Abstract
Background and Aims
Plant survival under extreme drought events has been associated with xylem vulnerability to embolism (the disruption of water transport due to air bubbles in conduits). Despite the ecological and economic importance of herbaceous species, studies focusing on hydraulic failure in herbs remain scarce. Here, we assess the vulnerability to embolism and anatomical adaptations in stems of seven herbaceous Brassicaceae species occurring in different vegetation zones of the island of Tenerife, Canary Islands, and merged them with a similar hydraulic–anatomical data set for herbaceous Asteraceae from Tenerife.
Methods
Measurements of vulnerability to xylem embolism using the in situ flow centrifuge technique along with light and transmission electron microscope observations were performed in stems of the herbaceous species. We also assessed the link between embolism resistance vs. mean annual precipitation and anatomical stem characters.
Key Results
The herbaceous species show a 2-fold variation in stem P50 from –2.1 MPa to –4.9 MPa. Within Hirschfeldia incana and Sisymbrium orientale, there is also a significant stem P50 difference between populations growing in contrasting environments. Variation in stem P50 is mainly explained by mean annual precipitation as well as by the variation in the degree of woodiness (calculated as the proportion of lignified area per total stem area) and to a lesser extent by the thickness of intervessel pit membranes. Moreover, mean annual precipitation explains the total variance in embolism resistance and stem anatomical traits.
Conclusions
The degree of woodiness and thickness of intervessel pit membranes are good predictors of embolism resistance in the herbaceous Brassicaceae and Asteraceae species studied. Differences in mean annual precipitation across the sampling sites affect embolism resistance and stem anatomical characters, both being important characters determining survival and distribution of the herbaceous eudicots.
Keywords: Canary Islands, drought, embolism resistance, herbaceous species, stem anatomy, thickness of intervessel pit membranes, woodiness, xylem hydraulics
INTRODUCTION
Hydraulic failure is one of the main physiological mechanisms associated with reductions in forest productivity and drought-induced tree mortality (Choat et al., 2012; Anderegg et al., 2016; Adams et al., 2017). Water movement inside the conduits is prone to dysfunction due to negative xylem pressures generating metastable conditions (Tyree and Sperry, 1989; Tyree and Zimmermann, 2002). With increasing drought stress, embolisms could propagate from a gas-filled conduit to a neighbouring functional conduit through interconduit pit membranes, potentially generating lethal levels of embolisms (Tyree and Zimmermann, 2002; Brodribb et al., 2010; Brodersen et al., 2013). The vulnerability to xylem embolism can be measured by vulnerability curves, in which the percentage loss of hydraulic conductivity is plotted against the xylem pressure (Cochard et al., 2010, 2013). The P50 value, referring to the negative pressure associated with 50 % loss of hydraulic conductivity, is an oft-cited proxy for plant drought resistance, although it does not present a critical threshold value for angiosperms (Urli et al., 2013; Adams et al., 2017).
There is considerable interspecific variation in P50 across plant species, from –0.5 MPa up to –19 MPa, and the majority of studies show that species from dry environments are generally more resistant to embolism (more negative P50) than species from wet environments (Choat et al., 2012; Lens et al., 2013, 2016; Larter et al., 2015). Knowledge about intraspecific variation in P50 remains scarce and provides contradictory results: it seems to be species specific, but it can vary either considerably (Kolb and Sperry, 1999; Choat et al., 2007; Corcuera et al., 2011; Nolf et al., 2014, 2016; Volaire et al., 2018; Cardoso et al., 2018) or subtly (Holste et al., 2006; Martínez-Vilalta et al., 2009; Lamy et al., 2013; Ahmad et al., 2017), or may even be absent (Maherali et al., 2009; Wortemann et al., 2011) for woody as well as for herbaceous species.
There is a vast body of literature available focusing on hydraulic conductivity and safety for hundreds of woody species (Maherali et al., 2004; Pittermann et al., 2010; Choat et al., 2012; Bouche et al., 2014; Gleason et al., 2016). Herbs, on the other hand, remain poorly investigated: P50 values of stems are available for <30 species, of which a minority are eudicots while most species are grasses (e.g. Mencuccini and Comstock, 1999; Stiller and Sperry, 2002; Kocacinar and Sage, 2003; Holste et al., 2006; Maherali et al., 2009; Rosenthal et al., 2010; Lens et al., 2013, 2016; Nolf et al., 2014, 2016; Skelton et al., 2017; Dória et al., 2018; Volaire et al., 2018). Based on this limited data set, most herbaceous species studied so far are sensitive to embolism formation in their stems, with a P50 of around –2.5 MPa. However, some of the grass stems studied are remarkably resistant to embolism formation (up to –7.5 MPa), implying that both herbs and trees share the ability to support very negative water potentials without embolism formation during drought stress (Lens et al., 2016).
In this study, we focus on the research field of xylem hydraulics in herbaceous stems which has been largely neglected, despite the overwhelming occurrence of economically important herbaceous food crops (Monfreda et al., 2008) and the dependency on grazed grasslands for our livestock. The main reason for neglecting herb hydraulics is that their fragile stems and often low hydraulic conductance make vulnerability curves technically more challenging. However, recent fine-tuning of the high-throughput in situ flow centrifuge method (cavitron; Lens et al., 2016; Dória et al., 2018) and the new optical vulnerability technique (Skelton et al., 2017) have yielded stem P50 data of herbaceous species, which opens up new opportunities to boost the virtually neglected aspect of herb hydraulics and predict future crop productivity and survival (Challinor et al., 2009), especially in a world facing climate change (Rahmstorf and Coumou, 2012; Dai, 2013).
In addition to the understudied aspect of herb hydraulics, we also investigate stem anatomical characters to assess poorly known structure–function relationships in herbaceous stems. Plant sensitivity to drought-induced embolism is determined by a whole suite of stem anatomical characters in woody trees (Hacke and Jansen, 2009; Lens et al., 2011; Jacobsen et al., 2012; Pivovaroff et al., 2016; Pereira et al., 2017; O’Brien et al., 2017), of which the thickness of intervessel pit membranes is probably one of the most hydraulically relevant anatomical features, altering both water flow efficiency and the spread of potential lethal levels of embolism in the xylem (Jansen et al., 2009; Lens et al., 2011; Li et al., 2016; Gleason et al., 2016; Dória et al., 2018). Furthermore, vessel diameter is an informative character determining xylem area-specific conductivity (Ks) (Hacke et al., 2016), but also correlates with plant height, environmental constraints and, potentially, embolism resistance (Davis et al., 1999; Olson and Rosell, 2013; Schreiber et al., 2015; Hacke et al., 2016; Olson et al., 2018). Mechanical characters such as wood density, total degree of lignification, thickness-to-span ratio of vessels and thickness of the intervessel wall have also been linked to increasing drought stress resistance (Hacke et al., 2001; Jacobsen et al., 2005, 2007; Chave et al., 2009; Hoffman et al., 2011; Pratt and Jacobsen, 2017). These mechanical characters are often reported as indirectly linked to embolism resistance, since embolism formation and spread occur at the pit level (Bouche et al., 2014; Pereira et al., 2017; Dória et al., 2018).
In herbaceous eudicots, an increase in embolism resistance is linked to an increase in wood formation, which reflects an increase in the proportion of lignified area per total stem area (Lens et al., 2013, 2016; Tixier et al., 2013; Dória et al., 2018), and also grasses that are more resistant to embolism formation have more lignified stems compared with the more vulnerable species (Lens et al., 2016). Wood formation has been observed in many herbaceous eudicots, especially at the base of the stem, and several studies show a continuous range in the degree of wood formation between stems of herbaceous eudicot species (Dulin and Kirchoff, 2010; Schweingruber et al., 2011; Lens et al., 2012a; Kidner et al., 2016; Dória et al., 2018). This highlights the fuzzy boundaries between woodiness and herbaceousness, leading to intermediate life forms such as ‘woody herbs’ or ‘half shrubs’ (Lens et al., 2012a), but species with these intermediate life forms do not form a wood cylinder that extends towards the upper parts of the stem and are therefore considered as herbaceous (Kidner et al., 2016).
In this study, we combine hydraulic measurements with detailed stem anatomical characteristics and climatic variables (from meteorological stations near the sampling sites) to investigate structure–function relationships in stems of seven herbaceous species belonging to the Brassicaceae family from the island of Tenerife (Canary Islands, Spain), and merged this data set with a similar data set for four herbaceous Asteraceae species that were sampled on the same island for a previous publication (Dória et al., 2018). The main reason for selecting Tenerife is the huge range of climatic conditions in a small area of 2034 km2, ranging from the humid northern laurel forests of Anaga to the dry southern desert-like region around El Médano, separated by the tall Teide volcano (approx. 3700 m asl) generating different altitudinal vegetation types (del-Arco et al., 2006). We address the following questions. (1) Do herbaceous species growing in drier environments have more embolism-resistant stems, both across and within species? (2) What are the stem anatomical characters that explain the variation in embolism resistance amongst the species studied? (3) Is there any relationship between precipitation and both xylem vulnerability to embolism and anatomical characters?
MATERIALS AND METHODS
Plant material and climate data
We collected the Brassicaceae specimens throughout the island of Tenerife, in different vegetation zones with different mean annual precipitation and aridity indices. The climatic data of precipitation and temperature for each of the sampling sites were provided by Agencia Estatal de Meteorología (AEMET, Spanish Government), covering a period from 110 to 30 years depending on the meteorological station. We received the data from five different meteorological stations (Anaga San Andrés, Arico Bueno, Arafo, Laguna Instituto and Vilaflor) matching the five sampling sites (Supplementary Data Fig. S1). We used the mean annual precipitation for each site, and calculated the potential evapotranspiration using the Thornthwaite equation (1948). The aridity indices were calculated as a ratio of mean annual precipitation to mean annual potential evapotranspiration (UNEP, 1997). Since this aridity index is highly correlated with mean annual precipitation (P < 0.001, r = 0.993) we opted to select the former in the statistical models.
The collection trip was carried out in March 2017, matching with the wet, flowering period of the herbaceous species. We harvested seven annual Brassicaceae species: Hirschfeldia incana (L.) Lagr.-Fossat, Raphanus raphanistrum L., Rapistrum rugosum L. All., Sinapis alba L., Sinapis arvensis L., Sisymbrium erysimoides Desf. and Sisymbrium orientale L. The time of germination is similar for all species studied and it is linked to the arrival of the rains in autumn and winter. However, there can be small differences between populations, amongst and within species: populations growing on the northern slopes of the island generally germinate earlier than plants growing on the southern slopes due to the moist north-eastern trade winds, and populations from higher altitudes usually germinate later than plants from lower altitudes.
The specimens of H. incana and S. orientale were collected from two different populations occurring in contrasting environments. The northern area of La Laguna (mean annual precipitation = 526.9 mm; aridity index = 0.68) and the southern area of Vilaflor (mean annual precipitation = 396.3 mm; aridity index = 0.53) were the wetter collection sites for H. incana and S. orientale populations, respectively. The drier sites were the southern areas of Guímar (mean annual precipitation = 311.8 mm; aridity index = 0.39) and the region of Arico Bueno (mean annual precipitation = 264.3 mm; aridity index = 0.34), for H. incana and S. orientale, respectively (Supplementary Data Fig. S1).
The four annual species of Asteraceae, Cladanthus mixtus (L.) Oberpr. & Vogt., Coleostephus myconis (L.) Cass., Glebionis coronaria (L.) Cass ex Spach and Glebionis segetum (L.) Fourr. included in this study were investigated by Dória et al. (2018), during the spring of 2016 in Tenerife in the area of La Laguna (mean annual precipitation = 526.9 mm; aridity index = 0.68), following the same methodological procedures described below. For both the Brassicaceae and Asteraceae species, we harvested 10–20 individuals per species. All the species studied are annual herbaceous species, but some species (especially S. alba and S. arvensis) show a tendency to become biannual, which may be a consequence of the release of seasonality compared with the European mainland (Carlquist, 1974).
All individuals were collected from the soil, with roots still attached, quickly wrapped in wet tissues and sealed in plastic bags. Afterwards, the stems were stored in a cold room (around 5 ºC) for a maximum of 5 d at the University of La Laguna, Tenerife. The sealed plastic bags were shipped by plane and immediately stored in a fridge for a maximum of 2 weeks at the caviplace facility to perform the hydraulic measurements (University of Bordeaux, France).
Xylem vulnerability to embolism
One to three stems per individual from at least ten individuals per species were used to measure vulnerability to embolism. Prior to measurements, all the stems were cut under water in the lab with a razor blade into a standard length of 27 or 42 cm in order to fit the two cavitron rotors used, and we confirmed that the vessels were shorter than the stem segments using the air pressure technique at 0.2 MPa. The cavitron is a modified centrifuge allowing the negative pressure in the central part of the stem segment to be lowered by spinning the stems at different speeds while simultaneously measuring the water transport in the vascular system (Cochard, 2002; Cochard et al., 2013). First, the maximum hydraulic conductance of the stem in its native state (Kmax in m2 MPa–1 s–1) was calculated under xylem pressure close to zero MPa using a reference ionic solution of 10 mm KCl and 1 mm CaCl2 in deionized ultrapure water. The rotation speed of the centrifuge was then gradually increased by –0.5 or –1 MPa to lower xylem pressure. The percentage loss of conductivity (PLC) of the stem was determined at each pressure step following the equation:
| (1) |
where Kmax represents the maximum conductance of the stem and K represents the conductance associated at each pressure step.
The vulnerability curves, showing the change in percentage loss of conductivity according to the xylem pressure, were obtained using the Cavisoft software (Cavisoft v1.5, University of Bordeaux, Bordeaux, France). A sigmoid function (Pammenter and Van der Willigen, 1998) was fitted to the data from each sample, using the following equation with SAS 9.4 (SAS 9.4, SAS Institute, Cary, NC, USA):
| (2) |
where S (% MPa–1) is the slope of the vulnerability curve at the inflexion point, P is the xylem pressure value used at each step, and P50 is the xylem pressure inducing 50 % loss of hydraulic conductivity. The parameters S and P50 were averaged for each species.
Stem anatomy
Light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were performed at Naturalis Biodiversity Center, the Netherlands, based on the samples for which we had obtained suitable vulnerability curves. The samples were taken from three individuals per species for LM and SEM, and from two individuals per species for TEM, from the middle part of the stem, where the negative pressure caused embolism formation during the cavitron experiment. The lab protocols for LM, SEM and TEM followed Dória et al. (2018). All the anatomical measurements were done using ImageJ (National Institutes of Health, Bethesda, MD, USA), largely following the suggestions of Scholz et al. (2013) and the IAWA Committee (1989).
Amongst the anatomical characters measured using LM, several indicators for lignification were calculated using a cross-section, such as the proportion of lignified area per total stem area [PLIG, measuring the sum of primary xylem area, secondary xylem (= wood) area and fibre caps area in the cortex and dividing it by the total stem area], the proportion of xylem fibre wall area per fibre area (PFWFX, at the level of a single cell), and the thickness-to-span ratio of vessels (TWDV). The diameter of vessels (DV) was calculated based on the lumen area that was considered to be a circle according to the equation:
| (3) |
where DV is the vessel diameter and A is the vessel lumen area. The hydraulically weighted vessel diameter (DH) was calculated following the equation:
| (4) |
where DV is the vessel diameter as measured in eqn (3).
The ultrastructure of intervessel pits was observed using a field emission scanning electron microscope (Jeol JSM-7600F, Tokyo, Japan) and a JEOL JEM 1400-Plus transmission electron microscope (JEOL, Tokyo, Japan), as described in Dória et al. (2018). Since we observed intervessel pit membranes from the central stem segment parts where centrifugal force was applied, our measurements provide a relative estimation of intervessel pit membrane thickness.
Statistical analyses
We tested the effect of both species and mean annual precipitation on the various hydraulic parameters (P12, P50, P88 and slope) using an analysis of covariance (ANCOVA). A log transformation, when necessary, was applied to the predictive variables to deal with heteroscedasticity and/or non-normality (Zuur et al., 2007). A post-hoc Tukey’s HSD test, from the R package Agricolae (Mendiburu, 2017), was used to test whether hydraulic parameters differ amongst species. To test the difference in P50 between the two Brassicaceae populations growing in contrasting environments (H. incana and S. orientale), we used linear mixed effects model, with the factor species as random effect, from the nlme R package (Pinheiro et al., 2018).
We applied simple linear regressions to test for the relationship between P50, climate data and anatomical variables. A log transformation, when necessary, was performed on the predictive variables to deal with heteroscedasticity and/or non-normality (Zuur et al., 2007).
In order to evaluate which anatomical variables explain embolism resistance, we performed a multiple linear regression with P50 as response variable and stem anatomical characters as predictive variables. We selected a priori the predictive variables using biological knowledge based on previously published studies in combination with a pairwise scatterplot to detect the presence of correlations and collinearities. Then, we conducted a variance inflation factor (VIF) analysis, keeping only variables with a VIF value <2 (Zuur et al., 2010). Subsequently, we followed the model simplification removing each time the least significant variable, until all the remaining terms in the model were significant (Crawley, 2007). The regression or differences were considered significant if P < 0.05. Next, we calculated the hierarchical partitioning (Chevan and Sutherland, 1991) for the variables retained in the model in order to assess their relative importance to explain P50.
Independent t-tests were used to compare stem anatomical differences between the two populations of Brassicaceae species collected in contrasting environments.
To test whether differences in mean annual precipitation for each sampling site (PR) explained the combined variation of P50 and the anatomical characters, including also these characters that were not retained in the multiple regression analysis (the proportion of xylem fibre wall area per fibre area as observed in a cross-section, the thickness-to-span ratio of vessels and the hydraulically weighted vessel diameter), we performed a permutational multivariate analysis of variance (PERMANOVA). The anatomical characters and P50 are the response variables (rank transformed) and the mean annual precipitation is the predictive variable. PERMANOVA was performed using the adonis function in the Vegan R package (Oksanen et al., 2015), based on Euclidean distances and 999 permutations. Later, a principal component analysis (PCA) was conducted using the function rda in the package Vegan, to observe simultaneously the relationships amongst the species, the main stem anatomical variables, the physiological variable (P50) and the mean annual precipitation (PR). We tested the relationship between some of the stem anatomical variables used in PCA with Pearson’s coefficient correlation.
All analyses were performed using R version 3.4.3 (R Core Team, 2017) in R Studio version 1.1.414 (R Studio Team, 2016). All the differences were considered significant when P was <0.05.
RESULTS
Interspecific and intraspecific vulnerability to xylem embolism in the herbaceous stems
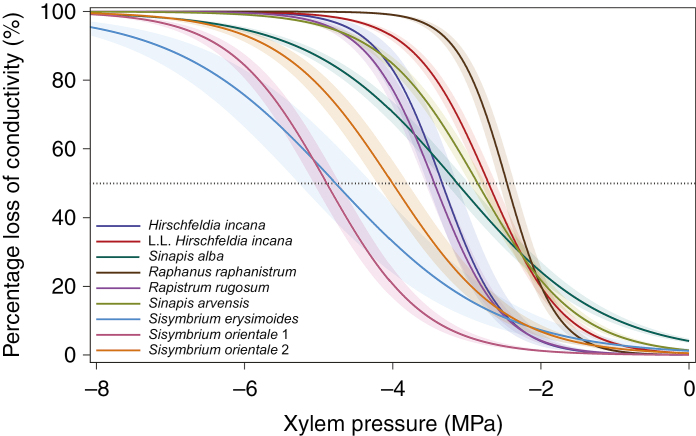
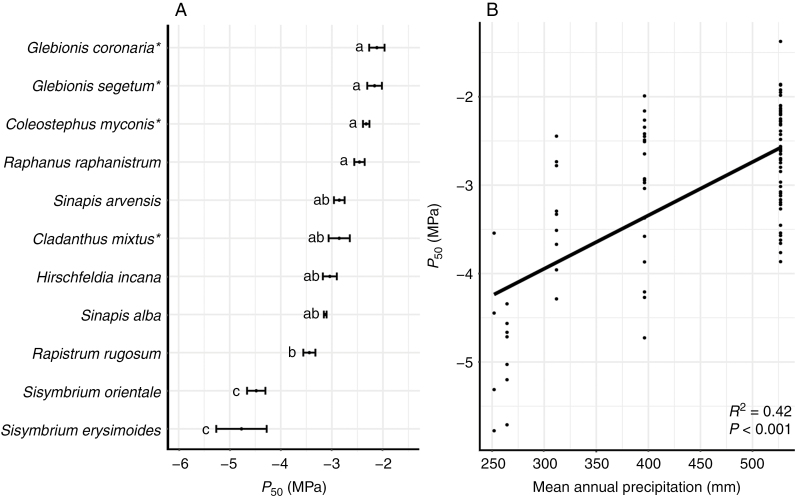
The 11 herbaceous species studied show stem P50 values varying 2-fold from –2.1 MPa to –4.9 MPa (Figs 1 and 2A; see Dória et al., 2018 for the vulnerability curves of Asteraceae species) (Supplementary Data Table S1). The range of stem P50 shows significant interspecific variation (F = 27.161, P < 0.001; Fig. 2A), with no interaction between species and mean annual precipitation (F = 2.948, P = 0.0901) (Supplementary Data Table S3). Species explain 70 % of the variance, regardless of the variation in mean annual precipitation for the sampling sites, while the mean annual precipitation (PR) explains 30 % of the variance, regardless of the variation in species (F = 16.689, P < 0.001; Fig. 2B) (Supplementary Data Table S3). Likewise, significant interspecific variations are also observed for P88 and P12 (F = 22.507, P < 0.001; F = 7.868, P < 0.001, respectively) with part of both variations explained by PR (F = 6.506, P < 0.05; F = 4.439, P < 0.05 for P88 and P12, respectively). Variation in slope amongst the species studied is also significant (F = 4.940, P < 0.001), but the mean precipitation is not significant for this parameter (F = 0.138, P = 0.712).
Fig. 1.
Mean vulnerability curves for each of the seven herbaceous Brassicaceae species studied native to different vegetation zones of Tenerife (Canary Islands), with reference to the sampling localities for Hirschfeldia incana and Sisymbrium orientale. Shaded bands represent P50 standard errors, and 50 % percentage loss of conductivity (PLC) is indicated by the horizontal dotted line. L.L. refers to the more humid population of H. incana collected in the city of La Laguna. The numbers 1 and 2 of Sisymbrium orientale refer to the populations collected in drier and more humid sites, respectively. See Supplementary Data Fig S1.
Fig. 2.
Range of stem P50 amongst seven herbaceous Brassicaceae and four Asteraceae (represented with an asterisk; data from Dória et al., 2018) species from different vegetation zones in Tenerife (Canary Islands, Spain), and its relationship to mean annual precipitation. (A) Mean values of stem P50 of the herbaceous Brassicaceae and Asteraceae species studied. Standard errors are represented by bars. Different letters indicate differences between species at P < 0.05. (B) Relationship between P50 and mean annual precipitation at the individual level (on average six individuals per species). The adjusted R2 and level of significance is given.
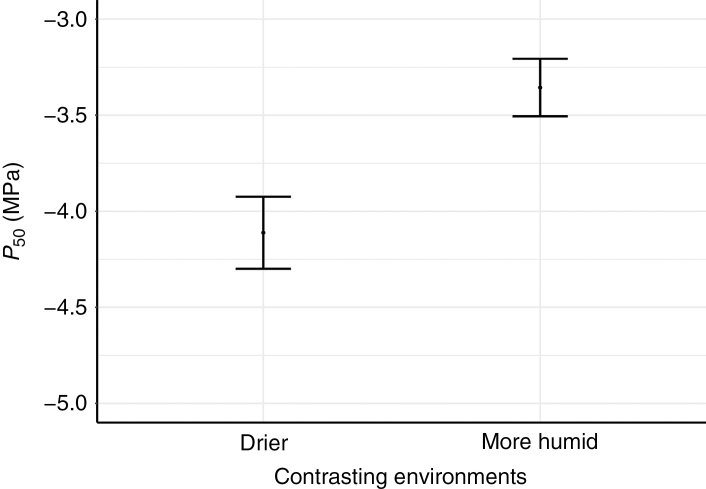
The two Brassicaceae populations of H. incana and S. orientale show significant intraspecific variation in P50 (P < 0.001, F = 17.6083), demonstrating that the contrasting environments are important to explain the intraspecific variation in P50 (Fig. 3). For H. incana, the drier site receives on average 311.8 mm of mean annual precipitation (aridity index = 0.39), while the more humid site receives on average 526.9 mm (aridity index = 0.68). For S. orientale, the drier site has on average 264.3 mm of mean annual precipitation, and the more humid site 396.3 mm for the same period (aridity index = 0.34 and 0.53, respectively) (Supplementary Data Fig. S1).
Fig. 3.
Intraspecific differences of mean stem P50 between the two populations of the Brassicaceae, Hirschfeldia incana and Sisymbrium orientale, collected in contrasting environments (H. incana: mean annual precipitation = 311.8 mm; aridity index = 0.39 for the drier site, and mean annual precipitation = 526.9 mm; aridity index = 0.68 for the more humid site. S. orientale: mean precipitation = 264.3 mm; aridity index = 0.34 for the drier site, and mean annual precipitation = 396.3 mm; aridity index = 0.53 for the more humid site.)
Structure–function relationships in the herbaceous stems show correlation between embolism resistance and anatomy
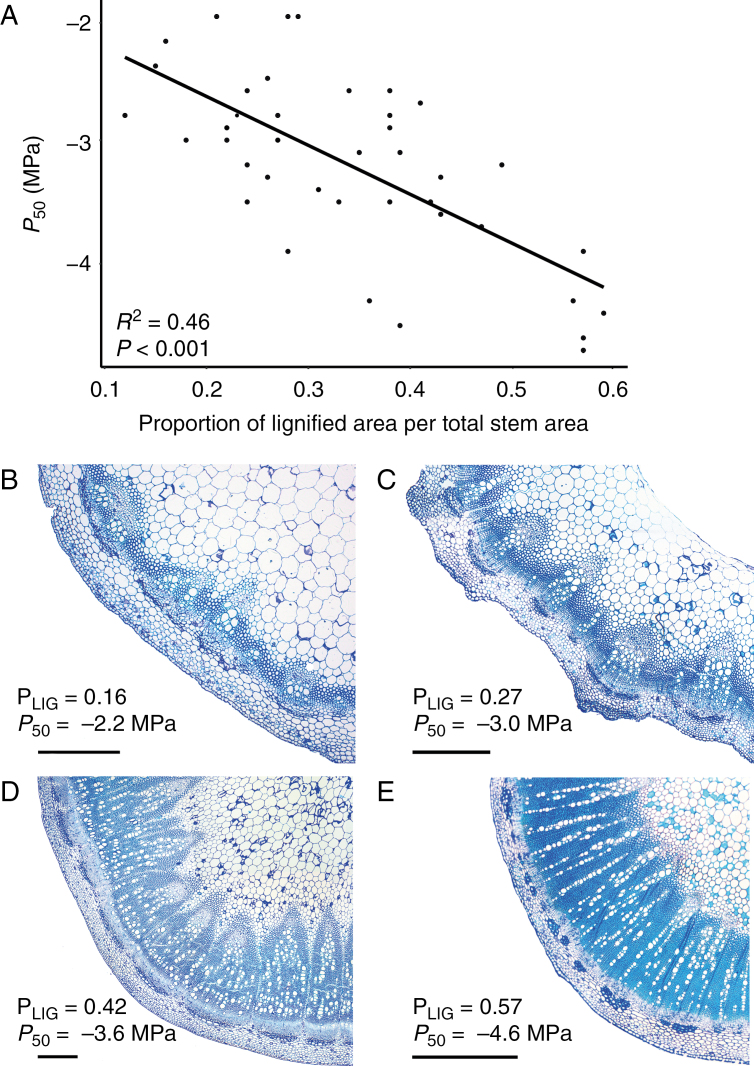
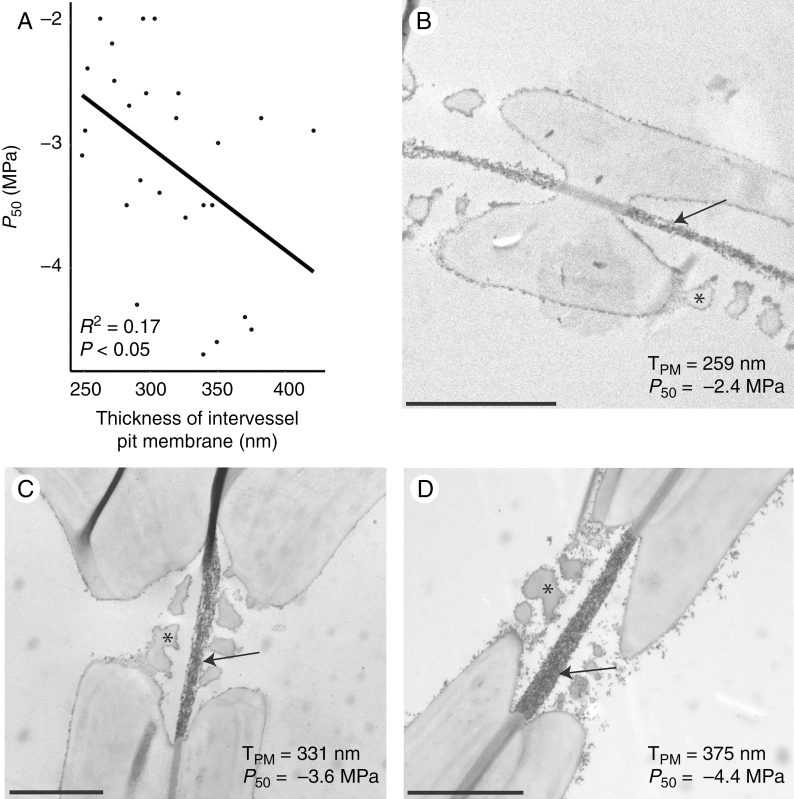
The stem anatomical variables that best explain the variation in P50 are the proportion of lignified area per total stem area (PLIG; which is a measure of stem woodiness) (Fig. 4) and the thickness of the intervessel pit membrane (TPM) (Fig. 5) (P < 0.001; R2 = 0.6783) (Supplementary Data Tables S2 and S4). The P50–PLIG relationship remains significant for the separate data sets (P < 0.001; R2 = 0.58 for Brassicaceae and P < 0.01; R2 = 0.48 for Asteraceae), while the P50–TPM correlation disappears when analysing the Brassicaceae and Asteraceae data sets separately (P = 0.2164, R2 = 0.040 vs. P = 0.6175, R2 = –0.099, respectively). In addition, PLIG is the main variable explaining 69 % of the P50 variation, while TPM explains the remaining 31 % (Supplementary Data Tables S4).
Fig. 4.
Relationships between stem P50 and the proportion of lignified area per total stem area (PLIG). (A) Linear regression between P50 and PLIG. The adjusted R2 and the level of significance are given. Each dot represents one individual (on average three individuals per species). (B–E) Light microscope images of cross-sections through the stem of Brassicaceae species showing an increase of PLIG matching with an increase in embolism resistance. (B) Raphanus raphanistrum. (C) Sinapis alba. (D) Rapistrum rugosum. (E) Sisymbrium orientale from the drier sampling site. The scale bars represent 500 μm.
Fig. 5.
Relationships between stem P50 and thickness of the intervessel pit membrane (TPM). (A) Linear regression between P50 and TPM. The adjusted R2 and the level of significance are given. Each dot represents one individual (on average two individuals per species). (B–D) Transmission electron microscope images of intervessel pits of Brassicaceae species showing thicker pit membranes (arrows) in species that are more embolism resistant; all the herbaceous Brassicaceae species studied have vestures (asterisks). (B) Raphanus raphanistrum. (C) Rapistrum rugosum. (D) Sisymbrium erysimoides. Scale bars represent 2 μm.
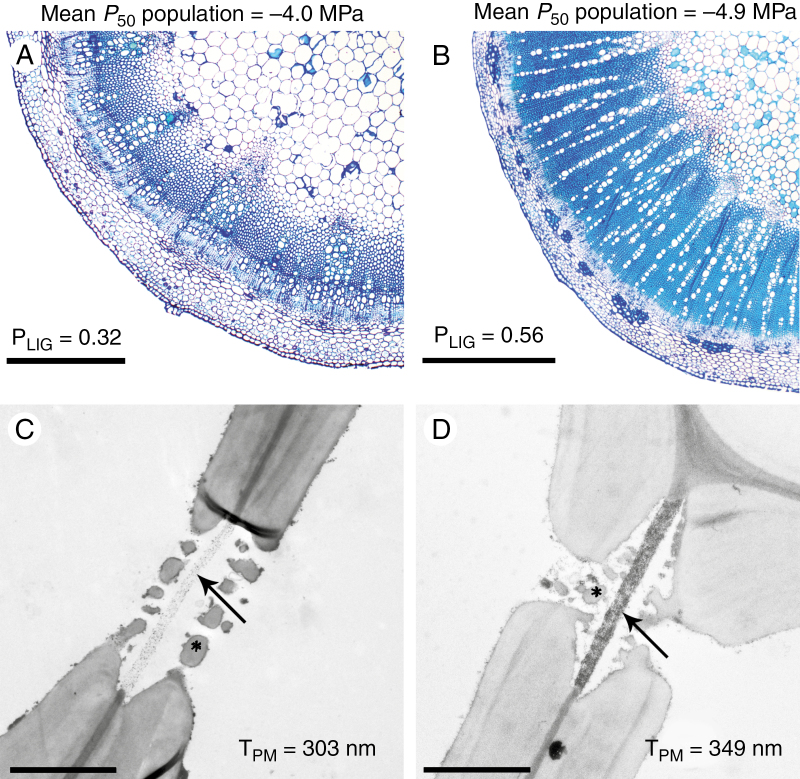
The S. orientale population growing in the drier sampling site shows a higher proportion of lignified area per total stem area (PLIG), thicker intervessel pit membranes (TPM) and thicker intervessel walls (TVW) than the population growing in the more humid sampling site (Fig. 6; Table 1) (Supplementary Data Table S2). No significant anatomical differences were found between the two populations of H. incana growing in contrasting environments.
Fig. 6.
Intraspecific differences between two populations of Sisymbrium orientale growing in the more humid habitat (A, C) vs. the drier sampling site (B, D). (A, B) Light microscope image of cross-sections through the stems showing the population mean P50 values and proportion of lignified area per total stem area (PLIG). Scale bars represent 500 μm. (C, D) Transmission electron microscope images of intervessel pits showing the population mean of thickness of the intervessel pit membrane (TPM) (arrows). Vestures are marked with an asterisk. Scale bars represent 2 μm.
Table 1.
Stem anatomical variables that showed significant t-test differences between the two populations of Sisymbrium orientale growing in contrasting environments
| Stem anatomical variable | Mean for S. orientale from the drier site | Mean for S. orientale from the more humid site | t-test (P-value) |
|---|---|---|---|
| Proportion of lignified area per total stem area | 0.57 | 0.32 | 0.00763 |
| Thickness of intervessel pit membrane (nm) | 349.14 | 303.43 | 0.04231 |
| Thickness of intervessel wall (μm) | 3.70 | 3.31 | 0.01194 |
Mean annual precipitation for the drier site is 264.3 mm and for the more humid site is 396.3 mm; the aridity indexes are 0.34 and 0.53, respectively.
All Brassicaceae observed have vestured pits (Fig. 5B–D and 6C, D), while these are absent in the Asteraceae species. No differences in the level of vesturing are observed amongst the embolism-resistant vs. vulnerable Brassicaceae species.
Relationship between mean precipitation (PR), stem anatomy and P50
The PERMANOVA test shows that the mean annual precipitation explains the variation in both stem anatomical characters and P50 (F = 3.8098, R2 = 0.14, P < 0.05) (Supplementary Data Table S5).
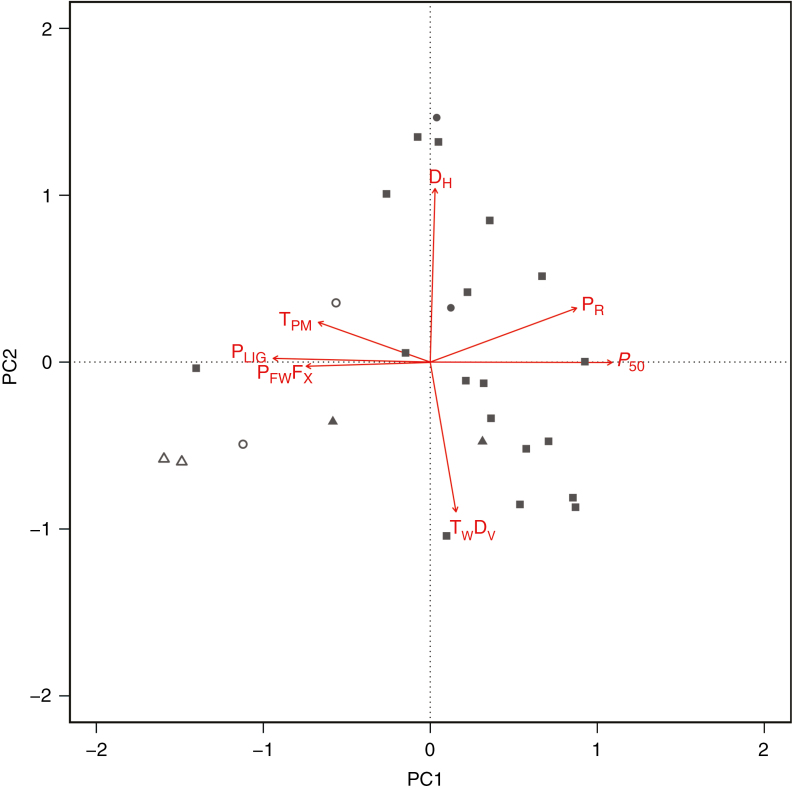
When analysing the association amongst stem anatomical characters, mean annual precipitation and P50 using a PCA, the first axis of the PCA explains 40 % of the total variance observed, while the second axis explains 21 %. The first principal component has large positive associations with P50 and with mean annual precipitation (PR), and negative associations with the proportion of lignified area per total stem area as observed in a cross-section (PLIG), the proportion of xylem fibre wall area per fibre area as observed in a cross-section (PFWFX) and the thickness of intervessel pit membranes (TPM) (Fig. 7). Along this first axis, the proportion of xylem fibre wall per fibre is correlated with P50 (P < 0.01, r = –0.45). The second principal component has a large positive association with the hydraulically weighted vessel diameter (DH) and a negative association with the thickness-to-span ratio of vessels (TWDV). These two variables are negatively correlated with each other (P < 0.01, r = –0.51), but neither of them is correlated with embolism resistance (P = 0.7608, r = –0.0525; P = 0.5662, r = –0.0988). The thickness of the vessel is also not correlated with TWDV (P = 0.2811, r = 0.1846). The individuals distributed at the right side of the multivariate PCA space are associated with less negative values of P50 and higher mean annual precipitation. Some of these individuals present higher values of the thickness-to-span ratio of vessels, while others have higher hydraulically weighted vessel diameters. In contrast, the individuals at the left side of the multivariate PCA space are associated with more negative values of P50, more pronounced lignification characters, thicker intervessel pit membranes and lower mean annual precipitation (Fig. 7).
Fig. 7.
Principal component analysis of stem anatomical characters, mean annual precipitation and P50 on the first two axes. PLIG = proportion of lignified area per total stem area as observed in a cross-section; PFWFX = proportion of xylem fibre wall area per fibre area as observed in a cross-section; PR = mean annual precipitation; TWDV = thickness-to-span ratio of vessels; P50 = pressure inducing 50 % loss of hydraulic conductivity; DH = hydraulically weighted vessel diameter; TPM = thickness of intervessel pit membrane. Circles represent individuals of H. incana from humid (filled) and dry (open) sampling sites, while triangles refer to individuals of S. orientale from the humid (filled) and dry (open) sites. The squares represent the other individuals of Brassicaceae and Asteraceae studied.
Individuals of the two Brassicaceae populations of H. incana (represented by circles) and S. orientale (represented by triangles) occupy different areas of the multivariate space (Fig. 7). The individuals collected in drier sites (open circles for H. incana and open triangles for S. orientale) are associated with a higher degree of lignification characters, thicker intervessel pit membranes and lower values of mean annual precipitation (Fig. 7). The individuals collected in more humid sites (filled circles for H. incana and filled triangles for S. orientale) are associated with higher hydraulically weighted vessel diameter and higher values of the thickness-to-span ratio of vessels (Fig.7).
DISCUSSION
Interspecific and intraspecific stem P50 variation across herbaceous eudicots is strongly linked to precipitation
Our data set, comprising 11 herbaceous species of Brassicaceae and Asteraceae from five different habitats in Tenerife with a mean annual precipitation from 252 to 527 mm, shows a 2-fold range of stem P50 values that match the precipitation values of the sampling sites: the most vulnerable species (P50 –2.1 MPa) was collected from wetter environments and the most resistant species (P50 –4.9 MPa) was sampled from drier vegetation types (Figs 1 and 2). The explanatory power of mean annual precipitation towards stem P50 supports the functional relevance of resistance to xylem embolism as an adaptive response to water deficit, as has been repeatedly demonstrated for woody trees (Maherali et al., 2004; Blackman et al., 2012; Choat et al., 2012) and to a lesser extent also herbs (mainly grasses, Lens et al., 2016). Likewise, the intraspecific (between-population) differences in stem P50 for both S. orientale and H. incana (Fig. 3) are also explained by mean annual precipitation: for both species, the more embolism-resistant populations occur in areas with less annual precipitation. This suggests that differences in habitat amongst herbaceous populations from the same species can increase the intraspecific plasticity in P50.
Percentage of lignified area per total stem area (PLIG) outcompetes intervessel pit membrane (TPM) as the explanatory variable explaining variation in stem P50
The percentage of lignified area per total stem area (PLIG), which is mainly defined by the amount of woodiness in the herbaceous stems as observed in a cross-section, is the character that best explains the variation of embolism resistance in stems, with more lignified stems being more resistant to embolism (Fig. 4). Since the germination time of the herbaceous species on Tenerife does more or less converge after the arrival of the rains in autumn and winter, we believe that the differences in woodiness is species and/or niche specific rather than dependent on major differences in stem age between species. For example, the three species (Raphanus raphanistrum, Sinapis arvensis and the population of Sisymbrium orientale from the more humid area) collected in Vilaflor village (sampling site 4 of Supplementary Data Fig. S1) show a 2-fold difference in the degree of woodiness matching nicely with stem P50, despite the fact that these three populations occurred along the same road (Supplementary Data Tables S1 and S2). The relationship between characters related to higher stem lignification and higher absolute values of P50 has been recorded for different plant groups, both in woody (Hacke et al., 2001; Jacobsen et al., 2005; Jansen et al., 2009; Pereira et al., 2017) and in herbaceous lineages (Lens et al., 2012b, 2013, 2016; Tixier et al., 2013) and in closely related woody lineages that are derived from herbaceous relatives (Dória et al., 2018). Differences in the proportion of the lignified area in the stem are also found at the intraspecific level in this study, with the more resistant population of S. orientale showing thicker intervessel walls and higher PLIG values compared with those of the more vulnerable population (Fig. 6; Table 1). The higher PLIG values in the drier population could also be strengthened by the presumably earlier germination time in the area of El Escobonal (470 m asl), which is about 900 m lower than the colder (and wetter) site of Vilaflor (1400 m asl), making the stems of the drier (and lower) site older, enabling them to lignify more.
It is challenging to relate increased stem lignification functionally with embolism resistance, since most lignification characters do not directly influence embolism formation and spread in the 3-D network of angiosperm vessels. Indeed, the thickness of intervessel pit membranes (TPM) is more likely to affect the length of the tortuous and irregularly shaped pores that air–water menisci need to cross before air-seeding may occur, explaining the spread of embolism through intervessel pit membranes into adjacent conduits (Jansen et al., 2009; Lens et al., 2011, 2013; Li et al., 2016). Although the P50–TPM relationship is confirmed in our herbaceous eudicot data set (Fig. 5), TPM provides a much lower power to explain differences in P50 compared with the degree of woodiness as observed in a cross-section, calculated as the percentage of lignified area per total stem area (PLIG). This may seem surprising, but studies investigating the relationship between stem P50 and TPM amongst herbaceous species are scarce and the functional relevance of TPM in herbs might be less important compared with woody species. A few examples that suggest this poor P50–TPM relationship in herbs are: the P50 – TPM relationship disappears in our study when only including the Brassicaceae species; no link between P50 and TPM was found in a grass data set based on four species with contrasting P50 values (Lens et al., 2016); and a third study investigating closely related herbaceous and woody daisies showed that the P50–TPM relationship was retrieved only when the herbaceous data set was combined with the woody data set (Dória et al., 2018). Evidently, more work on stem P50 and additional anatomical measurements based on the same – properly fixated – herbaceous stems is needed to shed more light on the functional relevance of TPM in herbs, which should in theory match the hydraulic importance of TPM as observed in shrubs and trees (Li et al., 2016).
Relationships between increased lignification and thicker intervessel pit membranes have been reported, which could explain the indirect correlation between higher lignification and higher embolism resistance (Jansen et al., 2009; Li et al., 2016; Dória et al. 2018). These findings are in accordance with our results for the two populations of S. orientale collected in contrasting environments (Table 1; Fig. 6): the more resistant population shows a higher proportion of lignified area in the stem, thicker intervessel wall, and thicker intervessel pit membranes. However, the TPM–lignification correlation disappears in our entire data set (including Asteraceae and Brassicaceae species), showing that increased lignification characters are not necessarily linked to thicker intervessel pit membranes.
The mean precipitation explains both P50 and anatomical variation in stems of herbaceous eudicots
Mean annual precipitation explains both the variation in stem P50 and the variation in stem anatomical characters across the herbaceous species studied. It has been well documented that environmental factors influence P50 (Maherali et al., 2004; Choat et al., 2012; Trueba et al., 2017) as well as anatomical traits (Carlquist, 1975; Baas et al., 1983; Lens et al., 2004; Dória et al., 2016; O’Brien et al., 2017). In our study, populations from drier sites show stems with more negative P50 values and more pronounced lignification, such as the proportion of lignified area per total stem area (a measure of the amount of woodiness) and the proportion of xylem fibre wall area per fibre area as observed in a cross-section. These characters are most associated with the first PCA axis (Fig. 7).
Our results show that the common pattern observed for woody species, i.e. a shift in rainfall patterns associated with survival and distribution of trees and shrubs (Engelbrecht et al., 2007; Allen et al., 2010; Trueba et al., 2017), and drought-induced tree mortality associated with substantial loss of hydraulic conductivity across taxa and biomes (Adams et al., 2017), is also true for herbaceous species (see also the first section of the Discussion). At the same time, different environment conditions also impact stem anatomical characters allowing plants to adapt to changing climates (Carlquist, 1975; Baas et al., 1983; Martinez-Vilalta et al., 2010; Kattge et al., 2011).
Across woody trees, a lineage-specific sub-set of stem anatomical traits can be linked to drought-induced embolism resistance, such as increased wood density (linked to fibre wall thickness in angiosperms; Chave et al., 2009; Zieminska et al., 2013), increased thickness-to-span ratio of conduits (Hacke et al., 2001; Bouche et al., 2014), thicker intervessel pit membranes (Jansen et al., 2009; Lens et al., 2011; Li et al., 2016; Dória et al., 2018) and narrower vessel diameters (Poorter et al., 2010; Hacke et al., 2016; Olson et al., 2018). Amongst herbaceous species, fragile stems also need to be reinforced by a suite of mechanical characters, as shown in our study: individuals occurring in drier areas show a higher degree of lignification/woodiness (PLIG) and thicker intervessel pit membranes (Fig. 7) (see previous section). The increment of cellular support against implosion is often cited as the reason for this hydraulic–mechanical trade-off, which can result from either an increase in vessel wall to lumen ratio (Hacke et al., 2001; Jacobsen et al., 2007; Cardoso et al., 2018) or an increase in fibre matrix support (more and thicker walled xylem fibres) (Jacobsen et al., 2005, 2007; Pratt and Jacobsen, 2017; Dória et al., 2018). For the herbaceous species studied here, we found the latter relationship, demonstrated by the correlation between a higher proportion of xylem fibre cell wall per fibre (PFWFX) and more negative P50. Both kinds of cellular reinforcements, due to either vessel wall reinforcements or a more pronounced surrounding fibre matrix, would result in increasing xylem density offering support against implosion. In accordance with this hydraulic–mechanical trade-off, collapse of xylem conduits was only observed in cells that lack a robust support of the fibre matrix, for instance in leaves (Cochard et al., 2004; Brodribb and Holbrook, 2005; Zhang et al., 2016) and in low-lignin stems of poplar mutants (Kitin et al., 2010). Our study confirms that increasing the mechanical strength of fragile herbaceous stems using a suite of lignification characters may be highly relevant to acquire a higher level of embolism resistance.
Another aspect of the hydraulic–mechanical relationship in our data set is highlighted by the negative correlation between the thickness-to-span ratio of vessels (TWDV), determining the resistance to implosion of the conduit, and the hydraulically weighted vessel diameter (DH). Since there is a significant relationship between TWDV and DH, but not between TWDV and the thickness of the vessel wall (TVW), it can be concluded that vessel diameter impacts much more the variation of TWDV than the thickness of vessel wall. It is known that larger vessel lumina increase hydraulic conductivity (Tyree and Zimmerman, 2002) and, because in our data set vessel wall thickness remains more or less the same, it gives rise to larger vessels that become mechanically weaker and potentially more vulnerable (Preston et al., 2006; Zanne et al., 2010; Pratt and Jacobsen, 2017). However, in our data set, P50 is not correlated with DH, with TVW or with TWDV, meaning that the vessel diameter and thickness-to-span ratio of vessels do not impact embolism resistance in our herbaceous data set.
In conclusion, this study investigated structure–function relationships in stems of seven herbaceous Brassicaceae occurring in different vegetation zones across the island of Tenerife and merged the data set produced with a similar data set for herbaceous Asteraceae growing on the same island. The 2-fold difference in embolism resistance found here shows that stems of herbaceous eudicots are able to deal with a range of negative pressures inside xylem conduits, although the P50 range in woody trees remains considerably higher. In addition, mean annual precipitation is the major determinant influencing both embolism resistance and anatomical characters in the herbaceous stems, demonstrating the predictive value of both characters with respect to survival and distribution of herbs along environmental gradients. This improves our understanding of the evolutionary and ecological significance of embolism resistance in non-woody species. Our results also show that the degree of woodiness (PLIG) outcompetes the thickness of intervessel pit membranes (TPM) as the most powerful character determining embolism resistance in stems of herbaceous eudicots studied. This may question the hydraulic relevance of TPM in herbs, although many more observations on embolism resistance and anatomical observations on herbaceous plants need to be carried out before a final conclusion can be reached.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: map of Tenerife with the five sampling sites, each corresponding to unique aridity indices. Table S1: hydraulic parameters of the herbaceous Brassicaceae species studied. Table S2: stem anatomical measurements of the herbaceous Brassicaceae species studied, along with the aridity indices and values for mean annual precipitation. Table S3: analysis of covariance of species and mean precipitation explaining the variance in P50 of the herbaceous Brassicaceae and Asteraceae species studied. Table S4: multiple regression model of anatomical features explaining the variance in P50 of the herbaceous Brassicaceae and Asteraceae species studied. Table S5: permutational multivariate analysis of variance of mean annual precipitation explaining the variance in P50 and in the main stem anatomical characters of the herbaceous Brassicaceae and Asteraceae species studied.
ACKNOWLEDGEMENTS
We thank the Cabildo de Tenerife (AFF 147/13 no. Sigma, 2013-00748; AFF 429/13 no. Sigma, 2013–02030; AFF 149/15 no. Sigma, 2015-00925; AFF 85/16 no. Sigma, 2016-00838) and Teide National Park (no. 152587, REUS 27257, 2013; no. 536556, REUS 83804, 2013; Res. no. 222/2015) for the collection permits, and the AEMET - Agencia Estatal de Meteorología, Spanish Government, for providing meteorological data. We also acknowledge the technical support of R. Langelaan, W. Star and G. Capdeville. This work was supported by the CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (PROC. no. 206433/2014-0), the Alberta Mennega Stichting, the Cluster of Excellence COTE (ANR-10-LABX-45, within the DEFI project) and the programme ‘Investments for the Future’ (ANR-10-EQPX-16, XYLOFOREST) funded by the French National Agency for Research.
LITERATURE CITED
- Adams HD, Zeppel MJB, Anderegg WRL, et al. . 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature, Ecology and Evolution 1: 1285–1291. [DOI] [PubMed] [Google Scholar]
- Ahmad HB, Lens F, Capdeville G, Burlett R, Lamarque LJ, Delzon S. 2017. Intraspecific variation in embolism resistance and stem anatomy across four sunflower (Helianthus annuus L.) accessions. Physiologia Plantarum 163: 59–72. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. . 2010. A global overview of drought and heat- induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M, et al. . 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences, USA 113: 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P, Werker E, Fahn A. 1983. Some ecological trends in vessel characters. IAWA Bulletin 4: 141–159. [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. 2012. Leaf hydraulic vulnerability influences species’ bioclimatic limits in a diverse group of woody angiosperms. Oecologia 168: 1–10. [DOI] [PubMed] [Google Scholar]
- Bouche PF, Larter M, Domec JC, et al. . 2014. A broad survey of xylem hydraulic safety and efficiency in conifers. Journal of Experimental Botany 65: 4419–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Lee EF, Shackel KA, Matthews MA. 2013. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiology 161: 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Holbrook NM. 2005. Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology 173: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Bowman D, Nichols S, Delzon S, Burlett R. 2010. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist 188: 533–542. [DOI] [PubMed] [Google Scholar]
- Cardoso AA, Brodribb TJ, Lucani CJ, DaMatta FM, McAdam SAM. 2018. Coordinated plasticity maintains hydraulic safety in sunflower leaves. Plant, Cell & Environment 41: 2567–2576. [DOI] [PubMed] [Google Scholar]
- Carlquist S. 1974. Insular woodiness. In: Carlquist SJ, ed. Island biology. New York: Columbia Univeristy Press, 350–428. [Google Scholar]
- Carlquist S. 1975. Ecological strategies of xylem evolution. Berkeley, CA: University of California Press. [Google Scholar]
- Challinor AJ, Ewert F, Arnold S, Simelton E, Fraser E. 2009. Crops and climate change: progress, trends, and challenges in simulating impacts and informing adaptation. Journal of Experimental Botany 60: 2775–2789. [DOI] [PubMed] [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Chevan A, Sutherland M. 1991. Hierarchical partitioning. American Statistical Association 45: 90–96. [Google Scholar]
- Choat B, Sack L, Holbrook NM. 2007. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytologist 175: 686–698. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. . 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752–756. [DOI] [PubMed] [Google Scholar]
- Cochard H. 2002. A technique for measuring xylem hydraulic conductance under high negative pressures. Plant, Cell & Environment 25: 815–819. [Google Scholar]
- Cochard H, Nardini A, Coll L. 2004. Hydraulic architecture of the leaf blades: where is the main resistance? Plant, Cell & Environment 27: 1257–1267. [Google Scholar]
- Cochard H, Herbette S, Barigah T, Badel E, Ennajeh M, Vilagrosa A. 2010. Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant, Cell & Environment 33: 1543–1552. [DOI] [PubMed] [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S. 2013. Methods for measuring plant vulnerability to cavitation: a critical review. Journal of Experimental Botany 64: 4779–4791. [DOI] [PubMed] [Google Scholar]
- Corcuera L, Cochard H, Gil-Pelegrin E, Notivol E. 2011. Phenotypic plasticity in mesic populations of Pinus pinaster improves resistance to xylem embolism (P50) under severe drought. Trees 25:1033–1042. [Google Scholar]
- Crawley MJ. 2007. The R book. Chichester, UK: John Wiley & Sons Ltd. [Google Scholar]
- Dai AG. 2013. Increasing drought under global warming in observations and models. Nature Climate Change 3: 52–58. [Google Scholar]
- Davis SD, Sperry JS, Hacke UG. 1999. The relationship between xylem conduit diameter and cavitation caused by freezing. American Journal of Botany 86:1367–1372. [PubMed] [Google Scholar]
- del-Arco M, Pérez-de-Paz PL, Acebes JR, et al. . 2006. Bioclimatology and climatophilous vegetation of Tenerife (Canary Islands). Annales Botanici Fennici 43: 167–192. [Google Scholar]
- Dória LC, Podadera DS, Batalha MA, Lima RS, Marcati RM. 2016. Do woody plants of the Caatinga show a higher degree of xeromorphism than in the Cerrado? Flora 224: 244–251. [Google Scholar]
- Dória LC, Podadera DS, del Arco M, et al. . 2018. Insular woody daisies (Argyranthemum, Asteraceae) are more resistant to drought-induced hydraulic failure than their herbaceous relatives. Functional Ecology 32: 1467–1478. [Google Scholar]
- Dulin MW, Kirchoff BK. 2010. Paedomorphosis, secondary woodiness and insular woodiness in plants. Botanical Review 76: 405–490. [Google Scholar]
- Engelbrecht BMJ, Comita LS, Condit R, et al. . 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80–82. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Westoby M, Jansen S, et al. . 2016. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytologist 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Jansen S. 2009. Embolism resistance of three boreal conifer species varies with pit structure. New Phytologist 182: 675–686. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Spice R, Schreiber SG, Plavcova L. 2016. An ecophysiological and developmental perspective on variation in vessel diameter. Plant, Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Hoffman WA, Marchin RM, Abit P, Lau LO. 2011. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biology 17: 2731–2742. [Google Scholar]
- Holste EK, Jerke MJ, Matzner SL. 2006. Long-term acclimatization of hydraulic properties, xylem conduit size, wall strength and cavitation resistance in Phaseolus vulgaris in response to different environmental effects. Plant, Cell & Environment 29: 836–843. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. 1989. IAWA list of microscopic features for hardwood identification. IAWA Bulletin 10: 219–332. [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis D. 2005. Do xylem fibers affect vessel cavitation resistance? Plant Physiology 139: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD. 2007. Cavitation resistance among 26 chaparral species of Southern California. Ecological Monographs 77: 99–115. [Google Scholar]
- Jacobsen AL, Pratt RB, Tobin MF, Hacke UG, Ewers FW. 2012. A global analysis of xylem vessel length in woody plants. American Journal of Botany 99: 1583–1591. [DOI] [PubMed] [Google Scholar]
- Jansen S, Choat B, Pletsers A. 2009. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. American Journal of Botany 96: 409–419. [DOI] [PubMed] [Google Scholar]
- Kattge J, Diaz S, Lavorel S, et al. . 2011. TRY – a global database of plant traits. Global Change Biology 17: 2905–2935. [Google Scholar]
- Kidner C, Groover A, Thomas D, Emelianova K, Soliz-Gamboa C, Lens F. 2016. First steps in studying the origins of secondary woodiness in Begonia (Begoniaceae): combining anatomy, phylogenetics, and stem transcriptomics. Biological Journal of the Linnean Society 117: 121–138. [Google Scholar]
- Kitin P, Voelker SL, Meinzer FC, Beeckman H, Strauss SH, Lachenbruch B. 2010. Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: study by cryo-fluorescence microscopy. Plant Physiology 154: 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocacinar F, Sage RF. 2003. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant, Cell & Environment 26: 2015–2026. [DOI] [PubMed] [Google Scholar]
- Kolb KJ, Sperry JS. 1999. Differences in drought adaptation between subspecies of Sagebrush (Artemisia tridentata). Ecology 7: 2373–2384. [Google Scholar]
- Lamy J, Delzon S, Bouche PS, et al. . 2013. Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytologist 201: 874–886. [DOI] [PubMed] [Google Scholar]
- Larter M, Brodribb T, John PS, Burlett R, Cochard H, Delzon S. 2015. Extreme aridity pushes trees to their physical limits. Plant Physiology 168: 804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Luteyn JL, Smets E, Jansen S. 2004. Ecological trends in the wood anatomy of Vaccinioideae (Ericaceae s.l.). Flora 199: 309–319. [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190: 709–723. [DOI] [PubMed] [Google Scholar]
- Lens F, Eeckhout S, Zwartjes R, Smets E, Janssens S. 2012a The multiple fuzzy origins of woodiness within Balsaminaceae using an integrated approach. Where do we draw the line? Annals of Botany 109: 783–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Smets E, Melzer S. 2012b Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytologist 193: 12–17. [DOI] [PubMed] [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S. 2013. Embolism resistance as a key mechanism to understand adaptive plant strategies. Current Opinion in Plant Biology 16: 287–292. [DOI] [PubMed] [Google Scholar]
- Lens F, Picon-Cochard C, Delmas C, et al. . 2016. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiology 172: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lens F, Espino S, et al. . 2016. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA Journal 37: 152–171. [Google Scholar]
- Maherali H, Pockman WT, Jackson R. 2004. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199. [Google Scholar]
- Maherali H, Walden AE, Husband BC. 2009. Genome duplication and the evolution of physiological responses to water stress. New Phytologist 184: 721–731. [DOI] [PubMed] [Google Scholar]
- Martinez-Vilalta J, Cochard H, Mencuccini M, et al. . 2009. Hydraulic adjustment of Scots pine across Europe. New Phytologist 184: 353–364. [DOI] [PubMed] [Google Scholar]
- Martinez-Vilalta J, Muncuccini M, Vayreda J, Retana J. 2010. Interspecific variation in functional traits, not climatic differences among species ranges, determines demographic rates across 44 temperate and Mediterranean tree species. Journal of Ecology 98: 1462–1475. [Google Scholar]
- Mencuccini M, Comstock J. 1999. Variability in hydraulic architecture and gas exchange of common bean (Phaseolus vulgaris) cultivars under well-watered conditions: interactions with leaf size. Australian Journal of Plant Physiology 26: 115–124. [Google Scholar]
- Mendiburu F. 2017. Agricolae: statistical procedures for agricultural research R package version 1.2–8. https://CRAN.R-project.org/package=agricolae (18 June 2018).
- Monfreda C, Ramankutty N, Foley JA. 2008. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Global Biogeochemical Cycles 22: Gb1022. [Google Scholar]
- Nolf M, Pagitz K, Mayr S. 2014. Physiological acclimation to drought stress in Solidago canadensis. Physiologia Plantarum 150: 529–539. [DOI] [PubMed] [Google Scholar]
- Nolf M, Rosani A, Ganthaler A, Beikircher B, Mayr S. 2016. Hydraulic variation in three Ranunculus species. Plant Physiology 170: 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MJ, Engelbrecht BMJ, Joswig J, et al. . 2017. A synthesis of tree functional traits related to drought induced mortality in forests across climatic zones. Journal of Applied Ecology 54: 1669–1686. [Google Scholar]
- Oksanen J, Guillaume Blanchet F, Kindt R, et al. . 2015. Vegan: community ecology package R package version. http://CRAN.R-project.org/package=vegan (11 June 2018).
- Olson ME, Rosell JA. 2013. Vessel diameter–stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytologist 197: 1204–1213. [DOI] [PubMed] [Google Scholar]
- Olson ME, Soriano D, Rosell JA, et al. . 2018. Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences, USA 115: 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter NW, van der Willigen C. 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology 18: 589–593. [DOI] [PubMed] [Google Scholar]
- Pereira L, Domingues-Junior AP, Jansen S, Choat B, Mazzafera P. 2017. Is embolism resistance in plant xylem associated with quantity and characteristics of lignin? Trees 32: 349–358. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2018. nlme: linear and nonlinear mixed effects models R package version 3.1–137. https://CRAN.R-project.org/package=nlme (26 June 2018).
- Pittermann J, Choat B, Jansen S, Stuart SA, Lynn L, Dawson TE. 2010. The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: the evolution of pit membrane form and function. Plant Physiology 153: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovaroff AL, Pasquini SC, Guzman ME, Alstad KP, Stemke JS, Santiago LS. 2016. Multiple strategies for drought survival among woody plant species. Functional Ecology 30: 517–526. [Google Scholar]
- Poorter L, McDonald I, Alarcón A, et al. . 2010. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytologist 185: 481–492. [DOI] [PubMed] [Google Scholar]
- Pratt RB, Jacobsen AL. 2017. Conflicting demands on angiosperm xylem: tradeoffs among storage, transport and biomechanics. Plant, Cell & Environment 40: 897–913. [DOI] [PubMed] [Google Scholar]
- Preston KA, Cornwell WK, Denoyer J. 2006. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytologist 170: 807–818. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.R-project.org (11 June 2018). [Google Scholar]
- R Studio Team. 2016. RStudio: integrated development for R. Boston: RStudio, Inc; http://www.rstudio.com/ (11 June 2018). [Google Scholar]
- Rahmstorf S, Coumou D. 2012. A decade of weather extremes. Nature Climate Change 2: 491–496. [Google Scholar]
- Rosenthal DM, Stiller V, Sperry JS, Donovan LA. 2010. Contrasting drought tolerance strategies in two desert annuals of hybrid origin. Journal of Experimental Botany 61: 2769–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Klepsch M, Karimi Z, Jansen S. 2013. How to quantify conduits in wood? Frontiers in Plant Science 56: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SG, Hacke UG, Hamann A. 2015. Variation of xylem vessel diameter in a wide-spread boreal forest tree in western Canada: insights from a large-scale reciprocal transplant experiment. Functional Ecology 29: 1392–1401. [Google Scholar]
- Schweingruber FH, Borner A, Schulze ED. 2011. Atlas of stem anatomy in herbs, shrubs and trees, Vol. 1 Heidelberg: Springer. [Google Scholar]
- Skelton RP, Brodribb TJ, Choat B. 2017. Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytologist 214: 561–569. [DOI] [PubMed] [Google Scholar]
- Stiller V, Sperry JS. 2002. Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.). Journal of Experimental Botany 53: 1155–1161. [DOI] [PubMed] [Google Scholar]
- Thornthwaite CW. 1948. An approach toward a rational classification of climate. Geographical Review 38: 55–94. [Google Scholar]
- Tixier A, Cochard H, Badel E, Dusotoit-Coucaud A, Jansen S, Herbette S. 2013. Arabidopsis thaliana as a model species for xylem hydraulics: does size matter? Journal of Experimental Botany 64: 2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba S, Pouteau R, Lens F, et al. . 2017. Vulnerability to xylem embolism as a major correlate of the environmental distribution of rainforest species on a tropical island. Plant, Cell & Environment 40: 277–289. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. 1989. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology 40: 19–38. [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap, 2nd edn. Berlin: Springer-Verlag. [Google Scholar]
- UNEP. 1997. World atlas of desertification. Nairobi, Kenya: United Nations Environmental Program. [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S. 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology 33: 672–683. [DOI] [PubMed] [Google Scholar]
- Volaire F, Lens F, Cochard H, et al. . 2018. Embolism and mechanical resistances play a key role in dehydration tolerance of a perennial grass Dactylis glomerata L. Annals of Botany 122: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortemann R, Herbette S, Barigah TS, et al. . 2011. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiology 31: 1175–1182. [DOI] [PubMed] [Google Scholar]
- Zanne AE, Westoby M, Falster DS, et al. . 2010. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. American Journal of Botany 97: 207–215. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Rockwell FE, Graham AC, Alexander T, Holbrook M. 2016. Reversible leaf collapse: a potential ‘circuit breaker’against cavitation. Plant Physiology 172: 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieminska K, Butler DW, Gleason SM, Wright IJ, Westoby M. 2013. Fibre wall and lumen fractions drive wood density variation across 24 Australian angiosperms. Annals of Botany 5: 1–14. [Google Scholar]
- Zuur AF, Ieno EN, Smith GM. 2007. Analysing ecological data. New York: Springer Science. [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.