Abstract
Apoptosis-inducing factor (AIF) serves a crucial role in cell death and is involved in several types of cancer, including kidney cancer. The present study aimed to explore the association between AIF expression and patient survival based on tumor grades. AIF expression in 96 patients with renal cell carcinoma (RCC) was investigated using immunohistochemistry. Negative AIF expression was determined in 80 patients (83.3%). mRNA expression of AIF was analyzed in RCC and adjacent tissue samples from 15 patients. AIF mRNA expression in RCC tissues were significantly lower compared with that in adjacent tissues. Analysis of histopathological grades revealed that AIF expression was negatively associated with RCC grade, with AIF expression in Grade II tumors being lower than Grade I types, but higher than Grade III. Finally, 68 patients were followed up for 6–118 months, and it was revealed that the overall postoperative survival rate of patients with negative AIF expression was significantly lower compared with those those with positive AIF expression. These results suggest that decreased AIF expression could be associated with worsening RCC grade. Therefore, reduced AIF expression may potentially help diagnose RCC and distinguish tumor grades.
Keywords: RCC, immunohistochemistry, diagnostic marker, postoperative survival, AIF
Introduction
Apoptosis-inducing factor (AIF) is a mitochondrial oxidoreductase that plays a role in oxidative phosphorylation and redox control in normal cells (1). AIF was originally cloned and identified as a mitochondrial effector of caspase-independent apoptotic cell death (2). Programmed cell death is a fundamental requirement for embryogenesis, organ metamorphosis and tissue homeostasis (3). In mammals, the release of mitochondrial cytochrome c results in the cytosolic assembly of the apoptosome, a caspase activation complex involving apoptotic protease-activating factor 1 (APAF1) and caspase-9, which induce apoptosis (4). However, there are mitochondria-regulated cell-death pathways that are independent of APAF1/caspase-9. Similar to cytochrome c, under normal conditions, AIF is usually confined to the mitochondrial intermembrane space and released in response to death stimuli, and following the induction of apoptosis, AIF is translocated to the nucleus, where it influences chromosome condensation and fragmentation (1,5). In addition, AIF can induce mitochondria to release the apoptogenic proteins including cytochrome c and caspase-9 (1). Joza et al (3) showed that the genetic inactivation of AIF renders embryonic stem cells resistant to cell death after serum deprivation. Moreover, AIF is essential for programmed cell death during the cavitation of embryoid bodies, which is the very first wave of cell death indispensable for mouse morphogenesis (3). AIF-dependent cell death exhibits structural features similar to those of apoptosis and can be genetically uncoupled from APAF1 and caspase-9 expression (5). Joza et al (3) concluded that their data provide genetic evidence for a caspase-independent pathway of programmed cell death that controls early morphogenesis.
In addition to its role in cell death, Shen et al (6) reported that AIF could influence tumor invasion and metastasis by physically interacting with and protecting phosphatase and tensin homolog on chromosome ten (PTEN) from oxidation. PTEN is a tumor suppressor that is susceptible to oxidation-mediated inactivation (7–10). A study by Tsai et al (11) demonstrated that an asthma medication, montelukast, induced lung cancer cell death via the nuclear translocation of AIF. Ohyama et al (12) observed a similar phenomenon where phosphatidylinositol derivatives induced caspase-independent apoptosis in gastric cancer cells through the accumulation of AIF and AIF-homologous mitochondrion-associated inducer of death in cancer cell nuclei. These findings indicated that AIF is involved in cell homeostasis and tumor development.
The AIF gene encodes a mitochondrial flavin adenine dinucleotide-dependent oxidoreductase that serves a role in oxidative phosphorylation and redox control in healthy cells by triggering chromatin condensation and DNA fragmentation (2). Xu et al (13) reported that owing to AIF deletion and the methylation of its promoter, AIF was downregulated in a majority of renal cell carcinomas (RCC) and that AIF overexpression in RCC cell lines could considerably induce apoptosis. Further investigations by Xu et al (13) demonstrated that AIF interacts with STK3, a known apoptosis regulator, and enhances its phosphorylation at Thr180. Despite these previous findings of the role of AIF in cancer, further studies are warranted to investigate the association between AIF expression and RCC grade.
RCC is a high-risk and high-mortality kidney cancer originating in the lining of the proximal convoluted tubules, and accounts for ~2–3% of human cancers worldwide (14,15). In China, RCC is the second most frequent genitourinary malignant cancer, demonstrating a steady increase in frequency (16). Since 2005, following a clearer understanding of the molecular mechanisms underlying RCCs, targeted therapies with small molecules have replaced cytokines in treating metastatic RCC. The survival time has increased from 10–12 months to 20–22 months (17,18). Considering the mortality rate associated with RCC and the challenging therapy, the identification of novel molecular markers for kidney cancer is vital for decreasing mortality and enhancing quality of life for patients. In addition, the identification of novel markers can facilitate the evaluation of individual risk, prognosis and help to predict the effects of therapy and advocacy for personalized treatment (19).
Although AIF was reported to decrease apoptosis in press kidney cells, the expression of AIF in RCC and the association between AIF expression and prognosis of RCC required further study. In the present study, AIF expression was investigated in RCC using immunohistochemistry (IHC), and the association between AIF expression and the prognosis of RCC was evaluated.
Materials and methods
Patients and tissue samples
In total, 96 pairs (78 of clear cell carcinoma, 13 of papillary carcinoma and 5 of other tissue types) of RCC and adjacent tissue specimens and a second set of 15 pairs of RCC and adjacent fresh tissue were collected from patients who attended the Haikou Municipal Hospital, which is affiliated to the Xiangya School of Medicine of the Central South University (Haikou, China). The inclusion criteria were patients undergoing radical nephrectomy or nephrosparing surgery. The exclusion criteria were patients receiving chemotherapy or targeted drug therapy and patients with distant RCC metastasis (T4) that did not undergo surgical treatment patients with grade IV RCC were excluded due to the very small number of patients, which was not suitable for statistical analysis. The 96 pairs of RCC and adjacent tissue specimens were collected by surgical removal between June 2004 and June 2014 from 63 males and 33 females aged 25–82 (mean, 64) years. All specimens were fixed in 10% formalin at 4°C for 12 h within 1 h of surgery, and then embedded in paraffin. Among these, 68 patients were followed up for 6–118 months (mean follow-up time, 47 months). None of the patients received chemotherapy or any other medications prior to surgery. The present study was approved by the Ethics Committee of Haikou Municipal Hospital, which is affiliated to the Xiangya School of Medicine of the Central South University. Written informed consent was obtained from all patients.
IHC
Paraffin-embedded tissue sections were dewaxed, hydrated and placed in EDTA antigen-repair solution (pH 9.0) at 95°C for 10 min for antigen repair. Endogenous peroxidase was blocked using 3% H2O2 for 10 min at room temperature. Subsequently, anti-human AIF antibody (1:100 in PBS; cat. no. 200-401-985; Rockland Immunochemicals Inc.) was added, and the tissue sections were incubated at 4°C in a humidity chamber overnight. The sections were then treated with horseradish peroxidase-conjugated secondary antibodies (1:200 in PBS; cat. no. GB23303; Servicebio) at 37°C for 30 min and diaminobenzidine reagent according to the manufacturer's instructions (DAB Horseradish Peroxidase Color Development Kit; cat. no. P0203; Beyotime Institute of Biotechnology). For the negative control group, PBS buffer without primary antibody was used. The results of the IHC were imaged at 20× and 40× on an Olympus Corporation light microscope and subjected to a double-blind analysis by two pathologists. Each specimen was randomized to count the number of positive cells in five high-power fields and was scored based on the average percentage of positive cells: ≤5%, 0 points; 6–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; and >75%, 4 points. In addition, the specimens were scored according to the intensity of color: No coloring, 0 points; light yellow, 1 point; brown, 2 points; light brown, 3 points; and dark brown, 4 points. Finally, the two scores were added and categorized as follows: 0–2, negative; 3–5, weak positive; and 6–8, strong positive. To facilitate statistical analysis, a score of ≥3 points was classified as positive. To quantify AIF expression in Figs. 1B and 3B, Image Pro plus version 6.0 software (Media Cybernetics, Inc.) was used to quantify the intensity of staining.
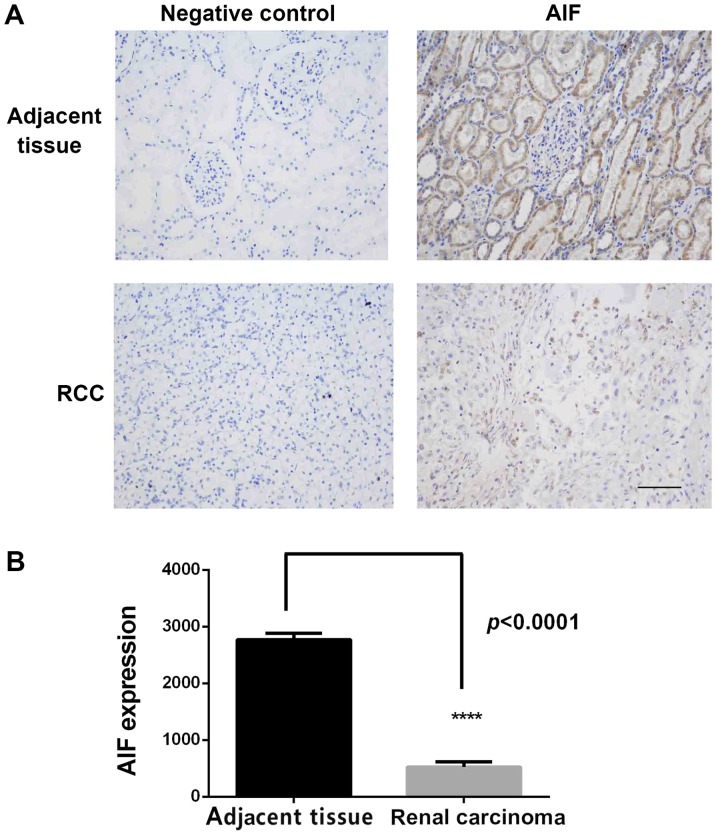
Figure 1.
AIF expression in RCC and adjacent normal tissues. (A) IHC staining of AIF expression in RCC and adjacent tissues. Strong staining of AIF was observed in normal kidney sections compared with weak staining in the adjacent tissues. (B) Quantification of AIF staining in IHC images of RCC and adjacent normal tissues. AIF expression was significantly decreased in RCC tissues. ****P<0.0001, n=96. Scale bar, 200 µm. AIF, apoptosis-inducing factor; RCC, renal cell carcinoma; IHC, immunohistochemistry.
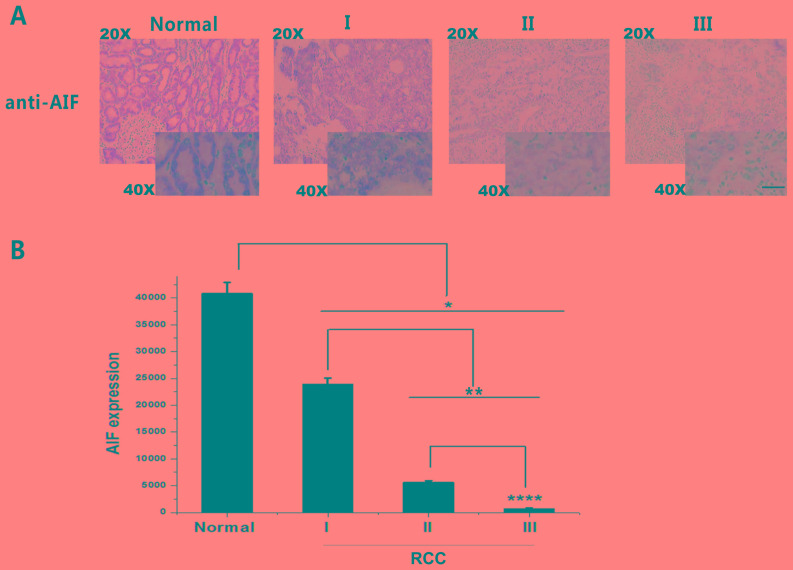
Figure 3.
AIF expression in RCC grades I, II and III and adjacent normal tissue. (A) IHC staining of AIF (brown) revealed a decrease in AIF expression with tumor grade. (B) Quantification of AIF expression in RCC grades I, II and III. AIF expression was significantly decreased in RCC tissues compared with that in adjacent normal tissues. *P<0.05, **P<0.01, ****P<0.0001. Scale bar, 400 µm. Normal, n=96; grade I, n=36; grade II, n=24; and grade II, n=36). AIF, apoptosis-inducing factor; RCC, renal cell carcinoma.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
A second set of 15 pairs of RCC and adjacent fresh tissue specimens were collected by surgical removal between April 2015 and April 2017 including 11 males and 4 females aged 25–82 (mean, 63±8.7) years. Total RNA from 15 pairs of RCC and adjacent tissues, which were stored in liquid nitrogen, was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Briefly, 2 µg of RNA was added to a solution of random primers (2 ml; Beijing ComWin Biotech Co., Ltd.) and water to a final volume of 15 µl. The mix was incubated at 70°C for 10 min, and then placed on ice immediately for 5 min. Subsequently dNTPs (0.25 µl; Takara Bio, Inc.), M-MLV Reverse Transcriptase (1 ml; Promega Corporation) and RNase inhibitor (0.5 ml; Promega Corporation) were added to the 15-µl mix. Finally, water was added to a final volume of 25 µl and the solution was incubated at 37°C for 1 h to synthesize cDNA. qPCR was performed using 10 µl SYBR® Green PCR Master mix (Roche Diagnostics), with specific forward and reverse primers and cDNA on an ABI QuantStudio version 5 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions for the qPCR were: 95°C For 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The following primers were obtained from Tsingke Biological Technology: AIF forward, 5′-AAGAAGTGGTCTGACCTCAAGA-3′ and reverse, 5′-AGGTTGCAGATACGTTGTTGC-3′; and GAPDH forward, 5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse, 5′-GGGGTCATTGATGGCAACAATA-3′. GAPDH was used as the reference gene. The results were quantified using the 2−ΔΔCq as described previously (20).
Immunofluorescence assay
The 96 pairs of RCC and adjacent tissues (grades I, II and III) were incubated with a primary AIF monoclonal antibody (1:50; cat. no. MA5-15880; Invitrogen; Thermo Fisher Scientific, Inc.). The tissues were incubated with a voltage-dependent anion-selective channel 1 (VDAC1) rabbit polyclonal antibody (1:50; cat. no. 10866-1-AP; Proteintech Group, Inc.) at 4°C overnight, which served as a mitochondrial marker. The tissues were subsequently incubated at 37°C for 1 h with an Alexa Fluor 488 goat anti-mouse immunoglobulin G (IgG) (H+L) antibody (1:20,000; cat. no. A32723; Thermo Fisher Scientific, Inc.) and an Alexa Fluor 555 goat anti-rabbit IgG (H+L) antibody (1:500; cat. no. A21428; Thermo Fisher Scientific, Inc.). Nuclei were counterstained using DAPI (Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for 10 min, and images were obtained using a FV1000 confocal microscope at 60× magnification. The signal values of AIF (green), VDAC1 (red), Merge (yellow) and DAPI (blue) were counted from 6 randomly selected with Image Pro Plus6.0 for quantification. The ratio of Merge/AIF was the percentage of mitochondria, and the rest were considered nuclei.
Statistical analysis
SPSS version 17.0.1 (SPSS, Inc.) and GraphPad Prism 6 (GraphPad Software, Inc.) were used for statistical analyses. The differences between two groups were compared using a paired Student's t-test, and ≥3 groups were compared using one-way ANOVA with a Tukey's post hoc test. Survival rates were compared using the Kaplan-Meier method and a log rank test was used to compare the survival curves of patients in the two groups. The association between clinicopathological features and AIF expression was analyzed using a Pearson's χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
AIF staining is decreased in RCC tissues
To investigate the role of AIF in RCC, 96 pairs of RCC and adjacent normal tissues were collected and AIF expression was determined using IHC. As shown in Fig. 1A, AIF was highly expressed in adjacent normal tissues, primarily in renal tubular epithelial cells with less expression in glomerular membrane cells. AIF expression in RCC tissue was low. Quantification of staining confirmed that AIF expression was significantly lower in RCC tissues compared with normal renal tissue (Fig. 1B and Table I).
Table I.
Association of AIF expression in renal cell carcinoma and adjacent normal tissue.
| AIF expression | ||||
|---|---|---|---|---|
| Tissue type | Positive, n | Negative, n | χ2 value | P-value |
| Renal cell carcinoma | 16 | 80 | 96.5 | <0.001 |
| Adjacent | 84 | 12 | ||
AIF, apoptosis-inducing factor.
AIF mRNA expression is decreased in RCC tissues
To further compare AIF expression in RCC and adjacent normal tissues, AIF mRNA expression was determined in 15 pairs of RCC and adjacent tissues. The results revealed that AIF expression was significantly decreased in RCC tissues compared with that in adjacent normal tissues (Fig. 2).
Figure 2.
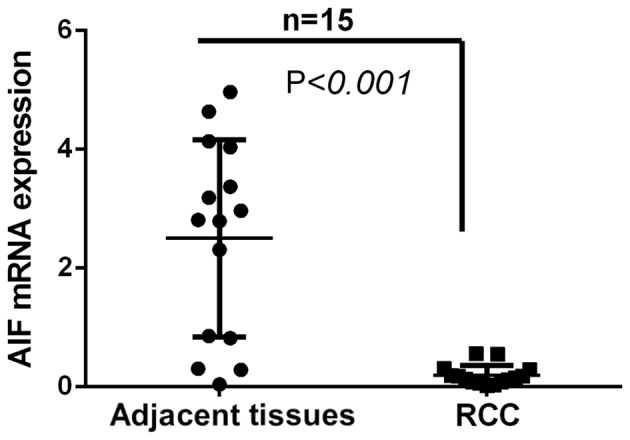
AIF mRNA expression in RCC and adjacent normal tissues. Total RNA extracted from 15 pairs of RCC and adjacent normal tissues was used for reverse transcription quantitative polymerase chain reaction. AIF expression in RCC tissues was significantly lower compared with that in adjacent normal tissues. (n=15). AIF, apoptosis-inducing factor; RCC, renal cell carcinoma.
AIF expression decreases with RCC progression
IHC findings demonstrated that reduced AIF expression was associated with increasing tumor grade. As shown in Table II, 87.5% of the adjacent normal tissue exhibited positive AIF expression. Conversely, 27.8, 16.7 and 5.6% of Grades I, II and III RCC samples exhibited positive AIF expression, respectively (Table II). The results revealed that AIF expression was associated with RCC progression (Table II). Quantification of AIF expression based on IHC demonstrated that AIF expression was significantly decreased in Grade I, II and III RCC compared with that in adjacent normal tissues (Fig. 3). Additionally, AIF expression in Group III was significantly lower than that in Group II, which was also lower than that in Group I. These results suggest that AIF expression is negatively associated with RCC grade. Thus, AIF downregulation may be a useful biomarker for diagnosing RCC and distinguishing tumor grades.
Table II.
Apoptosis-inducing factor expression in renal cell carcinoma grades I, II and III and adjacent normal tissue.
| Group | Samples, n | Positive, % | Negative, % |
|---|---|---|---|
| Normal tissue | 96 | 87.5 | 12.5 |
| RCC (total) | 96 | 16.7 | 83.3 |
| Grade I | 36 | 27.8 | 72.2 |
| Grade II | 24 | 16.7 | 83.3 |
| Grade III | 36 | 5.6 | 94.4 |
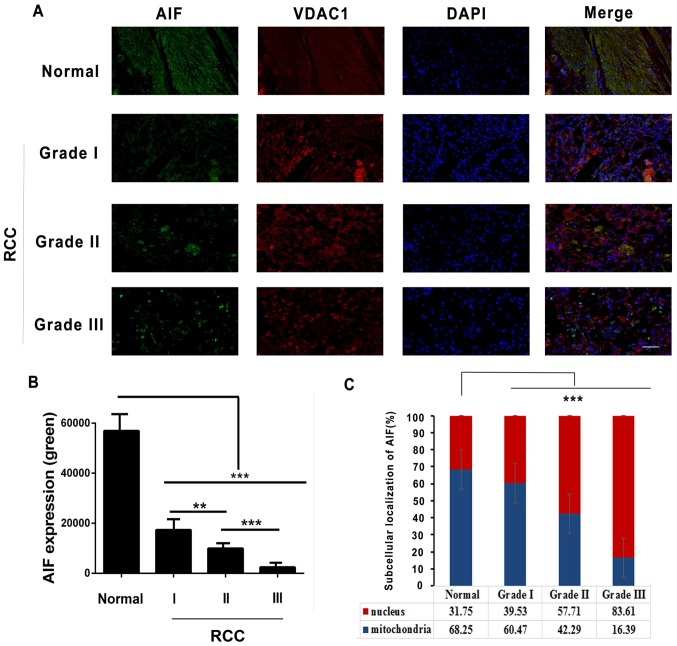
Reportedly, translocation of AIF from the mitochondrial intermembrane space in normal embryonic stem cells to the nucleus induces apoptosis (3). To examine the subcellular localization of AIF in RCCs, immunofluorescence staining was performed. As presented in Fig. 4A and B, AIF was found to be downregulated in RCC tissues according to tumor grade. Additionally, it was observed that AIF was primarily present in mitochondria (62.25%) in adjacent tissues, and less AIF was present in the nuclei (37.75%). In RCC tissues, more AIF was present in nuclei compared with mitochondria (Grade I, 39.53%; Grade II, 57.71% and Grade III, 83.61%) (Fig. 4A and C). These results suggest that AIF may translocate from the mitochondria to nucleus in RCC tissues.
Figure 4.
Subcellular localization of AIF in RCC cells. (A) Immunofluorescence staining of RCC grades I, II and III and adjacent normal tissue with antibodies against AIF (green), VDAC1 (red; mitochondrial marker) and with DAPI (blue; nuclear stain). (B) Quantification of AIF expression in RCC and adjacent normal tissue. (C) Quantification of AIF staining in mitochondrial and nuclear subcellular compartments in RCC grades I, II and III and adjacent normal tissue. **P<0.01, ***P<0.001 (n=6). Scale bar, 500 µm. AIF, apoptosis-inducing factor; RCC, renal cell carcinoma; VDAC1, voltage-dependent anon-selective channel 1.
Association between AIF expression and clinicopathological features in patients with RCC
Positive AIF expression rate in RCC was associated with age, tumor (T) stage and grade (P<0.05; Table III). The incidence of positive AIF expression in individuals aged <60 years was higher than that in individuals aged ≥60 years. Based on clinical T stage, the frequency of AIF-positive expression in stage T3 RCCs was significantly lower than that in stage T1 RCCs. Histopathological grade also indicated that the AIF-positive expression rate in patients with G3 grade RCCs was significantly lower than that in patients with G1 grade RCCs. These results suggest that decreased AIF expression is associated with RCC progression and grade. More severe malignancy was associated with decreased AIF-positive expression. AIF expression did not significantly differ between patients with lymph node metastasis and those without lymph node metastasis (P>0.05).
Table III.
Association between AIF expression and clinicopathological data of patients with renal cell carcinoma.
| AIF expression | ||||
|---|---|---|---|---|
| Clinicopathological feature | Positive, n | Negative, n | χ2 value | P-value |
| Age, years | ||||
| <60 | 10 | 24 | 6.157 | 0.013a |
| ≥60 | 6 | 56 | ||
| Sex | ||||
| Male | 10 | 53 | 0.083 | 0.773 |
| Female | 6 | 27 | ||
| T stage | ||||
| T1 | 9 | 21 | 6.583 | 0.037a |
| T2 | 3 | 39 | ||
| T3 | 4 | 20 | ||
| Grade | ||||
| I | 10 | 26 | 6.400 | 0.041a |
| II | 4 | 20 | ||
| III | 2 | 34 | ||
| Metastasis | ||||
| With | 2 | 19 | 0.987 | 0.32 |
| Without | 14 | 61 | ||
P<0.05. AIF, apoptosis-inducing factor; T, Tumor.
Negative AIF expression is associated with a worse survival rate
Of the 96 patients who underwent surgery, 68 were followed up for 6–118 months (mean follow-up time, 47 months). As presented in Fig. 5, the overall postoperative survival (OS) rate of the patients with negative AIF expression was significantly lower compared with that of patients with positive AIF expression (P=0.003). However, as the number of patients with positive AIF expression in the group was low, progression-free survival was not comparable between the two groups.
Figure 5.
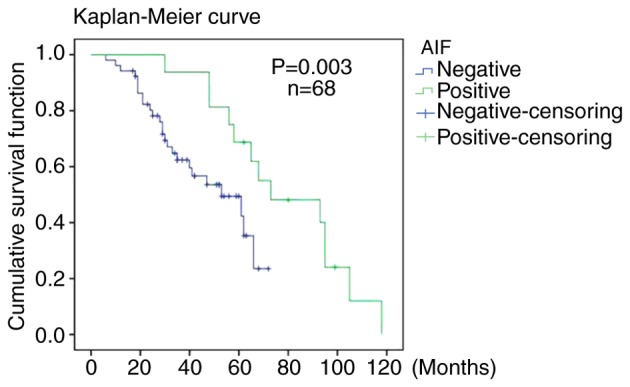
Postoperative survival rate of renal cell carcinoma patients with positive or negative AIF expression. Kaplan-Meier curves were used to analyze the postoperative survival rates. P=0.003 (n=68). AIF, apoptosis-inducing factor.
Discussion
AIF is thought to be a tumor suppressor and serves a critical role in caspase-independent cell apoptosis by triggering chromatin condensation and DNA fragmentation (21,22). Following an apoptotic signal, AIF is translocated from mitochondria to the nucleus where it initiates caspase-independent programmed cell death (3,23). However, AIF exhibits cell-type specificity in the induction of apoptosis. For example, in colon, breast and lung cancer cell lines, the mitochondria-to-nuclear localization of AIF was induced by peroxides and certain drugs used to treat cancer (24,25). Of note, in several colon and squamous carcinomas, AIF expression was significantly upregulated (26,27).
In the present study, 96 pairs of RCC and adjacent tissues were collected. Using IHC, it was demonstrated that AIF expression was significantly lower in RCC tissues compared with that in adjacent tissues. Although the IHC images in Fig. 3 appear notably different from the images in Fig. 1, this is due to the difference in the graphed background, and, as such, the quantification of AIF expression in Figs. 1B and 3B are very different due to the difference in staining color. In addition, immunofluorescent localization of the protein suggested that AIF translocated from mitochondria to the nucleus in RCC tissues (Fig. 4). AIF mRNA expression in RCC tissues, analyzed using RT-qPCR, was lower compared with that in normal renal tissues (Fig. 1). These results suggested that AIF downregulation in RCC tissue is associated with kidney tumorigenesis. Previous studies have reported that AIF was downregulated by factors such as hypoxia, microRNAs and epigenetic effectors (28–30). In addition, after 6–118 months of follow-up, the OS rates of patients with negative AIF expression were significantly worse compared with those of patients with positive AIF expression. Notably, a study by Xu et al (13) reported that AIF was significantly downregulated in kidney tumor specimens and that AIF overexpression induced apoptosis in RCC cells. The results of the present study are consistent with these findings reported by Xu et al (13).
In conclusion, Xu et al (13) suggested that AIF served a role in cell survival and kidney tumorigenesis, whilst the present study demonstrated an association between AIF expression and the progression of RCC. Therefore, AIF may serve as biomarker for RCC and determining AIF expression levels may have diagnostic value in patients with kidney tumors. Targeting AIF may be an effective treatment strategy for patients with RCC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AIF
apoptosis-inducing factor
- RCC
renal cell carcinoma
- OS
overall postoperative survival
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
Funding
This study was supported by the Hubei Polytechnic University School Talent Introduction Project (grant no. 180104), General project of Hainan Natural Science Foundation (grant no. 819MS136), Hubei Natural Science Foundation (grant no. 2014CFC1094), Huangshi Science and Technology Plan Project (grant no. 2014A069-1), Key Laboratory of Hubei Province 2014 Open Fund (grant no. SB201402) and Hubei Polytechnic University School Innovation Research Project (grant no. 14×jz05Q).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
ZW designed and performed experiments. CY designed and performed experiments, analyzed data and drafted the manuscript. YH and ZL performed certain experiments. XY and CL analyzed the data, provided valuable assistance and critically revised the manuscript. ZS designed and supervised the project, assisted with data analysis and interpretation, and critically revised the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committees of the Hubei Polytechnic University School (Huangshi, China) and that of the Haikou Hospital of Xiangya Medical School of Central South University (Haikou, China). Written informed consent was obtained from all patients.
Patient consent for publication
Written informed consent was obtained from all examined patients for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ghezzi D, Sevrioukova I, Invernizzi F, Lamperti C, Mora M, D'Adamo P, Novara F, Zuffardi O, Uziel G, Zeviani M. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis inducing factor. Am J Hum Genet. 2010;86:639–649. doi: 10.1016/j.ajhg.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 3.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 4.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 5.Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: Not just an apoptosis-inducing factor. Ann N Y Acad Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- 6.Shen SM, Guo M, Xiong Z, Yu Y, Zhao XY, Zhang FF, Chen GQ. AIF inhibits tumor metastasis by protecting PTEN from oxidation. EMBO Rep. 2015;16:1563–1580. doi: 10.15252/embr.201540536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of Poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 8.Leslie NR, Yang X, Downes CP, Weijer CJ. PtdIns(3,4,5)P(3)-dependent and independent roles for PTEN in the control of cell migration. Curr Biol. 2007;17:115–125. doi: 10.1016/j.cub.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CX, Li S, Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2015;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Suh J, Surh YJ, Na HK. Regulation of the tumor suppressor PTEN by natural anticancer compounds. Ann N Y Acad Sci. 2017;1401:136–149. doi: 10.1111/nyas.13422. [DOI] [PubMed] [Google Scholar]
- 11.Tsai MJ, Chang WA, Tsai PH, Wu CY, Ho YW, Yen MC, Lin YS, Kuo PL, Hsu YL. Montelukast induces apoptosis-inducing factor-mediated cell death of lung cancer cells. Int J Mol Sci. 2017;18:E1353. doi: 10.3390/ijms18071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohyama M, Tsuchiya A, Kaku Y, Kanno T, Shimizu T, Tanaka A, Nishizaki T. Phosphatidylinositol derivatives induce gastric cancer cell apoptosis by accumulating AIF and AMID in the nucleus. Anticancer Res. 2015;35:6563–6571. [PubMed] [Google Scholar]
- 13.Xu S, Wu H, Nie H, Yue L, Jiang H, Xiao S, Li Y. AIF downregulation and its interaction with STK3 in renal cell carcinoma. PLoS One. 2014;9:e100824. doi: 10.1371/journal.pone.0100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, Ding Z. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42:343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuusk T, Grivas N, de Bruijn R, Bex A. The current management of renal cell carcinoma. Minerva Med. 2017;108:357–369. doi: 10.23736/S0026-4806.17.05058-3. [DOI] [PubMed] [Google Scholar]
- 16.Ye DW, Zhang HL. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. Onco Targets Ther. 2014;7:925–935. doi: 10.2147/OTT.S41828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XL, Chen XQ, Zhang MN, Chen N, Nie L, Xu M, Gong J, Shen PF, Su ZZ, Weng X, et al. SOX9 was involved in TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling pathway. Int J Clin Exp Pathol. 2015;8:3871–3881. [PMC free article] [PubMed] [Google Scholar]
- 18.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, et al. External validation and comparison with other models of the International metastatic renal-cell carcinoma database consortium prognostic model: A population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Huang JH, Qu QH, Xia Q, Wang DS, Jin L, Sheng C. Evaluating the microRNA-target gene regulatory network in renal cell carcinomas, identification for potential biomarkers and critical pathways. Int J Clin Exp Med. 2015;8:7209–7219. [PMC free article] [PubMed] [Google Scholar]
- 20.Bustin S, Nolan T. Talking the talk, but not walking the walk: RT-qPCR as a paradigm for the lack of reproducibility in molecular research. Eur J Clin Invest. 2017;47:756–774. doi: 10.1111/eci.12801. [DOI] [PubMed] [Google Scholar]
- 21.Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): A ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/S0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 22.Ohiro Y, Garkavtsev I, Kobayashi S, Sreekumar KR, Nantz R, Higashikubo BT, Duffy SL, Higashikubo R, Usheva A, Gius D, et al. A novel p53 inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF) FEBS Lett. 2002;524:163–171. doi: 10.1016/S0014-5793(02)03049-1. [DOI] [PubMed] [Google Scholar]
- 23.Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal E, Chaudhuri A, Leiphrakpam PD, Haferbier KL, Brattain MG, Chowdhury S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer. 2014;14:145. doi: 10.1186/1471-2407-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JY, Zheng ZH, Son YO, Shi X, Jang YO, Lee JC. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol In Vitro. 2011;25:1654–1663. doi: 10.1016/j.tiv.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Jeong EG, Lee JW, Soung YH, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. Immunohistochemical and mutational analysis of apoptosis-inducing factor (AIF) in colorectal carcinomas. APMIS. 2006;114:867–873. doi: 10.1111/j.1600-0463.2006.apm_502.x. [DOI] [PubMed] [Google Scholar]
- 27.Skyrlas A, Hantschke M, Passa V, Gaitanis G, Malamou-Mitsi V, Bassukas ID. Expression of apoptosis-inducing factor (AIF) in keratoacanthomas and squamous cell carcinomas of the skin. Exp Dermatol. 2011;20:674–676. doi: 10.1111/j.1600-0625.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Zhang Y, Wang X, Blomgren K, Zhu C. Apoptosis-inducing factor downregulation increased neuronal progenitor, but not stem cell, survival in the neonatal hippocampus after cerebral hypoxia-ischemia. Mol Neurodegener. 2012;7:17. doi: 10.1186/1750-1326-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Z, Guo M, Yu Y, Zhang FF, Ge MK, Chen GQ, Shen SM. Downregulation of AIF by HIF-1 contributes to hypoxia-induced epithelial-mesenchymal transition of colon cancer. Carcinogenesis. 2016;37:1079–1088. doi: 10.1093/carcin/bgw089. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Li J, Zhao Y, Liu D. TNFα-induced downregulation of microRNA-186 contributes to apoptosis in rat primary cardiomyocytes. Immunobiology. 2017;222:778–784. doi: 10.1016/j.imbio.2017.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.