Abstract
Background and Aims
Floral colour in angiosperms can be controlled by variations in the expression of the genes of the anthocyanin pathway. Floral colour shifts influence pollinator specificity. Multiple shifts in floral colour occurred in the diversification of the genus Erica (Ericaceae), from plesiomorphic pink to, for example, red or white flowers. Variation in anthocyanin gene expression and its effects on floral colour in the red-, pink- and white-flowered Erica plukenetii species complex was investigated.
Methods
Next generation sequencing, reverse transcriptase PCR and real-time reverse transcriptase quantitative PCR were used to quantify anthocyanin gene expression.
Key Results
Non-homologous mutations causing loss of expression of single genes were found, indicating that the cause was likely to be mutations in transcription factor binding sites upstream of the 5′-untranslated region of the genes, and this was confirmed by sequencing.
Conclusions
Independent evolution and subsequent loss of expression of anthocyanin genes may have influenced diversification in the E. plukenetii species complex. The approach developed here should find more general application in studies on the role of floral colour shifts in diversification.
Keywords: Anthocyanin biosynthesis, gene expression, Erica, evolution, floral colour, RT-qPCR
Introduction
Flower colour is an important factor in pollinator specificity in angiosperms, and shifts in colour are important in pollinator-mediated speciation (Rausher, 2008; Carlson and Holsinger, 2013). The anthocyanin biosynthesis pathway (Grotewold, 2006) forms the anthocyanin pigments that colour plant tissues in general and are a major determinant of floral colour. The pathway consists of at least six enzymes, in order: chalcone synthase (CHS), chalcone isomerase (CHI), flavanone hydroxylase (F3H), dihydroflavanol-4-reductase (DFR), anthocyanin synthase (ANS) and UDP 3-O-glucosyltransferase (UDP-GST) to produce pelargonidin. The addition of flavanoid 3′ hydroxylase (F3′H) or flavanoid 3′ 5′ hydroxylase (F3′5 ′H) after F3H produces cyanidin or delphinidin, respectively. Transcription of the enzymes’ genes is collectively regulated by a complex of three transcription factors, Ip-WDR, Ip-MYB and Ip-bHLH in Ipomoea purpurea (Zhu et al., 2015), that bind to the MYB recognition element (MRE) and the bHLH recognition element (BRE) site upstream of the 5′ untranslated region (UTR) of each of the genes. Shifts in floral colour occur as a result of functional mutations in the transcription factor genes, loss of function mutations in the genes of the anthocyanin pathway themselves or mutations to the upstream transcription factor binding sites (Wessinger and Rausher, 2012).
To investigate the general process of shifts in flower colour associated with changes in anthocyanin biosynthesis, it is important to examine multiple such shifts in a biologically comparable system. To that end, we have been investigating a group of flowering plants in which shifts in flower colour have been frequent during the origins of a vast species diversity: the genus Erica (Ericaceae). Erica is the most species-rich genus in the Cape Floristic Region (CFR) with ~690 species (Oliver 1989, 2000; Linder, 2003), and hence a model group for investigating the factors driving evolutionary diversification.
Phylogenetic hypotheses for Erica based on nuclear and plastid DNA sequence markers and up to 60 % of the known species diversity (Pirie et al., 2011, 2016; Mugrabi De Kuppler et al., 2015) have been used to infer the age and diversification rate of the clade, as well as patterns of character evolution. The results showed that speciation in the most species-rich Cape clade was rapid (Pirie et al., 2016), and that multiple shifts in floral morphology had occurred (Pirie et al., 2011; Le Maitre, 2017). These shifts led to changes between wind-, insect- and bird pollination syndromes and hence restriction of gene flow between populations that may have played a role in driving speciation (Pirie et al., 2011). Along with the shifts in overall pollination syndrome, frequent changes in floral colour between the plesiomorphic pink [predominant in the older European lineages (Mugrabi De Kuppler et al., 2015)] and derived colours ranging from white (observed in species across the genus) through green, yellow and red (restricted to those of the diverse Cape clade) were inferred (Le Maitre, 2017).
Anthocyanins are the primary determinants of floral colour in Erica species (Crowden and Jarman, 1976). Only cyanidin is present in Erica plukenetii, giving it its red/pink coloration. The sequences of the anthocyanin genes and their trans-acting regulatory factors in Erica have not been investigated before. We chose to focus initially on one species, E. plukenetii, because it shows floral colour polymorphism both within and between populations, it has subspecies that are constitutively red or white, and it is hypothesized that the shifts in floral colour may influence pollinator shifts between bird- and insect pollination (van der Niet et al., 2014).
To study the molecular changes underlying these shifts in floral colour, first the anthocyanin genes in E. plukenetii had to be characterized. To this end, a next generation sequencing (NGS) approach was used to sequence the genome, in which the anthocyanin genes could be found. Primers could be designed on the exons of the genes for real time reverse transcriptase quantitative PCR (RT-qPCR) determinations of the expression of the genes of the anthocyanin pathway in differently coloured flowers. If expression of the genes was identical in red- and white-flowered plants, it would point to a loss of function mutation in a gene of the pathway. If expression of all the genes of the pathway was knocked down in the white-flowered plants, it would point to a transcription factor mutation. If expression of a single gene in the white-flowered plants was knocked down, it would point to a mutation in the transcription factor binding sites regulating that specific gene. The establishment of which mechanism underlies the floral colour shift in the E. plukenetii complex may give insights into the mechanism underlying the other independent floral colour shifts in the genus.
MATERIAL AND METHODS
DNA was extracted from a red-flowered E. plukenetii, collected from Table Mountain, Cape Town, South Africa, by Plant Research International at Wageningen University, the Netherlands, using their in-house CTAB extraction protocol. The Illumina library was prepared and one Illumina HiSeq2500 paired-end run with 250-bp reads and two Illumina MiSeq paired-end runs with 2×300-bp reads were performed and the data were assembled using CLC Bio. A local BLAST database was created in Geneious R9 (Kearse et al., 2012) from the contiguous sequences. Exon sequences are highly conserved and are therefore ideal for finding homologous sequences via local tBLASTx searches. The presence of non-conserved intron sequences in complete gene sequences, or the concatenation of exon sequences in mRNA transcripts of genes, lowers the overall sequence homology and makes finding genes much less likely, especially when genes potentially span more than one contiguous sequence. Therefore, it was necessary to use sequences of the anthocyanin pathway genes and their transcription factors in which exons were annotated and thus could be used individually in tBLASTx searches of the local BLAST database. For each gene, the fully annotated sequence from the most closely related species that could be identified on GenBank was used. In some cases these were represented by a species in the same family: Ericaceae (Rhododendron simsii); in others we were obliged to compare across the wider order Ericales (Camellia sinensis, Camellia nitidissima, Diospyros kaki, Vaccinium corymbosum and Actinidia chinensi); or, in the case of ANS, to a distantly related angiosperm clade, Rosids [Vitis vinifera; APG IV (Chase et al., 2016)]. Transcription factor binding sites were found using the Motif Finder in Geneious R9 and a Perl script (Zhu et al., 2015). Primer pairs were designed to amplify overlapping regions from the upstream regulatory regions of each gene to the end of the last exon based on the aligned exons from the tBLASTx searches. Where contiguous sequences had gaps, or a gene spanned multiple contiguous sequences, a chromosome walking approach was used to complete the sequences. DNA was directly amplified (Bellstedt et al., 2010) from the leaves of the same sample of red-flowered E. plukenetii subsp. plukenetii that was used for the Illumina sequencing. PCR amplifications were carried out using the Kapa Biosystems 3G Plant PCR kit. Each 25-µL PCR mix contained 12.5 µL buffer, 2 µL 25 mm MgCl2, 0.75 µL 20 mm forward and 0.75 µL 20 mm reverse primers (Table 1), 0.2 µL 2.5 U μL–1 3G PlantTaq, 0.2 µL 5 % DMSO, 1 µL extracted DNA and 8.6 µL milliQ water in an Applied Biosystems Veriti PCR thermal cycler. The PCR programme was 2 min at 95 °C; followed by 35 cycles of 95 °C for 30 s, annealing at the temperature specified in Table 1 for 30 s, and extension at 72 °C for 30 s; followed by a final extension step of 72 °C for 5 min. Sanger sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fischer Scientific) in an Applied Biosystems PCR thermal cycler using the STeP sequencing protocol (Platt et al., 2007). Each 10-µL sequencing reaction contained 5 µL sequencing buffer, 1 µL 0.8 mm forward primer or reverse primer (Table 1), 1 µL Big Dye, 1 µL of PCR product and 2.5 µL milliQ water. Sequencing electrophoresis was performed at the Central Analytical Facility at Stellenbosch University. Primers for RT-qPCR (Table 2) were designed on the sequences of the exons of the anthocyanin pathway genes. Paratubulin 1 (Ep-PBT1) was identified as a candidate reference gene, as it has been shown to be expressed stably in C. sinensis (Hao et al., 2014) and its expression was tested for consistency in floral tissue using the same methods as in Hao et al. (2014). Total RNA was extracted from 50 mg of floral tissue using the Qiagen RNeasy Power Plant Kit with the Qiagen RNase-Free DNase Set from corollas of eight different lengths (Supplementary Data Fig. S1) of the collected red-, pink- and white-flowered E. plukenetii subsp. plukenetii samples from a population on Du Toits Kloof Pass (DP), red- and white-flowered E. plukenetii subsp. plukenetii from Franschhoek Pass (FP) (Supplementary Data Fig. S2), as well as from corollas of three different lengths of white-flowered E. plukenetii subsp. breviflora in the Witzenberg Valley (WV), South Africa. Only three corolla lengths of E. plukenetii subsp. breviflora were collected as only three growth points were easy to differentiate, due to its reduced corolla length. RNA quality was verified using gel electrophoresis. RNA was quantified using a Nanodrop ND-1000 spectrophotometer, and the absorbance ratios at 260/280 nm were measured to check RNA quality.
Table 1.
The primer sequences used to re-sequence the contiguous sequences from the NGS data and also to amplify the region where transcription factors bind upstream of the 5′ UTR of the relevant gene of the anthocyanin biosynthesis pathway.
| Name | Sequence | Annealing temperature (°C) |
|---|---|---|
| ANS-352F | GTCATTGACTTCCTCTTGCGC | 60 |
| ANS-1,126R | TTGGGAATGGACTGGATGCC | |
| ANS-1,023F | ATTTCGGGTACTGCCTGCAA | 66 |
| ANS-2,269R | GAGTTCGTCCTTGCCACCA | |
| ANS-2,175F | GAGGCCTAACGACCAAGGTC | 60 |
| ANS-2,944R | AAGAGACAATCACAAGAGAAGTAGA | |
| CHI-25F | AACCGACACAGCATCCAGAG | 60 |
| CHI-878R | CGTGAAAGAAAGAACAAGAGGGT | |
| CHI-632F | ACGGGCAAGCAATACTCAGA | 60 |
| CHI-1,766R | CTTTGGGGAGGTCTTTGGCT | |
| CHI-1,601F | ATTCCCAGTGAGCAACCACC | 60 |
| CHI-2,522R | CAGGCTTTGAGTCCTCAGGG | |
| CHI-2,411F | ACCACGGCAGCTTTATTCCA | 60 |
| CHI-3,723R | GCCATGCTTCCCAACAATCG | |
| CHI-3,646F | CCTGAGAAGGGCAAAGTGGT | 60 |
| CHI-4,546R | CAAAGCTTCCACAGTATGCCA | |
| CHS-9F | ATACGTTCCTGGCTACCCCT | 60 |
| CHS-1,097R | ACTCCTTGATGGCCTTCACG | |
| CHS-992F | AATGTGTGCGCATACATGGC | 60 |
| CHS-2,020R | ACTGCAAACAACGGGCCTAA | |
| DFR-2,142F | GTGGGAGTAGAGTAGCCCCA | 62 |
| DFR-2,866R | TTGGTTGAGGGCACAAACCT | |
| DFR-2,720F | AGGATAACGTGAACGGCTCG | 66 |
| DFR-3,500R | CCGGACGGAGGGAGTAGTTA | |
| DFR-3,351F | AGCTTTGACGAGGCCATTGA | 66 |
| DFR-4,242R | GGTTCAAGTGCTGCCCTACT | |
| DFR-4,722F | ACGATCTCAAACTCAGGGCC | 56 |
| DFR-5,823R | TGCTCTTGGACATTGACGGT | |
| DFR-5,714F | AAGCCGACGATCAATGGTGT | 60 |
| DFR-6,920R | CCCCATCCCTTGCAACTTCT | |
| DFR-6,044F | GAGCATCCTGAAGCAGAGGG | 62 |
| DFR-6,996R | TTGTTTGAGACTGCTTTATATTTTCG | |
| F3′5′H-261F | CGGAGATGCTCACGTACTCC | 60 |
| F3′5′H-1,158R | AGCTCGTGCAAACTGACCAT | |
| F3′5′H-1,077F | GGTTGTGGAAAGTGAACGCA | 60 |
| F3′5′H-2,274R | ACCACCACATTGACCATCGA | |
| F3′H-1,335F | TCGGGCAAATATAACCTCGGA | 60 |
| F3′H-2,134R | AAGTCGTCCAAGGCCTTAGC | |
| F3′H-2,009F | CTCCGGGGCCAAGCATATT | 60 |
| F3′H-3,130R | ACAATAATGCTAGCCCCGGG | |
| F3′H-3,050F | TGGAAAACGAACCATGCGTTC | 60 |
| F3′H-4,027R | ACCGATGAGTTAAGTTGGCGA | |
| F3′H-3,926F | TGGTGAACAGACTTTTCAACTGG | 60 |
| F3′H-5,083R | GGTGCACAGGTTAAGGAGCT | |
| F3′H-5,000F | GAAGTAGCCATTCTGACGCG | 60 |
| F3′H-6,088R | CCCATTACGGTTGAGCACGA | |
| F3′H-6,070F | CGTGCTCAACCGTAATGGGA | 60 |
| F3′H-6,487R | AGGAGCGCATGAAACCGTAT | |
| F3′H-6,287F | CGATCTGAGACCCAACCCAA | 60 |
| F3′H-7,426R | GGAGAGAGAGTGGGGTGGAT | |
| F3′H-7,230F | CTCGTCCAGCACAGTGGAAT | 60 |
| F3′H-8,391R | AAGTGAACTTTTCATCCCCTTTTT | |
| F3H-528F | CGTTTAGACCTTCTCTCGAGCA | 60 |
| F3H-1,519R | ACAATCTTAAGTTTCCCATATTGACCT | |
| F3H-1,060F | CAGTCAAAGTTCGTCCGGGA | 60 |
| F3H-2,139R | GCGATGCGATCCGGTTTAAC | |
| F3H-2,023F | AAGCGGTCCCACAGTTTTGA | 56 |
| F3H-3,740R | ATGACCATGGTCGCCCAAAT | |
| F3H-2,810F | GAGCGAGAGTAAGTTGCCGT | 50 |
| F3H-3,740R | ATGACCATGGTCGCCCAAAT | |
| F3H-3,562F | AATGTCCACAACCCGACCTC | 60 |
| F3H-4,617R | ACGTACGGGATTGGTGCTTT | |
| F3H-4,448F | GCCCAGGACAGCCCAATTAT | 60 |
| F3H-5,794R | TGGTGGGTAGCAAATCTCGG | |
| UDP-GST-38F | TCGGGGTAGGTTTTCGTGTG | 60 |
| UDP-GST-839R | GTTGAACTTTGCGGCGACTT | |
| UDP-GST-720F | TTGAAGGCCACCATGATGCT | 60 |
| UDP-GST-1,884R | CGTTGTCATCTCACGCCTGA | |
| MYB-26F | GCGTCCACTTGTGTGTTTCC | 60 |
| MYB-1,395R | AACACGTACGGCTAACACAA | |
| bHLH-353F | GACCCATTGACGTGTTTGCC | 60 |
| bHLH-1,360R | ACAGACAACAGAGCTTCACA | |
| WDR-707F | CAGGTTGCCTTGTTTGCAGT | 60 |
| WDR-2,220R | TGTGATGAACAATGTGGGGC |
Table 2.
The primer and probe sequences used for RT-PCR and RT-qPCR that were used to determine expression of the genes of the anthocyanin biosynthesis pathway in Erica plukenetii.
| Name | Label | Quencher | Sequence | Expected product size (bp) |
|---|---|---|---|---|
| ANS-1,281F | 3′ Iowa Black® FQ | AGTCCTCTCCCTAGGCTTGG | 167 | |
| ANS-1,328Probe | 6-FAM | AAGTTGGTGGCAAGGACGAA | ||
| ANS-1,448R | ATGAAGGTGAGGGCGCTTAC | |||
| CHI-632F | 3′ Iowa Black® FQ | ACGGGCAAGCAATACTCAGA | 184 | |
| CHI-657Probe | Hex | TGGTGGAAAACTGTGTTGCC | ||
| CHI-816R | CTAACCGTTAGCGACCCCAG | |||
| CHS-153F | 3′ Iowa Black® RQ-Sp | CCGTCATGGCTATCGGGAC | 109 | |
| CHS-188Probe | Cy5 | TGCGTTGATCAGGCCACTTA | ||
| CHS-262R | CTCCTTCAACTCGGCCTTGT | |||
| DFR-112F | 3′ Iowa Black® RQ-Sp | AGGATAACGTGAACGGCTCG | 117 | |
| DFR-175Probe | Cy5 | GCTCCTGGCTCATCATGAGG | ||
| DFR-229R | ACGGTGGCTCGAACAACATA | |||
| F3′5′H-261F | 3′ Iowa Black® FQ | CGGAGATGCTCACGTACTCC | 139 | |
| F3′5′H-360Probe | Hex | ACATGGTGGTGGAGCTCATG | ||
| F3′5′H-400R | GTTGAATAAACCGGCCGACG | |||
| F3′H-406F | 3′ Iowa Black® RQ-Sp | CTCCGGGGCCAAGCATATT | 125 | |
| F3′H-471Probe | Cy5 | GGCGGATGCTCAGGAAGATA | ||
| F3′H-531R | AAGTCGTCCAAGGCCTTAGC | |||
| F3H-259F | 3′ Iowa Black® FQ | GATATCGCTAGCCGGGATCG | 127 | |
| F3H-338Probe | 6-FAM | TGGGGGATATTCCAGGTGGT | ||
| F3H-386R | TAATCAGACCGGCATCCACG | |||
| UDP-GST-480F | 3′ Iowa Black® RQ-Sp | AAGTCGCCGCAAAGTTCAAC | 135 | |
| UDP-GST-512Probe | Cy5 | GTTTTCCACGGCATCAGCTT | ||
| UDP-GST-615R | GGCACCAAAAAGGGTTCGTC | |||
| PTB1-1,416F | TTCATCAGAACCGGCTCAGG | 148 | ||
| PTB1-1,500Probe | Texas Red | 3′ Iowa Black® RQ-Sp | CGCTGATGTCGCTGGAAATG | |
| PTB1-1,564R | TGCTGACAAGACGTGCATCA | |||
| MYB-998F | ATAACCCAAAGCCCACGAGG | 136 | ||
| MYB-1,134R | CACCCGATCAACCTCAGCTT | |||
| bHLH-645F | AGTTGCGGAGGGATAGGCTA | 151 | ||
| bHLH-796R | GTCTGTTCTGGGAGGCCTTC | |||
| WDR-1,605F | CAGGACCCCAGGTATACGGA | 151 | ||
| WDR-1,756R | CCTCACTCGCACTGTGGAAT |
In the samples collected from the DP population, expression was tested using reverse transcriptase PCR (RT-PCR) and agarose gel electrophoresis to test the feasibility of the approach. The population was later burnt in a fire, precluding the collection of further samples for RT-qPCR analysis. The Kapa Plant 3G Plant PCR kit was used. Each RT-PCR mix contained 10 µL PCR buffer; 0.4 µL 10 mm forward and 0.4 µL 10 mm reverse primer (see Table 2); 0.4 µL 50× reverse transcriptase; 3 µL extracted RNA 5.8 µL milliQ water to make up a total of 20 µL. RT-PCR was performed in a Applied Biosystems Veriti PCR machine, with a single pre-incubation step of 42 °C for 5 min then 95 °C for 3 min, followed by 45 cycles of 95 °C for 3 s, 60 °C for 20 s and 72 °C for 2 s. PCR products were separated and visualized on a 1.5 % agarose gel.
In the samples collected from the FP population, expression of the genes of the anthocyanin biosynthesis pathway enzymes was determined using RT-qPCR with three biological replicates per corolla growth point, using the Kapa Probe Fast One Step RT-qPCR kit. Each multiplex RT-qPCR mix contained 10 µL buffer; two or three sets of: 0.4 µL 10 mm forward and 0.4 µL 10 mm reverse primer (see Table 1), 0.4 µL 10 mm probe (see Table 2); 0.4 µL 10 mm PBT1-1,416F, 0.4 µL 10 mm PBT1-1,564R and 0.4 µL 10 mm PBT1-1500Probe; 0.2 µL 2.5 U μl–1 3G Plant Taq; 1 µL extracted RNA and sufficient milliQ water to make up a total of 20 µL. Up to four reactions were multiplexed together in a single reaction. RT-qPCR was performed in a Roche LightCycler 96, with a single pre-incubation step of 42 °C for 5 min and then 95 °C for 3 min, followed by 45 cycles of 95 °C for 3 s, 60 °C for 20 s and 72 °C for 2 s.
In the E. plukenetii subsp. breviflora WV population, only some of the probes bound to their respective templates successfully, so quantifications were performed using the Kapa SYBR Fast Universal Kit. Each RT-qPCR mix contained 10 µL PCR buffer; 0.4 µL 10 mm forward and 0.4 µL 10 mm reverse primer (see Table 2); 0.4 µL 50× reverse transcriptase; 3 µL extracted RNA and 5.8 µL milliQ water to make up a total of 20 µL. RT-qPCR was performed in a Roche LightCycler 96, with two pre-incubation steps of 61 °C for 5 min and then 95 °C for 30 s, followed by 50 cycles of 95 °C for 3 s, 58 °C for 10 s and 72 °C for 2 s. A melting step followed at 95 °C for 10 s, 65 °C for 1 min and 95 °C for 1 s, verifying the presence of a single amplicon per reaction.
No-template and no-reverse transcriptase controls were included with each batch of samples analysed in all assays. Expression of the trans-acting transcription factor genes was determined using conventional RT-PCR and expression levels were assessed using gel electrophoresis. For the RT-qPCRs, expression of the genes of the anthocyanin biosynthesis pathway enzymes was measured relative to background fluorescence and was normalized to the expression of the reference gene, Ep-PBT1, using the Roche LightCycler 96 software. The RT-qPCR assays were optimized to ensure that all the genes had similar amplification efficiency, slope and Cq values. Melting curve analysis was used to check for the presence of a single amplicon with the SYBR Green RT-qPCRs.
RESULTS
The assembled Illumina NGS data of the red-flowered E. plukenetii contained 602 million reads, totalling more than eight billion bases. These reads were assembled using CLC Bio into 400 000 contiguous sequences. Actual coverage of the genome was around 20×. Of the assembled 4443 321 contiguous sequences, only 3.75 % were larger than 1000 bp, with an N50 of 1673.
All eight genes of the anthocyanin biosynthesis pathway enzymes and their trans-acting transcription factor genes (Grotewold, 2006; Zhu et al., 2015) were found in the NGS data using the tBLASTx approach. Exons of a particular gene (from GenBank) were aligned to the contiguous sequence(s), and the annotations were transferred to the contiguous sequence(s). If the aligned exons spanned multiple contiguous sequences, the contiguous sequences were concatenated. Start codons were identified and the stop codon at the end of the final exon was assumed to be the end of the gene. The cis BRE and MRE motifs upstream of the transcription start site were found in all of the contiguous sequences, except for that of Ep-CHI, where the region upstream of the start of the first exon of the gene was not present in the NGS data. Sequence identity in the aligned exonic regions approached 90 %. Chromosome walking using Sanger sequencing was successfully used to obtain any missing sequence data in or between contiguous sequences (Le Maitre, 2017). The sequences of the anthocyanin biosynthesis pathway genes and their trans-acting transcription factor genes from E. plukenetii subsp. plukenetii were deposited on GenBank, with accession numbers: MG948576 (Ep-ANS), MG948577 (Ep-bHLH), MG948578 (Ep-CHI), MG948579 (Ep-CHS), MG948580 (Ep-DFR), MG948582 (Ep-F3′H), MG948583 (Ep-F3H), MG948584 (Ep-R2R3-MYB), MG948585 (Ep-UDP-GST), MG948586 (Ep-WD40) and MG948587 (Ep-PTB1).
RNA was successfully isolated from the samples collected from the DP, FP and WV populations. RNA quality was verified using gel electrophoresis. Contamination with genomic DNA was minimal. Ep-PBT1 was found to be stably expressed in floral tissues and was therefore used to normalize expression of the genes. The three trans-acting genes that regulate expression of the anthocyanin genes Ep-R2R3 MYB, Ep-bHLH and Ep-WDR were expressed at comparable levels in all samples from all three populations.
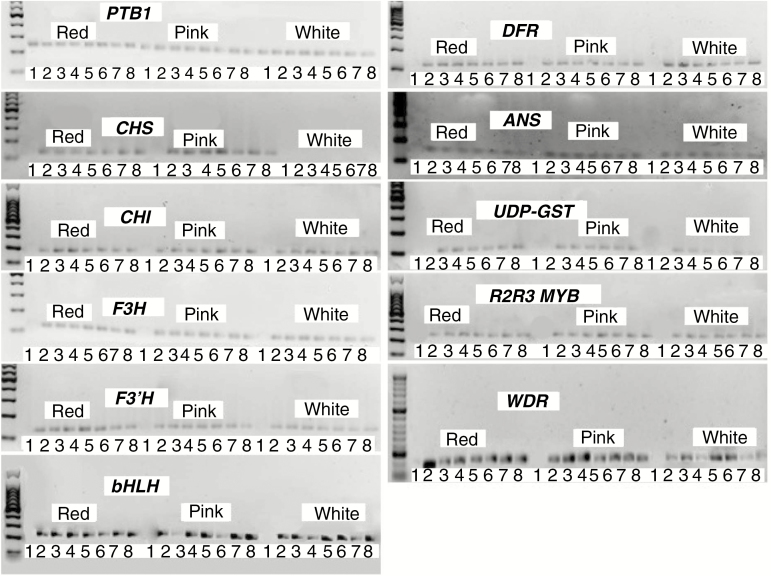
The anthocyanin genes were expressed in a coordinated manner. Expression levels increased with increasing corolla growth point and then declined as the flowers became mature. Red- and pink-flowered E. plukenetii subsp. plukenetii from the DP (Fig. 1) and FP (Fig. 2) populations expressed all the anthocyanin genes. In the white-flowered E. plukenetii subsp. plukenetii from DP, chalcone synthase (Ep-CHS) (Fig. 1) was not expressed and in the white-flowered E. plukenetii subsp. plukenetii from FP (Fig. 3), anthocyanin synthase (Ep-ANS) was not expressed above the background fluorescence. In E. plukenetii subsp. breviflora from WV, UDP-3-0-glucosyltransferase (Ep-UDP-GST) was not expressed above the background fluorescence and dihydroflavanol-4-reductase (Ep-DFR) was very low relative to both Ep-PTB1 and the other genes (Fig. 4), but it was expressed above the background fluorescence at growth point 2. Variation in individual gene expression levels in E. plukenetii subsp. breviflora was greater than in E. plukenetii ssp. plukenetii.
Fig. 1.
Chalcone synthase (Ep-CHS), chalcone isomerase (Ep-CHI), flavanone hydroxylase (Ep-F3H), flavanoid 3′ hydroxylase (Ep-F3′H), dihydroflavanol-4-reductase (Ep-DFR), anthocyanin synthase (Ep-ANS) and UDP 3-O-glucosyltransferase (Ep-UDP-GST) are the transcripts of the genes of the anthocyanin biosynthesis pathway enzymes required to produce red colour. Transcription of the enzymes’ genes is collectively regulated by a complex of three transcription factors, Ep-WDR, Ep-MYB and Ep-bHLH. Expression of the genes of the anthocyanin biosynthesis pathway enzymes in eight growth points (1–8) of red-, pink- and white-flowered Erica plukenetii subsp. plukenetii from the Du Toits Kloof Pass population as determined with reverse transcriptase PCR. Ep-CHS is not expressed in the white-flowered individual. Expression of the other genes of the pathway and the transcription factors is otherwise essentially identical between red-, pink- and white-flowered individuals. Samples were loaded on gels in the order of corolla length. A 1000-bp ladder was used.
Fig. 2.
Expression of the genes of the anthocyanin biosynthesis pathway enzymes in red-flowered Erica plukenetii subsp. plukenetii from the Franschhoek Pass population as determined by real time reverse transcriptase quantitative PCR. Expression has been normalized to the expression of Paratubulin 1. Three biological replicates of each corolla growth point were tested.
Fig. 3.
Expression of the genes of the anthocyanin biosynthesis pathway enzymes in white-flowered Erica plukenetii subsp. plukenetii from the Franschhoek Pass population as determined by real time reverse transcriptase quantitative PCR. Expression has been normaliszed to the expression of Paratubulin 1. Three biological replicates of each corolla growth point were tested. Expression of Ep-ANS is greatly reduced relative to the red-flowered E. plukenetii subsp. plukenetii from the Franschhoek Pass population in Fig. 2.
Fig. 4.
Expression of the genes of the anthocyanin biosynthesis pathway enzymes in white-flowered Erica plukenetii subsp. breviflora from the Witzenberg Valley population as determined by real time reverse transcriptase quantitative PCR. Expression has been normalized to the expression of Paratubulin 1. Three biological replicates of each corolla growth point were tested. Expression of Ep-UDP-GST and Ep-DFR is greatly reduced relative to the red-flowered E. plukenetii subsp. plukenetii from the Franschhoek Pass population in Fig. 2.
The 5′ upstream regions of the Ep-CHS gene from white-flowered E. plukenetii subsp. plukenetii (DP), the Ep-ANS gene from white-flowered E. plukenetii subsp. plukenetii (FP) and the Ep-DFR and Ep-UDP-GST genes from white-flowered E. plukenetii subsp. breviflora (WV) were sequenced to find any mutations that could account for the lack of expression observed. A deletion was found in the BRE motif of Ep-CHS in E. plukenetii subsp. plukenetii (DP), a CC to AA mutation was found in the MRE motif of Ep-ANS in E. plukenetii subsp. plukenetii (FP) and a C to A mutation was found in the MRE motif of Ep-UDP-GST of E. plukenetii subsp. breviflora (WV) (Fig. 5). No mutations were found in the BRE or MRE motifs of the Ep-DFR gene of E. plukenetii subsp. breviflora (WV).
Fig. 5.
Mutations in the binding sites for the trans-acting regulatory elements that may account for the observed differences in gene expression between the red- and white-flowered individuals. (A) The single base deletion in the bHLH recognition element of the Ep-CHS gene of the white-flowered E. plukenetii subsp. plukenetii from the Du Toits Kloof Pass population. (B) The double mutation (CC to AA) in the MYB recognition element (MRE) of the Ep-ANS gene of the white-flowered E. plukenetii subsp. plukenetii from the Franschhoek Pass population. (C) The single point mutation (C to A) in the MRE of the Ep-UDP-GST gene of white-flowered E. plukenetii subsp. breviflora from the Witzenberg Valley population.
Discussion
The NGS approach was successful but contiguous sequence lengths were typically too short to contain entire genes and exons often had to be located on separate contiguous sequences and concatenated. Using flow cytometry, the Erica plukenetii genome has been shown to have a 2C value of 0.91, which equates to around 900 Mb (T. van der Niet, pers. comm.). This fits well with the known genome sizes from related genera of Ericaceae that are ~695 Mb [Rhododendron delavayi; Ericoideae (the same subfamily as Erica) (Zhang et al., 2017)] and ~420 Mb [Vaccinium macrocarpum; more distantly related Vaccinioideae (Polashock et al., 2014)]. There are no known polyploids of Erica species, with known counts from wild species restricted to 2n = 24 in most species (Nelson and Oliver, 2005). Our coverage was not sufficient for a complete genome assembly, and hence for a precise estimate of genome size in Erica, but this was to be expected given the number of reads we obtained and a genome size similar to, or larger than, that known from other Ericaceae.
All of the eight genes of the anthocyanin biosynthesis pathway enzymes and their three trans-acting transcription factor genes were found in the NGS data generated from E. plukenetii subsp. plukenetii. The tBLASTx approach to locating the genes of interest in the NGS data will have broader application to other studies where little to no genetic data are available on the genes of interest.
The exons of the eight anthocyanin genes and the transcription factor genes are highly similar to the exons (exon sequence homology approached 90 %) of those same genes in the most closely related species for which data were available, from the same family: Rhododendron simsii (Ericaceae); same order: Camellia sinensis, Camellia nitidissima, Diospyros kaki, Vaccinium corymbosum, Actinidia chinensis (Order: Ericales); and Vitis vinifera (Order: Rosids), indicating that their functions are both conserved and important.
Clustering of the expression levels of all the genes at each corolla length indicates that expression of genes coding for enzymes of the anthocyanin biosynthesis pathway is regulated by a common method of transcriptional regulation, with expression of all the genes being roughly equal at a given time. This is notably similar to the results of Zhu et al. (2015). The loss of pigmentation in the DP, FP and WV populations appears to be linked to mutations in the cis motifs that are involved in the regulation of expression. Alternative explanations, such as mutations in the transcription factors themselves (which would lead to global knockdown of anthocyanin gene expression) or a loss of function mutation in one of the genes of the pathway, were not supported. While mutations in the cis motifs to which the trans-acting transcription factors bind are not the most common cause of the loss of red pigmentation, they have been observed in 12 % of studies documented by Streisfeld and Rausher (2011), and site-directed mutagenesis of the BRE and MRE motifs in I. purpurea was found to significantly reduce expression of anthocyanin pathway genes (Zhu et al., 2015).
Existence of the BRE and MRE motifs upstream of the anthocyanin genes, the loss of gene expression associated with mutations in the motifs and the coordinated expression of the genes of the anthocyanin biosynthesis pathway would seem to indicate that the mechanism of control of anthocyanin biosynthesis in E. plukenetii is the same as in Ipomoea (Zhu et al., 2015), Mimulus (Yuan et al., 2014) and Arabidopsis (Koes et al., 2005; Grotewold, 2006).
Point mutations in the cis motifs of the Ep-CHS, Ep-ANS and Ep-UDP-GST genes could disrupt binding of the trans-acting Ep-MYB–Ep-bHLH–Ep-WDR complex. Disruption of the binding of the trans-acting factors to the motifs could lead to a reduction or total knockdown of the expression of the Ep-CHS, Ep-ANS and Ep-UDP-GST genes, as has been observed in I. purpurea (Zhu et al., 2015). Consequently, the Ep-CHS, Ep-ANS and Ep-UDP-GST enzymes would not be produced.
If Ep-CHS is not produced, chalcone is not synthesized (Wessinger and Rausher, 2012; Zhu et al., 2015). The loss of chalcone would have implications for plant fitness, as it is not only a precursor for the anthocyanin pathway but also forms part of the immune system (Dao et al., 2011; Rozmer and Perjési, 2016) and plays a role in reducing the effects of heat stress and UV damage (Coberly and Rausher, 2003). If Ep-ANS and Ep-UDP-GST are not produced, the direct consequences are less obvious, as the intermediates are committed to the pathway (Wessinger and Rausher, 2012). However, there would be implications for the plant beyond the loss of colour, as anthocyanins play multiple roles in plant tissues, including protection of photosynthetic molecules and chelation of photosynthetic by-products (Gould et al., 1995; Smillie and Hetherington, 1999; Landi et al., 2015), and protection of other photolabile compounds in leaves (Gould et al., 2010).
The mutations associated with the observed colour shifts appear to have independent origins as they differ between both the white-flowered populations of E. plukenetii subsp. plukenetii and also from the population of E. plukenetii subsp. breviflora.
Neither the heritability nor any fitness effects of these mutations have been tested, nor has the incidence of these mutations within the sampled populations been quantified. However, they appear to be tolerable across generations, as white-flowered plants of various ages occur in the populations. Hence the same mutations may occur in other closely related white-flowered E. plukenetii populations, whilst similar mutations could be independently derived in more distantly related ones.
From a phylogeny of E. plukenetii (van der Niet et al., 2014), it appears that the plesiomorphic form, similar to that of E. plukenetii subsp. plukenetii, has pink or red, medium-length, unscented corollas; its also has an upright growth form with sturdy branches suitable for perching on by birds. Erica plukenetii subsp. breviflora, by contrast, has white, short, scented corollas attractive to its moth pollinators, with flowers presented on long thin branches that discourage bird perching (van der Niet et al., 2014). Although the latter differences in habit represent part of the overall pollination syndrome, they may primarily reflect adaptations to the differing habitats of the subspecies: mountains in E. plukenetii subsp. plukenetii and sandy plains in E. plukenetii subsp. breviflora. The major pollinator of the E. plukenetii species complex, the orange breasted sunbird (Anthobaphes violacea), is present in both habitats. In this scenario, the shift to moth pollination would not be related to the distribution of the pollinator, but instead a consequence of adaptation to the differing environment, followed by reinforcement through further pollinator-specific adaptations, including reduction in flower size and production of scent (van der Niet et al., 2014). It is not clear at what stage in this process white flower colour would have become fixed.
The loss of red flower colour is therefore just one of a suite of adaptations contributing to the shift from bird to insect pollination in E. plukenetii, and potentially it was neither the first nor the most critical factor involved in the process. However, the importance of flower colour changes in pollinator-mediated diversifications has been shown in a range of plant groups (Bradshaw and Schemske, 2003; Hoballah et al., 2007; Wessinger and Rausher, 2012). Amongst the wide variation in flower colour in Cape Erica, many taxa are either white flowered, or show white-flowered forms, including within species complexes such as in the E. abietina/E. viscaria clade [e.g. white-flowered E. viscaria subsp. pendula; white forms of E. regia subsp. regia and of E. vestita (Pirie et al., 2017)]. In general, the kind of complex scenario apparent for E. plukenetii, involving an interplay of adaptations to differing ecological and pollination niches, may drive elevated rates of speciation in Erica and other species-rich genera in the CFR (Linder, 2003; Pirie et al., 2011, 2016). Importantly, the simple general mechanism underlying the change from pink to white flowers may explain both the regular occurrence of white flower colour in Erica species and its contribution to the prevalence of pollinator shifts in the radiation of the Erica clade.
CONCLUSION
This is the first study to identify the genes and their putative trans-regulatory factors, of the anthocyanin biosynthesis pathway in a species of Erica, and to propose a mechanism for floral colour changes observed in Erica. This study represents an important step towards understanding specific mechanisms that may have contributed to the rapid speciation inferred in Erica, particularly in the Cape clade. The techniques and approaches developed will allow testing of the mechanisms of floral colour shifts in other Erica species complexes or species pairs in which floral colour shifts from the plesiomorphic pink- to red- or white-flowered species have occurred and identify the commonalities between them.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Photographs of the different corolla growth points of Erica plukenetii subsp. plukenetii at which anthocyanin synthesis enzyme gene expression levels were tested. Fig. S2: (A) Red- and white-flowered Erica plukenetii subsp. plukenetii flowering in the Franschhoek Pass population. (B) Erica plukenetii subsp. breviflora flowering in the Witzenberg Valley population.
FUNDING
This work was supported by the South African National Research Foundation [grant number 98867]. M.P. is supported by the Heisenberg programme of the Deutsche Forschungsgemeinschaft [PI 1169/3-1].
ACKNOWLEDGEMENTS
The authors would like to thank South African National Parks and Cape Nature for allowing sample collection and the University of Stellenbosch, Department of Biochemistry, for the facilities. N.L.M. collected samples, performed all the molecular analyses except the NGS, did all the data analysis and wrote the first draft of the paper. D.B. collected samples, conceptualized the project and edited the drafts. M.P. provided substantial editorial input and support.
LITERATURE CITED
- Bellstedt DU, Pirie MD, Visser JC, de Villiers MJ, Gehrke B. 2010. A rapid and inexpensive method for the direct PCR amplification of DNA from plants. American Journal of Botany 97: e65–e68. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2013. Direct and indirect selection on floral pigmentation by pollinators and seed predators in a color polymorphic South African shrub. Oecologia 171: 905–919. [DOI] [PubMed] [Google Scholar]
- Chase MW, Christenhusz MJM, Fay MF, et al. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Coberly LC, Rausher MD. 2003. Analysis of a chalcone synthase mutant in Ipomoea purpurea reveals a novel function for flavonoids: amelioration of heat stress. Molecular Ecology 12: 1113–1124. [DOI] [PubMed] [Google Scholar]
- Crowden RK, Jarman SJ. 1976. Anthocyanins in the genus Erica. Phytochemistry 15: 1796–1797. [Google Scholar]
- Dao TTH, Linthorst HJM, Verpoorte R. 2011. Chalcone synthase and its functions in plant resistance. Phytochemistry reviews : Proceedings of the Phytochemical Society of Europe 10: 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, Kuhn DN, Lee DW, Oberbauer SF. 1995. Why leaves are sometimes red. Nature 378: 241–242. [Google Scholar]
- Gould KS, Dudle DA, Neufeld HS. 2010. Why some stems are red: cauline anthocyanins shield photosystem II against high light stress. Journal of Experimental Botany 61: 2707–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology 57: 761–780. [DOI] [PubMed] [Google Scholar]
- Hao X, Horvath D, Chao W, Yang Y, Wang X, Xiao B. 2014. Identification and evaluation of reliable reference genes for Quantitative Real-Time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). International Journal of Molecular Sciences 15: 22155–22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoballah ME, Gübitz T, Stuurman J, et al. 2007. Single gene-mediated shift in pollinator attraction in Petunia. The Plant Cell Online 19: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, England) 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. 2005. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends in Plant Science 10: 236–242. [DOI] [PubMed] [Google Scholar]
- Landi M, Tattini M, Gould KS. 2015. Multiple functional roles of anthocyanins in plant–environment interactions. Environmental and Experimental Botany 119: 4–17. [Google Scholar]
- Linder HP. 2003. The radiation of the Cape flora, southern Africa. Biological Review 78: 597–638. [DOI] [PubMed] [Google Scholar]
- Le Maitre NC. 2017. Phylogenetics of the genus Erica and anthocyanin synthesis gene expression in Erica plukenetii. PhD Thesis, Stellenbosch University; http://scholar.sun.ac.za/handle/10019.1/101060 [Google Scholar]
- Mugrabi De Kuppler AL, Fagúndez J, Bellstedt DU, Oliver EGH, Léon J, Pirie MD. 2015. Testing reticulate versus coalescent origins of Erica lusitanica using a species phylogeny of the northern heathers (Ericeae, Ericaceae). Molecular Phylogenetics and Evolution 88: 121–131. [DOI] [PubMed] [Google Scholar]
- Nelson E, Oliver EGH. 2005. Chromosome numbers in Erica - an updated checklist In: Nelson EC, ed. Heathers 2. Southampton: The Heather Society, 57–58. [Google Scholar]
- van der Niet T, Pirie MD, Shuttleworth A, Johnson SD, Midgley JJ. 2014. Do pollinator distributions underlie the evolution of pollination ecotypes in the Cape shrub Erica plukenetii? Annals of Botany 113: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver EGH. 1989. The Ericoideae and the southern African heathers. Botanical Journal of the Linnean Society 101: 319–327. [Google Scholar]
- Oliver EGH. 2000. Systematics of Ericeae (Ericaceae: Ericoideae): species with indehiscent and partially dehiscent fruits. Contributions from the Bolus Herbarium 19: 1–483. [Google Scholar]
- Pirie MD, Oliver EGH, Bellstedt DU. 2011. A densely sampled ITS phylogeny of the Cape flagship genus Erica L. suggests numerous shifts in floral macro-morphology. Molecular Phylogenetics and Evolution 61: 593–601. [DOI] [PubMed] [Google Scholar]
- Pirie MD, Oliver EGH, Mugrabi de Kuppler A, et al. 2016. The biodiversity hotspot as evolutionary hot-bed: spectacular radiation of Erica in the Cape Floristic Region. BMC Evolutionary Biology 16: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie MD, Oliver EGH, Gehrke B, et al. 2017. Underestimated regional species diversity in the Cape Floristic Region revealed by phylogenetic analysis of the Erica abietina/E. viscaria clade (Ericaceae). Botanical Journal of the Linnean Society 184: 185–203. [Google Scholar]
- Platt AR, Woodhall RW, George AL. 2007. Improved DNA sequencing quality and efficiency using an optimized fast cycle sequencing protocol. BioTechniques 43: 58–62. [DOI] [PubMed] [Google Scholar]
- Polashock J, Zelzion E, Fajardo D, et al. 2014. The American cranberry: first insights into the whole genome of a species adapted to bog habitat. BMC Plant Biology 14: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD. 2008. Evolutionary transitions in floral color. International Journal of Plant Sciences 169: 7–21. [Google Scholar]
- Rozmer Z, Perjési P. 2016. Naturally occurring chalcones and their biological activities. Phytochemistry Reviews 15: 87–120. [Google Scholar]
- Smillie RM, Hetherington SE. 1999. Photoabatement by anthocyanin shields photosynthetic systems from light stress. Photosynthetica 36: 451–463. [Google Scholar]
- Streisfeld MA, Rausher MD. 2011. Population genetics, pleiotropy, and the preferential fixation of mutations during adaptive evolution. Evolution 65: 629–642. [DOI] [PubMed] [Google Scholar]
- Wessinger CA, Rausher MD. 2012. Lessons from flower colour evolution on targets of selection. Journal of Experimental Botany 63: 5741–5749. [DOI] [PubMed] [Google Scholar]
- Yuan Y-W, Sagawa JM, Frost L, Vela JP, Bradshaw HD. 2014. Transcriptional control of floral anthocyanin pigmentation in monkeyflowers (Mimulus). New Phytologist 204: 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xu P, Cai Y, et al. 2017. The draft genome assembly of Rhododendron delavayi Franch. var. delavayi. GigaScience 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Wang H, Wang Y, et al. 2015. Characterization of the cis elements in the proximal promoter regions of the anthocyanin pathway genes reveals a common regulatory logic that governs pathway regulation. Journal of Experimental Botany 66: 3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.