Abstract
Background and Aims
Future shifts in precipitation regimes and temperature are expected to affect plant traits dramatically. To date, many studies have explored the effects of acute stresses, but few have investigated the consequences of prolonged shifts in climatic conditions on plant growth and chemistry.
Methods
Plant size and metabolite profiles were assessed on naturally occurring Plantago lanceolata plants growing under different precipitation (ambient, 50 % less than ambient = drought) and temperature (ambient, +0.8, +2.4 and +4.0 °C above ambient) treatments at the Boston Area Climate Experiment (constructed in 2007).
Key Results
The analysis of primary and secondary metabolites revealed pronounced effects of drought, and a precipitation × temperature interaction. Strikingly, the effects of precipitation were minimal at the two lower temperatures but marked at the two higher temperatures. Compared with the ambient condition, plants in the drought plots had lower concentrations of foliar nitrogen, amino acids and most sugars, and higher concentrations of sorbitol, citrate and malate, common stress-induced metabolites. This pattern was especially evident at high temperatures. Moreover, drought-exposed plants showed lower concentrations of catalpol, an iridoid glycoside.
Conclusions
While the effect of warming on the metabolite profiles was less pronounced, differences were marked when combined with drought. Given the interactive effect of environmental variables on leaf chemistry, and the fact that woody and herbaceous plants seem to differ in their responses to temperature and precipitation, future studies should account for the direct and indirect effects of the community response to multifactorial field conditions.
Keywords: Amino acids, Boston Area Climate Experiment, climate warming, drought, foliar chemistry, iridoid glycosides, metabolite profiling, Plantago lanceolate, precipitation, sugars
INTRODUCTION
Global air temperatures are expected to increase by an average of 2–4 °C by the end of the century, especially at higher latitudes, and this increase will probably be accompanied by changes in precipitation (IPCC, 2014). Warmer temperatures and drought are expected to dramatically affect ecosystem processes, and alter morphological and chemical plant traits that affect the performance of plants. To date, many studies have explored the effects of acute or short-term individual stresses on plant growth and chemistry, but relatively few have investigated the consequences of sustained long-term (>5 years) shifts in certain abiotic conditions (Metz et al., 2014) and even fewer have manipulated multiple climatic factors simultaneously (Suseela et al., 2014, 2015; Van De Velde et al., 2015). This is a critical gap, since the effects of two or more climatic variables, such as temperature and drought, are typically non-additive and may shift with time (Dieleman et al., 2012; Gargallo-Garriga et al., 2015).
Short-term drought generally inhibits photosynthesis, suppresses shoot growth and triggers rapid changes in leaf chemistry, including increased concentrations of metabolites with osmoregulatory function, a loss of nutrients, changes in C/N/P/K stoichiometry and decreased or enhanced concentrations of secondary metabolites (Selmar and Kleinwächter, 2013; Moradi, 2016; Goufo et al., 2017). The short-term effects of warming are less pronounced, but higher temperatures generally lead to increased growth, while temperature-induced changes in the concentrations of primary and secondary metabolites vary among genotypes and depending on the metabolite (Maenpaa et al., 2013; Virjamo et al., 2014). The combination of warmer temperatures and drought can exacerbate moisture deficits and alter both morphological and chemical traits (Selmar et al., 2017; Zandalinas et al., 2018).
Although considerably less explored, the long-term effects of stress are often distinct and may function either directly on the plant or indirectly via changes in the environment. For instance, sustained drought stress causes shifts in traits that enhance avoidance and/or changes in the concentrations of stress-related metabolites, such as osmoregulators and antioxidants that increase tolerance to drought (Rodgers et al., 2012; Moradi, 2016). In contrast, mild warming leads to increased plant growth and concentrations of certain primary metabolites, i.e. sugars, amino acids and other organic acids, but not of stress-related metabolites (Hu et al., 2013; Zhang et al., 2016). The combination of drought and warming can be even more pronounced (Tharayil et al., 2011; Hoeppner and Dukes, 2012; Gargallo-Garriga et al., 2015; Song et al., 2016). For example, drought and high temperatures can substantially reduce plant biomass (Song et al., 2016) and alter the foliar concentrations of both primary and secondary metabolites (Tharayil et al., 2011; Suseela et al., 2015). In particular, foliar concentrations of metabolites that function as osmoregulators and antioxidants increase under the combination of drought and warming (Suseela et al., 2015). These changes may help plants to mitigate the effects of climatic stresses (Suseela et al., 2015; Moradi et al., 2017). Yet the combined effects of long-term drought and warming on metabolites important for plant stress tolerance and plant–herbivore interactions are poorly understood. Since shifts in chemicals that influence the balance between foliar nutrients and secondary metabolites may impact plant growth, and affect the interactions among plants, their enemies and even higher trophic levels, such studies are needed.
Plantago lanceolata L. (Plantaginaceae) is an excellent study system to explore the effects of prolonged drought and warming on plant size and leaf chemistry because it is an annual or facultative perennial cosmopolitan herbaceous weed with a wide tolerance to temperature and water limitation (Cavers et al., 1980; Prudic et al., 2005; Rodgers et al., 2012; Cranston et al., 2016; USDA, NRCS, 2017). Plantago also has a well-characterized chemistry that includes both osmoregulators such as proline, and two iridoid glycosides, aucubin and catalpol (Patel and Vora, 1985; Bowers et al., 1992a; Janković et al., 2012; Schweiger et al., 2014a). These metabolites of P. lanceolata are quite responsive to various environmental factors (Schweiger et al., 2014b; Pankoke et al., 2015), and differences in concentrations probably modify plant stress tolerance (Backhaus et al., 2014) and their interactions with herbivores (Bowers et al., 1992a,b).
In this study, we leveraged the long-running Boston Area Climate Experiment (BACE, constructed in 2007, Hoeppner and Dukes, 2012) to examine the consequences of prolonged shifts in climate on plant size and primary and secondary leaf metabolites. Specifically, we quantified the effects of sustained precipitation reduction and warming on growth- and chemistry-related traits in naturally occurring P. lanceolata plants. We measured plant size, foliar N, foliar C and the C/N ratio, and profiled diverse plant metabolites (including sugars, di- and tricarboxylic acids, the sugar alcohol sorbitol, the cyclic polyol myo-inositol, amino acids and iridoid glycosides). We compared plants growing under ambient precipitation and drought and under four temperatures. We expected that traits of plants in plots with prolonged exposure to the combination of drought and warming would be distinct from those of plants in plots exposed to only one stress. For each stressor separately, we expected that the effects of drought would be more pronounced than those of warming, given the species’ shallow rooting system (Tsialtas et al., 2001; Mommer et al., 2010) and high abundance at latitudes that differ greatly in temperature (USDA, NRCS, 2017). Specifically, we predicted that plants would be smaller in drought plots, and would have lower leaf N due to limited water and nutrient uptake. At the metabolome level, we expected drought-induced decreases in the concentrations of N-containing metabolites and iridoid glycosides but increases in stress-responsive osmoregulatory metabolites. Our study provides new insights into the consequences of prolonged water deficits and warming to plant metabolism and its potential effects on plant–herbivore interactions.
MATERIALS AND METHODS
Study site
The Boston Area Climate Experiment (BACE) was constructed in 2007 in an old-field ecosystem in Waltham, MA, USA (42°23.1′N, 71°12.9′W). Mean annual precipitation and temperature in nearby Boston are approx. 1000 mm and approx. 10 °C, respectively. The study site has a loam topsoil (Mesic Typic Dystrudept; Haven series) with 45 % sand, 46 % silt and 9 % clay (gravel content: 7 %) and a gravelly sandy loam sub-soil (Auyeung et al., 2013; Suseela et al., 2014). Prior to construction, the site was maintained by periodic mowing. In 2016, the most common plant species in the experimental plots, in addition to P. lanceolata, were Achillea millefolium, Asclepias syriaca, Chenopodium album, Lepidium virginicum, Linaria vulgaris, Poa trivialis, Solidago canadensis, Verbascum thapsus and Veronica arvensis.
The BACE consists of three replicate blocks with three levels of precipitation and four levels of temperature that are manipulated in a full-factorial, split-plot design (for a total of 36 experimental plots). The soil around each 2 × 2 m plot had been trenched to 60 cm depth and plots were lined with polyethylene sheets to prevent the movement of water and nutrients between plots. The three precipitation regimes per block were ambient, –50 % (hereafter ‘drought’) and +50 %, and were achieved using rainout shelters and a sprinkler system, respectively. Clear, corrugated polycarbonate slats (Rooflite®, Rimol Greenhouse Systems) removed 50 % of incoming precipitation in the drought plots. Such rainout shelters are widely used to study plant responses to water deficits (Kreyling et al., 2017). In this study, plants were collected from two precipitation treatments: ambient and drought.
Within each precipitation treatment group, there were four temperature treatment levels, unwarmed (ambient), and low (+ approx. 0.8 °C), medium (+ apporx. 2.4 °C) and high (+approx. 4 °C) warming (Suseela et al., 2015). Each temperature treatment was applied to a 2 × 2 m plot and all four treatments were repeated in each precipitation regime. Infrared heaters of different wattages were installed 1 m above the ground at each plot corner of the low (200 W), medium (600 W) and high (1000 W) warming treatments and faced towards the centre of the plots at a 45° downward angle to provide relatively uniform warming. Infrared radiometers measured canopy temperatures in the unwarmed (ambient) and high warming plots, and a control system (LabView National Instruments, Austin, TX, USA) provided active feedback control to maintain the target temperatures of the other warming treatment plots. Further details of the treatments and their consequences can be found in Suseela et al. (2012), Hoeppner and Dukes (2012) and Auyeung et al. (2013).
Plant species
Plantago lanceolata was introduced into North America >200 years ago and is now common in lawns and gardens, in agricultural fields and in hayfields. Although exotic, it is fed upon by several herbivore species native to North America (Bowers, 1983; Thomas et al., 1987). Plantago lanceolata grows naturally at BACE and in the surrounding landscape, and the population includes both seedlings and older vegetative clones. We only sampled from vegetative clones to avoid confounding the effects of precipitation and warming treatments with those of plant ontogeny (Quintero and Bowers, 2012). Aucubin and catalpol, the characteristic secondary metabolites in P. lanceolata (Bowers and Stamp, 1992, 1993), typically deter generalist herbivores, but attract specialist herbivores and even aid in their defence against predators upon sequestration (Bower, 1983; Theodoratus and Bowers, 1999; Dobler et al., 2011).
Water availability
Soil moisture was determined volumetrically (v/v) using time domain reflectrometry (TDR) sensors placed in the upper 10 cm of soil (see Auyeung et al., 2013). TDR sensors were permanently installed (at both 10 and 30 cm soil depth) to provide integrated measures of volumetric soil moisture. Measurements were taken weekly during the growing season using a portable TDR-100 (Campbell Scientific, Logan, UT, USA). We report soil moisture at 10 cm 2 d before harvest.
Plant harvest and determination of morphological and chemical plant traits
Plants were sampled on 1 June 2016. This date was chosen so that these data would inform our future experiments examining the consequences of any chemical changes to the performance of the Baltimore checkerspot butterfly (Euphydryas phaeton; Lepidoptera: Nymphalidae), which feed on P. lanceolata in Massachusetts. It is important to note that all sampled plants were free of herbivores. We also selected plants that had no close plant neighbours. This was done to ensure that any potential effects of shading would be minimized, and also to ensure that clonal individuals were only sampled once. With the exception of the ambient precipitation × low warming treatment (n = 4), there were six plants per treatment. The lower sample size in the ambient × warming treatment was due to the limited number of plants in that treatment.
Plant size and leaf harvest
For each plant, we measured the total number of leaves and the length of the longest leaf. To determine effects of the long-term drought and warming treatments on leaf chemistry, we collected leaves from each plant between 08.30 and 09.30 h. We sampled the three youngest fully expanded leaves of each plant, to control for the effects of leaf ontogeny on chemistry (Quintero and Bowers, 2012) and to ensure sufficient material for chemical analyses. The three leaves were put into Falcon tubes and immediately placed in a cooler filled with dry ice to stop any enzymatic degradation. Samples were then lyophilized and ground in a KLECO ball mill (Garcia Machine, Visalia, CA, USA). Dried samples were stored in sealed Eppendorf tubes in desiccated chambers until chemical analyses.
Carbon and nitrogen
We determined foliar %C and %N by dry combustion with a CHN analyser at Tufts University. In brief, 5 ± 0.5 mg of leaf powder per sample were analysed using a vario MICRO cube (Elementar Americas, Mt. Laurel, NJ, USA).
Metabolite profiling of primary and secondary leaf metabolites
Polar leaf metabolites were analysed by targeted metabolite profiling using two analytical platforms. On both platforms, blanks (without biological material) as well as several reference standards (from Sigma-Aldrich, Steinheim, Germany; AppliChem, Darmstadt, Germany; Merck, Darmstadt, Germany; Roth, Karlsruhe, Germany; Macherey-Nagel, Düren, Germany; Agilent Technologies, Waldbronn, Germany; and Phytoplan Diehm & Neuberger, Heidelberg, Germany) were analysed.
Concentrations of sugars, di- and tricarboxylic acids, the sugar alcohol sorbitol, the cyclic polyol myo-inositol and iridoid glycosides were determined using a gas chromatograph coupled to a flame ionization detector (GC-FID). GC analysis of derivatized compounds is commonly applied both for primary metabolites (Pankoke and Müller, 2013; Schweiger et al., 2014a) and for iridoid glycosides (Bowers and Stamp, 1992, 1993; Quintero and Bowers, 2012; Pankoke and Müller, 2013). Leaf powder (4 mg) was extracted and derivatization performed using a modified protocol after Schweiger et al. (2014a). Samples were extracted at room temperature with a 1:2.5:1 (by vol.) chloroform:methanol:Millipore H2O mixture (360 μL; chloroform, HPLC grade, AppliChem; methanol, LC-MS grade, Fisher Scientific, Loughborough, UK) containing ribitol (99 %, Sigma-Aldrich) as internal standard by vortexing and centrifugation. Phase separation was induced by addition of 140 μL of Millipore H2O, followed by vortexing and centrifugation. Aliquots of the methanol–water phases were dried under nitrogen. Samples were derivatized at 37 °C with O-methylhydroxylamine hydrochloride (≥98 %, Sigma-Aldrich; 20 mg mL–1 in pyridine) and N-methyl-N-trimethylsilyltrifluoracetamide (≥95 %, Macherey-Nagel) for 90 and 30 min, respectively. Metabolite concentrations were determined via GC-FID (GC-2010 Plus equipped with an AOC-20s auto sampler and AOC-20i auto injector, Shimadzu, Kyoto, Japan) using a VF-5 ms column (30 m × 0.25 mm i.d., 10 m guard column, Varian, Palo Alto, CA, USA) with 225 °C inlet temperature and 1.12 mL min–1 carrier gas (H2) column flow rate. The oven temperature was 80 °C (hold for 3 min) then ramped (5 °C min–1) to 325 °C. For the peaks that were absent in the blanks, Kováts retention indices (RIs; Kováts, 1958) were determined based on measurements of n-alkanes (C8–C40, Sigma-Aldrich) and used for peak identifications via comparison with RIs of reference standards. Peaks were integrated after file conversion using Xcalibur (1.4.SR1, Thermo Electron, Rodano, Italy). Thereby, peak areas of analytes belonging to the same metabolite were added together.
Amino acids were analysed by ultra-high performance liquid chromatography coupled to fluorescence detection (UHPLC-FLD) modified after Jakobs and Müller (2018). Leaf powder (4 mg) was extracted three times with 80 % methanol (LC-MS grade, Fisher Scientific) containing norvaline and sarcosine (Agilent Technologies) as internal standards by vortexing and centrifugation at room temperature. Supernatants were pooled, filtered (0.2 μm polytetrafluorethylene filters, Phenomenex, Torrance, CA, USA), and analysed via UHPLC-FLD (1290 Infinity UHPLC with 1260 Infinity FLD, Agilent Technologies, Santa Clara, CA, USA). Samples were mixed with borate buffer and pre-column derivatized by addition of o-phthaldialdehyde (OPA) reagent (10 mg mL–1 in 0.4 m borate buffer and 3-mercaptoproprionic acid, Agilent Technologies) and subsequently 9-fluorenyl-methyl chloroformate (FMOC) reagent (2.5 mg mL–1 in acetonitrile, Agilent Technologies). Amino acids were separated at 40 °C on a ZORBAX Eclipse Plus C18 column (250 × 4.6 mm, 5 μm particle size, with a guard column, Agilent Technologies) using a gradient of mobile phase A [1.4 g of Na2HPO4 (>99.5 %, AppliChem), 3.8 g of Na2B4O7·10 H2O (≥99.5 %, Sigma-Aldrich) and 32 mg of NaN3 (≥98 %, Roth) in 1 L of Millipore H2O, pH 8.2] to mobile phase B [4.5:4.5:1 (by vol.) mixture of methanol (LC-MS grade, Fisher Scientific), acetonitrile (LC-MS grade, VWR International, Fontenay-sous-Bois, France) and Millipore H2O] with a flow rate of 1.5 mL min–1. The gradient was ramped from 2 to 57 % B within 43.4 min, followed by column equilibration. The FLD excitation and emission wavelengths were set to 340 and 450 nm, respectively, for the OPA-derivatized primary amino acids and to 260 and 325 nm, respectively, for the FMOC-derivatized secondary amino acids. Those metabolites that were absent in the blanks were identified by comparison of retention times with those of reference standards. Peaks were integrated in OpenLab ChemStation (C.01.06, Agilent Technologies).
Statistical analyses
Univariate statistical analyses were conducted in R version 3.5.1 (R Core Team, 2018). A Shapiro–Wilk goodness-of-fit test was used to ensure normality. Because some TDR sensors were broken, we could not test the effects of temperature on volumetric water availability. We tested the effects of precipitation by pooling across temperature treatment levels (n = 4 for drought and n = 7 for ambient) using a one-tailed t-test. For plant traits, precipitation was a categorical variable (ambient vs. drought) and temperature was a continuous variable (0, 0.8, 2.4 and 4 °C). To determine the effects of these treatments on the number of leaves per plant, the length of the longest leaf, foliar %C, %N, C/N, aucubin concentration and catalpol concentration, analyses of covariance were used.
The peak areas of the metabolites were related to those of the internal standards (GC-FID, ribitol; UHPLC-FLD, norvaline for primary, sarcosine for secondary amino acids) and the dry weights of the samples (leaf powder), yielding relative concentrations. For the iridoid glycosides, absolute concentrations were additionally calculated to be able to compare these values with those in the literature. For that, response factors between ribitol and the iridoid glycosides were determined using the same ribitol concentration as in the samples (see above) and four concentrations of the iridoid glycosides in the linear range. Response factors were (averaged over concentrations and technical duplicates) 1.5 and 1.4 for aucubin and catalpol, respectively. Only those metabolites that occurred in >50 % of the replicates of at least one treatment (precipitation × temperature) group were retained.
Multivariate analyses were conducted in R version 3.5.1 (R Core Team, 2018). All data were autoscaled (i.e. mean centring and scaling to unit variance) and then analysed by redundancy analysis (RDA) using the vegan package (Oksanen et al., 2018) as described in Hervé et al. (2018). This approach allowed us to determine the percentage variation that is attributable to the effects of precipitation, temperature and their interaction. Type II permutation tests were conducted on the RDA models to determine the significance of these effects on metabolite profiles using the MVA.anova function from the RVAideMemoire package (Hervé et al., 2018).
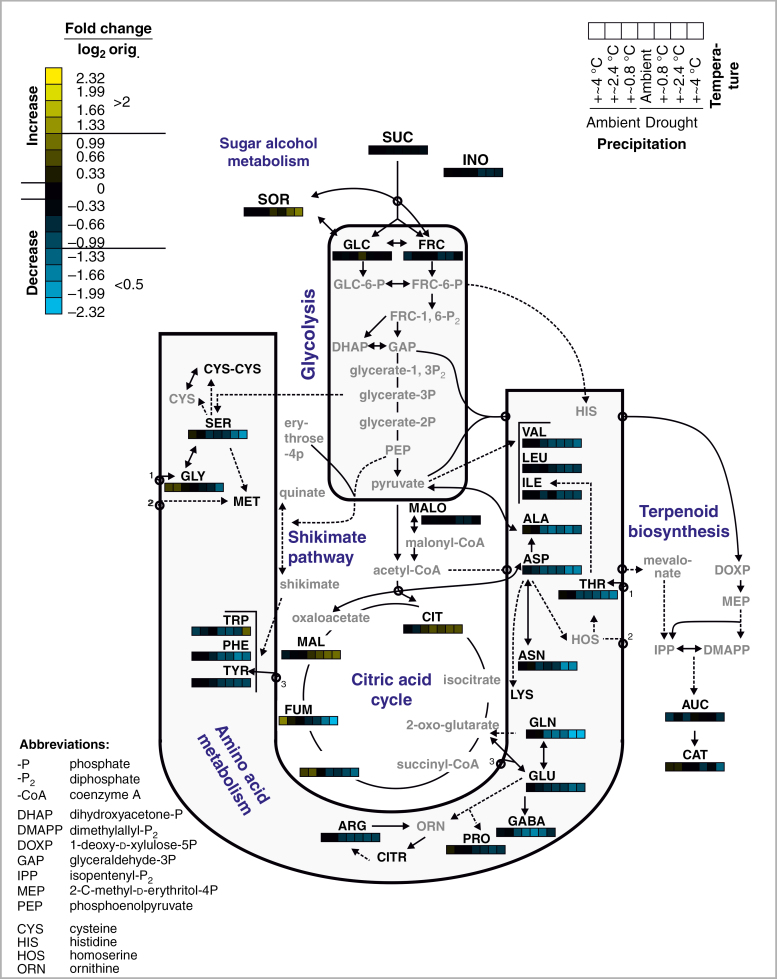
Fold changes (mean metabolite concentrations in treatment groups divided by the mean metabolite concentrations in the common control group, i.e. ambient precipitation and ambient temperature) were calculated for metabolites that occurred in >50 % of the replicates of all treatment groups and log2-transformed for scale symmetry. Metabolite pool sizes were considered to be decreased by the treatment (compared with the common control group) if fold changes were <0.5 (< – 1 on a log2 scale) and considered to be increased if fold changes were >2 (>1 on a log2 scale). Clustering of treatment groups and metabolites was performed based on mean fold changes (see above) using the average linkage hierarchical clustering method based on Pearson correlations in Cluster 3.0 (de Hoon et al., 2004). The heatmap was constructed with Java TreeView 1.1.6r4 (Saldanha, 2004). Heatmap stripes were mapped on a metabolic pathway map that was modified after Schweiger et al. (2014a) and relies on the KEGG PATHWAY database (Kaneshia and Goto, 2000; http://www.genome.jp/kegg/).
RESULTS
Soil moisture
At the time of harvest, the volumetric water (θv) availability in the top 10 cm of soil was different between the two precipitation treatments (t = 2.35, P = 0.03). Specifically, the volumetric water availability was about four times higher in the ambient (0.211 ± 0.03 θv, mean ± s.e., n = 7) than in the drought (0.047 ± 0.01 θv, n = 4) plots. Although statistical analyses could not be done, water availability was similar across the temperature treatment levels.
Plant size
The size of P. lanceolata plants was similar across all treatments (Table 1). For the number of leaves, there was a marginal effect of precipitation (F1,42 = 3.38, P = 0.07), but no effect of temperature (F1,42 = 1.22, P = 0.28) or their interaction (F1,42 = 1.41, P = 0.24). Surprisingly, plants growing in the drought plots tended to have more leaves. For the length of the longest leaf, there was no effect of precipitation (F1,42 = 1.43, P = 0.24), but a marginal effect of temperature (F1,42 = 3.38, P = 0.07) and no significant interaction (F1,42 = 0.01, P = 0.9).
Table 1.
Effects of precipitation and temperature treatments on the number of leaves and length of the longest leaf of Plantago lanceolata plants
| Precipitation | Temperature | No. of leaves | Leaf length (cm) |
| Ambient | Ambient | 5.7 ± 0.5 | 20.2 ± 1.6 |
| + approx. 0.8 °C | 6.0 ± 1.7 | 19.6 ± 0.3 | |
| + approx. 2.4 °C | 6.7 ± 0.9 | 18.2 ± 1.5 | |
| + approx. 4 °C | 5.7 ± 1.1 | 17.3 ± 0.9 | |
| Drought | Ambient | 10.3 ± 2.2 | 20.4 ± 1.3 |
| + approx. 0.8 °C | 5.5 ± 0.8 | 15.2 ± 1.6 | |
| + approx. 2.4 °C | 8.8 ± 0.5 | 18.1 ± 2.3 | |
| + approx. 4 °C | 5.8 ± 0.5 | 16.0 ± 1.9 |
Values are given as the mean ± s.e. of n = 4–6.
There were no significant effects of precipitation, temperature and their interaction on these plant traits, but a trend for more leaves in the drought treatment (see the Results for statistics).
Carbon and nitrogen
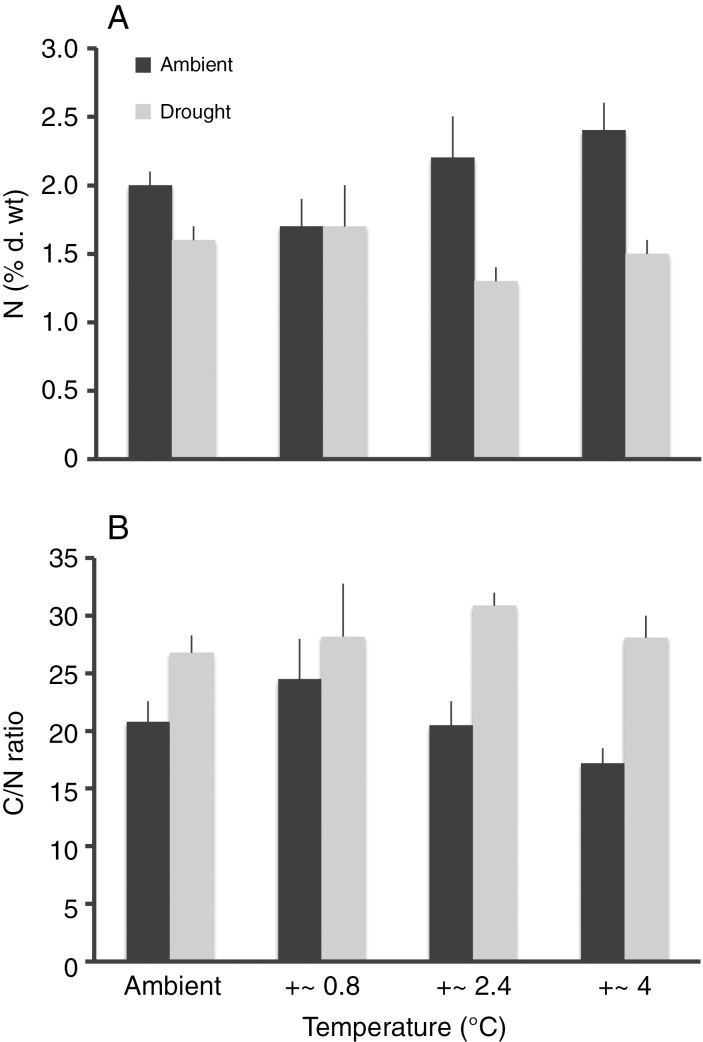
The %N and the C/N ratio were significantly influenced by precipitation (%N, F1,42 = 18.12, P < 0.001; C/N, F1,42 = 22.76, P < 0.001) but not by temperature (%N, F1,42 = 0.76, P = 0.39; C/N, F1,42 = 0.40, P = 0.53). For %N but not C/N, there was a significant interaction (%N, F1,42 = 4.0, P = 0.05; C/N, F1,42 = 2.10, P = 0.15) (Fig. 1). Since there were no effects of precipitation (F1,42 = 0.32, P = 0.58), temperature (F1,42 = 0.13, P = 0.72) or their interaction (F1,42 = 0.84, P = 0.36) on %C (data not shown), this indicates that shifts in N were driving the difference in C/N. Overall, %N was about 40 % higher in the ambient (2.1 ± 0.1 %; mean ± s.e. across all temperature levels) compared with the drought treatment (1.5 ± 0.1 %).
Fig. 1.
Effects of different precipitation and temperature treatments on (A) percentage nitrogen and (B) carbon/nitrogen ratios in Plantago lanceolata leaves; mean ± s.e. of n = 4–6.
Metabolite responses
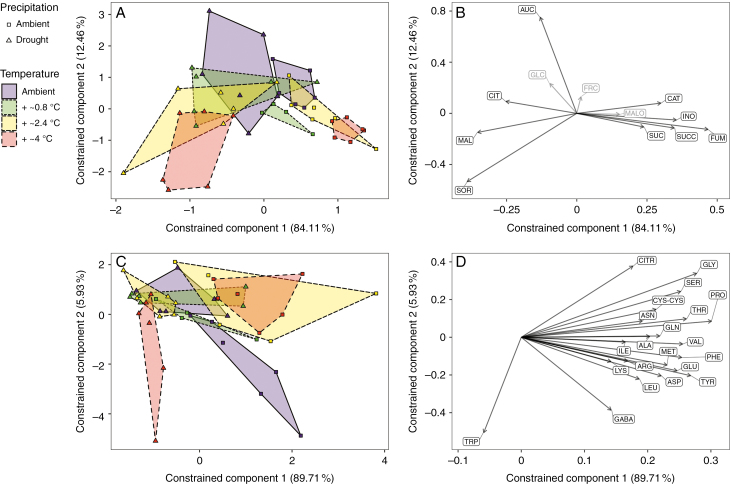
In our redundancy analysis, the experimental factors (precipitation, temperature and their interaction) explained 26.1 % of the total variance in the metabolites analysed by GC. A total of 96.6 % of the constrained variance was explained by the first two components. There was a significant effect of precipitation (F1,42 = 10.64; P < 0.001), no effect of temperature (F1,42 = 1.70, P = 0.10), but a significant precipitation × temperature interaction effect (F1,42 = 2.44, P = 0.02) (Fig. 2A, B).
Fig. 2.
Redundancy analysis of autoscaled leaf metabolite concentrations of Plantago lanceolata subjected to different precipitation and temperature treatments. Concentrations of sugars, di- and tricarboxylic acids, the sugar alcohol sorbitol, the cyclic polyol myo-inositol and iridoid glycosides (A and B), and amino acids (C and D). Score plots (A and C) with the percentage total variance explained by the first two constrained components in parentheses and convex hulls surrounding each treatment group. Loadings plots (B and D) with loadings depicted as arrows and metabolites abbreviated as in Supplementary data Table S1. n = 4–6. Metabolites in grey are those whose concentrations did not significantly correlate with either of the first two constrained components.
For amino acids, the redundancy analysis showed that precipitation, temperature and their interaction explained 32.2 % of the total variation. Of the constrained variance, 95.6 % was explained by the first two components. There was a significant effect of precipitation (F1,42 = 16.63; P < 0.001), no effect of temperature (F1,42 = 0.91, P = 0.38) and a marginal precipitation × temperature effect (F1,42 = 2.44, P = 0.07) (Fig. 2C, D). Across all temperatures, the metabolite profiles of plants grown under drought clustered separately from samples taken from plants grown under ambient precipitation mainly along the first constrained components (Fig. 2A, C). Drought effects on metabolite profiles were, however, strongest in plants grown under the two highest temperature levels, indicating that warming reinforced the effects of drought (Fig. 2A, C).
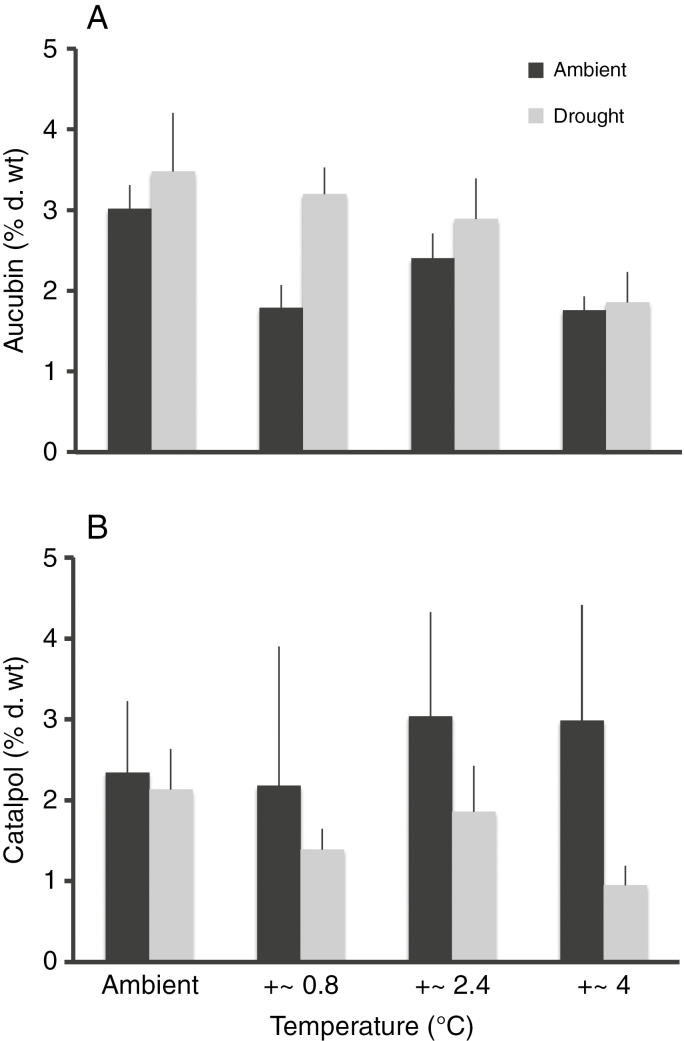
Compared with plants grown under ambient precipitation, plants grown in drought plots had higher foliar concentrations of malate, citrate and sorbitol (Fig. 2B) but lower concentrations of most other metabolites including proline and the majority of amino acids (Fig. 2D). The concentrations of the two iridoid glycosides aucubin and catalpol ranged from 0.9 to 6.5 % d. wt and from 0.4 to 6.6 % d. wt, respectively, and these two metabolites responded differently to the treatments (Figs 2B and 3; Supplementary data Table S1). Aucubin concentrations were influenced slightly by precipitation (F1,42 = 3.48, P = 0.07) and strongly by temperature (F1,42 = 10.73, P < 0.01) but not by their interaction (F1,42 = 0.69, P = 0.41). In contrast, concentrations of catalpol were only influenced by precipitation (F1,42 = 9.88, P < 0.01; temperature, F1,42 = 0.01, P = 0.93; interaction, F1,42 = 3.46, P = 0.07). Aucubin concentrations decreased with temperature and were slightly elevated under drought (Fig. 3). In contrast, catalpol had about 40 % lower concentrations in plants subjected to drought compared with plants grown under ambient precipitation (averaged across all temperature levels).
Fig. 3.
Effects of different precipitation and temperature treatments on the concentrations of the iridoid glycosides (A) aucubin and (B) catalpol in Plantago lanceolata leaves; mean ± s.e. of n = 4–6.
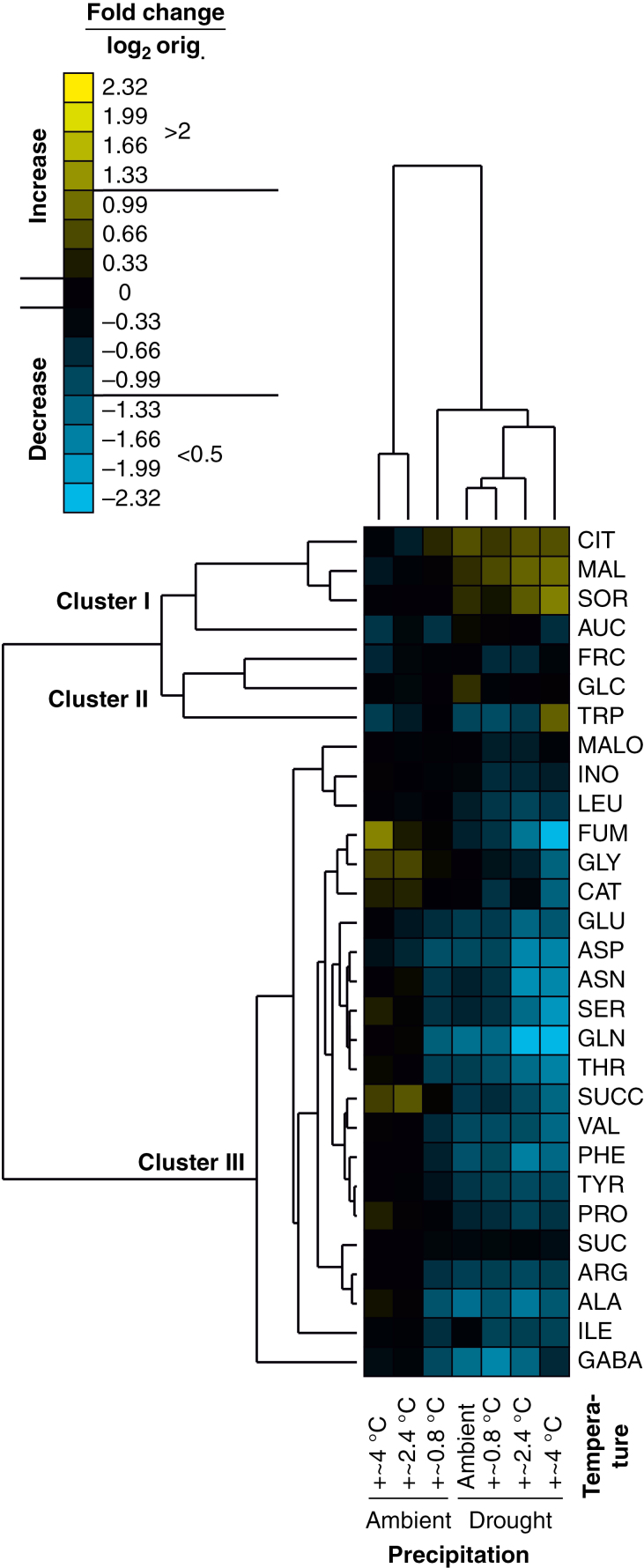
Cluster analysis confirmed that the effects on the foliar metabolite profiles were stronger for the drought than for the warming treatment, but that warming reinforced the effects of drought, as seen in the fold changes and clustering of treatment groups predominantly according to the precipitation treatment (Fig. 4). Moreover, two distinct clusters of treatment-responsive metabolites were found, confirming that most metabolites were reduced (Cluster III) and only some (Cluster I; malate, citrate, sorbitol and, to a lesser extent, aucubin) were increased in concentrations under drought. The drought-responsive metabolites were spread across the major plant primary and secondary metabolic pathways (Fig. 5). Metabolites specifically increased under drought were related to sugar and sugar alcohol metabolism (sorbitol) and part of the citric acid cycle (malate and citrate), while those decreased under drought were related to amino acid metabolism and to a lesser degree terpenoid biosynthesis.
Fig. 4.
Cluster heatmap based on fold changes of leaf metabolite concentrations of Plantago lanceolata subjected to different precipitation and temperature treatments. Clustering was performed based on mean fold changes (i.e. mean metabolite concentrations in treatment groups divided by those in the common control group; means of n = 4–6). Both treatment groups and metabolites were clustered using the average linkage hierarchical clustering method based on Pearson correlations. Only those 29 metabolites were included that occurred in >50 % of the replicates of all treatment groups. The colour code for log2-scaled fold changes is given at the top. On this colour bar, fold change thresholds (orig., untransformed) of <0.5 (considerable decrease in metabolite pool sizes) and >2 (considerable increase in metabolite pool size), respectively, are indicated. Abbreviations of metabolites are as in Supplementary data Table S1.
Fig. 5.
Metabolic pathway map showing fold changes of leaf metabolite concentrations of Plantago lanceolata subjected to different precipitation and temperature treatments. The map shows a part of the primary metabolism as well as the biosynthesis of iridoid glycosides. Only some major pathway intermediates are shown; dashed arrows indicate that intermediates were omitted. The names of the metabolites that were found in P. lanceolata leaves in this study are written in black, whereas others are given in grey. The heatmap stripes (mean log2-scaled fold changes compared with the common control group) were derived from the cluster heatmap (Fig. 4); the corresponding colour bar (log2 scale and original scale) and order of treatments (same order as derived by clustering, see Fig. 4) are given in the keys at the top. Full names of metabolites are given in the lower key and in Supplementary data Table S1.
DISCUSSION
Striking differences in the foliar leaf metabolite profiles were observed, despite no differences in plant size at the time of sampling. While effects of the drought treatment were strong, the effects of warming were weak. However, we found a significant precipitation × temperature treatment interaction. Specifically, differences between ambient and drought treatments were minimal at lower temperatures but quite distinct at higher temperatures. This key finding demonstrates that the combination of climatic variables more strongly influences phenotypic traits than either one alone (see also Zandalinas et al., 2018). In the season when plants were harvested, water is generally less limiting and temperatures are lower, but later in the season, when it is both drier and hotter, phenotypic differences would be expected to be even more pronounced (e.g. Rodgers et al., 2012). Thus, the chemical changes reported in the current study provide a conservative estimate of the effects of prolonged precipitation deficits and warming on plant traits.
Effects of drought and warming on the leaf metabolome
There were large differences in the foliar concentrations of primary and secondary metabolites in response to reduced water availability. Foliar N, amino acids and the iridoid glycoside catalpol were reduced under drought, while sorbitol, two acids of the citric acid cycle (malate and citrate) and aucubin were increased. We had expected drought-induced increases of sorbitol, malate, citrate and proline since they are all stress-responsive osmoregulatory metabolites (Venekamp, 1989; Rai, 2002; Reddy et al., 2004; Singh et al., 2015). We observed partial support for this expectation. Sorbitol, malate and citrate all increased, but proline concentrations were lower in plants grown under drought.
The response of proline to drought was quite unexpected given that many studies indicate that proline is associated with plant tolerance to drought and extreme temperatures (Wang et al., 2003; Reddy et al., 2004; Verbruggen and Hermans, 2008; Moradi, 2016). While other free amino acids may also play a major role in a plant’s osmotic adjustment capacity (Rai, 2002; Hu et al., 2015), none of the amino acids was higher in this condition. Rather, most amino acids were lower or unchanged in concentration in the plants grown in the drought treatment. Several factors may have contributed to the reduced leaf amino acid concentrations under drought. First, foliar N levels were approx. 40 % higher in the ambient precipitation regime, suggesting that plants in the drought plots may have been N limited which could have limited the biosynthesis of amino acids. The capacity to take up N-containing nutrients from the soil is typically lower under water deficits because drought-induced stomatal closure reduces the transpiration stream and nutrient mobility (da Silva et al., 2011). In this way, warming could result in more rapid desiccation and thus enhance the effects of drought. In addition, desiccation-induced suppression of the soil microbial community can slow litter decomposition and thus the release of nutrients (Suseela et al., 2014). In contrast to N-containing osmolytes, the production of non-N-containing compounds with an osmoregulatory function such as sorbitol, citrate and malate may be less constrained by drought and N nutrition.
Secondly, proline may be a less important osmolyte for P. lanceolata. In the closely related species Plantago major, sorbitol but not proline, was increased in response to salt stress (Hassan et al., 2016), and in general sorbitol concentrations are strongly increased by drought and salt stress in many Plantago species (Pommerrenig et al., 2007; Hassan et al., 2016). Sorbitol may be especially important when N is limiting. Thus we suggest that under N limitation, there may be a shift from accumulation of N-containing osmolytes, such as proline, to non-N-containing osmolytes such as sorbitol.
As we found, aucubin and catalpol can make up high proportions (>2–4 % for each iridoid glycoside) of the leaf dry matter of P. lanceolata (Bowers and Stamp, 1992, 1993; Bowers et al., 1992a; Quintero and Bowers, 2012; Pankoke and Müller, 2013). Although the effects of drought on secondary metabolites such as iridoid glycosides can be variable (Chaves et al., 2003; Jamieson et al., 2013; Selmar and Kleinwächter, 2013; Metz et al., 2014), we had predicted that an increase in compounds involved in osmoregulation would be associated with decreased iridoid glycoside concentrations. Indeed, catalpol concentrations were approx. 40 % lower in leaves of plants grown in the drought plots. In contrast, aucubin concentrations were slightly higher in these plants. Similar to our study, catalpol concentrations have been shown to be more responsive to many factors including low mineral N availability, interspecific competition (Pankoke et al., 2015) and arbuscular mycorrhiza (Schweiger et al., 2014a) than those of aucubin. Since aucubin is the biosynthetic precursor of catalpol (Damtoft, 1994), it is possible that the biosynthetic conversion of aucubin to catalpol is impaired in more stressed plants.
While the responses of individual compounds are informative, our multivariate analyses of the polar leaf metabolites provide a more comprehensive perspective (Figs 2, 4 and 5). Of particular interest is the precipitation × temperature interaction effect, whereby the effects of precipitation were most pronounced at higher temperatures. This provides further evidence that the combined effects of drought and warming can be especially marked (see also Suseela et al., 2015). In addition, our cluster analyses revealed that plants in the drought treatment were clustered together and that warming magnified the effects of drought (Fig. 4 top). The chemistry of the plants also clustered in intriguing ways (Fig. 4 bottom). While most chemicals responded similarly (Cluster III) with lower concentrations under drought, those in Cluster I (especially malate, citrate and sorbitol) had higher concentrations. These results imply that metabolic shifts are correlated. From Fig. 5 it is apparent that metabolites related to sugar and sugar alcohol metabolism (sorbitol) and those that are part of the citric acid cycle (malate and citrate) increased under drought. In contrast, amino acid metabolism and, to a lesser degree, terpenoid metabolism decreased under drought. While the effects of our experimental factors were significant, they did not explain a high proportion of metabolite variation (26 and 32 % for the two analytical platforms). Which other factors are contributing to variation in foliar metabolite profiles deserves further study. There might be, for example, plot-level differences in soil nutrients that might affect chemistry (Jamieson et al., 2013).
Comparing our results with previous work at BACE focused on the response of a woody plant species, Quercus rubra, suggests that woody plants may respond differently (Suseela et al., 2015). While Suseela and colleagues also found that (1) oaks in dry-warm plots were most distinct; (2) levels of citric acid were higher in the drought + warming treatment; and (3) foliar N was lower in drought-exposed plants, several metabolites responded differently. In contrast to our results, the concentrations of amino acids were quite variable in Q. rubra. Some amino acids, such as proline, were higher in the drought treatment, while other amino acids were lower. Perhaps woody plants, with their deeper roots, are less affected by drought-induced nutrient deficiency and more buffered against shifts in climate.
The underlying mechanisms for the chemical shifts we observed deserve further study. These shifts could reflect plasticity to changes in the environment or may be a result of genetic differentiation after years of prolonged exposure. The chemical differences may be a direct consequence of the effects of precipitation or warming on plant growth and development, or could be an indirect consequence of changes in soil nutrients, plant–microbe interactions or the surrounding plant community (sensuBowers et al., 1992; Schweiger et al., 2014a). While we tried to control for plant ontogeny and selected plants that were isolated, we are unable to determine the cause of the differences in our sampling/experimental design. A long-term manipulative transplant experiment, which controls for above- and below-ground competition, for example, would be necessary to differentiate among plasticity, evolutionary shifts, and direct and indirect effects. Future experiments might also, given that these treatments can lead to secondary oxidative stress, measure the response of other metabolites, such as flavonoids and phenylpropanoid glycosides (Janković et al., 2012), and enzymes, which function as antioxidants.
Besides chemical adjustments to drought in individual plants, other external factors probably modify the severity of drought effects on plant traits in natural communities. Our results suggest that periods of high temperature will exacerbate the effects of drought. In addition, the composition of the surrounding plant community may affect water availability in the soil, depending on root architectures and water uptake efficiencies. Moreover, plant associations with mycorrhizal fungi may improve the plant’s drought tolerances (Ruiz-Lozano et al., 2012) and affect plant chemistry (Asensio et al., 2012). Thus, to understand long-term effects of drought on plant traits, it is important to study drought effects under multifactorial field conditions.
Implications for plant–herbivore interactions
Most herbivores are N limited (Mattson, 1980), so changes in nutritional profiles of leaves can impact herbivore feeding, growth, reproduction and survival. Likewise, changes in plant secondary metabolites may have similar direct effects on herbivores, and indirectly affect their susceptibility to predators (Bowers, 1983; Theodoratus and Bowers, 1999; Tomczak and Müller, 2017). While plant size and thus the quantity of leaf material available to herbivores was similar across treatments in early June, the lower foliar concentrations of N, amino acids and catalpol in drought-stressed P. lanceolata plants probably have important consequences for herbivores. The decreased concentrations of N and amino acids under drought and warming are expected to affect herbivores negatively (Mattson, 1980). It is, however, the balance between nutritional and defence compounds that determines how well herbivores survive, develop and reproduce on the plants. We suggest that the effects of the reduced catalpol concentrations in the drought treatment would depend on the dietary breadth of the herbivore. June coincides with the occurrence of the last two larval instars of Euphyrdryas phaeton prior to adult emergence in late June and early July (Bowers et al., 1992b). Euphydryas phaeton and other North American specialist herbivore species evolved on native plant species that produce iridoid glycosides and subsequently have incorporated P. lanceolata into their diet (Bowers et al., 1992b). The native host of E. phaeton is Chelone glabra (Plantaginaceae) which produces high concentrations of catalpol and very little aucubin. Since catalpol may act as an oviposition stimulant as shown for another specialist butterfly species (Pereyra and Bowers, 1988), reductions in catalpol might hamper the ability of adult female butterflies to detect P. lanceolata in drought-prone habitats. Moreover, since iridoid glycosides are sequestered as a defence against predators by some specialist herbivores, lower concentrations of catalpol may make the larvae and emerging adults in drought-prone habitats more susceptible to their enemies (Bowers, 1980; Theodoratus and Bowers, 1999; Dobler et al., 2011), but potentially better defended against parasitoids (Smilanich et al., 2009; Quintero et al., 2014). In contrast, generalist herbivores are predicted to perform better on drought-stressed plants with lower concentrations of catalpol. Future studies are needed to examine the consequences of the chemical responses to stress observed in P. lanceolata plants and should also consider the effects of precipitation and warming on both iridoid glycoside concentrations and β-glucosidase activity, since both traits form a dual defence system (Pankoke et al., 2013).
Conclusion
Climate projections indicate more variable precipitation and higher air temperatures. Compared with warming, we found that the reduction of precipitation exerts a stronger influence on the polar leaf metabolome of P. lanceolata that might influence plant tolerance to further stress and suitability for associated herbivores, pathogens and members of higher trophic levels. While warming had less of an effect, the magnitude of drought effects was reinforced by warming. These effects may, in part, reflect the direct and indirect impacts of prolonged exposure to drought and temperature. They probably also reflect rapid shifts (plasticity) in chemistry as environmental conditions change. If the severity of drought and warming increases over the season, then the consequences are likely to be even more pronounced. Clearly, more frequent drought periods and warming will have profound impacts on the metabolism of plants and will be likely to alter the behaviour and performance of herbivores. This will probably feed back to affect the performance of the plants, since damage to resource-limited plants is likely to have a greater effect on their long-term performance. How these shifts in plant traits will affect the ecological outcome of plant–herbivore–predator/parasitoid and plant–pathogen interactions remains unknown.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Table S1: metabolites, grouped according to their chemical class, in Plantago lanceolata leaf tissue.
FUNDING
This work was supported by funds from Tufts University and Bielefeld University. The BACE has been supported by grants to J.S.D. from the National Science Foundation (DEB-0546670); the US Department of Energy’s Office of Science (BER), through the Northeastern Regional Center of the National Institute for Climatic Change Research and the Terrestrial Ecosystem Sciences program; and the United States Department of Agriculture’s National Institute of Food and Agriculture (USDA-NIFA 2015-67003-23485).
ACKNOWLEDGEMENTS
We thank Risa McNellis and Annie Nguyen for help with fieldwork, Ruth Jakobs for assistance with the chemical analyses, and anonymous reviewers for their insights. We also thank the University of Massachusetts’ Extension Division for leasing land to the BACE.
LITERATURE CITED
- Asensio D, Rapparini F, Peñuelas J. 2012. AM fungi root colonization increases the production of essential isoprenoids vs. nonessential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry 77: 149–161. [DOI] [PubMed] [Google Scholar]
- Auyeung DSN, Suseela V, Dukes JS. 2013. Warming and drought reduce temperature sensitivity of nitrogen transformations. Global Change Biology 19: 662–676. [DOI] [PubMed] [Google Scholar]
- Backhaus S, Kreyling J, Grant K, Beierkuhnlein C, Walter J, Jentsch A. 2014. Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 17: 1068–1081. [Google Scholar]
- Bowers MD. 1980. Unpalatability as a defense strategy of Euphydryas phaeton (Lepidoptera: Nymphalidae). Evolution 34: 586–600. [DOI] [PubMed] [Google Scholar]
- Bowers MD. 1983. The role of iridoid glycosides in host-plant specificity of checkerspot butterflies. Journal of Chemical Ecology 9: 475–493. [DOI] [PubMed] [Google Scholar]
- Bowers MD, Stamp NE. 1992. Chemical variation within and between individuals of Plantago lanceolata (Plantaginaceae). Journal of Chemical Ecology 18: 985–995. [DOI] [PubMed] [Google Scholar]
- Bowers MD, Stamp NE. 1993. Effects of plant age, genotype, and herbivory on Plantago performance and chemistry. Ecology 74: 1778–1791. [Google Scholar]
- Bowers MD, Collinge SK, Gamble SE, Schmitt J. 1992a Effects of genotype, habitat, and seasonal variation on iridoid glycoside content of Plantago lanceolata (Plantaginaceae) and the implications for insect herbivores. Oecologia 91: 201–207. [DOI] [PubMed] [Google Scholar]
- Bowers MD, Stamp NE, Collinge SK. 1992b Early stage of host range expansion by a specialist herbivore, Euphydryas phaeton (Nymphalidae). Ecology 73: 526–536. [Google Scholar]
- Cavers PB, Bassett IJ, Crompton CW. 1980. The biology of Canadian weeds. 47. Plantago lanceolata L. Canadian Journal of Plant Science 60: 1269–1282. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought – from genes to the whole plant. Functional Plant Biology 30: 239–264. [DOI] [PubMed] [Google Scholar]
- Cranston LM, Kenyon PR, Morris ST, Lopez-Villalobos N, Kemp PD. 2016. Morphological and physiological responses of plantain (Plantago lanceolata) and chicory (Cichorium intybus) to water stress and defoliation frequency. Journal of Agronomy and Crop Science 202: 13–24. [Google Scholar]
- Damtoft S. 1994. Biosynthesis of catalpol. Phytochemistry 35: 1187–1189. [Google Scholar]
- Dieleman WIJ, Vicca S, Dijkstra FA, et al. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology 18: 2681–2693. [DOI] [PubMed] [Google Scholar]
- Dobler S, Petschenka G, Pankoke H. 2011. Coping with toxic plant compounds – the insect’s perspective on iridoid glycosides and cardenolides. Phytochemistry 72: 1593–1604. [DOI] [PubMed] [Google Scholar]
- Gargallo-Garriga A, Sardans J, Pérez-Trujillo M, et al. 2015. Warming differentially influences the effects of drought on stoichiometry and metabolomics in shoots and roots. New Phytologist 207: 591–603. [DOI] [PubMed] [Google Scholar]
- Goufo P, Moutinho-Pereira JM, Jorge TF, et al. 2017. Cowpea (Vigna unguiculata L. Walp.) metabolomics: osmoprotection as a physiological strategy for drought stress resistance and improved yield. Frontiers in Plant Science 8: 586 10.3389/fpls.2017.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MA, Pacurar A, López-Gresa MP, et al. 2016. Effects of salt stress on three ecologically distinct Plantago species. PLoS One 11: e0160236 10.1371/journal.pone.0160236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé MR, Nicolè F, Lê Cao K-A. 2018. Multivariate analysis of multiple datasets: a practical guide for chemical ecology. Journal of Chemical Ecology 44: 215–234. [DOI] [PubMed] [Google Scholar]
- Hoeppner SS, Dukes JS. 2012. Interactive responses of old-field plant growth and composition to warming and precipitation. Global Change Biology 18: 1754–1768. [Google Scholar]
- de Hoon MJL, Imoto S, Nolan J, Miyano S. 2004. Open source clustering software. Bioinformatics 20: 1453–1454. [DOI] [PubMed] [Google Scholar]
- Hu B, Simon J, Rennenberg H. 2013. Drought and air warming affect the species-specific levels of stress-related foliar metabolites of three oak species on acidic and calcareous soil. Tree Physiology 33: 489–504. [DOI] [PubMed] [Google Scholar]
- Hu B, Simon J, Günthardt-Goerg MS, Arend M, Kuster TM, Rennenberg H. 2015. Changes in the dynamics of foliar N metabolites in oak saplings by drought and air warming depend on species and soil type. PLoS One 10: e0126701 10.1371/journal.pone.0126701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC 2014. Climate change 2014: synthesis report. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- Jakobs R, Müller C. 2018. Effects of intraspecific and intra-individual differences in plant quality on preference and performance of monophagous aphid species. Oecologia 186: 173–184. [DOI] [PubMed] [Google Scholar]
- Jamieson MA, Quintero C, Blumenthal DM. 2013. Interactive effects of simulated nitrogen deposition and altered precipitation patterns on plant allelochemical concentrations. Journal of Chemical Ecology 39: 1204–1208. [DOI] [PubMed] [Google Scholar]
- Janković T, Zdunić G, Beara I, et al. 2012. Comparative study of some polyphenols in Plantago species. Biochemical Systematics and Ecology 42: 69–74. [Google Scholar]
- Kaneshia M, Goto S. 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováts E. 1958. Gaschromatographische charakterisierung organischer verbindungen. 1. Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helvetica Chimica Acta 41: 1915–1932. [Google Scholar]
- Kreyling J, Arfin Khan MAS, Sultana F, et al. 2017. Drought effects in climate change manipulation experiments: quantifying the influence of ambient weather conditions and rain-out shelter artifacts. Ecosystems 20: 301–315. [Google Scholar]
- Maenpaa M, Ossipov V, Kontunen-Soppela S, Keinanen M, Rousi M, Oksanen E. 2013. Biochemical and growth acclimation of birch to night temperatures: genotypic similarities and differences. Plant Biology 15: 36–43. [DOI] [PubMed] [Google Scholar]
- Mattson WJ. 1980. Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics 11: 119–161. [Google Scholar]
- Metz J, Ribbers K, Tielbörger K, Müller C. 2014. Long- and medium-term effects of aridity on the chemical defence of a widespread Brassicaceae in the Mediterranean. Environmental and Experimental Botany 105: 39–45. [Google Scholar]
- Mommer L, van Ruijven J, de Caluwe H, et al. 2010. Unveiling below-ground species abundance in a biodiversity experiment: a test of vertical niche differentiation among grassland species. Journal of Ecology 98: 1117–1127. [Google Scholar]
- Moradi P. 2016. Key plant products and common mechanisms utilized by plants in water deficit stress responses. Botanical Sciences 94: 657–671. [Google Scholar]
- Moradi P, Ford-Lloyd B, Pritchard J. 2017. Metabolomic approach reveals the biochemical mechanisms underlying drought stress tolerance in thyme. Analytical Biochemistry 527: 49–62. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2018. https://cran.r-project.org/web/packages/vegan/vegan.pdf https://cran.r-project.org/web/packages/vegan/vegan.pdf vegan: Community Ecology Package.
- Pankoke H, Müller C. 2013. Impact of defoliation on the regrowth capacity and the shoot metabolite profile of Plantago lanceolata L. Plant Physiology and Biochemistry 71: 325–333. [DOI] [PubMed] [Google Scholar]
- Pankoke H, Buschmann T, Müller C. 2013. Role of plant β-glucosidases in the dual defense system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major. Phytochemistry 94: 99–107. [DOI] [PubMed] [Google Scholar]
- Pankoke H, Höpfner I, Matuszak A, Beyschlag W, Müller C. 2015. The effects of mineral nitrogen limitation, competition, arbuscular mycorrhiza, and their respective interactions, on morphological and chemical plant traits of Plantago lanceolata. Phytochemistry 118: 149–161. [DOI] [PubMed] [Google Scholar]
- Patel JA, Vora AB. 1985. Free proline accumulation in drought-stressed plants. Plant and Soil 84: 427–429. [Google Scholar]
- Pereyra PC, Bowers MD. 1988. Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia (Nymphalidae). Journal of Chemical Ecology 14: 917–928. [DOI] [PubMed] [Google Scholar]
- Pommerrenig B, Papini-Terzi FS, Sauer N. 2007. Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiology 144: 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudic KL, Oliver JC, Bowers MD. 2005. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143: 578–587. [DOI] [PubMed] [Google Scholar]
- Quintero C, Bowers MD. 2012. Changes in plant chemical defenses and nutritional quality as a function of ontogeny in Plantago lanceolata (Plantaginaceae). Oecologia 168: 471–481. [DOI] [PubMed] [Google Scholar]
- Quintero C, Lambert EC, Bowers MD. 2014. Time is of the essence: direct and indirect effects of plant ontogenetic trajectories on higher trophic levels. Ecology 95: 2589–2602. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Rai VK. 2002. Role of amino acids in plant responses to stresses. Biologia Plantarum 45: 481–487. [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. 2004. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology 161: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rodgers VL, Hoeppner SS, Daley MJ, Dukes JS. 2012. Leaf-level gas exchange and foliar chemistry of common old-field species responding to warming and precipitation treatments. International Journal of Plant Sciences 173: 957–970. [Google Scholar]
- Ruiz-Lozano J, Porcel R, Bárzana G, Azcón R, Aroca R. 2012. Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: state of the art. In: Aroca R, ed. Plant responses to drought stress: from morphological to molecular features. Berlin, Heidelberg: Springer Berlin Heidelberg, 335–362. [Google Scholar]
- Saldanha AJ. 2004. Java Treeview – extensible visualization of microarray data. Bioinformatics 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- Schweiger R, Baier MC, Persicke M, Müller C. 2014a High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nature Communications 5: 3886 DOI: 10.1038/ncomms4886. [DOI] [PubMed] [Google Scholar]
- Schweiger R, Heise A-M, Persicke M, Müller C. 2014b Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant, Cell & Environment 37: 1574–1585. [DOI] [PubMed] [Google Scholar]
- Selmar D, Kleinwächter M. 2013. Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant & Cell Physiology 54: 817–826. [DOI] [PubMed] [Google Scholar]
- Selmar D, Kleinwächter M, Abouzeid S, Yahyazadeh M, Nowak M. 2017. The impact of drought stress on the quality of spice and medicinal plants. In: Ghorbanpour M, Varma A, eds. Medicinal plants and environmental challenges. Cham: Springer, 159–175. [Google Scholar]
- da Silva EC, Nogueira R, da Silva MA, de Albuquerque MB. 2011. Drought stress and plant nutrition. Plant Stress 5: 32–41. [Google Scholar]
- Singh M, Kumar J, Singh S, Singh VP, Prasad SM. 2015. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Reviews in Environmental Science and Biotechnology 14: 407–426. [Google Scholar]
- Smilanich AM, Dyer LA, Chambers JQ, Bowers MD. 2009. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecology Letters 12: 612–621. [DOI] [PubMed] [Google Scholar]
- Song X, Wang Y, Lv X. 2016. Responses of plant biomass, photosynthesis and lipid peroxidation to warming and precipitation change in two dominant species (Stipa grandis and Leymus chinensis) from North China Grasslands. Ecology and Evolution 6: 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suseela V, Conant RT, Wallenstein MD, Dukes JS. 2012. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Global Change Biology 18: 336–348. [Google Scholar]
- Suseela V, Tharayil N, Xing B, Dukes JS. 2014. Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biology and Biochemistry 75: 102–112. [Google Scholar]
- Suseela V, Tharayil N, Xing B, Dukes JS. 2015. Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercus rubra. Global Change Biology 21: 4177–4195. [DOI] [PubMed] [Google Scholar]
- Tharayil N, Suseela V, Triebwasser DJ, Preston CM, Gerard PD, Dukes JS. 2011. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: climatic stress-induced tannins are more reactive. New Phytologist 191: 132–145. [DOI] [PubMed] [Google Scholar]
- Theodoratus DH, Bowers MD. 1999. Effects of sequestered iridoid glycosides on prey choice of the prairie wolf spider, Lycosa carolinensis. Journal of Chemical Ecology 25: 283–295. [Google Scholar]
- Thomas CD, Ng D, Singer MC, Mallet JLB, Parmesan C, Billington HL. 1987. Incorporation of a European weed into the diet of a North American herbivore. Evolution 41: 892–901. [DOI] [PubMed] [Google Scholar]
- Tomczak VV, Müller C. 2017. Influence of arbuscular mycorrhizal stage and plant age on the performance of a generalist aphid. Journal of Insect Physiology 98: 258–266. [DOI] [PubMed] [Google Scholar]
- Tsialtas JT, Handley LL, Kassioumi MT, Veresoglou DS, Gagianas AA. 2001. Interspecific variation in potential water-use efficiency and its relation to plant species abundance in a water-limited grassland. Functional Ecology 15: 605–614. [Google Scholar]
- USDA, NRCS 2017. The PLANTS database http://plants.usda.gov.
- Van De Velde H, Bonte D, AbdElgawad H, Asard H, Nijs I. 2015. Combined elevated CO2 and climate warming induces lagged effects of drought in Lolium perenne and Plantago lanceolata. Plant Ecology 216: 1047–1059. [Google Scholar]
- Venekamp JH. 1989. Regulation of cytosol acidity in plants under conditions of drought. Physiologia Plantarum 76: 112–117. [Google Scholar]
- Verbruggen N, Hermans C. 2008. Proline accumulation in plants: a review. Amino Acids 35: 753–759. [DOI] [PubMed] [Google Scholar]
- Virjamo V, Sutinen S, Julkunen-Tiitto R. 2014. Combined effect of elevated UVB, elevated temperature and fertilization on growth, needle structure and phytochemistry of young Norway spruce (Picea abies) seedlings. Global Change Biology 20: 2252–2260. [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14. [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R. Balfagón D, Arbona V, Gómez-Cadenas A. 2018. Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum 162: 2–12. [DOI] [PubMed] [Google Scholar]
- Zhang T, Yang S, Guo R, Guo J. 2016. Warming and nitrogen addition alter photosynthetic pigments, sugars and nutrients in a temperate meadow ecosystem. PLoS ONE 11. doi: 10.1371/journal.pone.0155375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.