Abstract
Background and Aims
Many plants exhibit a mixed mating system. Published models suggest that this might be an evolutionarily stable rather than a transitional state despite the presence of inbreeding depression, but there is little empirical evidence. Through field experimentation, we studied the role of inbreeding depression in eliminating inbred progeny from the reproductive cohort of the forest tree Eucalyptus regnans, and demonstrate a stable mixed primary mating system over two successive generations.
Methods
Two field experiments were conducted using seed from natural populations. We sowed open-pollinated seeds to simulate a natural regeneration event and determined isozyme genotypes of dominant and suppressed individuals over 10 years. We also planted a mixture of open-pollinated, outcross and selfed families with common maternal parentage; monitored survival of cross types over 29 years; and determined the percentage of outcrosses in open-pollinated seed from a sample of reproductively mature trees using microsatellite analysis.
Key Results
Both experiments demonstrated progressive competitive elimination of inbred plants. By 29 years, the reproductive cohort in the planted experiment consisted only of outcrosses which produced seed which averaged 66 % outcrosses, similar to the estimate for the parental natural population (74 %).
Conclusions
Selective elimination of inbred genotypes during the intense intra-specific competition characteristic of the pre-reproductive phase of the life cycle of E. regnans results in a fully outcrossed reproductive population, in which self-fertility is comparable with that of its parental generation. The mixed mating system may be viewed as an unavoidable consequence of the species’ reproductive ecology, which includes the demonstrated effects of inbreeding depression, rather than a strategy which is actively favoured by natural selection.
Keywords: Demography, Eucalyptus, forest trees, genetic structure, inbreeding, mixed mating, outcrossing rate
INTRODUCTION
The mating system is a major influence on the population genetic structure of plants. Selfing directly affects inbreeding coefficients of individuals within populations and reduces gene flow through pollen, in turn increasing genetic drift and thus population structure and differentiation (reviewed by Duminil et al., 2009). Plant mating systems vary from complete selfing to complete outcrossing, but mixed mating systems are relatively common (Goodwillie et al., 2005; Duminil et al., 2009; Winn et al., 2011). Many long-lived perennials, including trees of the genus Eucalyptus (Byrne, 2008), have a mixed mating system (meaning an intermediate outcrossing rate at the germination stage of the life cycle; this outcrossing rate is often called the primary rate). However, in such species, inbreeding depression (ID) may result in a large difference between the primary mating system and the effective or secondary mating system estimated from the population of reproductive individuals (Lande et al., 1994). Inbreeding levels therefore do not necessarily increase every generation (Duminil et al., 2009). ID appears to be most severe in specific life cycle stages (Husband and Schemske 1996), but few empirical studies of long-lived perennials cover the life cycle from seed production to reproductive maturity of the regenerating population (Koelewijn et al., 1999). Such data are important for evaluating the numerous models which attempt to define conditions under which mixed mating systems may or not be stable (Schemske and Lande, 1985; Charlesworth and Charlesworth, 1987; Morgan et al., 1997; Cheptou and Dieckmann, 2002; Cheptou and Schoen, 2003; Winn et al., 2011).
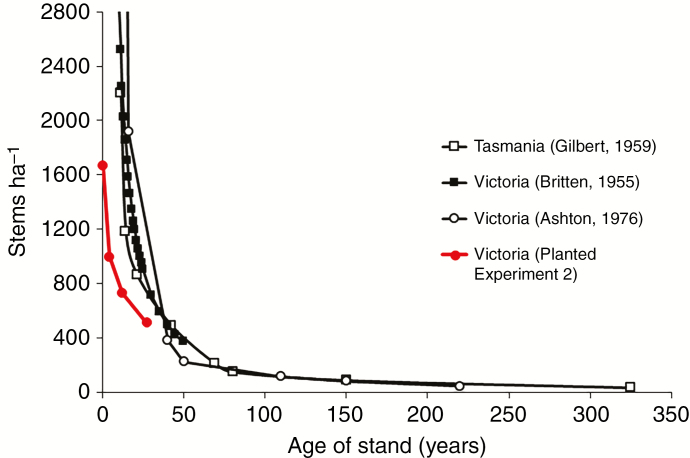
Our long-term research has focused on Eucalyptus regnans F.Muell, the tallest flowering plant species on Earth (Tng et al., 2012). This long-lived species is a dominant of many wet sclerophyll forests of Victoria and the island of Tasmania, south-eastern Australia (Turner et al., 2009; Nevill et al., 2010; Wood et al., 2010; Tng et al., 2012; Sillett et al., 2015). It is a mass flowering species, with small hermaphrodite flowers with white filaments attracting a diversity of insect pollinators (Griffin et al., 2009). Individual flowers are protandrous, but there is no temporal barrier to geitonogamous pollination (Griffin, 1980). The species has a mixed primary mating system (Moran et al., 1989) and, while trees are generally self-compatible, post-zygotic ID influences the proportion of selfed and outcrossed seed which successfully mature (Griffin et al., 1987). The crown of a mature tree may exceed 35 m in diameter and carry >1.5 million flowers, 50 % of which may set, with each capsule containing an average of 1.5 full seed (Griffin et al., 1987; unpubl. res.). The capsules are woody and are retained unopened on the tree for an average of 3 years (Cremer, 1965b), so a large tree may carry >3 million seeds at any one time. There is usually mass shedding of this canopy-stored seed after trees are damaged by wild fire (Cremer, 1965a). Since the seeds are small and not long lived once shed, there is no build-up of a seed bank within the soil and retention on the tree is critical for regeneration. Catastrophic disturbance by fire is required to produce the site conditions under which seed can germinate, often at extremely high density (Cunningham, 1960; Evans, 1976; Ashton, 1981). The timing of regeneration events following tree-killing fires is highly unpredictable, occurring on average at between 75 and 150 year intervals in the E. regnans forests of Victoria (McCarthy et al., 1999). However, the regular flowering and persistence of resulting capsule crops within the tree crown ensures a seed rain far exceeding the carrying capacity of the site whenever such an event does occur. Since E. regnans is an important forestry species, the reduction in population density with age in natural forests is well documented; its life cycle is characterized by very high density regeneration followed by severe, competition-driven, thinning with age (Fig. 1).
Fig. 1.
Competition-driven thinning curves for E. regnans for two studies of natural populations in Victoria and one in Tasmania, together with a plot of data from Experiment 2 reported herein showing convergence to the natural curve over time.
The experiments described herein are aimed at understanding the process of genetic structuring which occurs within an E. regnans population from germination onwards, as ID is expressed and inbred individuals fail to compete and die. Earlier publications (Griffin and Cotterill, 1988; Hardner and Potts, 1997) reported ID for growth and mortality to age 15 years in a planted experiment of selfed and outcrossed progeny. At that age, none of the trees was reproductively mature. Here we extend the observation period to 29 years, when seed production had started. We also determine the proportion of self and outcross progeny following open-pollinated (OP) mating within the experiment, to compare the primary mating system over two successive generations. We also studied the development of genetic structure over 10 years in a hand-sown field experiment which more closely mimics the regeneration scenario under natural conditions. These data provide a basis for discussion of implications for the maintenance of mixed mating systems.
MATERIALS AND METHODS
Parental populations and their genetic structure
Two field experiments were established, both based on seed from trees in two remnant natural populations of E. regnans on farmland in Gippsland, Victoria, Australia – Narracan (N) and Thorpdale (T). We designate these below as Generation (Gen) n. The Narracan population is described in Griffin (1980) and Fripp et al. (1987). The population at Thorpdale has a similar structure and history but is more extensive. A previous study (Moran et al., 1989) collected OP seed samples (Gen n + 1) from 41 Narracan trees; estimated outcrossing rates, tm, by multilocus isozyme genotyping using the method of Ritland and Jain (1981); and estimated the inbreeding coefficient (F) for the parental and progeny populations from observed (Ho) and expected (He) heterozygosities. For the Thorpdale stand, Gen n parameters were determined from isozyme genotyping of foliage samples from 113 trees, and samples of OP seed from the trees used in Experiment 2 were also assayed (J. C. Bell, unpubl. res.). Based on the combined Gen (n + 1) data, we collected OP seed from trees containing varying proportions of outcrosses and a high probability of outcross detection, for use in a hand sowing study (Experiment 1). Thirteen trees with accessible flowers (six from Narracan and seven from Thorpdale including three of the four for Experiment 1) were mated to derive the selfs and outcrosses planted in another field experiment together with their OP siblings (Experiment 2) (Griffin and Cotterill, 1988).
Experiment 1: hand sowing to mimic a natural regeneration event
A recently harvested natural E. regnans forest site near Jeeralang in the Strzelecki Ranges of Victoria (38°24ʹ S, 146°28ʹ E) was chosen for the experimental sowing in October 1979. All branch debris was removed by hand and the soil raked clear but not cultivated. Sixteen contiguous 9 × 9 ft square plots (7.52 m2) were marked out with spacing of at least 1 m between plots to allow access. Each plot was treated with methyl bromide 1 month before sowing to reduce pest and disease levels. The four OP seedlots (N5, T9, T11 and T13) were allocated to plots in a randomized block layout with four replicates. Seeds were cold stratified for 1 month before sowing to encourage rapid uniform germination, mixed with fine sand and hand sown at approx. 600 000 viable seed ha–1. Access paths were also sown to avoid edge effects. The site was fenced to exclude browsing animals. No further action was taken to control insects or fungal pathogens, but the plots were weeded throughout the first year to ensure that the major source of competition would be intra-specific. Each plot was pegged with a string grid at 1 ft (0.3 m) intervals so that the location of every seedling could be defined by row–column co-ordinates. After 26 months, each surviving plant was tagged with a number which was maintained for the remainder of the experiment.
Demographic information.
The number of surviving plants per plot was counted regularly (at 3, 5, 13, 26 36, 43, 54, 68 and 126 months after sowing). Heights of the largest (dominant) and three smallest (suppressed) trees in each row of each plot were measured at 43 months (3.6 years) and again for the dominants only at 54 (4.5 years) and 68 (5.7 years) months. Because of mortality, it was not possible to obtain an entire set of measurements from all rows. We followed the survival and relative dominance of individual trees between 3.6 and 10.5 years after sowing. At 3.6 years, plant density across the experiment averaged 131 000 trees ha–1 and the dominant cohort averaged 0.72 m in height. By 10.5 years, the plots had naturally thinned to 26 000 trees ha–1 and dominant height was 9.2 m. This growth rate is comparable with that reported for a series of natural regeneration studies of E. regnans elsewhere in Victoria (Van Der Meer and Dignan, 2007).
Determination of genetic structure with stand development.
We expected inbreeding depression for growth in the self progenies (Eldridge and Griffin, 1983) and postulated that, as size class structure developed with age, competition would result in the plots becoming genetically structured, with outcrosses dominating and proportionately more selfs in the suppressed stratum. To test this hypothesis, foliage samples were collected from nine dominant trees and 27 suppressed trees per plot after 3.6 years. Isozyme genotypes were determined for the same set of seven loci (MDH-2, GPI-2, SDH-1, LAP-2, GDH-2, AAT-2 and AAT-4) which were assayed to determine the maternal genotypes, permitting the proportion of detectable outcrosses in each progeny sample to be determined based on the presence of non-maternal alleles as well as the observed heterozygosity for each individual (Ho). A total of 135 dominants and 386 suppressed plants were assayed, and the proportion of detectable outcrosses among the survivors of each cohort was recalculated at 54, 68 and 126 months after sowing.
Experiment 2: planted comparison of selfed, outcrossed and open-pollinated progenies
Genetic structure of the experiment.
The origin of the seed and establishment of the field experiment on an ex-E. regnans site near Mount Worth Victoria (38°16ʹ S, 145°58ʹ E) are described in Griffin and Cotterill (1988). In brief, six trees from Narracan and seven from Thorpdale were mated in two disconnected within-population factorials to produce 21 controlled outcross families. Controlled self- and OP progeny were also obtained from all parents. Seed were germinated and plants grown for 7 months in the nursery. The experiment was laid out with 12 complete randomized blocks of all families irrespective of mating type, with each family represented in each replicate as a three-tree non-contiguous plot (Griffin and Cotterill, 1988). All families were well represented in the experiment, but in a few cases the full requirement of seedlings was unavailable and those planting spots were filled from another family. The entire experiment was surrounded by one row of unpedigreed E. regnans seedlings as a buffer. The spacing between trees was 2 × 3 m (1667 plants ha–1). In total, there were 425 selfed, 468 OP and 694 outcross trees planted. The outcrosses thus accounted for 0.62 of the combined selfed and outcrossed population, roughly approximating the ratio expected directly after seed germination for OP seed according to the outcrossing estimates for seed from the parental native stands (Hardner and Potts, 1997).
Assessment.
Measurements of height, diameter at breast height (DBH) and survival to age 4 years are reported in Griffin and Cotterill (1988) with additional information to age 15 years in Hardner and Potts (1997). At age 10 years, dominant height was 24.2 m, nearly three times greater than for Experiment 1 which was sown at high density into uncultivated ground. At age 27 years, diameter and survival were again recorded. At age 29, prior to commercial harvest of the trees, a final survival and diameter assessment was made and all surviving trees were scored for the presence or absence of capsules. The mean height of a sample of 12 dominant trees was 51.2 m at this time.
To examine the importance to reproductive success of attaining early dominance in the stand, the significance of the growth differences between reproductive and non-reproductive survivors was tested at age 4, 10, 15 and 29 years, using diameter and height data for just the open-pollinated and outcross populations. This was done by fitting a repeated measures linear model with the presence or absence of capsules at age 29, age and their interaction as fixed effects. Trees were the subject, and the model was parameterized to fit the full residual variance/covariance structure. Only the sub-set of trees surviving 29 years was used, and diameter and height were tested in separate analyses. The analyses were done using PROC GLIMMIX of SAS (version 9.4) with Type III sum of squares, assuming a Gaussian distribution of residuals. The significance of model terms was based on Walds F test. Least-squares means and their standard errors were estimated, and the difference between reproductive and non-reproductive trees tested at each age using the ‘slice’ option. Cross type was not included in the model as preliminary analyses indicated that it was not significant as a main or interactive effect.
Determination of mating system in the seed crop.
At age 29 years, 22 of the 26 OP and outcross families in the experiment contained at least one capsule-bearing tree. Seventeen dominant trees representing 13 different families were climbed at this stage, and an average of approx. 1800 capsules were collected from each tree. A total of 11 of the 13 original parental trees were represented in the pedigrees of the sampled set. Capsules were dried to extract the seed and a 0.5 g sample of seed + chaff per tree was stratified on moist filter paper for 25 d at 4 °C to promote uniform germination. After germination, 100 seedlings per family were pricked out into containers and grown on in a glasshouse for 7 weeks when these Gen (n + 2) seedlings had between one and three expanded leaf pairs. Leaves were collected and DNA extracted from 32 seedlings per family selected at random. The DNA was assayed with five nuclear microsatellite loci (CRC6, EL13, ES140, ES211 and ES255) as detailed in Bloomfield et al. (2011). Maternal genotypes were determined from the progeny arrays, and outcrosses were detected by exclusion based on the presence of non-maternal alleles at one or more loci. The population of mother trees averaged 6.2 alleles per locus across all loci. Based on the maternal allelic frequencies, we calculated that the average probability of misclassifying an outcross as a self was 2.2 %, comparable with (1) the proportion of outcrosses which were poorly identified in that they had only one minor (2 bp) microsatellite allelic difference from a maternal allele (2.3 %) and (2) our error rate in microsatellite repeatability scoring of 2.4 %.
RESULTS
Experiment 1: hand sown
Survival of the dominant and suppressed cohorts of seedlings and related estimates of the percentage of outcrosses in the survivors of each cohort to 10.5 years for individual families are shown in Supplementary Data Table S1, and pooled data treating the experiment as a single population, approximating the reality when seed rain from multiple parents falls in a natural regeneration, are illustrated in Fig. 2. Of the estimated 600 000 viable seed ha–1 sown, the mean density at 5 months was 215 000 ha–1, a reduction of 64 %. Thereafter, the stand suffered ongoing mortality from unspecified environmental factors and from natural, competition-driven, thinning. By 3.6 years, each plot had developed a clear size class structure, and samples from both dominant and suppressed cohorts were genotyped. For the dominant cohort, there was 27 % mortality between 3.6 and 10.5 years, but 94 % mortality for the suppressed cohort. For the total experiment, only 4 % of the estimated seeds sown and 12 % of the seedlings observed at 5 months survived to 10.5 years, so there was clearly also very high mortality of intermediate sized trees.
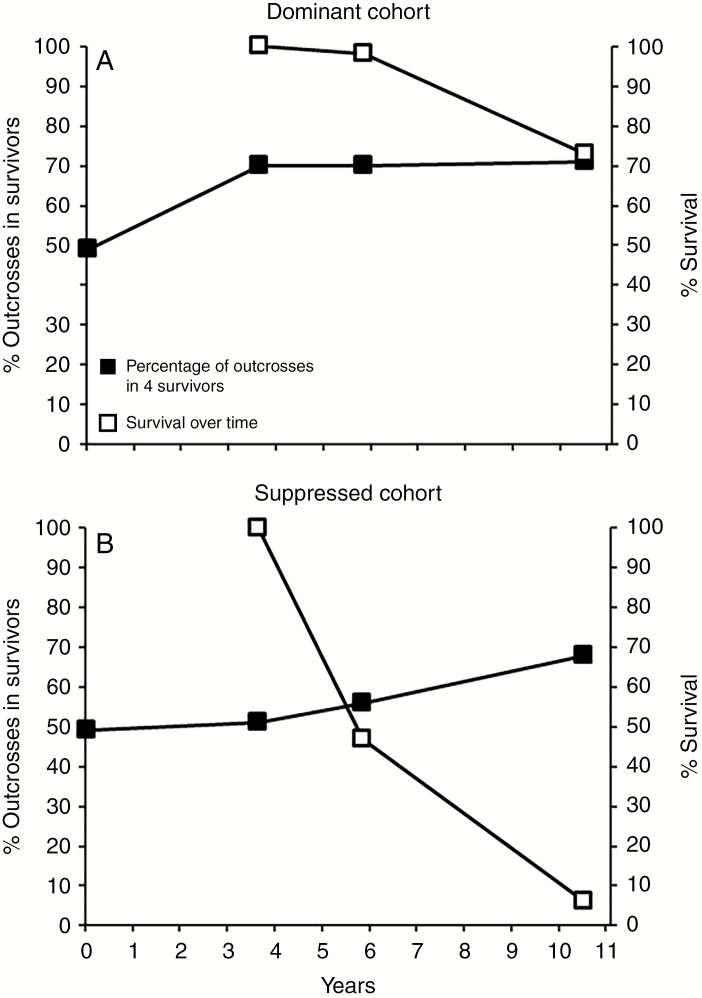
Fig. 2.
The percentage of outcrosses in the survivors and survival over time for samples of the dominant (A) and suppressed (B) cohorts of E. regnans seedlings in Experiment 1 alive 3.6 years after sowing. Both cohorts developed from a common population of seedlings containing an estimated 48 % outcrosses at sowing. Data are from four families pooled (see Supplementary Data Table S1).
In freshly germinated seed, the weighted mean proportion of detectable outcrosses (tm) averaged 49 % over the four selected OP families (range from 0.19 to 0.84) (Supplementary Data Table S1). By age 3.6 years, the dominant cohort of seedlings contained 70 % outcrosses compared with 51 % in the suppressed cohort (Fig. 2). Between 3.6 and 10.5 years, the percentage of detectable outcrosses in the survivors of the dominant cohort was little changed at 71 %, reflecting the small reduction in survival, but, for the surviving sub-set of the suppressed cohort, outcrosses increased to 68 %. Corresponding changes occurred in Ho (data not shown) from a mean of 0.26 in the original OP seed to 0.27 in the dominant cohort and 0.23 in the suppressed cohort at 3.6 years. By 10.5 years, Ho was unchanged at 0.27 in the dominant cohort but there was a tendency for the more heterozygous plants in the suppressed cohort preferentially to survive to that age as inferred from the increase in the Ho estimate to 0.25. At the family level, there was a direct positive relationship between the primary outcrossing rate and the survivorship in the family plots from the first direct count at 5 months and the final assessment at 10.5 years (n = 4; Pearsons r = 1.0, P = 0.003; data in Supplementary Data Table S1). This trend was evident (but not significant) as early as age 3.6 years (r = 0.83, P = 0.172).
Experiment 2: planted pedigreed field experiment
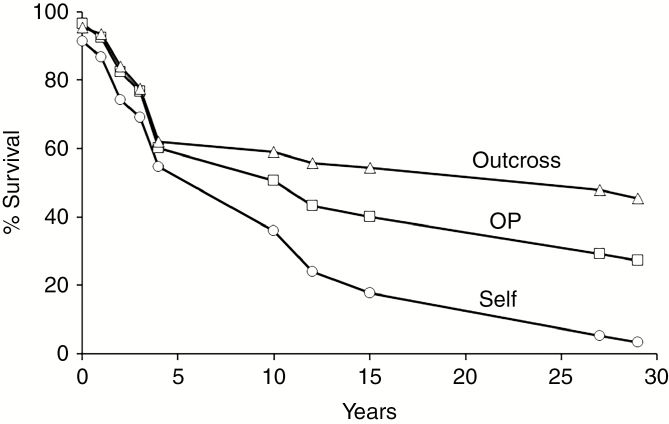
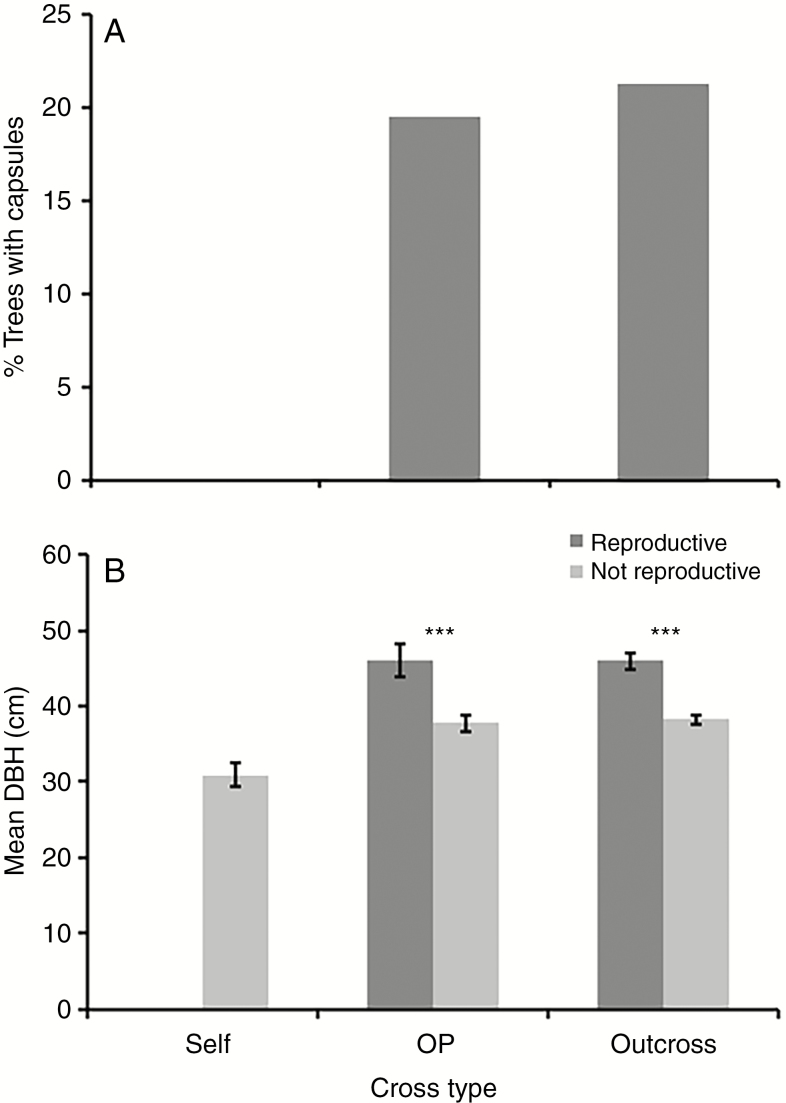
In the long-term pedigreed field experiment, the differential survival of the three mating types (selfing, outcrossing and OP) to age 15 years was reported by Hardner and Potts (1997). Here we extend the observation period to 29 years, at which time only 3 % of the selfs planted were still alive (Fig. 3). Survival of the OP population was intermediate and significantly less than that of the outcross population (27 % OP and 45 % outcrossed trees planted survived; contingency χ2P < 0.001). However, the proportion of the surviving trees which were reproductive did not differ significantly (19.5 % OP and 21.2 % outcrosses – Fig. 4; contingency χ2P = 0.73). This is consistent with the surviving OPs being predominantly outcrosses (Fig. 5). When comparing just the OP and outcross populations in a two-way fixed effects model for age 29 year DBH, the reproductive status (with vs. without capsules) was highly significant (F1,434 = 39.5, P < 0.001) and the cross type effect (OP vs. outcrosses; F1,434 = 0.03, P = 0.853) and interaction term (F1,434 = 0.09, P = 0.770) were non-significant. The reproductive trees were significantly larger than surviving non-reproductive trees in both the outcrossed and OP populations (Fig. 4B), arguing that in a competitive stand exemplified by this 29-year-old experiment, reproductive success is related to tree size. The few surviving selfs not only failed to reproduce at this age but were significantly smaller than even the non-reproductive OP and outcrossed trees, and were presumably doomed to be outcompeted and die if the experiment had not been stopped at this age (refer to Fig. 1). Retrospective analyses indicated that by 4 years of age trees that subsequently flowered had greater stem diameter, but not height, than non-reproductive trees. However, by year 10, the larger trees in terms of both diameter and height clearly had a higher probability of bearing capsules (Table 1).
Fig. 3.
Percentage of planted seedlings surviving over time for progeny from self (n planted = 425), open-pollinated (OP, n = 468) and outcrossed (n = 694) progeny (Gen n + 1) derived from 13 E. regnans parents (Gen n) and grown in a common environment field experiment (Experiment 2). The initial assessment was at 3 months after planting.
Fig. 4.
The percentage and size (DBH) of reproductive trees from the self, open-pollinated (OP) and outcross populations (Gen n + 1) of E. regnans in Experiment 2. (A) Percentage of trees bearing capsules by cross type at age 29 years. None of the surviving selfs was reproductive. (B) The stem diameter (DBH) of reproductive (with capsules) and non-reproductive (no capsules) trees of each cross type at age 29 years. *** indicates that contrasts between reproductive and non-reproductive trees are significant at the P < 0.001 level in a two-factor fixed effect model.
Fig. 5.
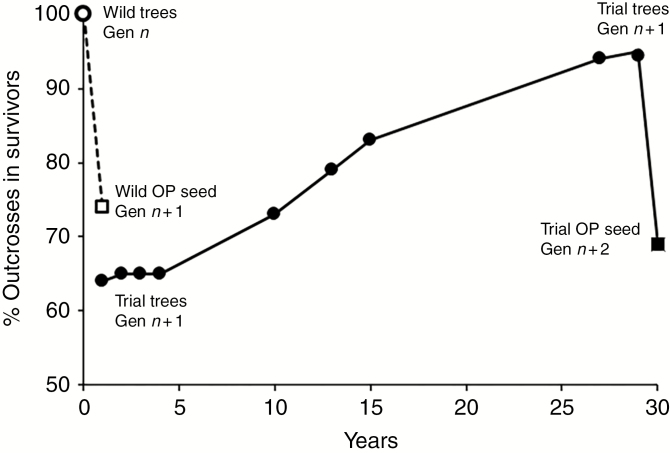
Multigenerational variation in estimated percentage of outcrossed genotypes alive at different stages of the life cycle of E. regnans. The figure shows the estimate for the wild parental trees, generation n (see text); open-pollinated (OP) seed from those trees representing the start of generation n + 1 (Narracan stand only; see Moran et al., 1989); the change over time in the proportion of surviving outcrosses in an experimental n + 1 population comprising a known mix of self and outcross progeny (Experiment 2); and the estimate derived from microsatellite assay of seed from that population, forming the basis of the n + 2 population. The complete outcrossed status of the population of wild parental trees at generation n is derived from estimated inbreeding coefficients from allozyme studies presented in Moran et al. (1989) (Narracan) and the present study (Thorpdale). The estimates over time for generation n + 1 are derived from Hardner and Potts (1997, fig. 3) and extended to 29 years in the present study. The seed estimates for generation n + 2 are derived from the OP seed assayed in the present study that were harvested from reproductive Gen n + 1 trees at age 29 years (see Supplementary Data Table S2).
Table 1.
Least-square means (Lsmean) and standard errors (s.e.) for diameter and height for reproductive (with capsules) and non-reproductive (no capsules) Eucalyptus regnans trees at age 29 years pooled across the open-pollinated and outcross cross types in the field experiment
| Age (years) | Trait | Non-reproductive | Reproductive | |||||
|---|---|---|---|---|---|---|---|---|
| Lsmean | s.e. | Lsmean | s.e. | F 1,459 | Pr > F | % difference | ||
| 4 | Diameter (cm) | 12.8 | 0.11 | 13.3 | 0.21 | 4.3 | 0.039 | 3.9 |
| 4 | Height (m) | 10.9 | 0.07 | 10.9 | 0.14 | 0.2 | 0.669 | 0 |
| 10 | Diameter (cm) | 25.2 | 0.21 | 27.3 | 0.41 | 21.6 | 0 | 8.3 |
| 15 | Height (m) | 31.5 | 0.21 | 32.9 | 0.41 | 9.8 | 0.002 | 4.4 |
| 15 | Diameter (cm) | 30.0 | 0.28 | 33.6 | 0.55 | 34.9 | 0 | 12.0 |
| 29 | Diameter (cm) | 37.7 | 0.51 | 45.7 | 1.01 | 49.6 | 0 | 21.2 |
Also shown are the Walds F statistic and its probability for the difference between the two groups, and the percentage difference in the means relative to the non-reproductive mean. The reproductive partition was based on the presence of capsules at age 29 years.
Molecular estimates of F and outcrossing rate
The dominance of the mature forest by outcrosses is confirmed by our previous isozyme-based estimates of the inbreeding coefficient (F) and surviving outcrosses in the original native populations (Gen n in Fig. 5). The fully outcrossed status of the mature stands was inferred from F estimates (F = 1 – Ho/He) derived from analysis of the isozyme genotypic arrays for the two stands. Based on sample size weighted estimates of Ho and He averaged across loci, we predicted F to be –0.11 ± 0.058 in the Narracan population (41 mother trees Ho = 0.31 and He = 0.28 across five variable loci) and 0.10 ± 0.050 in Thorpdale (113 mother trees Ho = 0.31 He = 0.35 across seven variable loci). Two-tailed t-tests using the F estimates for each locus as replication indicated that neither of these average F estimates was significantly different from zero (P > 0.05). Following Duminil et al. (2015), these values equate to effective outcrossing rate estimates of 1.2 and 0.8, respectively (i.e. tm = 1–2 × F assuming the adult population is in Hardy–Weinberg equilibrium as selfing is countered by inbreeding depression), which average 1.0, consistent with expectations from Experiment 2. These estimates contrast with the isozyme-based estimate of 0.74 ± 0.025 in the OP seed (Gen n +1) from the Narracan stand (Moran et al., 1989) which aligns with the microsatellite estimates of outcrossing rate in the OP (Gen n + 2) seed collected from the reproductively mature cohort in Experiment 2. This averaged 0.66 ± 0.041, with an individual tree range of 0.41–0.93 (Supplementary Data Table S2). Allelic frequencies in the parental (Gen n + 1) population from which this Gen n + 2 estimate was derived are given in Supplementary Data Table S3.
DISCUSSION
Outcrossing taxa generally show stronger expression of ID than selfers during the seed production, germination and pre-reproductive growth phases of the life cycle (Husband and Schemske, 1996), and Winn et al. (2011) concluded that taxa with mixed mating systems, such as E. regnans, have similar ID to outcrossers at each life cycle stage. Our study accords with this general conclusion, since we have shown that ID effectively eliminated inbred progeny over the germination and juvenile growth stages (the first 29 years of the life cycle of this long-lived woody perennial) (Fig. 5). A previous study showed that expression of the relative competitive advantage of outcrosses had already begun during seed maturation (Griffin et al., 1987).
Winn et al. (2011) observed that most studies of ID reviewed involved laboratory germination of seed and subsequent progeny growth under environmental conditions likely to be less variable and more benign than natural field conditions. Our hand sowing experiment (Experiment 1) was specifically designed to mimic regeneration conditions at a germination density approximating that reported from the natural forest (Cunningham, 1960; Evans, 1976; Ashton, 1981). Convergence of the stand density curve for the planted Experiment 2 with that observed in the natural forest (Fig. 1) gives confidence that our experimental results reflect the dynamics of natural populations. For example, by interpolation in Fig. 1, survival in the three natural data sets ranged from 714 to 1145 stems ha–1 at age 29 compared with 677 ha–1 in the planted experiment. The competitive thinning of E. regnans stands (Fig. 1) is far from unique. In dense even-aged populations of a wide variety of plant species, the relationship between log number of survivors and time is nearly linear (Harper and White, 1974), as was shown by Barber (1965) for the Gilbert (1958) data in Fig. 1.
In Experiment 1, selfed and outcrossed progeny were not identified until age 3.6 years, by which time the average survival had reduced to 22 % of the estimated viable seed sown (Supplementary Data Table S1). However, we were able to infer that differential elimination of selfs had started earlier since (1) the proportion of detectable outcrosses increased from 0.49 in the seed to 0.56 in the extant seedling population and (2) the family outcrossing rate correlated with survivorship from 5 months to 3.6 years. Furthermore, the greater proportion of detectable outcrosses in the sample of the dominant cohort (0.70) than the suppressed cohort (0.51) by 3.6 years also suggests that the surviving outcrosses were on average more vigorous than surviving selfs. Competitive interactions established early in stand development have been shown to persist to later ages (Costa e Silva et al., 2017) and, consistent with size-dependent mortality (Koelewijn et al., 1999; Costa e Silva et al., 2011), 94 % of the tagged suppressed plants in the sowing experiment had died by 10.5 years, compared with only 27 % of the dominants.
The four E. regnans families sown were selected to cover the full range of outcrossing rates observed in the natural parental populations. All showed similar self-thinning patterns (Supplementary Data Table S1), but the density of surviving plants within family plots was strongly dependent on the primary outcrossing rate, consistent with expected higher mortality of selfed than outcrossed progeny. Multiple factors may affect the timing of such differential mortality, including the competitive environment (Cheptou and Schoen, 2003), site productivity (Costa e Silva et al., 2011) and environmental stress (Armbruster and Reed, 2005; Nickolas et al., 2019; but see Waller et al., 2008). Nevertheless, even plots of our most inbred family (t = 0.19) contained an estimated 112 200 ha–1 outcrossed seed at sowing and carried an estimated 15 964 ha–1 outcrossed trees after 10.5 years, greatly in excess of the number which could survive to reproductive age (Fig. 1). Competitive mortality must mean that many potentially viable genotypes will be eliminated, dependent upon stochastic effects as well as density-dependent selection (Barber, 1965). Our finding in Experiment 2 that stem diameter at age 4 years was positively associated with seed production at age 29 years shows the critical advantage of early establishment of dominance and accords with the general finding that reproductive competence is related to plant size rather than age per se (Hackett, 1985; Lacey, 1986; Meinzer et al., 2011). This advantage is likely to persist through the life cycle since modelling by Ashton (1976) showed that trees constituting the mature E. regnans forest at age 200 years were probably present in the dominant class at 40 years. From comparison of our two experiments and data presented in Fig. 1, we suggest that over a wide range of initial population densities (from 215 000 germinants ha–1 in the sowing experiment to 1667 ha–1 in the planted experiment), intra-specific competition will result in similar densities as the trees reach reproductive maturity. Reproduction is then confined to outcrossed individuals, since selfs have been eliminated by this stage of the life cycle or even earlier under the most competitive conditions, as argued by Costa e Silva et al (2011).
Stability of the mating system
We have shown that the mixed mating system of E. regnans is repeatable over two successive generations. Because of the differential elimination of selfs, the reproductive cohort of Gen n + 1 trees in Experiment 2 included only outcrossed individuals, but their open pollinated Gen n + 2 seed had a similar mix of selfs and outcrosses to that found in the Gen n population (Fig. 5). While this is clearly not sufficient to demonstrate evolutionary stability of the mating system, the review of 23 eucalypt species by Byrne (2008) strongly suggests that mixed mating is the norm (with a mean allozyme-derived tm of 0.74) and provides compelling circumstantial evidence of stability. Eucalyptus is a large genus which radiated rapidly throughout Australia during the Mid-Cenozoic (25–10 million years ago) period of climatic change (Crisp et al., 2004) and if mating systems were evolving we might expect greater diversity among species.
How can the mixed mating system remain stable?
Given that most plant taxa which have a mixed mating system also show inbreeding depression for growth rate (Winn et al., 2011), differential elimination of selfs under intra-specific competition seems likely to be a common phenomenon. ID for growth and survival in our experiments is comparable with those reported for other eucalypt species (E. globulus – Hardner and Potts, 1995; López et al., 2000; Costa e Silva et al., 2010, 2011; Nickolas et al., 2019; E. nitens – Hardner and Tibbits, 1998). A diversity of other angiosperm families show similar life cycle dynamics. In experimental mixed populations of Fuchsia excorticata (Onagraceae) and Sophora microphylla (Fabaceae), few selfs remained after 11 years and none was flowering or appeared likely to do so (Robertson et al., 2011), while ID combined with a mixed mating system is also reported in different genera of Fabaceae (Kittelson and Maron, 2000; Harwood et al., 2004; Yuan et al., 2013), Dipterocarpaceae (Naito et al., 2008) and Ericaceae (Delmas et al., 2014). Of the gymnosperms, Winn et al. (2011) referenced seven species, and Sorensen and Miles (1982) reviewed results from field experiments of an additional six species covering four genera in all. Why is purging of genetic load and a loss of ID not a more common phenomenon in these woody perennials? As discussed by Winn et al. (2011), modelling by Lande et al. (1994) showed that when the effects of deleterious mutations at multiple loci combine to prevent any selfs from surviving to reproduce, there is no opportunity for selection against homozygous deleterious mutations and, provided that the rate of selfing is below a threshold value dependent upon the number of lethal alleles, purging will not occur. This phenomenon is termed selective interference. Eucalyptus regnans meets the condition that selfs are eliminated by reproductive age; the demonstrated level of selfing and indeed the average of 26 % for all Eucalyptus taxa reviewed by Byrne (2008) is sufficiently low; and, while the number of deleterious mutations in E. regnans is unknown, it is likely to be high since large woody perennials are expected to accumulate somatic mutations in the many mitotic cell divisions which occur between meiotic events (Klekowski, 1988). A recent genome-wide study (nearly 10 000 loci) of a selfed (S1) family of E. grandis found an average of 65.5 % heterozygotes (range 52–79 %), much higher than the 50 % expected under random segregation, supporting the occurrence of strong selection against homozygosity of deleterious alleles and/or overdominance at many loci (Hedrick et al., 2016). The quantitative genetic architecture of an inbred population of E. globulus is consistent with ID for growth arising from rare partially recessive deleterious alleles rather than overdominance (Costa e Silva et al., 2010).
Features of the reproductive ecology of E. regnans help explain why partial selfing and high ID for survival and growth may have few direct adverse effects on the fitness of the maternal genotypes. A mature stand is capable of producing an annual seed rain in the order of 7.5 million seed ha–1, yet no regeneration occurs until such time as there is a tree-killing fire event (Cunningham, 1960; Evans, 1976; Ashton and Martin, 1996; McCarthy et al., 1999). The timing of regeneration is highly unpredictable, occurring on average at between 75 and 150 year intervals in the E. regnans forests of Victoria (McCarthy et al., 1999). The probability of any one seed successfully developing into a reproductively mature plant is extremely small since only around 100 plants ha–1 will survive the competitive thinning process to full maturity (Fig. 1) and, as we have shown, ID ensures that those will be outcrosses in spite of floral and pollination biology which results in significant production of selfed seed. The impact of selection favouring maternal genotypes with high outcrossing rates is further reduced by the limited seed dispersal of E. regnans (Cremer, 1966), favouring the establishment of family groups (Jones et al., 2007) during regeneration which would reduce the competitive interactions among progeny derived from different maternal trees (potentially differing in their outcrossing rates) and favour maternal tree replacement by one of its outcrossed progeny. The selective differential between trees of varying outcrossing rates may also be overestimated due to trade-offs between high outcrossing rate and total seed output. We know that selection against selfs commences during seed development (Griffin et al., 1987) which means that fitness differentials based on the primary outcrossing rate assessed at dispersal may overestimate differences in maternal fitness through a reduction in total seed production in highly outcrossed trees. It is therefore not necessary to look for evidence of direct selection pressure in the mating system per se. Rather, the primary mating system may simply be a consequence of other life cycle traits of a species (Duminil et al., 2009), in this case the most important is the unavoidable production of large numbers of selfed seeds by these large mass flowering plants and the periodic high density regeneration within which intra-specific competition and ID act to ensure a fully outcrossed reproductive population. There are possible reasons why partial selfing may have a selective advantage in some conditions. These include reproductive assurance (Lloyd, 1992; Herlihy and Eckert, 2002) which might be important at low population densities in old or fragmented stands, and also predator satiation (Ghazoul and Satake, 2009). However, the life cycle-related explanation offered above best fits the ecological and genetic traits of the species and helps explain why there is no apparent selection pressure to diverge from the observed mixed mating system.
CONCLUSIONS
Our results demonstrate that high ID is combined with a repeatable mixed mating system across successive generations in this long-lived forest tree species. Selective elimination of inbred genotypes during the intense intra-specific competition characteristic of the pre-reproductive phase of the life cycle of E. regnans results in a fully outcrossed reproductive population, in which self-fertility is comparable with that of its parental generation. This selective elimination of selfs is shown to occur over a wide range of initial plant densities, and the early establishment of dominance is a strong determinant of reproductive success. While two generations is insufficient to demonstrate stability of the mixed primary mating system, the life cycle biology of E. regnans accords well with the conclusion of Winn et al. (2011) that ‘strong cumulative inbreeding depression … and an average inbreeding coefficient for parents that is close to zero (supports) the potential for selective interference to contribute to stable mixed mating in self-compatible taxa that cannot prevent the receipt of self pollen’. Inbreeding depression is an important component of the suite of life cycle attributes which are likely to favour a stable mixed primary mating system.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: experiment 1 – survival of seedlings from sowing to 10.5 years for four individual open-pollinated families and changes in proportion of outcrosses in the dominant and suppressed cohorts over time. Table S2: experiment 2 – percentage of outcrosses in OP Gen n + 2 seed collected from 17 dominant Gen n + 1 trees at age 29 years. Table S3: experiment 2 – microsatellite allele frequencies in the sample of 17 Gen n + 1 trees from which Gen n + 2 seed was collected for estimation of outcrossing rates.
FUNDING
The majority of the work was carried out when A.R.G. and J.C.B. were employed by the CSIRO Divison of Forestry and Forest Products.
Supplementary Material
ACKNOWLEDGEMENTS
The field experiments were established on land currently owned by Hancock Victoria Plantations who permitted access for final assessments. We thank Martin Lavery for seed collection, Des Stackpole for final measurement of the trees, Jane Harbard for raising seedlings, Sascha Wise for assistance with genotyping, and Paul Tilyard for assistance with figures and data management. We also thank the anonymous referees for their useful suggestions for improvement of the manuscript. The authors have no conflict of interest to declare.
LITERATURE CITED
- Armbruster P, Reed DH. 2005. Inbreeding depression in benign and stressful environments. Heredity 95: 235–242. [DOI] [PubMed] [Google Scholar]
- Ashton DH. 1976. Development of even-aged stands of Eucalyptus regnans F Muell in Central Victoria. Australian Journal of Botany, 24: 397–414. [Google Scholar]
- Ashton DH. 1981. Fire in tall open forests (wet sclerophyll forests). In: Gill AM, Groves RH, Noble IR, eds. Fire and the Australian biota. Canberra: Australian Academy of Science, 339–366. [Google Scholar]
- Ashton D, Martin D. 1996. Regeneration in a pole-stage forest of Eucalyptus regnans subjected to different fire intensities in 1982. Australian Journal of Botany 44: 393–410. [Google Scholar]
- Barber HN. 1965. Selection in natural populations. Heredity 20: 551–572. [Google Scholar]
- Bloomfield J, Nevill P, Potts B, Vaillancourt R, Steane D. 2011. Molecular genetic variation in a widespread forest tree species Eucalyptus obliqua (Myrtaceae) on the island of Tasmania. Australian Journal of Botany 59: 226–237. [Google Scholar]
- Britten P. 1955. Eucalyptus regnans regrowth data, Mnt. Disappointment and Toolangi Forests. Unpubl. Report, Victorian Forests Commission, Melbourne, Australia. [Google Scholar]
- Byrne M. 2008. Phylogeny, diversity and evolution of eucalypts; In: Sharma AK, Sharma A, eds. Plant genome: biodiversity and evolution. Volume 1, Part E, Phanerogams-Angiosperm. Enfield: Science Publishers, 303–346. [Google Scholar]
- Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–68. [Google Scholar]
- Cheptou PO, Dieckmann U. 2002. The evolution of self-fertilization in density-regulated populations. Proceedings of the Royal Society B: Biological Sciences 269: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheptou PO, Schoen D aniel J. 2003. Frequency‐dependent inbreeding depression in Amsinckia. The American Naturalist 162: 744–753. [DOI] [PubMed] [Google Scholar]
- Costa e Silva J, Hardner C, Tilyard P, Pires AM, Potts BM. 2010. Effects of inbreeding on population mean performance and observational variances in Eucalyptus globulus. Annals of Forest Science 67: 605. doi: 10.1051/forest/2010018. [DOI] [Google Scholar]
- Costa e Silva J, Hardner C, Tilyard P, Potts BM. 2011. The effects of age and environment on the expression of inbreeding depression in Eucalyptus globulus. Heredity 107: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa e Silva J, Potts BM, Gilmour AR, Kerr RJ. 2017. Genetic-based interactions among tree neighbors: identification of the most influential neighbors, and estimation of correlations among direct and indirect genetic effects for leaf disease and growth in Eucalyptus globulus. Heredity 119: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer KW. 1965a Effects of fire on seedshed from Eucalyptus regnans. Australian Forestry 29: 252–262. [Google Scholar]
- Cremer KW. 1965b How eucalypt fruits release their seed. Australian Journal of Botany 13: 11–16. [Google Scholar]
- Cremer KW. 1966. Dissemination of seed from Eucalyptus regnans. Australian Forestry 30: 33–37. [Google Scholar]
- Crisp M, Cook L, Steane D. 2004. Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about evolution of diversity in present-day communities? Philosophical Transactions of the Royal Society B: Biological Sciences 359: 1551–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. 1960. The natural regeneration of Eucalyptus regnans. Bulletin No. 1. Melbourne: School of Forestry, University of Melbourne. [Google Scholar]
- Delmas CEL, Cheptou PO, Escaravage N, Pornon A. 2014. High lifetime inbreeding depression counteracts the reproductive assurance benefit of selfing in a mass-flowering shrub. BMC Evolutionary Biology 14: 243. doi: 10.1186/s12862-014-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duminil J, Hardy OJ, Petit RJ. 2009. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evolutionary Biology 9: 177. doi: 10.1186/1471-2148-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duminil J, Daïnou K, Kaviriri DK, et al. 2015. Relationships between population density, fine-scale genetic structure, mating system and pollen dispersal in a timber tree from African rainforests. Heredity 116: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge KG, Griffin AR. 1983. Selfing effects in Eucalyptus regnans. Silvae Genetica 32: 216–221. [Google Scholar]
- Evans GC. 1976. A sack of uncut diamonds: the study of ecosystems and the future resources of mankind. Journal of Ecology 64: 1–39. [Google Scholar]
- Fripp YJ, Griffin AR, Moran GF. 1987. Variation in allele frequencies in the outcross pollen pool of Eucalyptus regnans F. Muell. throughout a flowering season. Heredity 59: 161–171. [Google Scholar]
- Ghazoul J, Satake A. 2009. Nonviable seed set enhances plant fitness: the sacrificial sibling hypothesis. Ecology 90: 369–377. [DOI] [PubMed] [Google Scholar]
- Gilbert JM. 1958. Forest succession in the Florentine Valley, Tasmania. Proceedings of the Royal Society of Tasmania 93: 129–151. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology Evolution and Systematics 36: 47–79. [Google Scholar]
- Griffin AR. 1980. Floral phenology of a stand of mountain ash (Eucalyptus regnans F. Muell.) in Gippsland, Victoria. Australian Journal of Botany 28: 393–404. [Google Scholar]
- Griffin AR, Cotterill PP. 1988. Genetic variation in growth of outcrossed, selfed and open-pollinated progenies of Eucalyptus regnans and some implications for breeding strategy. Silvae Genetica 37: 124–131. [Google Scholar]
- Griffin AR, Moran GF, Fripp YJ. 1987. Preferential outcrossing in Eucalyptus regnans F. Muell. Australian Journal of Botany 35: 465–475. [Google Scholar]
- Griffin AR, Hingston AB, Ohmart CP. 2009. Pollinators of Eucalyptus regnans (Myrtaceae), the world’s tallest flowering plant species. Australian Journal of Botany 57: 18–25. [Google Scholar]
- Hackett WP. 1985. Juvenility, maturation, and rejuvenation in woody plants. Horticultural Reviews 7: 109–155. [Google Scholar]
- Hardner C, Tibbits W. 1998. Inbreeding depression for growth, wood and fecundity traits in Eucalyptus nitens. Forest Genetics 5: 11–20. [Google Scholar]
- Hardner CM, Potts BM. 1995. Inbreeding depression and changes in variation after selfing Eucalyptus globulus subsp. globulus. Silvae Genetica 44: 46–54. [Google Scholar]
- Hardner CM, Potts BM. 1997. Post-dispersal selection following mixed-mating in Eucalyptus regnans. Evolution 51: 103–111. [DOI] [PubMed] [Google Scholar]
- Harper JL, White J. 1974. The demography of plants. Annual Review of Ecology and Systematics 5: 419–463. [Google Scholar]
- Harwood CE, Thinh HH, Quang TH, Butcher PA, Williams ER. 2004. The effect of inbreeding on early growth of Acacia mangium in Vietnam. Silvae Genetica 53: 65–69. [Google Scholar]
- Hedrick PW, Hellsten U, Grattapaglia D. 2016. Examining the cause of high inbreeding depression: analysis of whole-genome sequence data in 28 selfed progeny of Eucalyptus grandis. New Phytologist 209: 600–611. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. 2002. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416: 320–323. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DC. 1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54–70. [DOI] [PubMed] [Google Scholar]
- Jones TH, Vaillancourt RE, Potts BM. 2007. Detection and visualization of spatial genetic structure in continuous Eucalyptus globulus forest. Molecular Ecology 16: 697–707. [DOI] [PubMed] [Google Scholar]
- Kittelson PM, Maron JL. 2000. Outcrossing rate and inbreeding depression in the perennial yellow bush lupine, Lupinus arboreus (Fabaceae). American Journal of Botany 87: 652–660. [PubMed] [Google Scholar]
- Klekowski EJ. (1988). Genetic load and its causes in long lived plants. Trees 2: 195–203. [Google Scholar]
- Koelewijn HP, Koski V, Savolainen O. 1999. Magnitude and timing of inbreeding depression in Scots pine (Pinus sylvestris L.). Evolution 53: 758–768. [DOI] [PubMed] [Google Scholar]
- Lacey EP. 1986. Onset of reproduction in plants: size-versus age-dependency. Trends in Ecology & Evolution 1: 72–75. [DOI] [PubMed] [Google Scholar]
- Lande R, Schemske DW, Schultz ST. 1994. High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations. Evolution 48: 965–978. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370–380. [Google Scholar]
- López GA, Potts BM, Tilyard PA. 2000. F1 hybrid inviability in Eucalyptus: the case of E. ovata × E. globulus. Heredity 85: 242–250. [DOI] [PubMed] [Google Scholar]
- McCarthy MA, Gill AM, Lindenmayer DB. 1999. Fire regimes in mountain ash forest: evidence from forest age structure, extinction models and wildlife habitat. Forest Ecology and Management 124: 193–203. [Google Scholar]
- Meinzer FC, Lachenbruch B, Dawson TE. 2011. Size- and age-related changes in tree structure and function. Dordrecht: Springer Science & Business Media. [Google Scholar]
- Moran GF, Bell JC, Griffin AR. 1989. Reduction in levels of inbreeding in a seed orchard of Eucalyptus regnans F. Muell. compared with natural populations. Silvae Genetica 38: 32–36. [Google Scholar]
- Morgan MT, Schoen DJ, Bataillon TM. 1997. The evolution of self-fertilization in perennials. American Naturalist 150: 618–638. [DOI] [PubMed] [Google Scholar]
- Naito Y, Kanzaki M, Iwata H, et al. 2008. Density-dependent selfing and its effects on seed performance in a tropical canopy tree species, Shorea acuminata (Dipterocarpaceae). Forest Ecology and Management 256: 375–383. [Google Scholar]
- Nevill PG, Bossinger G, Ades PK. 2010. Phylogeography of the world’s tallest angiosperm, Eucalyptus regnans: evidence for multiple isolated Quaternary refugia. Journal of Biogeography 37: 179–192. [Google Scholar]
- Nickolas H, Harrison PA, Tilyard P, Vaillancourt RE, Potts BM. 2019. Inbreeding depression and differential maladaptation shape the fitness trajectory of two co-occurring Eucalyptus species. Annals of Forest Science 76: 10. [Google Scholar]
- Ritland K,, Jain S. 1981. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 47: 35–52. [Google Scholar]
- Robertson AW, Kelly D, Ladley JJ. 2011. Futile selfing in the trees Fuchsia excorticata (Onagraceae) and Sophora microphylla (Fabaceae): inbreeding depression over 11 years. International Journal of Plant Sciences 172: 191–198. [Google Scholar]
- Schemske DW, Lande R. 1985. The evolution of self-fertilisation and inbreeding depression in plants. II. Empirical observations. Evolution 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Sillett SC, Van P elt R, Kramer RD, Carroll AL, Koch GW. 2015. Biomass and growth potential of Eucalyptus regnans up to 100 m tall. Forest Ecology and Management 348: 78–91. [Google Scholar]
- Sorensen FC, Miles RS. 1982. Inbreeding depression in height, height growth, and survival of douglas-fir, ponderosa pine, and noble fir to 10 years of age. Forest Science 28: 283–292. [Google Scholar]
- Tng DYP, Williamson GJ, Jordan GJ, Bowman D. 2012. Giant eucalypts – globally unique fire-adapted rain-forest trees? New Phytologist 196: 1001–1014. [DOI] [PubMed] [Google Scholar]
- Turner PAM, Balmer J, Kirkpatrick JB. 2009. Stand-replacing wildfires? The incidence of multi-cohort and single-cohort Eucalyptus regnans and E. obliqua forests in southern Tasmania. Forest Ecology and Management 258: 366–375. [Google Scholar]
- Van Der Meer PJ, Dignan P. 2007. Regeneration after 8 years in artificial canopy gaps in Mountain Ash (Eucalyptus regnans F. Muell.) forest in south-eastern Australia. Forest Ecology and Management 244: 102–111. [Google Scholar]
- Waller DM, Dole J, Bersch AJ. 2008. Effects of stress and phenotypic variation on inbreeding depression in Brassica rapa. Evolution, 62: 917–931. [DOI] [PubMed] [Google Scholar]
- Winn AA, Elle E, Kalisz S, et al. 2011. Analysis of inbreeding depression in mixed-mating plants provides evidence for selective interference and stable mixed mating. Evolution 65: 3339–3359. [DOI] [PubMed] [Google Scholar]
- Wood SW, Hua Q, Allen KJ, Bowman DMJS. 2010. Age and growth of a fire prone Tasmanian temperate old-growth forest stand dominated by Eucalyptus regnans, the world’s tallest angiosperm. Forest Ecology and Management 260: 438–447. [Google Scholar]
- Yuan CQ, Li YF, Wang L, et al. 2013. Evidence for inbreeding depression in the tree Robinia pseudoacacia L. (Fabaceae). Genetics and Molecular Research 12: 6249–6256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.