation in its supply is a major abiotic stress restricting plant growth and crop yield (Stebbins, 1952; Boyer, 1982; Bohnert et al., 1995; Bray, 1997; Lambers et al., 1998; Bray et al., 2000). Water limitation is also a crucial determinant of the distribution, abundance and diversity of plant species (Hoffmann and Sgró, 2011).
All spermatophytes possess the molecular toolkit to tolerate intense cellular dehydration in seeds (Golovina et al., 1997; Kermode, 1997; Wehmeyer and Vierling, 2000). Adult plants can draw from this toolbox to tolerate a certain degree of dehydration in vegetative organs (Ludlow, 1989; Shinozaki and Yamaguchi-Shinozaki, 2007). This tolerance strategy relies on osmotic adjustment via the accumulation of an array of solutes, such as amino acids, sugars or dehydrins (Close, 1996). The expression of heat shock proteins, chaperones or late embryogenesis abundant (LEA) proteins can further help to protect the cell against damage imposed by low internal water potential (Ingram and Bartels, 1996; Reddy et al., 2004; Yue et al., 2006; Szabados, 2010).
However, plants have evolved additional strategies to handle drought stress: escape and avoidance (Ludlow, 1989; Fukai and Cooper, 1995; Verslues and Juenger, 2011; Fang and Xiong, 2015). The escape strategy is based on the adjustment of developmental transitions to elude direct exposure to drought. With an increase in the duration of seed dormancy or a shortening of the life cycle, the plant is simply not facing dry seasons (Fox, 1990; Bewley, 1997; Tonsor et al., 2005; Franks et al., 2007; Kronholm et al., 2012; Lovell et al., 2013). The avoidance strategy, instead, seeks to maintain water levels within tissues through a reduction of water loss and the enhancement of water uptake, so that the plant by-passes the damaging effects of drought (Levitt, 1980; Ludlow, 1989; Price et al., 2002; Farooq et al., 2009; Munemasa et al., 2015).
The relative importance of strategies to cope with drought stress is expected to be intimately linked to the life history and ecology of species. Indeed, tolerance, avoidance and escape strategies are not independent in evolution (Grime, 1977). Trade-offs between growth and tolerance can constrain their optimization (McKay et al., 2003; Steven, 2011). Annual species prioritize the escape strategy, which in turn can release the need for tolerance mechanisms (Kooyers, 2015). Perennial species, in contrast, must maintain tolerance mechanisms to increase long-term survival.
Dehydration triggers dramatic responses in plant cells, as indicated by the fast and extensive changes in gene transcript levels (Iuchi et al., 2001; Seki et al., 2001; Shinozaki and Yamaguchi-Shinozaki, 2007; Matsui et al., 2008; Harb et al., 2010). Part of this response is regulated by the key drought stress hormone abscisic acid (ABA), but ABA-independent transcriptional regulation also plays an important role (Iuchi et al., 2001; Seki et al., 2001; Sakuma et al., 2006; Yoshida et al., 2014; Urano et al., 2017). The complex architecture of gene regulatory responses to stress is believed to contribute to restricting the reactions at cell and whole-plant levels when the internal water potential drops (Bray, 1997; Szabados, 2010; Osakabe et al., 2014). By articulating growth and stress responses, transcriptomic changes take part in both the deployment of avoidance strategies and the promotion of recovery from stress, yet they also reveal the degree of stress sensed by the organisms. Distantly related annual species, such as rice and Arabidopsis, show common patterns of stress responses (Nakashima et al., 2009). Much less is known about how responses to stress are reshaped in closely related species with strongly divergent ecologies and life histories.
Comparison of Arabidopsis thaliana with its close relatives can help disentangle the molecular changes contributing to tolerance and avoidance mechanisms, because different species in the genus have evolved distinct ecologies with contrasting demands on tolerance and avoidance (Clauss and Koch, 2006). The model species A. thaliana shows a broad distribution range from north of Scandinavia to Africa (Hoffmann, 2005, Durvasula et al., 2017). The response of A. thaliana to severe or mild drought stress has been described in detail (Seki et al., 2002; Bray, 2004; Verslues and Juenger, 2011; Des Marais et al., 2012; Juenger, 2013; Bechtold et al., 2016; Lovell et al., 2015). Several studies point to the adaptive relevance of its variation (Kesari et al., 2012; Exposito-Alonso et al., 2018). This annual species can also rely on modifications of its life cycle to adjust the timing of escape and/or avoidance strategies to drought threats (McKay et al., 2003; Kronholm et al., 2012; Wolfe and Tonsor, 2014). The two sister species Arabidopsis lyrata and Arabidopsis halleri, in contrast, are less likely to rely on escape strategies because year to year survival is of major importance for these perennials. Arabidopsis lyrata is probably the most exposed of the two to natural selection by drought due to its preference for low competitive communities in soils that do not retain water (Clauss and Koch, 2006; Ellenberg and Leuschner, 2010; Sletvold and Agren, 2012). On the other hand, A. halleri must grow to out-compete other species in crowded habitats (Clauss and Koch, 2006; Ellenberg and Leuschner, 2010; Stein et al., 2017). Its specific ability to accumulate heavy metals enhances its defences against herbivores but sets strong constitutive demands on detoxifying systems which are important for re-establishing homeostasis after stress (Mittler, 2002; Becher et al., 2004; Krämer and Clemens, 2006; Stolpe et al., 2016). The contrasting ecologies of these three species thus predict major consequences on their strategies to face up to the challenges imposed by water limitations.
To test this prediction, we set up an experiment to infer the response strategy to drought of sets of accessions representative of the three species A. thaliana, A. halleri and A. lyrata. For this, we measured plant drought reaction at both phenotypic and transcriptomic levels in a dry-down experiment that mimics the progression of water depletion in natural conditions. Our data showed that species deploy different avoidance and tolerance strategies in response to decreasing levels of soil water content (SWC).
MATERIALS AND METHODS
Plant material and growth conditions
Altogether, 16–22 and 12–17 central European A. lyrata and A. halleri accessions, respectively, were included in the dry-down experiments. The accessions were taken from populations representative of the diversity described in these species (Supplementary Data Table S1; Pauwels et al., 2005; Ross-Ibarra et al., 2008; Novikova et al., 2016; Stein et al., 2017). They were compared with 16 A. thaliana accessions from Spain with a European genomic background (The 1001 Genomes Consortium, 2016). This sample was chosen because the populations (1) are among the most drought resistant in A. thaliana (Exposito-Alonso et al., 2018) and (2) are late flowering (Arapheno database, FT16, DOI: 10.21958/phenotype:262) so that the stress exposure cannot be circumvented by life cycle termination. For each accession, five replicates (vegetatively propagated clones for the self-incompatible species, single-descent seeds for A. thaliana) were distributed in five randomized complete blocks.
Plants were grown in 7 × 7 × 8 cm pots filled with 150 g of a well-homogenized mixture of VM soil (60–70 % peat and 30–40% clay), perlite and seramis (clay granules) in a CLF controlled growth chamber (Perkin Elmer, USA). Growth conditions were 10 h (20 °C): 14 h (16 °C), light:dark, at a photosynthetic photon flux density (PPFD) of 100 μmol m–2 s–1 supplemented with 10 min of dark-red light at the end of the day. Relative humidity was set to 60 %.
Dry-down experimental design
Plants were grown for 5 weeks in the greenhouse, re-potted in weighed pots filled with the initial soil mixture and transferred to the growth chamber. Soil moisture was quantified every day (Xt) by monitoring pot mass with a precision balance with an accuracy of 0.01 g. To calculate the soil moisture, several pots were fully dried down in an oven to estimate the weight of dry soil (X0) in the initial soil mixture and subsequently saturated with water to determine the weight of 100 % wet soil (Xf). The percentage of soil moisture was calculated as [(Xt – X0)/(Xf – X0)] × 100. For acclimation, plants were grown for 2 weeks in pots with 60 % soil moisture. After acclimation, plants were not watered until showing the first symptoms of wilting. Plants were re-watered 2 d after wilting. One to two weeks later, survival and symptoms of damage were scored.
Three independent biological experiments were performed. We discarded any plant that was not healthy and vigorously growing at the start of the experiment. Focusing on initially healthy plants thus resulted in slight differences in the number of replicates and/or accessions (for details, see Supplementary Data Tables S1–S3). The two first experiments were used for phenotypic characterization and the third for sampling of leaf material for RNA extraction. In the experiment, plants were re-watered on the day of wilting to allow the collection of leaf material after recovery.
Phenotypic trait measurements
Phenotypic differences between species in well-watered conditions.
Three phenotypes were measured on separate replicate cuttings of nine accessions of A. halleri and A. lyrata: stomatal density, stomatal length and carbon isotope discrimination (δ13C). These replicate cuttings were maintained in the glasshouse under well-watered conditions and were not used for the dry-down experiments (see below). Stomatal density and length were quantified following the protocol described by Paccard et al. (2014). δ13C in one fully developed leaf was quantified for four replicates of the same nine accessions of each species according to the method used by Gowik et al. (2011).
Phenotypic variation in response to soil dry-down.
Eight phenotypes were measured during the dry-down experiment. Rosette leaf area was quantified on day zero of the dry-down experiment, using ImageJ to separate green pixels from the background images, and RosetteTracker (De Vylder et al., 2012) to convert total green pixels into mm2. The first day on which we observed that leaves had lost their turgidity was scored as wilting day. Soil moisture was measured every day until the day of wilting. The rate of soil water loss was calculated for each pot over the first 7 d after water withdrawal. Leaf lamina thickness was measured on one ink-marked medium-size leaf every second day using a digital ruler (HOLEX, Hoffmann Group, Knoxville, TN, USA) with an accuracy of 0.03 mm. Efficiency of the photosynthetic light reaction was measured by pulse amplitude modulation (PAM) fluorometry (Schreiber et al., 1986) using the IMAGING-PAM-Series (M-Series-Maxi version, Heinz Walz GmbH, Effeltrich, Germany). In order to gain information on the intactness of photosystem II (PSII) and hence its potential photosynthetic capacity, the maximum quantum efficiency of open PSII reaction centres (Fv:Fm, i.e. the ratio of variable to maximum chlorophyll a fluorescence) was determined (Genty et al., 1989; Maxwell and Johnson, 2000). Before the application of a saturating light flash (duration 0.8 s), plants were dark-adapted for 30 min. Intact and non-stressed plants usually show an Fv:Fm ratio of around 0.8. Plants that developed new leaves within 2 weeks after re-watering were scored as having survived, and the damage caused by wilting was quantified visually on a damage severity scale from 1 to 5, reflecting the percentage of damaged leaf area, leaf colour and leaf strength. The number of days of tolerated wilting was scored on plants that survived the first dry-down experiment. For this, plants were dried down a second time until wilting and re-watered after 3, 4, 5 or 6 d of wilting. Despite previous exposure to drought stress, plants wilted at the same limiting SWC (e.g. approx. 20 %), suggesting that if a plant shows differences in stress memory, this effect is not detectable after 3 weeks. Photosynthetic activity and duration of tolerated wilting were measured in the first experiment, whereas rosette area and leaf thickness were measured only in the second experiment (Supplementary Data Table S2).
Statistical analysis of phenotypic variation
All plots were created using the CRAN-package ggplot2 (Wickham, 2009). We used generalized linear models (R function glm) and multiple comparison tests using the Simultaneous Inference in General Parametric Models package named multcomp, and Tukey’s honest significant difference test (Tukey HSD). For each phenotype, we ran several models. As we did not detect any block effect for the different measured traits, we removed it from our models. Following are the different tested models, and later, in the Results, we will mention which was the best model:
(M1) tests the accessions nested within species effect
(M2) tests only the species effect when the accession effect is not significant
(M3) tests the interaction between species and time effect
(M4) tests the effect of interaction between species and the cofactor of interest
Where: Y is a quantitative-dependent variable, e.g. measured phenotypic trait; μ is the overall mean; α, β and γ are regression coefficients; species, accession, time, cofactor (e.g. initial rosette size, desiccation rate, initial leaf thickness, damage scores, days after wilting etc.) are independent variables with the different levels i, j and k; and ε is the prediction error.
Different error distributions were specified for each phenotypic trait, depending on whether or not overdispersion was detected (i.e. whether the residual deviance was of the order of magnitude of the degrees of freedom). A negative binomial fitted best the number of days until wilting, soil moisture, initial rosette area, initial leaf thickness, damage scores, relative leaf water loss, stomatal density and stomatal length. A Gaussian distribution fitted better measures of desiccation rate and δ13C, a quasi-Poisson distribution was used for the photosynthetic activity and quasi-binomial distribution for survival rate. We performed an analysis of variance (ANOVA) using Fisher’s test (or a χ2 test for the binomial distribution of error) to identify the best model (P-value ≤0.05).
Analysis of transcriptome variation during dry-down
In the third dry-down experiment, 3–4 young leaves of ‘hal2.2’ and ‘Plech61.2a’, typical accessions of A. halleri and A. lyrata, respectively, were sampled from three replicate individuals at three time points: (1) before water withdrawal (soil moisture around 60 %); (2) before wilting symptoms appeared (20–25 % soil moisture); and (3) leaves formed during the recovery phase (10–15 d after re-watering). These two accessions are representative of the phenotypic diversity observed in the dry-down experiment. RNA extraction was performed using the PureLink™ RNA Ambion Mini Kit (Thermofisher, Darmstadt, Germany). RNA quality and quantity were checked by an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) using RNA nano chips. RNA of 18 leaf samples was sequenced on an Illumina HiSeq4000 by the Cologne Center for Genomics. Raw sequence data are available in the Sequence Read Archive (SRA) database under the accession number: SRP150056.
We used the fastx-tool-kits from the FastQC package (V0.11.4) for raw sequence quality trimming and filtering following He et al. (2016). Low quality nucleotides were removed from the 3’ ends of the sequences using 20 as a phred score threshold (t) and 50 as minimum length (l). Sequences were reverse complemented using fastx_reverse_complement to cut the other end as we did for the 3’ end. Reads with <90 % bases above the quality threshold and paired-end reads with a single valid end were discarded. We used the software package STAR with standard parameters (Dobin and Gingeras, 2015) to map trimmed and filtered reads to the A. lyrata reference genome V1 (Hu et al., 2011). Alternative transcripts were not considered because the current annotation of the A. lyrata genome does not describe alternative transcripts. Transcriptome sequencing yielded a total of 15 million read pairs per sample, with a read length of 75 bp. We used ‘samtools view -q 10’ to select the unique and high quality mapping reads with a probability of correct mapping of 90 %.
On average, >80 % of all reads and around 20 % of unmapped and multiple mapped reads were uniquely mapped (Supplementary Data Fig. S1). R scripts were used to verify that reads covered the whole length of genes (and to confirm that we had no sign of RNA degradation) and for counting the number of reads mapped to each. The DESeq2 Bioconductor package from R (Bioconductor version: Release 3.5) was used to find genes that were differentially expressed between the different conditions (Love et al., 2014). We used the Wald test to compute P-values and the following design: ~ species/sample point, with two levels for the factor species (A. halleri and A. lyrata) and three levels for the factor sample point (leaves sampled at 60 % of soil moisture, at 20–25 % of soil moisture and after recovery). Genes with a P-value <0.1 after Benjamini–Hochberg correction for false discovery rate (FDR) and log2-fold change ≤ –0.5 or ≥0.5 were considered as differentially expressed.
Gene Ontology analysis
Functional enrichments among differentially expressed genes were performed using the org.At.tair.db data package of Bioconductor, and the rank test of the TopGO package (Alexa and Rahnenfuhrer, 2010) was used to identify enriched Gene Ontology (GO) terms. The elim algorithm followed by a Fisher test were used with a cut-off of 0.01. As background, all expressed genes were used (around 12 220 genes). Enrichments were analysed separately for: (1) all responsive genes; (2) downregulated genes; and (3) upregulated genes. The hyper-geometric test was used to test for the significance of gene overlap with a set of stress-responsive genes (Matsui et al., 2008).
RESULTS
Interspecific differences in stomatal density and stomatal length but not in water-use efficiency
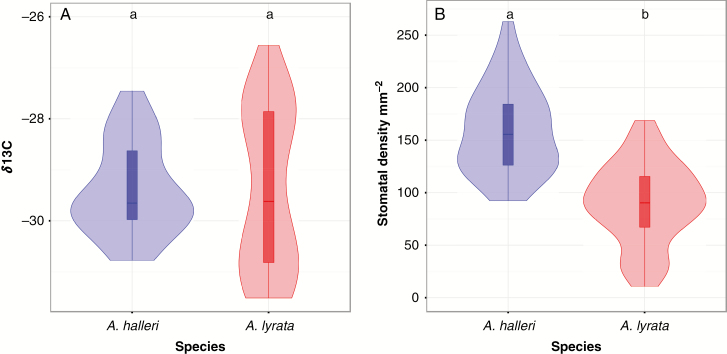
We evaluated whether, under well-watered conditions, constitutive physiological differences between A. lyrata and A. halleri can influence their potential to face limiting SWC. Variation in stomatal density on the leaf surface was explained by both within- and between-species variance (M1: F18, 469 = 36.15, P-value <2e-16 within species; F1, 487 = 256.59, P-value <2.2e-16, between species, Fig. 1A).
Fig. 1.
Stomatal density and δ13C measured in Arabidopsis halleri and A. lyrata grown under well-watered conditions. (A) Abaxial stomatal density. (B) δ13C measured for the same plants. Violin plots with the same letter are not significantly different according to Tukey’s HSD (P-value <0.05).
In A. lyrata, stomatal density on the abaxial leaf surface was lower than in A. halleri (on average 80 mm–2 in A. lyrata and 150 stomata mm–2 in A. halleri). By comparison, a recent and exhaustive analysis of stomatal density in A. thaliana reported that stomatal density varies from 87 to 204 stomata mm–2 and it is negatively correlated with stomatal length (Dittberner et al., 2018). Stomata were larger in A. lyrata compared with A. halleri (M1: P-value <2e-16) and the genetic variation in stomatal length was significant both within and between these two species (M1: F16, 1370 = 53.68, P-value <2e-16 within species; F1, 1386 = 3801.39, P-value <2.2e-16, between species). These differences however did not coincide with differences in carbon isotope discrimination (δ13C), a commonly used proxy for water-use efficiency (WUE; Farquhar and Richards, 1984; Farquhar et al., 1989; Lambers et al., 1998; Dawson et al., 2002). In non-stressed conditions, leaf δ13C showed significant genetic variation within species, but not between A. halleri and A. lyrata (–29.38 ‰ in A. lyrata and –29.37 ‰ in A. halleri, on average, M1: F16, 54 = 7.440, P-value = 9.76e-09 within species; and F1, 70 = 0.005, P-value = 0.969, between species Fig. 1B).
Wilting-related phenotypes revealed different drought response strategies
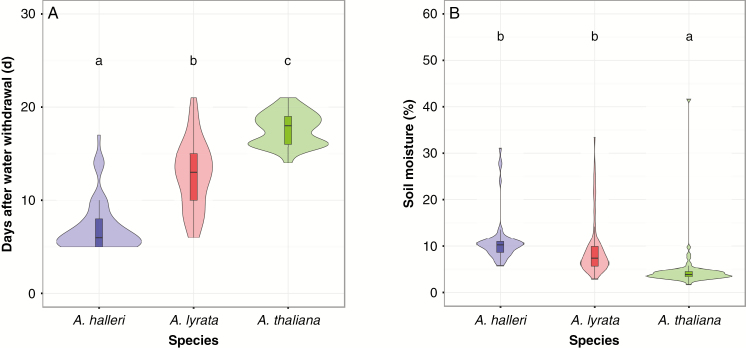
The day of the first appearance of wilting symptoms differed significantly between species in the first experiment, although accessions within species also differed (M1: F2, 214 = 316.48, P-value <2.2e-16 for species, Fig. 3A; F48, 166 = 3.51, P-value = 1.159e-09 for accessions within species). The same result was observed in the second experiment (M1: F2, 201 = 115.27, P-value <2.2e-16; F33, 168 = 1.97, P-value= 0.002, Supplementary Data Fig. S2A). Wilting manifested differently in the three species. In A. thaliana, leaves became pale and curled laterally, in A. lyrata, they curled apically and, in A. halleri, leaves changed to darker green and collapsed (Fig. 2). On average, A. halleri accessions wilted around 5–7 d after water withdrawal, A. lyrata accessions after 12 d and A. thaliana accessions after 18 d (Fig. 3A; Supplementary Data Table S4). Differences in the timing of wilting did not exactly coincide with SWC differences. At wilting, A. halleri and A. lyrata showed similar soil moisture (18–20 %), whereas A. thaliana only wilted after soil moisture dropped below 10 % (Fig. 3B; Supplementary Data Table S5). Again, these effects were consistent across experiments (Supplementary Data Fig. S2B). Significant differences were detected between species for soil moisture at wilting (M1: F2, 214 = 44.27, P-value = 3.982e-16; F2, 201 = 181.60, P-value <2.2e-16 for the first and second experiment, respectively), and within species (M1: F48, 166 = 1.52, P-value = 0.020; F33, 168 = 2.23, P-value = 1.07e-10 for the first and second experiment, respectively).
Fig. 3.
Wilting day and soil moisture at wilting for Arabidopsis halleri, A. lyrata and A. thaliana. (A) Number of days between initiation of soil dry-down treatment and wilting. (B) Soil moisture at wilting. Letters above violin plots indicate significant differences between species (Tukey’s HSD test, P-value <0.05). Results are shown for the first biological experiment.
Fig. 2.
Typical phenotypes of wilting observed in Arabidopsis halleri, A. lyrata and A. thaliana. Plant morphology before the water withdrawal treatment (top row) and at wilting (bottom row) for A. halleri (A, D), A. lyrata (B, E) and A. thaliana (C, F). All plants were grown in 7 cm pots. One single plant was grown in each 7 cm pot and no vegetative propagation had occurred at the time the experiment was performed.
Arabidopsis halleri plants exhaust SWC faster
To understand why A. halleri plants wilted around 1 week earlier than A. lyrata but at a similar soil moisture, we evaluated the rate of soil water loss for each species. We detected a significant interaction between species and time on soil moisture before wilting which showed that soil moisture decreased faster in pots where A. halleri accessions grew (Supplementary Data Fig. S3A, M3: F12, 1194 = 97.026, P-value <2.2e-16). Arabidopsis halleri thus consumed water significantly faster than A. thaliana and A. lyrata. Here again, this observation was replicated in the second biological experiment (M3: F4, 1224 = 761.07, P-value <2.2e-16, Supplementary Data Fig. S3B).
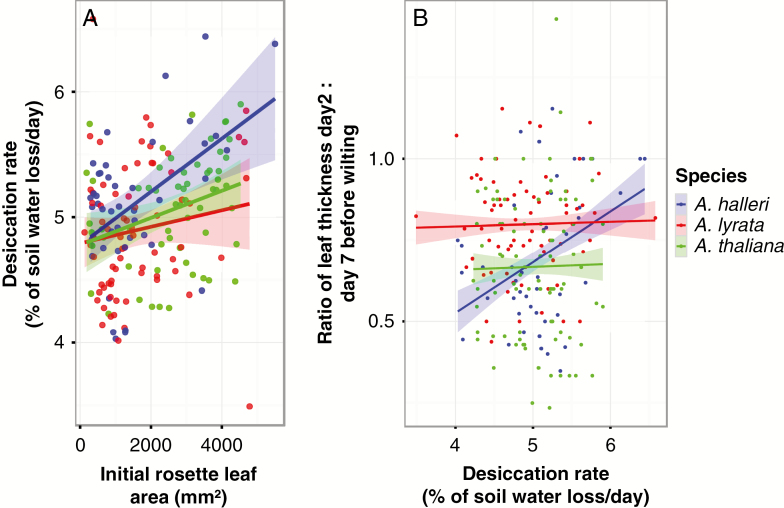
To examine the impact of plant size on the rate of soil water loss, we measured initial plant size and estimated the desiccation rate, defined as the rate of soil water loss per day over the 7 d following the water withdrawal in the second experiment of the dry-down experiment. Arabidopsis lyrata and A. halleri accessions started with a similar rosette size, but A. thaliana rosettes were initially larger (M2: F2, 173 = 10.85, P-value = 3.65e-05, Supplementary Data Fig. S4A; Table S6). We detected a significant effect of the initial rosette area on the pot desiccation rate (M4 F1, 170 =16.10, P-value = 8.97e-05). Significant correlations were detected between desiccation rate and initial rosette size in A. halleri, less so in A. thaliana but not in A. lyrata (Fig. 4A). Yet, the absence of a significant interaction term between initial rosette area and species (M4: F2, 170 = 1.89, P-value = 0.15) indicated that interspecific differences in plant size did not explain interspecific differences in the rate of soil water consumption.
Fig. 4.
Correlations between desiccation rate and initial leaf size and desiccation rate and the relative leaf water loss. (A) Correlation between the initial rosette leaf area (at 60 % of soil moisture) and the percentage of soil desiccation rate [Pearson correlation coefficients and P-values for: Arabidopsis thaliana (r = 0.32, P = 0.013); A. lyrata (r = 0.14, P = 0.22) and A. halleri (r = 0.48, P = 0.00072)]. (B) Correlation between the relative water loss in leaves before wilting (equivalent to the ratio of leaf thickness on day 2 vs. day 7 before wilting) and the desiccation rate [Pearson correlation coefficients and P-values for: A. thaliana (r = 0.018, P = 0.732); A. lyrata (r = 0.023, P = 0.692) and A. halleri (r = 0.39, P = 4.282.10-08)]. Results are shown for the second biological experiment. Lines represent a linear regression smoothing where the shaded ribbons represent the standard error.
Arabidopsis lyrata has the lowest relative loss of leaf water content before wilting
To estimate changes in leaf water content during the water-limited phase, we monitored leaf thickness (Lambers et al., 1998) during the soil dry-down phase in the second biological experiment. Initial leaf thickness was significantly higher in A. lyrata plants compared with A. thaliana and A. halleri (M1: F2, 140 = 9.38, P-value = 3.30e-10, Supplementary Data Fig. S4B; Table S7). We also detected a significant accession effect within A. lyrata on the initial leaf thickness (M1, F33, 140 = 1.642, P-value = 0.02548).
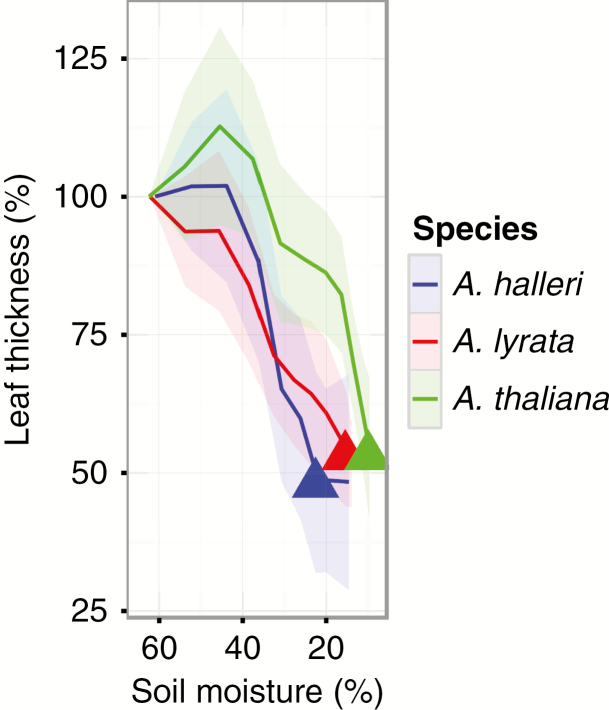
The significant interaction effect of soil desiccation rate and species (M4, F2, 818 = 11.15, P-value = 1.66e-05) on leaf thickness change over time revealed that the correlation between leaf thickness and soil desiccation rate was significant only for A. halleri (Fig. 4B; Supplementary Data Table S9). Furthermore, this analysis showed that A. thaliana leaves were able to hold higher amounts of water at lower soil moisture, compared with A. lyrata and A. halleri (Fig. 5), an indication that this species can effectively avoid the effects of drought by maintaining a comparatively higher water content in its leaves.
Fig. 5.
Leaf thickness in response to decrease of soil moisture for Arabidopsis thaliana, A. halleri and A. lyrata. Results were collected in the second biological experiment. Shaded ribbons represent the standard deviation. Filled triangles correspond to the average wilting soil moisture for the different species.
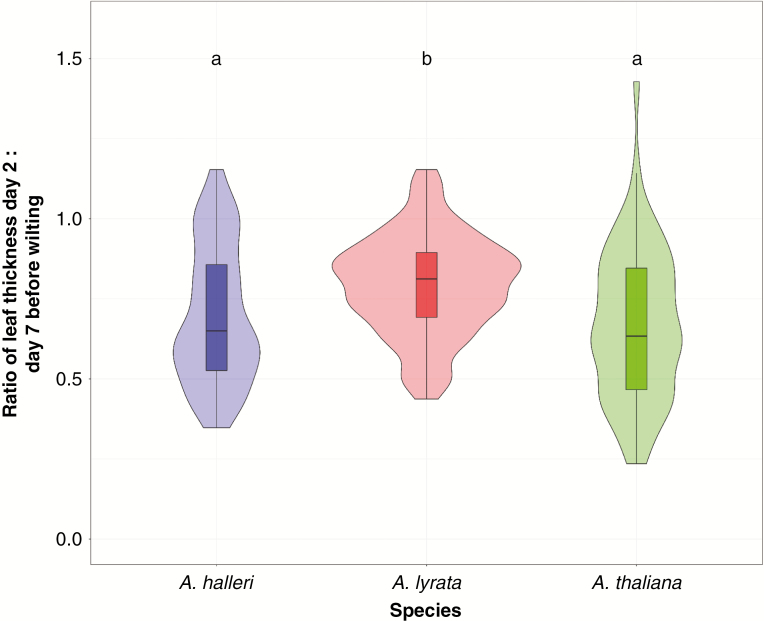
Arabidopsis thaliana and A. halleri, however, lost similar amounts of water in the days preceding wilting. The relative loss of leaf water content before wilting was calculated by the ratio of leaf thickness 2 d before wilting to leaf thickness 7 d before wilting (Fig. 6). There was no significant accession effect on the decrease of leaf thickness in the 7 d before wilting (M1: F33, 138 = 0.9401, P-value = 0.566) but the relative decrease before wilting was significantly higher in A. thaliana and A. halleri compared with A. lyrata (M1: F2,171 = 6.628, P-value = 5.00e-8, Fig. 6; Supplementary Data Table S8). This pattern indicates that leaf water content in the days preceding the onset of wilting decreased more slowly in A. lyrata plants compared with A. halleri and A. thaliana. This suggests that wilting A. lyrata leaves experience a lower loss of turgor.
Fig. 6.
Relative leaf water loss 7 d before wilting in Arabidopsis halleri, A. lyrata and A. thaliana. This is equivalent to the ratio of leaf thickness at day 2 vs. day 7 before wilting. Boxplots with the same letter are not significantly different (Tukey’s HSD, P-value <0.05). Results are shown for the second biological experiment.
High photosynthetic efficiency in wilted A. halleri and A. lyrata plants
Photosynthetic efficiency was measured to evaluate the physiological status of plants at wilting. We used the Fv:Fm ratio as an indicator for the potential capacity of non-cyclic electron flow in the photosynthetic light reaction. Despite the collapsed or rolled leaves observed at wilting in A. halleri and A. lyrata, respectively, both still had a high photosynthetic capacity: on average 83 and 90 %, respectively. In contrast, the photosynthetic capacity had significantly dropped in wilted A. thaliana rosettes (Supplementary Data Fig. S5; Table S10).
Arabidopsis thaliana has the lowest survival rate
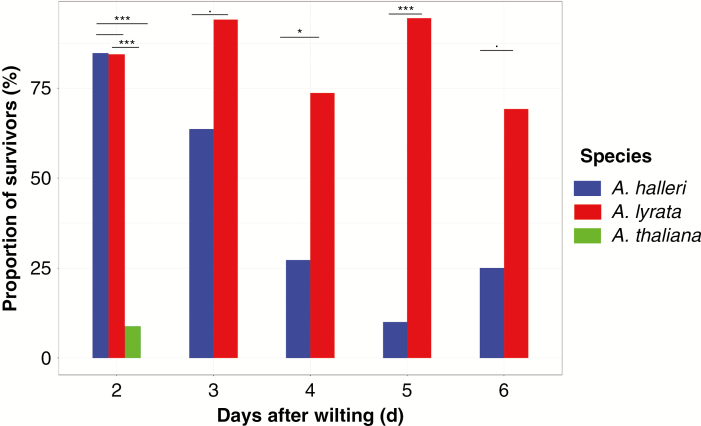
Individual plants were re-watered 2 d after observing symptoms of wilting. Two to three weeks after re-watering, we scored survival. The proportion of survivors was significantly lower in A. thaliana compared with A. halleri and A. lyrata (9 % in A. thaliana, 85 % in A. halleri and 84 % in A. lyrata, Fig. 7; Supplementary Data Table S11). These differences were consistent across the two experiments (Supplementary Data Fig. S6).
Fig. 7.
Average survival rate after re-watering following 2–6 d of wilting for Arabidopsis halleri, A. lyrata and A. thaliana. Results are shown for the first biological replicate. Barplots with one asterisk or more are significantly different (Tukey’s HSD, P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001).
To evaluate and compare the tolerance to wilting in A. lyrata and A. halleri, we ran an additional experiment examining whether extending the time from wilting to re-watering impacted survival. We detected a significant interaction effect of species and time to re-watering on survival (M4: χ2 = 234, d.f. = 1, d.f. residuals = 252, P-value = 1.615e-04). We observed that 70–85 % of A. lyrata plants survived 3 –6 day-long wilting periods (Fig. 7). In comparison, this percentage dropped to 10 % for A. halleri plants after 5 d of wilting, and this was significantly different between species (Fig. 7, M2: F1, 26 = 20.681, P-value = 2.44e-10). These results indicate that A. lyrata is more tolerant to wilting than its sister species A. halleri.
Efficient post-drought recovery in A. lyrata plants
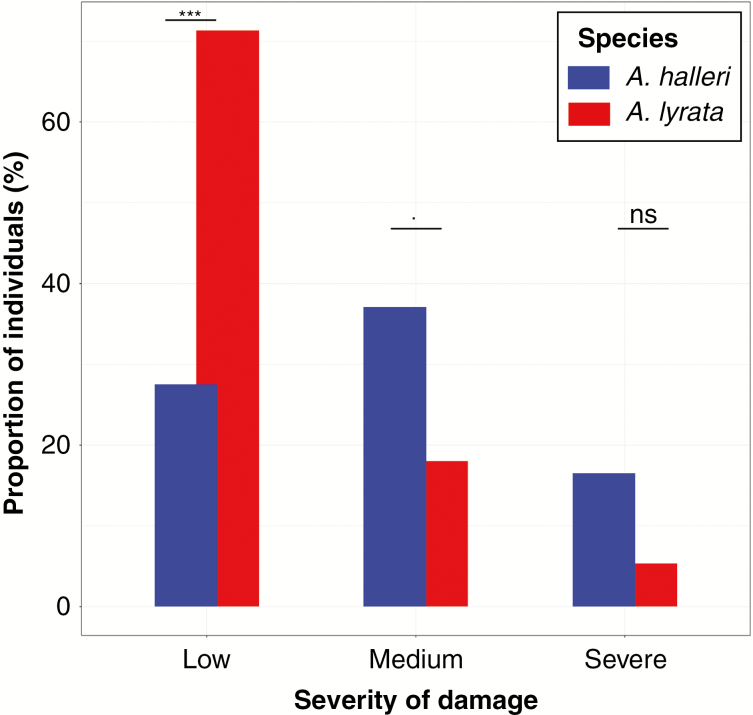
We further assessed the tolerance to wilting by comparing damage exhibited by plants that survived 2 d of wilting in A. lyrata and A. halleri. The interaction between species and the damage score were found to be significant (M4: F3, 100 = 2.96, P-value = 0.035). In A. lyrata, about 70 % of plants showed a very low degree of damage in leaves, whereas in A. halleri, only 30 % of plants had low damage levels (M4: Fig. 8, F1, 25 = 24.063, P-value = 4.761e-05). We did not include A. thaliana in the statistical analysis because only ten out of 60 plants survived wilting. These results confirmed that A. lyrata tolerates soil dehydration and wilting better than A. halleri.
Fig. 8.
Damage scored on survivors of 2 d of wilting after resuming growth for Arabidopsis halleri, A. lyrata and A. thaliana. Results are shown for the second biological experiment. Barplots with one asterisk or more are significantly different (Tukey’s HSD, P < 0.1; ***P < 0.001; ns, not significant).
Transcriptome analysis confirms that A. halleri is more sensitive to low SWC
Arabidopsis lyrata and A. halleri both wilted at the same SWC, but they differed in their survival following wilting. In order to gain insight into the molecular changes underpinning these differences, we performed a third dry-down experiment to collect leaf material in one representative accession of each of the sister species A. halleri and A. lyrata, and examined the reaction to stress and recovery at the transcriptome level.
For each species, we compared transcript abundance at three time points during the dry-down experiment, i.e. at soil moisture 60 %, soil moisture 20–25 % and after recovery. The two species wilted at around 18 % of soil moisture, as observed in the first two experiments, i.e. just below the soil moisture level at which leaf material was sampled. A total of 107 and 976 genes changed their expression level at 20–25 % vs. 60 % soil moisture in A. lyrata and A. halleri, respectively (FDR 0.1; fold change >1.6). Only three genes were responsive in both species to the decrease in SWC, and this was a random overlap (hypergeometric test, P-value = 0.993).
After recovery, 275 A. lyrata genes and 20 A. halleri genes had changed expression level compared with at 60 % SWC (Table 1). Since both species had similarly high survival rates upon 2 d of wilting and because new undamaged leaves were sampled, these differences are not due to survival differences. We conclude that A. halleri displayed a comparatively sharpened response to low SWC, whereas the transcriptome of A. lyrata was comparatively more altered after recovery.
Table 1.
Number of significantly differentially expressed genes in Arabidopsis halleri and A. lyrata during the dry-down experiment at 20 % soil moisture or after recovery compared with expression before stress (60 % soil moisture)
| A. halleri | A. lyrata | ||
|---|---|---|---|
| 20 % vs. 60 % soil moisture | Up | 253 | 36 |
| Down | 676 | 71 | |
| Recovery vs. 60 % soil moisture | Up | 8 | 111 |
| Down | 12 | 156 |
In a previous study, 2975 and 5445 genes were shown to be responsive to 2 and 10 h of dehydration in A. thaliana, respectively (Matsui et al., 2008). These drought-responsive genes were enriched in all sets of responsive genes identified in our study, either in A. halleri or in A. lyrata, at 20 % soil moisture or after recovery (Table 2, hypergeometric test, maximum P ≤8.77e-19). This confirmed that our protocol succeeded in activating dehydration-responsive genes. The list of significantly differentially expressed genes (including only AGI codes) is provided in Supplementary Data Table S12.
Table 2.
Percentage of differentially expressed genes that overlap with differentially expressed genes reported in Matsui et al. (2008) after 2 h (dh2) and 10 h (dh10) of dehydration stress
| dh2 expected: up 7.39 %, down 10 % | dh10 expected: up 10 %, down 7.5 % | ||
|---|---|---|---|
| A. halleri 20 % vs. 60 % soil moisture | Up (127 ATG genes) | 27.5 % P = 1.09e-12 | 47.2 % P = 7.82e-28 |
| Down (385 ATG genes) | 12.4 % P = 6.03e-23 | 36.3% P = 1.17e-59 | |
| A. halleri recovery vs. 60 % soil moisture | Up (6 ATG genes) | 0 ns | 0 ns |
| Down (7 ATG genes) | 0 ns | 28.5 % P = 1.20e-02 | |
| A. lyrata 20 % vs. 60 % soil moisture | Up (15 ATG genes) | 40 % P = 4.52e-05 | 46.6 % P = 3.34e-05 |
| Down (37 ATG genes) | 5.4 % ns | 18.9 % P = 5.7e-03 | |
| A. lyrata recovery vs. 60 % oil moisture | Up (61 ATG genes) | 63.9 % P = 1.06e-30 | 54 % P = 8–77e-19 |
| Down (90 ATG genes) | 11.1 % ns | 32.2 % P = 1.63e-12 |
ns, not significant.
The random expectation of percentage overlap is indicated in the top row.
Different GO categories are regulated by decreasing SWC in the two species
Analysis of enrichment in GO categories confirmed that different sets of genes were activated in the two species at each sampling stage. In A. halleri, many genes involved in growth and development were downregulated when SWC decreased to 20–25 % (Table 3). These functions were not enriched in A. lyrata samples collected at the same time; instead, genes involved in response to water deprivation and in ethylene and ABA signalling pathways were upregulated in A. lyrata after recovery (Table 3). Several of the GO terms enriched either in A. halleri at 20 % SWC or in A. lyrata after recovery have already been associated with drought stress. For example, GO categories such as isopentenyl diphosphate metabolic process, response to water deprivation, hyperosmotic salinity response, photosynthetic light reaction, response to chitin, photosystem II assembly and maltose metabolic process (Table 3) were also enriched among genes responding to mild drought stress in A. thaliana, although the direction of the gene expression change was not the same (Des Marais et al., 2012). We further observed that genes with altered expression in A. halleri were enriched for genes functioning in plastid organization, the pentose-phosphate shunt and photosystem II assembly. These three GO categories harbour an excess of cis-acting changes in the A. halleri lineage in response to dehydration stress (He et al., 2016).
Table 3.
GO categories showing a significant enrichment (P < 0.01) among differentially expressed genes between 20 and 60 % soil moisture and between recovery and 60 % soil moisture for Arabidopsis halleri and A. lyrata
| GO.ID | Term | P-value | Gene regulation | |
|---|---|---|---|---|
| A. halleri 20 % vs. 60 % soil moisture | GO:0015979 | Photosynthesis | 0.0011 | Down |
| GO:1901576 | Organic substance biosynthetic process | 0.0013 | Down | |
| GO:0044711 | Single-organism biosynthetic process | 0.0014 | Down | |
| GO:0051188 | Cofactor biosynthetic process | 0.0023 | Down | |
| GO:0008283 | Cell proliferation | 0.0035 | Down | |
| GO:0006098 | Pentose-phosphate shunt | 0.0041 | Down | |
| GO:0009965 | Leaf morphogenesis | 0.0048 | Down | |
| GO:0009657 | Plastid organization | 0.0059 | Down | |
| GO:0042254 | Ribosome biogenesis | 0.0059 | Down | |
| GO:0006084 | Acetyl-CoA metabolic process | 0.0064 | Down | |
| A. lyrata recovery vs. 60 % soil moisture | GO:0006098 | Pentose-phosphate shunt | 0.000043 | Down |
| GO:0010200 | Response to chitin | 0.000051 | Up | |
| GO:0010207 | Photosystem II assembly | 0.00007 | Down | |
| GO:0000023 | Maltose metabolic process | 0.00017 | Down | |
| GO:0009873 | Ethylene-activated signaling pathway | 0.0002 | Up | |
| GO:0019252 | Starch biosynthetic process | 0.00039 | Down | |
| GO:0009612 | Response to mechanical stimulus | 0.0015 | Up | |
| GO:0009414 | Response to water deprivation | 0.0029 | Up | |
| GO:0042538 | Hyperosmotic salinity response | 0.0043 | Up | |
| GO:0051707 | Response to other organism | 0.005 | Up | |
| GO:0009657 | Plastid organization | 0.00571 | Down | |
| GO:0050790 | Regulation of catalytic activity | 0.00763 | Down | |
| GO:0042742 | Defense response to bacterium | 0.00784 | Down | |
| GO:0009738 | Abscisic acid-activated signaling pathway | 0.0086 | Up |
DISCUSSION
In our experimental design, we have used several accessions per species as we were interested in comparing the drought stress response of the three related species, while accounting for variation within species. To exclude the possibility that our results are influenced by a previous history of stress, we discarded sick or slow growing plants and studied the drought response of vigorously growing individuals. Our results showed genotypic differences in initial leaf thickness, initial stomatal density or initial rosette area, but the response to depletion in SWC did not reveal significant differences between accessions. Differences in the response to water depletion therefore revealed fixed interspecific differences in avoidance and strategies of tolerance to drought stress.
Critical SWC does not reflect ecological differences between A. halleri and A. lyrata
The sister species A. lyrata and A. halleri have separated recently, and gene flow between the clades is still detectable (Novikova et al., 2016). Yet, the two species display marked differences in ecological preference (Clauss and Koch, 2006). Ellenberg indices, which are reliable estimates of ecological preferences in Central Europe, show that A. lyrata is found in very dry areas with a soil humidity index (F) of 3, while A. halleri occurs in habitats where water is less limiting (F = 6) (Ellenberg and Leuschner, 2010). We were therefore surprised to observe that A. halleri and A. lyrata individuals wilted at an identical SWC. In addition, contrary to our expectations, the ruderal species A. thaliana tolerated markedly lower SWC than its perennial relatives. Taken together, these observations show that the ecological preferences of A. lyrata, A. halleri and A. thaliana are not explained by the SWC threshold at which wilting symptoms appear.
Arabidopsis halleri is directly exposed to stress caused by low SWC
We observed that A. halleri was the fastest to consume the water contained in the soil. In pots where A. halleri individuals grew, SWC decreased significantly faster (Supplementary Data Fig. S3). Arabidopsis halleri also displayed the strongest correlation between plant size and the rate of water consumption, and an accelerated decrease in leaf thickness preceding the onset of wilting (Figs 4–6). At 25 % soil water content, i.e. shortly before the appearance of the first wilting symptoms, the rate of decrease in leaf thickness accelerated in A. halleri compared with A. lyrata. This turning point coincided with a change in the expression levels of a larger number of genes belonging to stress-repressed GO categories such as leaf morphogenesis, cell proliferation or photosynthesis. The downregulation of growth-related genes we observed, even before wilting symptoms appear, indicates that the plant experiences direct stress at the cellular level as SWC approaches the limiting threshold. In agreement with the high levels of stress it experienced, A. halleri also showed comparatively higher damage when survivors resumed growth after stress.
Although less tolerant to wilting than A. lyrata, A. halleri did display some level of tolerance, because it was comparatively more tolerant than A. thaliana as it survived 2 d of wilting. Yet, of the three species, A. halleri clearly displayed the weakest levels of drought avoidance, being almost directly exposed to stress caused by decreasing SWC. Arabidopsis halleri thrives in more competitive habitats than its relatives (Clauss and Koch, 2006; Stein et al., 2017), and competitive ability generally evolves in a trade-off with stress tolerance in plant species (Grime et al., 1977; Sreenivasulu et al., 2012). It is therefore possible that improved competitive ability was selected in this lineage at the expense of tolerance and avoidance mechanisms. Such evolutionary scenarios have been documented in several grass species (Fernández and Reynolds, 2000; Liancourt et al., 2005; Sugiyama, 2006). Interestingly, we have previously observed that an excess of cis-acting changes upregulating gene expression after 1 h of dehydration had accumulated in the A. halleri lineage in several functions that the more tolerant species, A. lyrata, downregulates during recovery (He et al., 2016). It is therefore possible that the decrease in tolerance and avoidance of drought stress was advantageous in the context of selection for increased competitive ability.
Arabidopsis lyrata displays avoidance and tolerance responses to soil dehydration
By comparison with A. halleri, A. lyrata displayed a more economical use of water. Arabidopsis lyrata plants displayed both a lower rate of water consumption and markedly lower damage levels after resuming growth. In addition, we observed that A. lyrata plants had the ability to survive longer durations of wilting than both A. halleri and A. thaliana (Fig. 7). It is also the only species that showed adaxial leaf rolling, a phenotype favouring drought avoidance in plants (Oppenheimer, 1960; O’Toole and Moya, 1978; Jones, 1979; Clarke, 1986). Leaf rolling indeed reduces the transpiration rate by reducing the effective leaf area. Altogether, this indicates that exposure to limiting SWC is comparatively less damaging in A. lyrata.
The transcriptome response to decreasing SWC corroborated this observation, by documenting lower levels of cellular stress in A. lyrata immediately before wilting, compared with A. halleri. Only a few genes changed expression before wilting in A. lyrata. We further observed that among genes downregulated after recovery, the most enriched GO category is ‘pentose-phosphate shunt’ (P < 5 × 10–5), a metabolic pathway involved in the scavenging of reactive oxygen intermediates that is strongly activated by abiotic stress (Mittler, 2002; Kruger and von Schaewen, 2003). Several additional GO functions associated with drought stress, such as ‘hyperosmotic salinity response’, ‘response to water deprivation’, ‘abscisic acid-activated signaling pathway’, ‘ethylene-activated signaling pathway’ and ‘response to chitin’ were upregulated in A. lyrata during recovery. These functions seem to have a dynamic role in drought stress. In A. thaliana, they were all upregulated by severe fast wilting (Matsui et al., 2008) but downregulated by mild stress (Des Marais et al., 2012). Their upregulation after recovery in A. lyrata, in the absence of obvious stress, shows that the reaction of this species to lowering SWC contrasts not only with that displayed by A. halleri but also with that known for A. thaliana. The absence of a strong modification of the expression of drought stress-responsive genes at SWC approaching critical levels in A. lyrata, combined with a high survival rate, further indicates that this species has the ability to (1) minimize its exposure to the stressful consequences of low soil water content and (2) maximize its ability to survive severe dehydration. It thus deploys both avoidance and tolerance strategies.
Whether the lower stomatal density observed in A. lyrata (Fig. 1A) contributes to its improved ability to cope with limiting water availability is difficult to evaluate with our data. Indeed, increased stomatal density has been associated with decreased WUE both within and between species (Reich, 1984; Muchow and Sinclair, 1989; Anderson and Briske, 1990; Pearce et al., 2006; Doheny-Adams et al., 2012; Liu et al., 2012; Carlson et al., 2016). Yet, in monkey flowers and in A. thaliana, lower stomatal density was associated with higher WUE (Wu et al., 2010; Dittberner et al., 2018). The consequences of modification in density and size on the plant’s ability to cope with limiting water supply are, in fact, not easily predictable. First, WUE can increase as a result of either increased stomatal density or increased stomatal size because larger stomata close more slowly (Raven, 2014). Secondly, the two traits generally correlate negatively (Hetherington and Woodward, 2003; Dittberner et al., 2018). Thirdly, parameters independent of stomatal patterning such as photosynthetic ability can also contribute to variation in WUE, as reported recently in A. thaliana (Farquhar et al., 1989; Dittberner et al., 2018). Fourthly, stomatal patterning changes in A. lyrata plants when exposed to limiting water supply (Paccard et al., 2014). Our data reveal that in well-watered greenhouse conditions, A. lyrata did not show a globally higher WUE than A. halleri (Fig. 1B), despite significant differences in stomatal density and size. Future work will have to investigate the impact of modifications in stomatal patterning on interspecific differences in tolerance and avoidance in the face of limiting SWC.
High levels of stress avoidance are associated with low tolerance to drought in A. thaliana
In annual species, seasonal drought can be a potent source of selection for accelerated flowering and faster cycling (Fitter and Fitter, 2002; Franks et al., 2007). Arabidopsis thaliana was also expected to maximize its resource investment into growth and reproduction and to show a lower level of stress tolerance compared with its perennial relatives. Here, we focused on late flowering A. thaliana accessions that in the conditions we imposed could not accelerate their development to escape drought. Thus, we cannot conclude on the relative investment of Arabidopsis species in escape strategies, but our experimental set up allowed an interspecific assessment of avoidance and tolerance to wilting. Contrary to expectations, we observed that our sample of accessions could persist at lower SWC than both of their perennial relatives, A. lyrata and A. halleri (Fig. 3A). In addition, the delayed decrease in leaf thickness observed in A. thaliana shows that, compared with the other two species, it is able to maintain its leaf water content at lower SWC (Fig. 5). This therefore suggests that the annual species A. thaliana also employs stress avoidance mechanisms. The ability of this annual species to escape stress by accelerating development has therefore not led to the loss of mechanisms favouring the maintenance of internal water potentials. Indeed, the production of proline, which is both an osmoprotectant and an anti-oxidant, δ13C, a proxy measuring WUE, as well as the maintenance of photosynthesis during terminal drought have been documented to play a role in local adaptation in this species (Verslues and Juenger, 2011; Kesari et al., 2012; Dittberner et al., 2018; Exposito-Alonso et al., 2018).
Arabidopsis thaliana, however, was not able to tolerate wilting. We observed a marked decrease in the photosynthetic capacity at wilting in this species, as previously reported in several species such as Hordeum vulgare, Hibiscus rosa-sinensis and Andropogon gerardii (Golding and Johnson, 2003; Muñoz and Quiles, 2013; Maricle et al., 2017). In addition, A. thaliana did not survive after 2 d of wilting, although its perennial relatives displayed markedly higher survival rates. The annual species therefore appears to have evolved lower levels of tolerance to wilting.
We detected no significant variation for the response to decreasing SWC between the A. thaliana accessions included in this study; however, we cannot conclude that the avoidance capacity and the low tolerance to wilting we observed is fixed in the species. The A. thaliana population we used consisted of a set of late-flowering accessions from Spain that could not accelerate flowering quickly enough to escape stress. This set of accessions is not necessarily representative of the whole species. Arabidopsis thaliana is broadly distributed and its accessions can form ecotypes with markedly different levels of stress resistance (May et al., 2017). Furthermore, two recent studies indicate that Swedish accessions have a comparatively greater capacity to face dry conditions, probably because the short favourable season of Scandinavia constrains them to face limiting water availability when it strikes (Dittberner et al., 2018; Exposito-Alonso et al., 2018).
Conclusion
This study documents the contrasting reactions deployed by Arabidopsis species in response to lowering SWC. In the face of their respective ecologies, these diversified reactions probably reflect the priority shifts imposed by divergent ecologies and life cycles. Future studies aiming at dissecting the genetic and molecular underpinning of these differences promise to teach us much about the processes accompanying ecological diversification in plant species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: summary of short read mapping to the A. lyrata reference genome V1. Figure S2: wilting day and soil moisture at wilting for the two first biological experiments of the drying-down experiments. Figure S3: soil water content during the first 7 d after water withdrawal. Figure S4: initial rosette area and leaf thickness of the plants used in the second biological experiments of the drying-down experiment. Figure S5: photosynthetic efficiency at wilting. Figure S6: proportion of surviving A. halleri, A lyrata and A. thaliana plants 2 d after re-watering for the two first biological experiments. Table S1: list of accessions used for the dry-down experiments. Table S2: phenotypes measured in the three drying-down experiments. Table S3: number of accessions used in the three drying-down experiments. Table S4: summary statistics of the multiple comparison of the wilting day between species. Table S5: summary statistics of the multiple comparison of the soil moisture at wilting between species. Table S6: summary statistics of the multiple comparison of the initial rosette area between species. Table S7: summary statistics of the multiple comparison of the initial leaf thickness between species. Table S8: summary statistics of the multiple comparison of the relative leaf water loss 7 d before wilting between species. Table S9: summary statistics of glm testing the effect of interaction between species and desiccation rate on the relative loss of leaf water content before wilting. Table S10: summary statistics of the multiple comparison of the photosynthetic efficiency at wilting between species. Table S11: summary statistics of the multiple comparison of the survival rate 2 d after re-watering between species. Table S12: differentially expressed genes identified for each of Arabidopsis halleri and A. lyrata between 20 and 60 % soil moisture and between recovery and 60 % soil moisture. Table S13: phenotypic data collected in this study.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Agustín Arce, Kim Steige, Ulrike Goebel, Hannes Dittberner, Veronica Preite, Julia Plewka, Nina Grisard, Martin Rippin and Kirsten Bell for their technical support, and Ute Krämer for providing A. halleri accessions and commenting on the experimental set up and on the manuscript draft. The Cologne Center for Genomics performed HiSeq4000 RNA sequencing. The phenotypic data are available as supplementary information (Supplementary Data Table 13). This work was supported by the German Research Foundation ‘Deutsche Forschungsgemeinschaft DFG [DFG priority program 1529 ‘ADAPTOMICS’] and by the Cluster of Excellence on Plant Sciences [EXC 1028].
LITERATURE CITED
- Alexa A, Rahnenfuhrer J. 2010. topGO: enrichment analysis for Gene Ontology. R package version 2.20.0. [Google Scholar]
- Anderson VJ, Briske DD. 1990. Stomatal distribution, density and conductance of three perennial grasses native to the southern true prairie of Texas. The American Naturalist 123: 152–159. [Google Scholar]
- Becher M, Talke IN, Krall L, Krämer U. 2004. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. The Plant Journal 37: 251–268. [DOI] [PubMed] [Google Scholar]
- Bechtold U, Penfold CA, Jenkins DJ, et al. . 2016. Time-series transcriptomics reveals that AGAMOUS-LIKE22 affects primary metabolism and developmental processes in droughtstressed Arabidopsis. The Plant Cell 28: 345–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9: 1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. 1995. Adaptations to environmental stresses. The Plant Cell 7: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 1982. Plant productivity and environment. Science 218: 443–448. [DOI] [PubMed] [Google Scholar]
- Bray EA. 1997. Plant responses to water deficit. Trends in Plant Science 2: 48–54. [Google Scholar]
- Bray EA. 2004. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany 55: 2331–2341. [DOI] [PubMed] [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists, 1158–1203. [Google Scholar]
- Carlson JE, Adams CA, Holsinger KE. 2016. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Annals of Botany 117: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JM. 1986. Effect of leaf rolling on leaf water loss in Trilicam spp. Canadian Journal of Plant Science 66: 885–891. [Google Scholar]
- Clauss MJ, Koch MA. 2006. Poorly known relatives of Arabidopsis thaliana. Trends in Plant Science 9: 449–459. [DOI] [PubMed] [Google Scholar]
- Close TJ. 1996. Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum 97: 795–803. [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annual Review of Ecology, Evolution, and Systematics 33: 507–559. [Google Scholar]
- Des Marais DL, McKay JK, Richards JH, Saunak S, Tierney W, Juenger TE. 2012. Physiological genomics of response to soil drying in diverse Arabidopsis accessions. The Plant Cell 24: 893–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vylder J, Vandenbussche F, Hu Y, Philips W, Van Der Straeten D. 2012. Rosette tracker: an open source image analysis tool for automatic quantification of genotype effects. Plant Physiology 160: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittberner H, Korte A, Mettler-Altmann T, Weber APM, Monroe G, de Meaux J. 2018. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Molecular Ecology 27: 4052–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Gingeras TR. 2015. Mapping RNA-seq reads with STAR. Current Protocols in Bioinformatics 51: 11.14.1–11.14.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula A, Fulgione A, Gutaker RM, et al. . 2017. African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 114:5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. 2010. Vegetation Mitteleuropas mit den Alpen, 6th edn. Stuttgart: Eugen Ulmer. [Google Scholar]
- Exposito-Alonso M, Vasseur F, Ding W, Wang G, Burbano HA, Weigel D. 2018. Genomic basis and evolutionary potential for extreme drought adaptation in Arabidopsis thaliana. Nature Ecology and Evolution 2: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Xiong L. 2015. General mechanisms of drought response and their application in drought resistance improvement in plants. Cellular and Molecular Life Sciences. 72: 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185–212. [Google Scholar]
- Farquhar GD, Richards A. 1984. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11: 539–552. [Google Scholar]
- Farquhar GD, Ehleringer J, Hubick K. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. [Google Scholar]
- Fernández TJ, Reynolds JF. 2000. Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia 123: 90–98 [DOI] [PubMed] [Google Scholar]
- Fitter AH, Fitter RSR.. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Fox GA. 1990. Drought and the evolution of flowering time in desert annuals. American Journal of Botany 77: 1508–1518. [Google Scholar]
- Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences, USA 104: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai S, Cooper M. 1995. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Research 40: 67–86. [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Golding AJ, Johnson GN. 2003. Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218: 107–114. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Wolkers WF, Hoekstra FA. 1997. Long-term stability of protein secondary structure in dry seeds. Comparative Biochemistry and Physiology 117A: 343–348. [Google Scholar]
- Gowik U, Brautigam A, Weber KL, Weber APM, Westhoff P. 2011. Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4? The Plant Cell 23 6: 2087–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary. The American Naturalist 111: 1169–1194. [Google Scholar]
- Harb A, Krishnan A, Ambavaram Madana MR, Pereira A. 2010. Molecular and physiological analysis of drought stress in arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology 154: 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Arce AL, Schmitz G, Koornneef M, Novikova P, Beyer A, de Meaux J. 2016. The footprint of polygenic adaptation on stress-responsive cis-regulatory divergence in the Arabidopsis genus. Molecular Biology and Evolution 8: 2088–2101. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424: 901–908. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 479: 479–485. [DOI] [PubMed] [Google Scholar]
- Hoffmann MH. 2005. Evolution of the realized climatic niche in the genus Arabidopsis (Brassicaceae). Evolution 59: 1425–1436. [PubMed] [Google Scholar]
- Hu TT, Pattyn P, Bakker E, et al. . 2011. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nature Genetics 43: 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J, Bartels D. 1996. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 377–403. [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, et al. . 2001. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. The Plant Journal 27: 325–333. [DOI] [PubMed] [Google Scholar]
- Jones HG. 1979. Visual estimation of plant water status in cereals. Journal of Agricultural Science 92: 83–89. [Google Scholar]
- Juenger TE. 2013. Natural variation and genetic constraints on drought tolerance. Current Opinion in Plant Biology 16: 274–281. [DOI] [PubMed] [Google Scholar]
- Kermode A. 1997. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Science Research 7: 75–95. [Google Scholar]
- Kesari R, Lasky JR, Villamor JG, et al. . 2012. Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proceedings of the National Academy of Sciences, USA 109: 9197–9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyers NJ. 2015. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science 234: 155–162. [DOI] [PubMed] [Google Scholar]
- Krämer U, Clemens S. 2006. Functions and homeostasis of zinc, copper and nickel in plants. In: Tamas MJ, Martinoia E, eds. Molecular biology of metal homeostasis and detoxification. Heidelberg: Springer-Verlag, 215–271. [Google Scholar]
- Kronholm I, Picó FX, Alonso-Blanco C, Goudet J, de Meaux JD. 2012. Genetic basis of adaptation in Arabidopsis thaliana: local adaptation at the seed dormancy QTL DOG1. Evolution 66: 2287–2302. [DOI] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A. 2003. The oxidative pentose phosphate pathway: structure and organization. Current Opinion in Plant Biology 6: 236–246. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 1998. Plant physiological ecology. New York: Springer-Verlag. [Google Scholar]
- Levitt J. 1980. Responses of plants to environmental stresses, 2nd edn. New York: Academic Press. [Google Scholar]
- Liancourt P, Callaway RM, Michalet R. 2005. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 86: 1611–1618. [Google Scholar]
- Liu J, Zhang F, Zhou J, Chen F, Wang B, Xie X. 2012. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Molecular Biology 78: 289–300. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JT, Juenger TE, Michaels SD, et al. . 2013. Pleiotropy of FRIGIDA enhances the potential for multivariate adaptation. Proceedings of the Royal Society B: Biological Sciences 280: 2013–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JT, Mullen JL, Lowry DB, et al. . 2015. Exploiting differential gene expression and epistasis to discover candidate genes for drought-associated QTLs in Arabidopsis thaliana. The Plant Cell 27: 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow MM. 1989. Strategies of response to water stress. In: Kreeeb KH, Richter H, Hinckley TM, eds. Structural and functional responses to environmental stresses. The Hague: SPB Academic Publishing, 269–281. [Google Scholar]
- Maricle BR, Caudle KL, Lindsey KJ, Baer SG, Johnson LC. 2017. Effects of extreme drought on photosynthesis and water potential of Andropogon gerardii (Big Bluestem) ecotypes in common gardens across Kansas. Transactions of the Kansas Academy of Science 120: 1–16. [Google Scholar]
- Matsui A, Ishida J, Morosawa T, et al. . 2008. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant & Cell Physiology 49: 1135–1149. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51: 659–668. [DOI] [PubMed] [Google Scholar]
- May RL, Warner S, Wingler A. 2017. Classification of intra-specific variation in plant functional strategies reveals adaptation to climate. Annals of Botany 119: 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. 2003. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology 12: 1137–1151. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T. 2001. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecology & Evolution 16: 693–700. [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7: 405–510. [DOI] [PubMed] [Google Scholar]
- Muchow RC, Sinclair TR. 1989. Epidermal conductance, stomatal density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant, Cell & Environment 12: 425–431. [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. 2015. Mechanisms of abscisic acid-mediated control of stomatal aperture. Current Opinion in Plant Biology 28: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz R, Quiles M J. 2013. Water deficit and heat affect the tolerance to high illumination in hibiscus plants. International Journal of Molecular Sciences 14: 5432–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Ito Y, Yamaguchi-Shinozaki K. 2009. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology 149: 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova PY, Hohmann N, Nizhynska V, et al. . 2016. Sequencing of the genus Arabidopsis identifies a complex history of nonbifurcating speciation and abundant trans-specific polymorphism. Nature Genetics 48: 077–1082. [DOI] [PubMed] [Google Scholar]
- O’Toole JC, Moya TB. 1978. Genotypic variation in maintenance of leaf water potential in rice. Crop Science 18: 873–876. [Google Scholar]
- Oppenheimer HR. 1960. Adaptation to drought: xerophytism. In: Plant–water relationships in arid and semi-arid conditions. Paris: United Nations Educational, Scientific and Cultural Organization, 105–138. [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP. 2014. ABA control of plant macro-element membrane transport systems in response to water deficit and high salinity. New Phytologist 202: 35–49. [DOI] [PubMed] [Google Scholar]
- Paccard A, Fruleux A, Willi Y. 2014. Latitudinal trait variation and responses to drought in Arabidopsis lyrata. Oecologia 175: 577–587. [DOI] [PubMed] [Google Scholar]
- Pauwels M, Saumitou-Laprade P, Holl AC, Petit D, Bonnin I. 2005. Multiple origin of metallicolous populations of the pseudometallophyte Arabidopsis halleri (Brassicaceae) in central Europe: the cpDNA testimony. Molecular Ecology 14: 4403–4414. [DOI] [PubMed] [Google Scholar]
- Pearce DW, Millard S, Bray DF, Rood SB. 2006. Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiology 26: 211–218. [DOI] [PubMed] [Google Scholar]
- Price AH, Cairns JE, Horton P, Jones HG, Griffiths H. 2002. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. Journal of Experimental Botany 53: 989–1004. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. 2004. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology 161: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Reich PB. 1984. Leaf stomatal density and diffusive conductance in three amphistomatous hybrid poplar cultivars. New Phytologist 98: 231–239. [Google Scholar]
- Ross-Ibarra J, Wright SI, Foxe JP, et al. . 2008. Patterns of polymorphism and demographic history in natural populations of Arabidopsis lyrata. PLoS One 5: 8. doi: 10.1371/journal.pone.0002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, et al. . 2006. Functional analysis of an arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant Cell 18: 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. 1986. Continuous recording of photochemical and non-photochemical chlorophyll flourescence quenching with a new type of modulation fluorometer. Photosynthesis Research 10: 51–62. [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, et al. . 2001. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. The Plant Cell 13: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, et al. . 2002. Monito ring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA micro-array. The Plant Journal 31: 279–292. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. 2007. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany 58: 221–227. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Agren J. 2012. Variation in tolerance to drought among Scandinavian populations of Arabidopsis lyrata. Evolutionary Ecology 26: 559–77. [Google Scholar]
- Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A. 2012. Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506: 265–273. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. 1952. Aridity as a stimulus to plant evolution. The American Naturalist 86: 33–44. [Google Scholar]
- Stein RJ, Hoereth S, Romário J, et al. . 2017. Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. New Phytologist 213: 1274–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven FJ. 2011. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytologist 190: 249–257. [DOI] [PubMed] [Google Scholar]
- Stolpe C, Krämer U, Müller C. 2016. Heavy metal hyper-accumulation in leaves of Arabidopsis halleri is accompanied by a reduced performance of herbivores and shifts in leaf glucosinolate and element concentrations. Environmental and Experimental Botany 133: 78–86. [Google Scholar]
- Sugiyama S. 2006. Responses of shoot growth and survival to water stress gradient in diploid and tetraploid populations of Lolium multiflorum and L. perenne. Grassland Science 52: 155–160. [Google Scholar]
- Szabados L, Savouré A. 2010. Proline: a multifunctional amino acid. Trends in Plant Science 15: 89–97. [DOI] [PubMed] [Google Scholar]
- The 1001 Genomes Consortium 2016. 1135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonsor SJ, Alonso-Blanko C, Koornneef M. 2005. Gene function beyond the single trait: natural variation, gene effects, and evolutionary ecology in Arabidopsis thaliana. Plant, Cell & Environment 28: 2–20. [Google Scholar]
- Urano K, Maruyama K, Jikumaru Y, Kamiya Y, Yamaguchi-Shinozaki K, Shinozaki K. 2017. Analysis of plant hormone profiles in response to moderate dehydration stress. The Plant Journal 90: 17–36. [DOI] [PubMed] [Google Scholar]
- Verslues PE, Juenger TE. 2011. Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Current Opinion in Plant Biology 14: 240–245. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. 2000. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiology 122: 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2009. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Wolfe MD, Tonsor SJ. 2014. Adaptation to spring heat and drought in northeastern Spanish Arabidopsis thaliana. New Phytologist 201: 323–334. [DOI] [PubMed] [Google Scholar]
- Wu CA, Lowry DB, Nutter LI, Willis JH. 2010. Natural variation for drought response traits in the Mimulus guttatus species complex. Oecologia 162: 23–33. [DOI] [PubMed] [Google Scholar]
- Yue B, Xue W, Xiong L, et al. . 2006. Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172: 121–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.