Abstract
Background and Aim
Quaternary glaciations strongly affected the distribution of species from arid and semi-arid environments, as temperature drops were accompanied by strong fluctuations in rainfall. In this study, we examined the response of xerophytic species to glacial cycles, determining the genetic patterns and climatic niche of Echinopsis chiloensis var. chiloensis, an endemic columnar cactus of arid and semi-arid regions of Chile.
Methods
We analysed 11 polymorphic microsatellites for 130 individuals from 13 populations distributed across the entire distribution of the species. We examined genetic diversity and structure, identified possible patterns of isolation by distance (IBD) and tested two competing population history scenarios using Approximate Bayesian Computation. The first scenario assumes a constant population size while the second includes a bottleneck in the southern population. The latter scenario assumed that the southernmost populations experienced a strong contraction during glaciation, followed by a postglacial expansion; by contrast, the area of the northernmost populations remained as a stable refugium. We also used ecological niche modelling (ENM) to evaluate the location and extension of suitable areas during the Last Glacial Maximum (LGM) and the mid-Holocene.
Key Results
We found a decline in genetic diversity towards high latitudes and a significant IBD pattern that together with ENM predictions suggest that E. chiloensis var. chiloensis experienced range contraction northwards during wet–cold conditions of the LGM, followed by expansion during aridification of the mid-Holocene. In addition to IBD, we detected the presence of a strong barrier to gene flow at 32°30′S, which according to coalescence analysis occurred 44 kyr BP. The resulting genetic clusters differed in realized climatic niche, particularly in the variables related to precipitation.
Conclusions
Our results suggest that the cactus E. chiloensis var. chiloensis experienced range contraction and fragmentation during the wet–cold conditions of the LGM, which may have facilitated ecological differentiation between northern and southern populations, promoting incipient speciation.
Keywords: Desert, Echinopsis chiloensis, ecological niche modelling, glacial fragmentation, microsatellites, post-glacial expansion, phylogeography
INTRODUCTION
Quaternary climatic oscillations strongly affected the distributions of plant species in the temperate and boreal regions of the Northern and Southern Hemispheres (Hewitt, 1996, 2000). Accordingly, genetic and fossil evidence show that during glacial periods many plant species contracted their distribution range to warm regions at lower latitudes and lower elevations, while others persisted at high latitudes and montane regions in small areas surrounded by unfavourable conditions (multiple or cryptic refugia) (Cruzan and Templeton, 2000; Bennett and Provan, 2008; Rull, 2009; Premoli et al., 2000). Less is known about the response of xerophytic species of arid and semi-arid regions where glaciers did not occupy large areas, but where temperature drops were accompanied by strong fluctuations in rainfall (but see Tarasov et al., 2000; Médail and Diadema, 2009).
One of the most conspicuous elements of the arid and semi-arid areas of the Americas are the columnar cacti (Fleming and Valiente-Banuet, 2002; Yetman, 2007), which belong to different lineages of the family Cactaceae. The largest species richness of columnar cacti is concentrated in Mexico, adjacent Central America, and south-western United States, but they are also prominent in eastern South America and the south-western Andean region (Gómez-Hinostrosa and Hernández, 2000; Hernández et al., 2001; Mauseth et al., 2002; Ortega-Baes and Godínez-Alvarez, 2006; Guerrero et al., 2011, Gurvich et al., 2014).
Cornejo-Romero et al. (2013) reviewed the research on genetic structure that can contribute to explain cactus speciation in North and South America. Some of these genetic studies incorporated a historical component in their analyses, and documented contrasting responses of columnar cacti to Quaternary climatic fluctuations. For example, in the Sonora Desert, where conditions were wetter than at present during the Last Glacial Maximum (LGM), the widespread cactus species Lophocereus schottii (Nason et al., 2002) and Pachycereus pringlei (Gutiérrez-Flores, 2015) show a marked decrease in genetic diversity with latitude and an isolation-by-distance pattern (IBD) concordant with the classical scenario of glacial contraction–postglacial expansion. In the Tehuacan-Cuitlán Valley, there is evidence of a bottleneck for Neobuxbaumia macrocephala, N. mezcalensis and N. tetetzo (Esparza-Olguín, 2005), which could also support the hypothesis of contraction–expansion. In contrast, high levels of population divergence have been documented for Pilosocereus machrisii of central South America that today grow in small and isolated patches of xerophytic vegetation (Moraes et al., 2005; Bonatelli et al., 2014) that are thought to have functioned as refugia during the wet–warm conditions of interglacial periods (interglacial refugia). There is little information about the genetic structure of cacti in southern South America. Only one study in Echinopsis terscheckii showed significant genetic structure, but this was not studied in relation to historical processes (Quipildor et al., 2017). However, in general terms, during the LGM the tropics of South America were drier than the present climate with the exception of north-eastern Brazil, while the subtropics were wetter (Wainer et al., 2005).
In Chile, the semi-arid region occurs from 30° to 34°S along a steep rainfall gradient that is controlled by the latitudinal position of the Southern Westerly Wind (SWW) belt and Southern Pacific Anticyclone (SPA). During the LGM, the SWW belt was probably stronger and displaced towards the equator, producing increased winter rains in north-central Chile (Heusser et al., 1999; Lamy et al., 1999; Kaiser et al., 2008). Accordingly, an increased abundance of humid-adapted C3 vegetation at 30°S and northward expansion of temperate forest taxa has been documented for the LGM (e.g. Nothofagus and Prumnopitys) (Heusser, 1990; Valero-Garcés et al., 2005; Kaiser et al., 2008). After 16 kyr BP, humidity started to decrease (Kaiser et al., 2008), with maximum aridity in the mid-Holocene (7-6 kyr BP), when vegetative cover was very low and dominated by xerophytic C3 and CAM plants (Valero-Garcés et al., 2005; Maldonado and Villagrán, 2006; Kaiser et al., 2008). Conditions became wetter during the Late Holocene and present day (Maldonado and Villagrán, 2006; Kaiser et al., 2008).
Echinopsis chiloensis (Colla) Friedrich & G.D. Rowley is a species complex formed by three varieties of endemic columnar cacti that inhabit the arid and semi-arid regions of north-central Chile (30°–34°S): E. chiloensis var. chiloensis with a wider inland distribution (VI to IV Region), E. chiloensis var. litoralis (Johow) M.Lowry, which grows along the coast from Valparaiso (V Region) to Los Vilos (IV Region), and E. chiloensis var. skottsbergii (Backeb. ex Skottsb.) G.J. Charles, which is restricted to the localities of Fray Jorge and Talinay (IV Region). In this study, we examined the genetic diversity patterns of E. chiloensis var. chiloensis (hereafter E. chiloensis) using 11 microsatellite markers and complemented these data with ecological niche modelling (ENM). We attempt to: (1) determine the climatically suitable areas for this cactus species during the LGM and the mid-Holocene (maximum aridity), (2) estimate genetic diversity and population divergence of E. chiloensis and (3) explore its historical demography by simulating alternative scenarios using coalescent-based Approximate Bayesian Computation (ABC).
Based on information for the glacial period of Chile, we expect to find evidence of northward contraction during the LGM and recent southward expansion, which are expected to have caused a north-to-south trend of decreasing genetic diversity and IBD.
MATERIALS AND METHODS
Present and past distribution ENM models
We modelled the present ENM of E. chiloensis from 50 unique georeferenced species records obtained from our fieldwork, taking precaution not to georeference populations on the western side of the coastal mountains, to avoid confounding with other varieties or hybrids. The present ENM was constructed using the maximum entropy method (Phillips et al., 2006) and ten bioclimatic variables contained in the dataset of Pliscoff et al. (2014, http://www.unil.ch/ecospat/home/menuguid/ecospat-resources/data.html) at 1-km2 resolution. Based on an initial set of models run using all 19 bioclimatic layers, by using a principal components analysis (PCA) we selected ten climatic variables that contributed most to the variance (Bio1, Bio5, Bio7, Bio9, Bio10, Bio14, Bio15, Bio17, Bio18 and Bio19). Precipitation variables had the highest eigenvalues in PC1, explaining 52 % of the variance, and temperature variables contributed the most in PC2, explaining an additional 31 % of the variance.
Background points were randomly chosen within the area enclosed by a minimum convex polygon including all records of the species. Occurrence data were partitioned 100 times into training and test data (75 % and 25 %, respectively) for model evaluation using the area under the operating characteristic curve (AUC). The relative contribution of each variable was analysed through MaxEnt’s jackknife tests. Finally, we used the average model of 50 ENM replicates.
To estimate the potential distribution of E. chiloensis during the LGM and mid-Holocene, we projected the average current ENM onto the reconstructed past climatic conditions for both periods available in the WorldClim dataset with a resolution of 4.5 km2 (Hijmans et al., 2005). We tested three general circulation models: the Community Climate System Model (CCSM4) (Gent et al., 2011), Model for Interdisciplinary Research on Climate (MIROC‐ESM 2010) (Watanabe et al., 2011) and the Max Planck Institute for Earth system model setup of orbit and vegetation (MPI-ESM-P). We selected the MPI-ESM-P model because it was more consistent with fossil evidence of the LGM palaeoclimate (Moreno et al., 1994; Kaiser et al., 2008; Maldonado and Rozas, 2008).
Niche equivalency was calculated by pooling all occurrence records and split into two random datasets to calculate Schöener’s D statistic (Schoener, 1992), where D = 1 means complete niche overlap and D = 0 means niche non-overlap, evaluating overlap between the original and random datasets. This process was replicated 100 times to calculate a P-value for statistical support in the test of the null hypothesis (niche equivalency). Analyses were made in R utilizing the ECOSPAT, ADE4, ADEHABITATHR and SP packages.
Microsatellite analysis
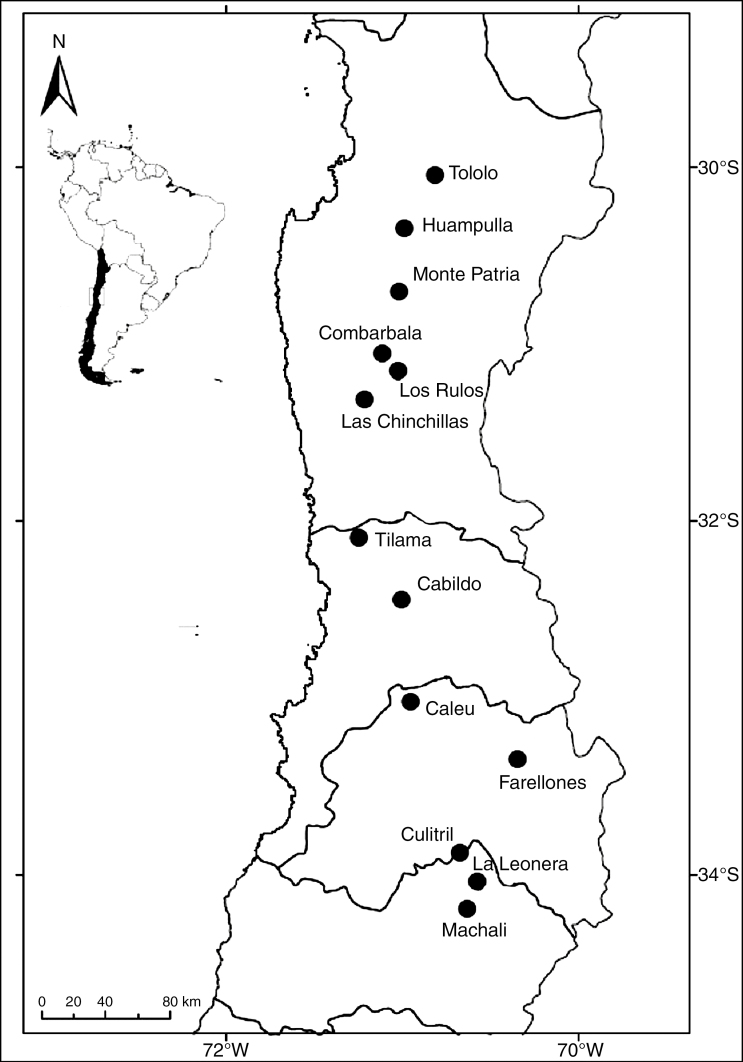
The material for this study consisted of 130 samples of stem tissue of E. chiloensis collected from 13 localities between 29° and 33°S (Fig. 1). Samples of ten individuals per locality were stored in silica gel; total genomic DNA was extracted following the method of Ossa et al. (2016), modified from the cetyltrimethyl ammonium bromide (CTAB) method of Doyle and Doyle (1987). Individuals were genotyped at 11 polymorphic microsatellite loci: Ech4, Ech5, Ech9, Ech16, Ech20, Ech21, Ech22, Ech23, Ech25, Ech28 and Ech29 (Ossa et al., 2016). Fragment lengths were sequenced using a 3130xl Genetic Analyser (Applied Biosystems, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA). Microsatellites were analysed using Geneious R8.0.5 (http://www.geneious.com;Kearse et al., 2012).
Fig. 1.
Map of north-central Chile showing the sampling sites of Echinopsis chiloensis.
To determine whether some loci are under selection we used BayesScan v.2.01 (Foll and Gaggiotti, 2008) implemented in R, to identify loci under divergent or uniform selection. We used the default parameters given in the program for this analysis. As recommended in the manual, we used an analysis of false discovery rate (FDR) in R.
Genetic diversity
The mean number of alleles (N), number of rare alleles (AR), expected heterozygosity (He), observed heterozygosity (Ho) and deviations from Hardy–Weinberg equilibrium (HWE) were estimated for each site using GenAlEx v.6.1 (Peakall and Smouse, 2006). Linkage disequilibrium between each pair of loci was examined using Genepop v.4.2 (Raymond and Roussett, 1995; Rousset, 2008) for each sampling location. The null hypothesis of linkage equilibrium was accepted for all comparisons (P > 0.06). To examine possible founder effects and explore the process of southward and eastward expansion, we tested the effect of latitude and longitude on within-population genetic diversity using a linear regression model in R (R Core Team, 2015).
Population divergence
To evaluate the population structure of E. chiloensis we used the spatially explicit Bayesian clustering program Geneland v.4.0.0 (Guillot et al., 2005), which uses multilocus genotypes and spatial coordinates of individuals to cluster them into populations at approximately HWE. We performed 1 000 000 Markov chain Monte Carlo (MCMC) iterations with 1–13 genetic clusters (K), a thinning interval of 100 generations and an uncorrelated allele frequency model. We also examined population structure using the Bayesian program Structure v.2.3.1 (Pritchard et al., 2000), which identifies the number of genetic clusters (K), and assigns each individual to these clusters probabilistically without sampling location information. We carried out ten independent runs with K values ranging from 1 to 13 using the MCMC method. Each run was estimated as 100 000 steps, with a 100 000-step burn-in. To determine the most likely number of clusters (K) we used the Structure Harvester v.0.6.93 tool (Earl and vonHoldt, 2012). We performed a hierarchical analysis of molecular variance (AMOVA) in GenAlEx to quantify the degree of differentiation between these clusters. To identify patterns of IBD, we estimated pairwise FST values for all pair of sites in GenAlEx and then performed a Mantel test with geographical distances. Given that IBD can introduce biases in clustering analyses (Meirmans., 2012), we constructed a pairwise cluster relatedness matrix, consisting of zeros (when populations belong to different genetic clusters) and ones (when populations belong to the same cluster). We then conducted a partial Mantel test between genetic distances and clusters using geographical distances as the covariate. In this way, we discount that clusters obtained by Structure and Geneland resulted from IBD.
Demographic history
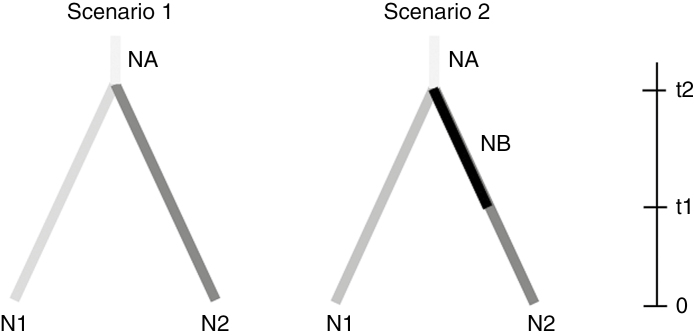
To examine whether E. chiloensis has undergone changes in effective population size over time, we used coalescent-based approximate Bayesian computation implemented in DIYABC v.2.0 (Cornuet et al., 2014). Based on the results of Structure and Geneland analyses, we defined two populations and constructed two scenarios. The first scenario assumes a constant population size, whereas the second includes a bottleneck in the southern population when it split from the northern population (Fig. 2). The latter scenario is based on the hypothesis that the southernmost populations of E. chiloensis experienced a strong contraction during glaciation, followed by a postglacial expansion, while the area of the northernmost populations remained as a stable refugium, based on the Pleistocene history of the Chilean Mediterranean flora (see Valero-Garcés et al., 2005; Maldonado and Villagrán, 2006; Kaiser et al., 2008), and in agreement with observations for North America. We assumed a uniform prior distribution of mean mutation rate from 10−5 to 10−3 and a generalized stepwise mutation model. We estimated the mean number of alleles, mean genetic diversity and mean allele size variance for each population. We ran 2 × 106 simulated datasets to estimate posterior probabilities of both scenarios using logistic regression. We selected the scenario with the highest posterior probability and estimated the values of the associated demographic parameters. The modelling was previously checked using PCA, implemented in DIYABC. Over 10 000 random simulations were made, in order to check if the prior distributions allowed simulating data sets to be close to the observed data whatever the scenario envisaged.
Fig. 2.
Scenarios explored in DIYABC. The first scenario assumes a constant population size. The second scenario includes a bottleneck in the southern population when it split from the ancestral population. The latter scenario is based on the hypothesis that the southernmost populations of Echinopsis chiloensis experienced a strong glacial contraction followed by a postglacial expansion, whereas the northernmost populations remained as a stable refugium. NA, N1, N2 and NB denote the population size of the ancestral population, the northern population at present, the southern population at the present and southern population during the bottleneck.
Niche overlap between genetic clusters
A posteriori, we estimated the niche overlap between the two genetic clusters obtained with the Structure and Geneland analyses using the approach of Broennimann et al. (2012). Based on the initial ten bioclimatic layers selected, we selected the six variables that contributed at least 4 % to model performance (Bio5, Bio7, Bio14, Bio15, Bio18, Bio19) through a Jackknife test of each variable’s importance for the ENM in MaxEnt; the other variables contributed less than 1 % to model performance. We estimated the density of occurrence of both genetic clusters and of climatic variables along the first two PCA axes. Niche overlap along these two axes was then measured using the D statistic (Warren et al., 2008), which varies from 0 (no overlap) to 1 (complete overlap). The observed D value was compared against a reference null distribution constructed by randomization with 100 replicates (i.e. all occurrences are pooled and randomly split into two datasets). The null hypothesis of niche equivalency is rejected when the observed value of D is lower than 95 % of the simulated values. Analyses were performed in R v.3.3.2 utilizing the ECOSPAT, ADE4, ADEHABITATHR and SP packages (Broennimann et al., 2014).
RESULTS
Present and past distribution ENM models
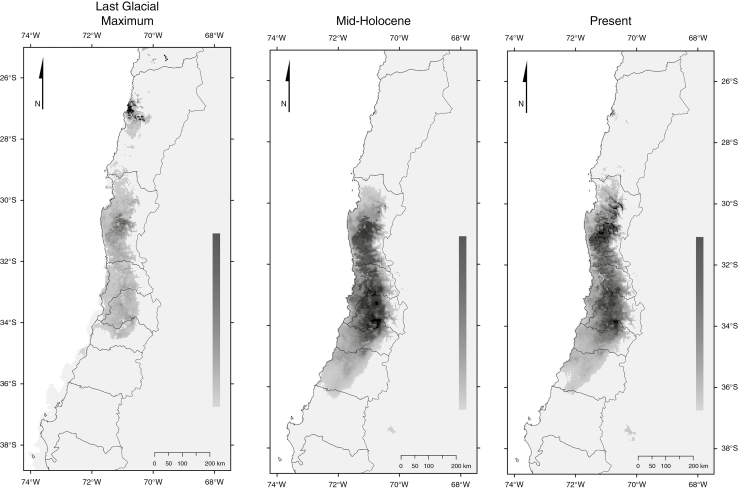
The ENM model showed high power of discrimination between presences and background (AUC values of 0.996) and predicted accurately the current distribution of E. chiloensis (Fig. 3). Two areas of high suitability were detected under current conditions, one at 31°S and other at 34°S, separated by an area of moderate suitability located around 32°S. Palaeoclimatic models indicated that the climatic space was more restricted and fragmented during the LGM than at present, with a clear decrease in probability of occurrence southward and the persistence of an area of high suitability at 31°S. We also detected a second disjunct area of high suitability outside the current distribution of the species at 27°S (Fig. 3). Climatic space during the mid-Holocene was more continuous than at present, but similar in extent. These results suggest a northward contraction of species during the wet–cold conditions of the LGM, followed by southward expansion during the extreme xeric conditions of the mid-Holocene and by an incipient fragmentation during the wetter conditions of the Late Holocene.
Fig. 3.
Geographical distribution of Echinopsis chiloensis according to MPI-ESM-P model consensus maps for the Last Glacial Maximum, mid-Holocene and present.
Genetic diversity
Analysis using BayeScan v.2.01 showed that all the microsatellites were neutral, the q-values were between 0.0038 and 0.8465, and the FDR was between 0.9962 and 0.08362.
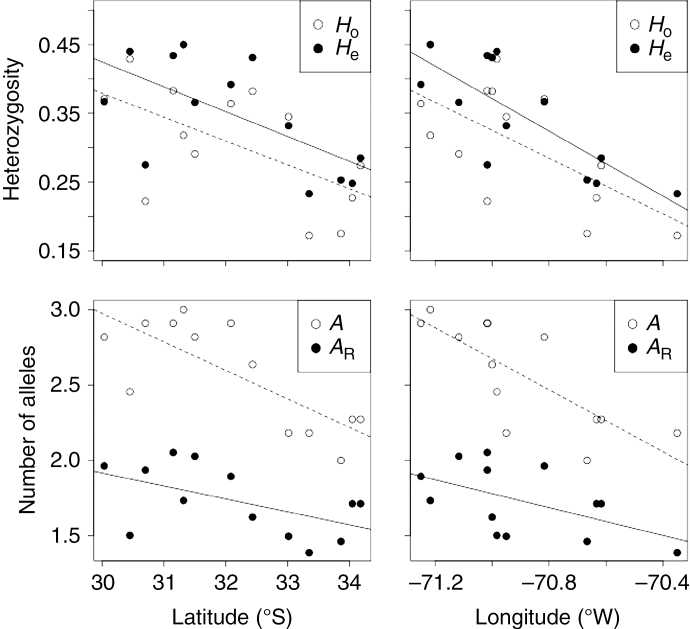
Echinopsis chiloensis showed a decreasing trend in genetic diversity southward and eastward (Table 1; Fig. 4). Linear models revealed a significant effect of latitude and longitude on allelic richness (Lat: F2,10 = 22.1, P < 0.001; Lon: F2,10 = 11.1, P = 0.007), the number of rare alleles (Lat: F2,10 = 5.6, P = 0.04; Lon: F2,10 = 5.5, P = 0.04) and expected heterozygosity (Lat: F2,10 = 12.4, P = 0.005; Lon: F2,10 = 6.7, P = 0.03); by contrast, observed heterozygosity was only significantly related to latitude (Lat: F2,10 = 6.2, P = 0.03; Lon: F2,10 = 2.2, P = 0.16). These results are consistent with a classical scenario of range contraction towards the north and coast during the LGM, followed by a postglacial expansion towards higher latitudes and elevations.
Table 1.
Geographical location, sample size (N), allelic richness (A), rare alleles (AR), observed (Ho) and expected (He) heterozygosity and inbreeding coefficient (FIS) for 13 sites of Echinopsis chiloensis based on 12 nuclear microsatellites. Asterisks indicate significant deviation from Hardy–Weinberg equilibrium
| Locality | Abbreviation | Latitude S | Longitude W | N | A | A R | H o | H e | F IS |
|---|---|---|---|---|---|---|---|---|---|
| Tololo | TO | 30°02′ | 70°49′ | 10 | 2.727 | 1.732 | 0.371 | 0.367 | 0.046 |
| Huampulla | HU | 30°27′ | 70°59′ | 10 | 2.818 | 1.963 | 0.429 | 0.440 | 0.079 |
| Monte Patria | MP | 30°42′ | 71°01′ | 9 | 2.455 | 1.503 | 0.222 | 0.275 | 0.248 |
| Combarbalá | CM | 31°09′ | 71°01′ | 10 | 2.909 | 1.936 | 0.383 | 0.434 | 0.171 |
| Los Rulos | LR | 31°19′ | 71°13′ | 10 | 2.909 | 2.053 | 0.318 | 0.450 | 0.340* |
| Las Chinchillas | LC | 31°30′ | 71°07′ | 10 | 3.000 | 1.734 | 0.291 | 0.366 | 0.255* |
| Tilama | TL | 32°05′ | 71°15′ | 11 | 2.818 | 2.028 | 0.364 | 0.392 | 0.121 |
| Cabildo | CB | 32°26′ | 71°00′ | 10 | 2.909 | 1.894 | 0.382 | 0.431 | 0.166 |
| Caleu | CL | 33°01′ | 70°57′ | 10 | 2.636 | 1.624 | 0.345 | 0.332 | 0.012 |
| Farellones | FA | 33°21′ | 70°21′ | 10 | 2.182 | 1.497 | 0.172 | 0.233 | 0.308* |
| Culitril | CU | 33°52′ | 70°40′ | 10 | 2.182 | 1.388 | 0.175 | 0.253 | 0.357* |
| La Leonera | LE | 34°03′ | 70°38′ | 10 | 2.000 | 1.463 | 0.227 | 0.248 | 0.136 |
| Machalí | MA | 34°11′ | 70°37′ | 10 | 2.273 | 1.713 | 0.274 | 0.285 | 0.094 |
| All populations | – | – | – | 130 | 2.601 | 1.733 | 0.304 | 0.347 | 0.165* |
Fig. 4.
Latitudinal and longitudinal variation in allelic richness (A), number of rare alleles (AR), observed heterozygosity (Ho) and expected heterozygosity (He) in Echinopsis chiloensis.
Population divergence
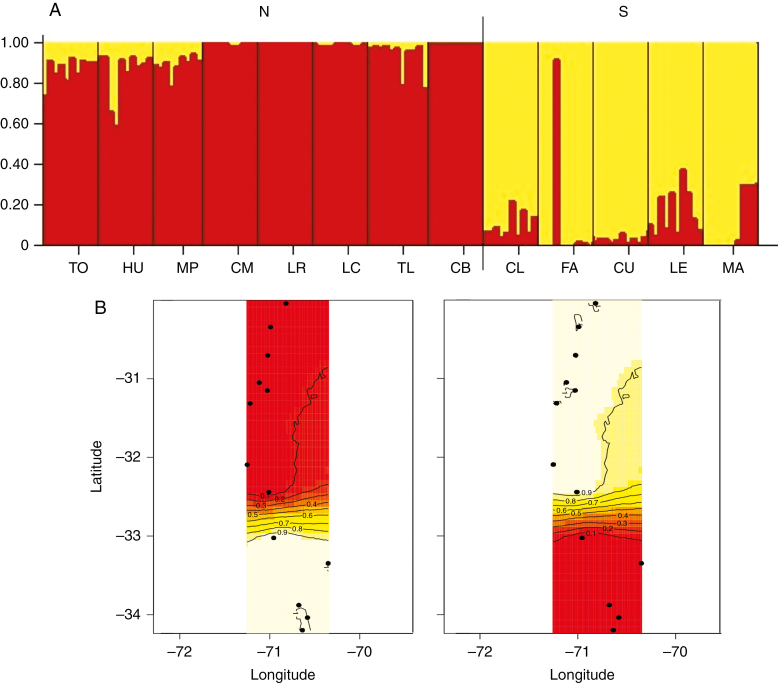
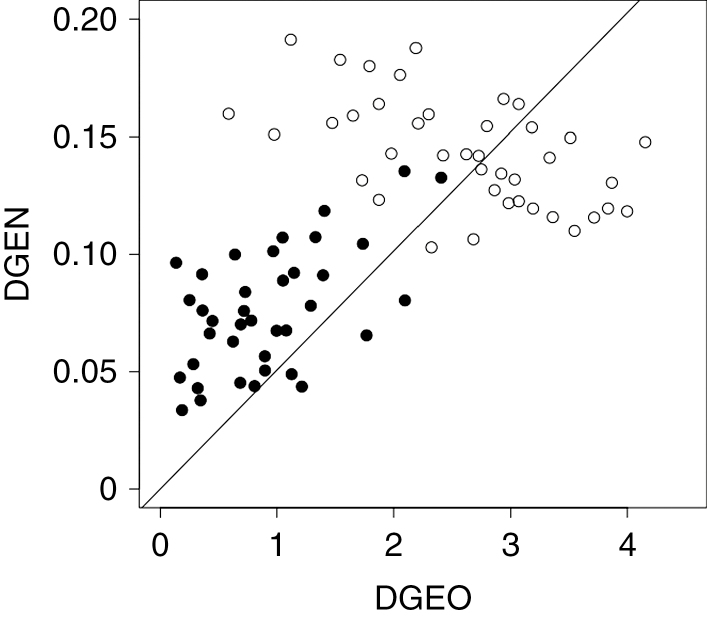
AMOVA revealed that 75 % of the genetic variation was partitioned within individuals and only 25 % among populations. Both Geneland and Structure analyses identified two geographical groups: north (TO, HU, MP, CM, LR, LC, TL, CB) and south (CL, FA, CU, LE, MA), with a boundary around 32°30′S (Fig. 5). Differentiation between these regions was significant (AMOVA; P = 0.001), with 22 % of the genetic variation corresponding to differences among regions and only 11 % to differences among populations. Genetic distance (DGEN) between populations was significantly associated with geographical distance (DGEO) (F2,75 = 71.32, P < 0.001), indicating a significant IBD pattern, as well as cluster relatedness (F2,75 = 70.32, P < 0.001), suggesting the presence of a strong barrier to gene flow in addition to IBD (Fig. 6).
Fig. 5.
Population structure of Echinopsis chiloensis inferred with Structure (A) and Geneland (B). (A) Bayesian clustering of sampled individuals. Each individual is represented by a vertical column partitioned into K = 2 coloured groups (North, red; South, yellow) according to its estimated membership coefficient. Sampling sites are separated by black lines (see abbreviations in Table 1). (B) Posterior probabilities of population membership and genetic discontinuities from the Geneland spatial model. Contour lines indicate the spatial position of genetic discontinuities and lighter colours indicate higher probabilities of population membership. Two genetic clusters were identified (left: North, right: South).
Fig. 6.
Pairwise genetic differentiation among sampling locations (FST) against geographical distances. Pairs of sampling locations belonging to the same and different genetic clusters are denoted with filled and open circles, respectively (DGEN: genetic distance; DGEO: geographical distance).
Demographic history
DIYABC analyses indicated that scenario 2, which included a bottleneck in the southern population, was more probable than scenario 1 (Fig. 2), which assumed a constant population size (scenario 2: probability = 0.93; scenario 1: probability = 0.07). According to scenario 2, the southern population diverged from the northern population 2200 generations ago [90 % confidence interval (CI): 542–7160], when it had an effective population size seven times smaller than the northern population (NB: median = 1110, 90 % CI = 143–3640; N1: median = 7230, 90 % CI = 3840–9560). The southern population would have experienced an expansion around 695 generations ago (90 % CI: 313–3540), reaching a Ne of 5450 (90 % CI: 2380–9370).
Niche overlap between genetic clusters
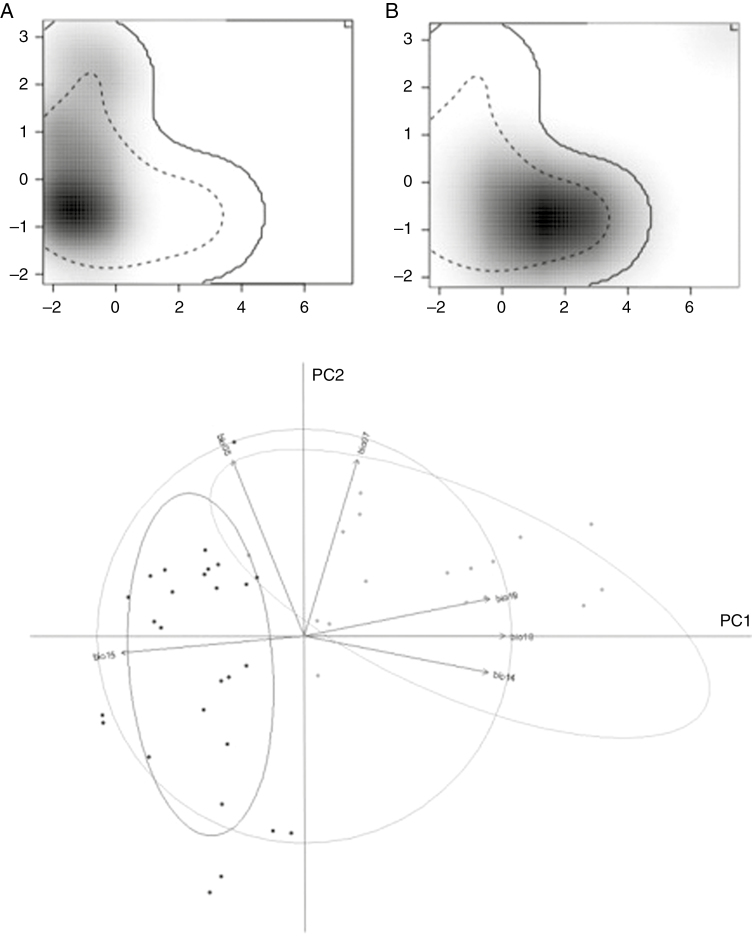
The two genetic clusters obtained with the Structure and Geneland analyses showed significant differences in the realized climatic niche. The D overlap index was lower than expected under the null hypothesis of niche equivalency (D = 0.21, P = 0. 02). According to PCA–environment analysis, clusters differ more in precipitation than in temperature dimensions of the niche (Fig. 7). The first principal component, which explained 58 % of the variance, clearly separated genetic clusters. This axis was positively correlated with summer and winter precipitation (Bio14, Bio18 and Bio19) and negatively correlated with precipitation seasonality (Bio15). In contrast, no segregation between clusters was observed with respect to the second component (PC2). This axis explained 25 % of the variance and was negatively correlated with temperature annual range (Bio7) and summer temperature (Bio5).
Fig. 7.
Environmental niche of the northern (A) and southern (B) genetic clusters of Echinopsis chiloensis along the first two axes of PCA–environment analyses. Grey shading shows the density of occurrence of the species by cell, whereas the solid and dashed lines correspond to 100 % and 50 % of the background environment, respectively. The contribution of the climatic variables on PCA axes is also shown.
DISCUSSION
Palaeo-reconstructions of the climatic niche of the columnar cactus E. chiloensis and its genetic patterns suggest that this species experienced range contraction and fragmentation during the last glaciation. Palaeoclimatic models predicted range contraction towards the north of Chile during the cold–wet conditions of the LGM, followed by a southward expansion during the warm–dry conditions of the mid-Holocene. Correspondingly, we detected a remarkable decline in genetic diversity inland and southward, together with a significant pattern of IBD, that are consistent with a recent demographic expansion in which successive population bottlenecks reduced diversity (Hewitt, 1996; Comps et al., 2001; Petit et al., 2002). In addition to IBD, we found the presence of a strong barrier to gene flow around 32°30′S that according to DIYABC analysis would have formed 2200 generations ago. These analyses also suggest that along with fragmentation, the southernmost populations of E. chiloensis experienced a bottleneck that lasted 1500 generations and was followed by a population expansion process that started 695 generations ago and involved a five-fold increase in population size.
The generation length of E. chiloensis and other columnar cacti of Chile remains unknown, but there are data for giant cacti of Mexico, including Cephalocereus columna-trajani that reaches reproductive maturity at 60–70 years when it has a mean height of 3.5 m, and Carnegiea gigantea at around 52 years with a height of 2.4 m (Zavala-Hurtado and Díaz-Solís, 1995). The generation time of E. chiloensis is probably shorter, because it reaches reproductive maturity at smaller sizes (1.0 m; C. G. Ossa, pers. observ.). Assuming a generation time of 20 years, divergence between northern and southern populations together with contraction of the latter would have occurred around 44 kyr BP, that is during the last glaciation. The same data suggest that the southward expansion of E. chiloensis would have started around 14 kyr BP. This time matches partially with the beginning of an arid period in north-central Chile (Kaiser et al., 2008). Based on terrestrial and marine organic proxies from marine sediments obtained at 30°S, Kaiser et al. (2008) suggested that humidity started to decrease in north-central Chile at 17–16 kyr BP, 2 kyr after the beginning of temperature warming and Antarctic sea ice retreat (19 kyr). Pollen reconstructions from Laguna Tagua Tagua located at 34°S also suggest a reduction in humidity starting around 15 kyr BP with the retraction southward of typical evergreen arboreal taxa (Heusser, 1990; Valero-Garcés et al., 2005). Maximum aridificaion, however, was reached in the mid-Holocene (7–6 kyr BP), when vegetative cover was very low and dominated by xerophytic C3 and CAM plants (Valero-Garcés et al., 2005; Maldonado and Villagrán, 2006; Kaiser et al., 2008).
Genetic evidence of postglacial population expansion has been documented for the columnar cacti Lophocereus schottii (Nason et al., 2002) and Pachycereus pringlei (Gutiérrez-Flores, 2015), as well for other xerophytic plants from the deserts of North America (Agave lechuguilla, Silva-Montellano and Aguiarte, 2003; Larrea tridentate, Duran et al., 2005; Hunnemannia fumariifolia, Sosa et al., 2009; Humifusa, Majure et al., 2012; Lindleya, Vásquez-Cruz and Sosa, 2016), as well as Asia (Juniperus sabina, Guo et al., 2010; Clematis sibirica, Zhang and Zhang, 2012; Nitraria, Su et al., 2016), where cold–wet conditions during glacial periods caused the range contraction of xerophytic plants. Likewise, other drought-adapted plants of northern and central Chile show genetic signals of postglacial range expansion, including the shrubs Nolana crasullifolia and N. incana (Ossa et al., 2013) and the shrub Colliguaya odorifera (Bull-Hereñe et al., 2005). However, none of these Chilean species shows evidence of a genetic break. Conversely, a genetic break around 31–33°S has been detected in the tree species Drymis winteri (Jara Arancio et al., 2012), Aextoxicon punctatum (Nuñez-Ávila and Armesto, 2006) and Myrceugenia correifolia (Pérez et al., 2017). All these species grow in the fog-dependent forests of northern and central Chile that are considered climatic relicts. It is thought that fragmentation and contraction of their distribution ranges was triggered by the increased warming and reduced rainfall that occurred during Late Tertiary (Nuñez-Ávila and Armesto, 2006) or later, during warm/dry cycles of the Pleistocene (Villagrán et al., 2004; Pérez et al., 2017). Recently, based on microsatellite data, Pérez et al. (2017) suggested that increased precipitation during glacial periods triggered northward expansion of the range of M. correifolia, with subsequent admixture between populations that remained separated during interglacial periods.
Range fragmentation in E. chiloensis triggered by glacial periods could have facilitated differentiation of ecological traits. As revealed by PCA–environment analyses, genetic clusters differ in realized climatic niche, which can reflect different climatic requirements and physiological tolerances. Also, it has been shown that floral morphology and pollination syndromes vary across the distribution range of E. chiloensis. Flowers of southern populations open at dusk and are pollinated by hawk moths, bees and dipterans (Walter, 2010; Lemaitre et al., 2014), whereas in northern populations flowers open in the morning and are pollinated only by bees and dipterans (Ossa and Medel, 2011). The limit found between gene clusters also matches the relative distribution of two different haplotypes of the holoparasite Tristerix aphyllus (Amico and Nickrent, 2009), which infects E. chiloensis and other columnar cacti of northern and central Chile (Follman and Mahú, 1964). Congruence between the genetic structure of parasites and their hosts has been found frequently in other systems (Mazé-Guilmo et al., 2016), and indicates that hosts and their parasites evolved in response to similar historical and environmental events (Nieberding and Olivieri, 2007).
Echinopsis chiloensis showed higher levels of population divergence (FST = 0.25) than many columnar cactus species from North America (Pachycereus schottii, Parker and Hamrick, 1992; Carnegiea gigantea, Pachycereus pringlei, Hamrick et al., 2002) and Venezuela (Stenocereus griseus, Cereus repandus, Pilosocereus lanuginosus, Nassar et al., 2003), in which 3–14 % of the genetic variation is explained by differences among populations. Most of these species are pollinated by bats. In contrast, E. chiloensis is pollinated by insects (Walter, 2010; Ossa and Medel, 2011; Lemaitre et al., 2014), which travel shorter distances than bats, reducing gene flow among populations. Levels of population differentiation similar to those of E. chiloensis were observed in Stenocereus thurberi (FST = 0.18), which also shows variation in phenology and pollination syndromes.
Our results suggest that the cactus E. chiloensis experienced range contraction and fragmentation during the wet–cold conditions of the last glaciation. Range fragmentation may have facilitated differentiation of southern and northern populations in several ecological traits, including climatic requirements, phenology and pollinators. Future studies are needed to examine whether differentiation in pollinators and flower phenology could drive incipient speciation in E. chiloensis.
ACKNOWLEDGMENTS
We thank PhD Mª José Peréz-Alvarez, PhD David Véliz and Paulo Zepeda for their help with some of the software, Daniela Aros for assisting with fieldwork and Lafayette Eaton for corrections to the English text. This work was supported by FONDECYT 3140354 to C.G.O. and FONDECYT 1171369 and 1141047 to F.P.
LITERATURE CITED
- Amico GC, Nickrent DL. 2009. Population structure and phylogeography of the mistletoes Tristerix corymbosus and T. aphyllus (Loranthaceae) using chloroplast DNA sequence variation. American Journal of Botany 96: 1571–1580. [DOI] [PubMed] [Google Scholar]
- Bennett KD, Provan J. 2008. What do we mean by ‘refugia’? Quaternary Science Reviews 27: 2449–2455. [Google Scholar]
- Bonatelli IA, Zappi DC, Taylor NP, Moraes EM. 2014. Interglacial microrefugia and diversification of a cactus species complex: phylogeography and palaeodistributional reconstructions for Pilosocereus aurisetus and allies. Molecular Ecology 23: 3044–3063. [DOI] [PubMed] [Google Scholar]
- Broennimann O, Fitzpatrick MC, Pearman PB, et al. . 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology Biogeography 21: 481–497. [Google Scholar]
- Broennimann O, Petitpierre B, Randin C, et al. 2014. https://cran.r-project.org/web/packages/ecospat/index.html https://cran.r-project.org/web/packages/ecospat/index.html R Package ‘ecospat’.
- Bull-Hereñu K, Martínez EA, Squeo FA. 2005. Structure and genetic diversity in Colliguaja odorifera Mol. (Euphorbiaceae), a shrub subjected to Pleisto-Holocenic natural perturbations in a mediterranean South American region. Journal of Biogeography 32: 1129–1138. [Google Scholar]
- Comps B, Ömöry DG, Letouzey J, Thiébaut B, Petit RJ. 2001. Diverging trends between heterozygosity and allelic richness during postglacial colonization in the European beech. Genetics 157: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo-Romero A, Vargas-Mendoza CF, Valverde P, Rendón-Aguilar B. 2013. Estructura genética y filogeografía en cactáceas. Cactus y Suculentas de México 58: 4–28 [Google Scholar]
- Cornuet JM, Pudlo P, Veyssier J, et al. . 2014. Diyabc v2.0: a software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics 30: 1187–1189. [DOI] [PubMed] [Google Scholar]
- Cruzan MB, Templeton AR. 2000. Paleoecology and coalescence: phylogeographic analysis of hypotheses from the fossil record. Trends in Ecology and Evolution 15: 491–496. [DOI] [PubMed] [Google Scholar]
- Duran KL, Lowrey TK, Parmenter RR, Lewis PO. 2005. Genetic diversity in Chihuahuan Desert populations of creosotebush (Zygophyllaceae: Larrea tridentata). American Journal of Botany 92: 722–729. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Earl DA, vonHoldt BM. 2012. Structure harvester: A website and program for visualizing structure output and implementing the Evanno method. Conservation Genetics Resources 4: 359–361. [Google Scholar]
- Esparza-Olguín LG, Valverde T, Mandujano MC. 2005. Comparative demographic analysis of three Neobuxbaumia species (Cactaceae) with differing degree of rarity. Population Ecology 47: 229–245. [Google Scholar]
- Fleming TH, Valiente-Banuet A, eds. 2002. Columnar cacti and their mutualists: evolution, ecology, and conservation. Tucson: University of Arizona Press. [Google Scholar]
- Foll M, Gaggiotti O. 2008. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: a Bayesian perspective. Genetics 180: 977–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follman G, Mahú M. 1964. Las plantas huéspedes de “Phrygilanthus aphyllus” (Miers) Eichl. Boletín de la Universidad de Chile, Ciencias 7: 39–4. [Google Scholar]
- Gent P, Danabasoglu G, Donner L, et al. . 2011. The Community Climate System Model Version 4. Journal of Climate 24: 4973–4991. [Google Scholar]
- Gómez-Hinostrosa C, Hernández HM. 2000. Diversity, geographical distribution, and conservation of Cactaceae in the Mier y Noriega region, México. Biodiversity and Conservation 9: 403–418. [Google Scholar]
- Guerrero PC, Durán AP, Walter HE. 2011. Latitudinal and altitudinal patterns of the endemic cacti from Atacama desert to Mediterranean Chile. Journal of Arid Environments 75: 991–997. [Google Scholar]
- Guo YP, Zhang R, Chen CY, Zhou DW, Liu JQ. 2010. Allopatric divergence and regional range expansion of Juniperus sabina in China. Journal of Systematics and Evolution 48: 153–160. [Google Scholar]
- Guillot G, Mortier F, Estoup A. 2005. Geneland: a computer package for landscape genetics. Molecular Ecology Notes 5: 712–715. [Google Scholar]
- Gurvich DE, Zeballos SR, Demaio PH. 2014. Diversity and composition of cactus species along an altitudinal gradient in the Sierras del Norte Mountains (Córdoba, Argentina). South African Journal of Botany 93: 142–147. [Google Scholar]
- Gutiérrez-Flores C. 2015. Filogeografía y estructura genética poblacional del cardón Pachycereus pringlei en el Noroeste de México. PhD Thesis, Centro de Investigaciones Biológicas del Norte, S.C. La Paz, Baja California Sur, México. [Google Scholar]
- Hamrick JL, Nason DJ, Fleming TH, Nassar JM. 2002. Genetic diversity in columnar cacti. In: Fleming TH, Valiente-Banuet A, eds. Columnar cacti and their mutualists: evolution, ecology and conservation. Tucson: University of Arizona Press, 122–133. [Google Scholar]
- Hewitt GM. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Botanical Journal of the Linnean Society 58: 247–276. [Google Scholar]
- Hewitt GM. 2000. The genetic legacy of the Quaternary ice ages. Nature 405: 907–913. [DOI] [PubMed] [Google Scholar]
- Hernández HM, Gómez-Hinostrosa C, Bárcenas RT. 2001. Diversity, spatial arrangement, and endemism of Cactaceae in the Huizache area, a hot-spot in the Chihuahuan Desert. Biodiversity and Conservation 10: 1097–1112. [Google Scholar]
- Heusser C. 1990. Ice age vegetation and climate of subtropical Chile. Palaeogeography, Palaeoclimatology, Palaeoecology 80: 107–127. [Google Scholar]
- Heusser CJ, Heusser LE, Lowell TV. 1999. Paleoecology of the southern Chilean Lake District – Isla Grande de Chiloé during middle-Late Llanquihue glaciation and deglaciation. Geografiska Annaler Series A Physical Geography 81A: 231–284. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Jara Arancio P, Carmona MR, Correa C, Squeo FA, Arancio G. 2012. Leaf morphological and genetic divergence in populations of Drimys (Winteraceae) in Chile. Genetics and Molecular Research 11: 229–243. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Schefuβ E, Lamy F, Mohtadi M, Hebbeln D. 2008. Glacial to Holocene changes in sea surface temperature and coastal vegetation in north central Chile: high versus low latitude forcing. Quaternary Science Reviews 27: 2064–2075. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. . 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy F, Hebbeln D, Wefer G. 1999. High-resolution marine record of climatic change in mid-latitude Chile during the last 28,000 years based on terrigenous sediment parameters. Quaternary Research 51: 83–89. [Google Scholar]
- Lemaitre AB, Pinto CF, Niemeyer HM. 2014. Generalized pollination system: are floral traits adapted to different pollinators? Arthropod–Plant Interactions 8: 261–272. [Google Scholar]
- Maldonado A, Rozas E. 2008. Clima y Paleoambientes durante el Cuaternario Tardío en la Región de Atacama. In: Squeo FA, Arancio G, Gutiérrez JR, eds. Libro Rojo de la Flora Nativa y de los Sitios Prioritarios para su Conservación: Región de Atacama. La Serena: Ediciones Universidad de La Serena, 293–304. [Google Scholar]
- Maldonado A, Villagrán C. 2006. Climate variability over the last 9900 cal yr BP from a swamp forest pollen record along the semiarid coast of Chile. Quaternary Research 66: 246–258. [Google Scholar]
- Majure LC, Judd WS, Soltis PS, Soltis DE. 2012. Cytogeography of the Humifusa clade of Opuntia s.s. Mill. 1754 (Cactaceae: Opuntioideae): correlations with geographic distributions and morphological differentiation of a polyploid complex. Comparative Cytogenetics 6: 53–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauseth JD, Kiesling R, Ostolaza C. 2002. A cactus odyssey: journeys in the wilds of Bolivia, Peru, and Argentina. Portland, OR: Timber Press. [Google Scholar]
- Mazé-Guilmo E, Blanchet S, McCoy KD, Loot G. 2016. Host dispersal as the driver of parasite genetic structure: a paradigm lost? Ecology Letters 19: 336–347. [DOI] [PubMed] [Google Scholar]
- Médail F, Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean basin. Journal of Biogeography 36: 1333–1345. [Google Scholar]
- Moreno PI, Villagran C, Marquet PA, Marshall LG. 1994. Quaternary paleobiogeography of northern and central Chile [Paleobiogeografía Cuaternaria del Norte y Centro de Chile]. Revista Chilena de Historia Natural 67: 487–502. [Google Scholar]
- Moraes EM, Abreu AG, Andrade SCS, Sene FM, Solferini VN. 2005. Population genetic structure of two columnar cacti with a patchy distribution in eastern Brazil. Genetica 125: 311–323. [DOI] [PubMed] [Google Scholar]
- Nassar JM, Hamrick JL, Fleming TH. 2003. Population genetic structure of Venezuelan chiropterophilous columnar cacti (Cactaceae). American Journal of Botany 90: 1628–1637. [DOI] [PubMed] [Google Scholar]
- Nason JD, Hamrick JL, Fleming TH. 2002. Historical vicariance and postglacial colonization effects on the evolution of genetic structure in Lophocereus, a Sonoran Desert columnar cactus. Evolution 56: 2214–2226. [DOI] [PubMed] [Google Scholar]
- Nieberding CM, Olivieri I. 2007. Parasites: proxies for host genealogy and ecology? Trends in Ecology and Evolution 22: 156–165. [DOI] [PubMed] [Google Scholar]
- Nuñez-Ávila M, Armesto JJ. 2006. Relict islands of temperate rainforest tree Aextoxicon punctatum (Aextoxicaceae) in semi-arid Chile: genetic diversity and biogeographic history. Australian Journal Botany 54: 733–743. [Google Scholar]
- Ortega-Baes P, Godínez-Alvarez H. 2006. Global diversity and conservation priorities in the Cactaceae. Biodiversity and Conservation 15: 817–827. [Google Scholar]
- Ossa CG, Medel R. 2011. Notes on the floral biology and pollination syndrome of Echinopsis chiloensis (Cactaceae) in a population of semiarid Chile. Gayana Bototánica 68: 213–218. [Google Scholar]
- Ossa CG, Larridon I, Peralta G, Asselman P, Pérez F. 2016. Development of microsatellite markers using next-generation sequencing for the columnar cactus Echinopsis chiloensis (Cactaceae). Molecular Biology Reports 43: 1315–1320. [DOI] [PubMed] [Google Scholar]
- Ossa PG, Perez F, Armesto JJ. 2013. Phylogeography of two closely related species of Nolana from the coastal Atacama Desert of Chile: post-glacial population expansions in response to climate fluctuations. Journal of Biogeography 40: 2191–2203. [Google Scholar]
- Parker KC, Hamrick JL. 1992. Genetic diversity and clonal structure in a columnar cactus, Lophocereus shottii. American Journal of Botany 79: 86–96. [Google Scholar]
- Peakall R, Smouse PE. 2006. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Hinojosa LF, Peralta G, Montenegro P, Irarrázabal C, Cossio M. 2017. Genetic patterns of Myrceugenia correifolia, a rare species of fog-dependent forests of Mediterranean Chile: is it a climatic relict? Frontiers in Plant Science 8: 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit RJ, Csaikl UM, Bordacs S, et al. . 2002. Chloroplast DNA variation in European white oaks – Phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management 156: 5–26. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- Pliscoff P, Luebert F, Hilger HH, Guisan A. 2014. Effects of alternative sets of climatic predictors on species distribution models and associated estimates of extinction risk: a test with plants in an arid environment. Ecological Modelling 288: 166–177. [Google Scholar]
- Pritchard JK, Stephens P, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli A, Kitzberger T, Veblem TT. 2000. Isozyme variation and recent biogeographical history of the long-lived conifer Fitzroya cupressoides. Journal of Biogeography 27: 251–260. [Google Scholar]
- Quipildor VB, Mathiasen P, Premoli AC. 2017. Population genetic structure of the giant cactus Echinopsis terscheckii in north-western Argentina is shaped by patterns of vegetation cover. Journal of Heredity 108: 469–478. [DOI] [PubMed] [Google Scholar]
- R Core Team 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/ [Google Scholar]
- Raymond M, Rousset F. 1995. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Journal of Heredity 86: 248–249. [Google Scholar]
- Rousset F. 2008. Genepop’007: a complete reimplementation of the Genepop software for Windows and Linux. Molecular Ecology Resources 8: 103–106. [DOI] [PubMed] [Google Scholar]
- Rull V. 2009. Microrefugia. Journal of Biogeography 36: 481–484. [Google Scholar]
- Schoener TW. 1992. Overdispersed niches on a crowded island – a citation-classic commentary on the anolis lizards of Bimini – resource partitioning in a complex fauna. Current Contents/Agriculture Biology & Environmental Sciences 6:10. [Google Scholar]
- Silva-Montellano A, Aguiarte LE. 2003. Geographic patterns in the reproductive ecology of Agave lechuguilla in the Chihuahuan desert. I. Floral characteristics, visitors, and fecundity. American Journal of Botany 90: 377–387. [DOI] [PubMed] [Google Scholar]
- Sosa V, Ruiz-Sanchez E, Rodriguez-Gomez FC. 2009. Hidden phylogeographic complexity in the Sierra Madre Oriental: the case of the Mexican tulip poppy Hunnemannia fumariifolia (Papaveraceae). Journal of Biogeography 36: 18–27. [Google Scholar]
- Su Z, Lu W, Zhang M. 2016. Phylogeographical patterns of two closely related desert shrubs, Nitraria roborowskii and N. sphaerocarpa (Nitrariaceae), from arid north‐western China. Botanical Journal of the Linnean Society 180: 334–347. [Google Scholar]
- Tarasov PE, Volkova VS, Webb T, et al. . 2000. Last glacial maximum biomes reconstructed from pollen and plant macrofossil data from northern Eurasia. Journal of Biogeography 27: 609–620. [Google Scholar]
- Valero-Garcés B, Jenny B, Rondanelli M, et al. . 2005. Palaeohydrology of Laguna de Tagua Tagua (34°30’ S) and moisture fluctuations in Central Chile for the last 46.000 yr. Journal of Quaternary Science 20: 625–641. [Google Scholar]
- Vásquez-Cruz M, Sosa V. 2016. New insights on the origin of the woody flora of the Chihuahuan Desert: the case of Lindleya. American Journal of Botany 103: 1694–1707. [DOI] [PubMed] [Google Scholar]
- Villagrán C, Armesto JJ, Hinojosa LF, Cuvertino J, Pérez C, Medina C. 2004. El Enigmático Origen del Bosque Relicto de Fray Jorge. In: Squeo FA, Gutiérrez JR, Hernández IR, eds. Historia Natural del Parque Nacional Bosque Fray Jorge. Serena: Ediciones Universidad de La Serena, 1–42. [Google Scholar]
- Wainer I, Clauzet G, Ledru MP, Brady E, Otto-Bliesner B. 2005. Last Glacial Maximum in South America: paleoclimate proxies and model results. Geophysical Research Letters 32: 1–4. [Google Scholar]
- Walter HE. 2010. Floral biology of Echinopsis chiloensis ssp. chiloensis (Cactaceae): evidence for a mixed pollination syndrome. Flora 205: 757–763. [Google Scholar]
- Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62: 2868–2883. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hajima T, Sudo K, et al. . 2011. MIROC-ESM: Model description and basic results of CMIP5-20c3m experiments. Geoscientific Model Development 4: 845–872. [Google Scholar]
- Yetman D. 2007. The great cacti. Tucson: University of Arizona Press. [Google Scholar]
- Zhang HX, Zhang ML. 2012. Identifying a contact zone between two phylogeographic lineages of Clematis sibirica (Ranunculeae) in the Tianshan and Altai Mountains. Journal of Systematics and Evolution 50: 295–304. [Google Scholar]
- Zavala-Hurtado JA, Díaz-Solís A. 1995. Repair, growth, age and reproduction in the giant columnar cactus Cephalocereus columna-trajani (Karwinski ex.Pfeiffer) Schumann (Cactaceae). Journal of Arid Environments 31: 21–31. [Google Scholar]