Abstract
Aims
Iron deficiency worsens symptoms, quality of life, and exercise capacity in chronic heart failure (CHF) and might do so by promoting fluid retention. We assessed whether iron repletion improved congestion in CHF and appraised the prognostic utility of calculated plasma volume status (PVS), a novel index of congestion, in the FAIR‐HF data set.
Methods and results
In FAIR‐HF, 459 iron deficient CHF patients were randomized to intravenous ferric carboxymaltose (FCM) or saline and assessed at 4, 12, and 24 weeks. Using weight and haematocrit, we calculated PVS in 436 patients. At baseline, PVS and weight were −5.5 ± 7.7% and 76.9 ± 14.3 kg, with peripheral oedema evident in 35% of subjects. Higher PVS values correlated to other congestion surrogates such as lower serum albumin. At 4 weeks, FCM was associated with greater reductions in weight (0.02) and PVS (P < 0.0001), and a trend for improved peripheral oedema at 24 weeks (0.07). Irrespective of treatment allocation, patients with a decrease in PVS from baseline to week 24 had higher increments in 6 min walking distance (61.4 m vs. 43.5 m, 0.02) and were more likely to improve their NYHA class (33.3% vs. 15.5%, 0.001). A PVS > −4% at baseline predicted worse outcomes even after adjustment for treatment assignment (hazard ratio 1.88, 95% confidence interval 1.01–3.51, 0.046).
Conclusions
Intravenous iron therapy with FCM is associated with early reductions in PVS and weight, implying that decongestion might be one mechanism via which iron repletion aids CHF patients. Calculated PVS is of prognostic utility in this cohort.
Keywords: Iron, Congestion, Plasma volume, Oedema
Introduction
Iron deficiency (ID) worsens symptoms, exercise capacity, and hospitalization rates in chronic heart failure (CHF),1, 2, 3 but the underlying mechanism(s) remain obscure. Because congestion drives dyspnoea, exercise intolerance, and hospital admissions in CHF,4, 5 ID might be detrimental by directly or indirectly augmenting fluid retention. In experimental animals and humans, ID has been shown to elevate circulating catecholamine levels that promote congestion in CHF,6, 7 and in the small FERRIC‐HF trial, intravenous (IV) iron sucrose significantly reduced body weight compared with placebo.8 To date, no study has appraised the impact of iron therapy on clinical congestion in a large CHF population, and none have addressed this interaction using newer and potentially more reliable metrics of fluid overload.
Plasma volume (PV) expansion underlies systemic congestion in CHF and can be objectively estimated using validated equations based on weight and haematocrit (Hct).9, 10 In a prior analysis, calculated PV values were shown to significantly mirror those measured using gold standard radioisotope assays. Moreover, in a trial data set of 5002 CHF patients, relative PV status (PVS), derived by subtracting calculated ideal from actual PV levels, predicted outcomes even after adjustment for 22 variables including natriuretic peptides.11
We sought to determine the distribution, correlates, and prognostic utility of calculated PVS in CHF patients enrolled in the large FAIR‐HF (Ferric carboxymaltose Assessment in patients with Iron deficiency and chronic Heart Failure) trial.12 Moreover, we investigated the impact of IV iron therapy with ferric carboxymaltose (FCM) on calculated PVS and clinical congestion in this cohort. Our co‐primary hypotheses were that PVS levels would be predictive of outcomes and that iron repletion would trigger early reductions in PVS and clinical congestion consistent with a possible direct interaction between ID and volume overload in CHF.
Methods
Study design and patients
The design and primary results of the FAIR‐HF trial have been reported previously.12 Briefly, FAIR‐HF was a multicentre, randomized, double‐blind trial including 459 ambulatory patients with NYHA class II or III CHF,13 an left ventricular ejection fraction (LVEF) ≤40% (NYHA II) or ≤45% (NYHA III), ID as defined by a ferritin <100 ug/L or 100 to 299 ug/L with a transferrin saturation <20%, and a haemoglobin (Hb) level between 9.5 and 13.5 g/L. Patients were randomized in a 2:1 ratio to IV iron as FCM or IV placebo (saline) weekly until iron repletion (correction phase), then 4 weekly until week 24 (maintenance phase). Regular assessments were done at weeks 4, 12, and 24. After 24 weeks of treatment, patients randomized to IV FCM had significant improvements in Patient Global Assessment, NYHA class, and 6 min walking distance (6MWD), compared with patients allocated to saline. The Hct and weight at randomization were used for PVS calculations and were available in 436 (95%) patients whose data were therefore used in this post hoc analysis. The FAIR‐HF trial was conducted in accordance with the Declaration of Helsinki, with independent Institutional Review Board or ethics committee approval at all participating centres, and with written informed consent from all patients.
Plasma volume equations
Actual PV was calculated with the following equation that was derived by curve‐fitting techniques using subjects' Hct and weight compared with PV values measured with gold standard radioisotope assays9:
where Hct is a fraction and a = 1530 in men and 864 in women, and b = 41 in men and 47.9 in women.
Ideal PV was calculated from the following well‐established formula10:
where c = 39 in men and 40 in women.
Relative PVS, an index of the degree to which patients have deviated from their ideal PV, was subsequently calculated from the following equation:
Clinical congestion
Patients were weighed and examined for signs and symptoms of congestion at randomization and at weeks 4, 12, and 24. The presence or absence of peripheral oedema, pulmonary congestion (crackles and rales), and an S3 gallop (third heart sound) were recorded, as was the actual jugular venous pressure (JVP).
Statistics
Data are presented as mean ± SD, frequencies (%), or medians (interquartile range). Intergroup comparisons were made using a Kruskall–Wallis analysis of variance, analysis of covariance, Student t‐test, Pearson χ2 test, or a Mann–Whitney U‐test as appropriate. Associations between variables were determined using logistic regression. A P‐value <0.10 was used to enter and retain covariates into stepwise multivariable regression models.
To assess the impact of treatment assignment on PVS, comparisons of changes in PVS from baseline at 4, 12, and 24 weeks between the placebo and FCM group were evaluated by comparing the least square means at each visit from a repeated‐measures model using an unstructured covariance structure and adjusting for the continuous baseline value and a visit × drug interaction. Changes over time for other variables were similarly determined.
For prognostic analyses, the key safety endpoint was the combined outcome of all‐cause death or hospitalization. The relation between safety endpoints and baseline PVS as a continuous or categorical (≤−4% vs. >−4%) variable was determined using Cox proportional hazards analyses. This categorical cut‐off was previously shown to best stratify outcomes in a large CHF cohort.11 In Cox analyses, the proportional hazard assumption was assessed by inspection of the log time–log hazard plot. The event rate using a person‐time ‘at risk’ denominator, hazard ratio (HR), 95% confidence interval (CI), and significance levels for χ2 (likelihood ratio test) were calculated. Kaplan–Meier cumulative survival plots were constructed for display and assessed using the log‐rank test.
Data were analysed using SAS version 9.2 (SAS Institute Inc, Cary, NC). A two‐tailed P‐value <0.05 was considered statistically significant.
Results
Calculated plasma volume status in FAIR‐HF and its baseline correlates
Baseline characteristics of the FAIR‐HF cohort have been previously published.12 In brief, the clinical and laboratory features of patients at enrolment were similar between those randomized to FCM (n = 304) and those randomized to placebo (n = 155). The baseline characteristics of the 436 patients included in this substudy are shown in Table 1. Their mean weight‐adjusted actual PV, weight‐adjusted ideal PV, and PVS were 37.3 ± 3 mL/kg, 3036.6 ± 573.2 mL/kg, and −5.5 ± 7.7%, respectively. Values of PVS ranged from −25% to +24%. A PVS ≤0 or ˃0 was evident in 350 (80.3%) and 86 (19.7%) patients, respectively (Figure 1). The mean JVP was 1.9 cm with 9.2% of patients having an elevated JVP (>4 cm), and 35%, 23%, and 12% of patients having peripheral oedema, pulmonary oedema, and an S3 gallop, respectively.
Table 1.
Baseline characteristics of FAIR‐HF patients stratified by PVS
| All (N = 436) | PVS ≤ 0% (N = 350) | PVS > 0% (N = 86) | P value | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 67.8 (10.71) | 67.1 (10.54) | 70.5 (11) | 0.008 |
| Male gender, n (%) | 203 (46.6) | 154 (44.0) | 49 (57.0) | 0.040 |
| NYHA III, n (%) | 361 (82.8) | 288 (82.3) | 73 (84.9) | 0.635 |
| Left ventricular ejection fraction, % | 32.3 (5.7) | 32.5 (5.61) | 31.2 (5.94) | 0.055 |
| Ischaemic aetiology, n (%) | 351 (80.5) | 283 (80.9) | 68 (79.1) | 0.761 |
| Prior CABG, n (%) | 46 (10.6) | 34 (9.7) | 12 (14.0) | 0.326 |
| Prior PTCA, n (%) | 61 (14.0) | 48 (13.7) | 13 (15.1) | 0.862 |
| Prior stroke, n (%) | 29 (6.7) | 24 (6.9) | 5 (5.8) | 0.815 |
| History of atrial fibrillation, n (%) | 131 (30.0) | 107 (30.6) | 24 (27.9) | 0.695 |
| History of hypertension, n (%) | 352 (80.7) | 291 (83.1) | 61 (70.9) | 0.014 |
| History of diabetes mellitus, n (%) | 120 (27.5) | 103 (29.4) | 17 (19.8) | 0.080 |
| Physical examination | ||||
| Systolic blood pressure, mm Hg | 126.1 (14.71) | 127 (14.52) | 122.4 (14.98) | 0.008 |
| Diastolic blood pressure, mm Hg | 76.4 (9.43) | 77.1 (8.92) | 73.5 (10.85) | 0.001 |
| Heart rate, bpm | 71.3 (11.58) | 71.5 (11.74) | 70.2 (10.92) | |
| Weight, kg | 76.9 (14.73) | 79.8 (14.19) | 65 (10.36) | <0.001 |
| Body mass index, kg/m2 | 27.9 (4.78) | 28.8 (4.62) | 24.3 (3.68) | <0.001 |
| Hip/waist ratio, cm | 1.1 (0.15) | 1.1 (0.15) | 1.1 (0.15) | 0.289 |
| Jugular venous pressure, cm | 1.9 (2.22) | 1.9 (2.16) | 1.8 (2.49) | 0.871 |
| Peripheral oedema, n (%) | 152 (34.9) | 127 (36.3) | 25 (29.1) | 0.256 |
| Pulmonary oedema, n (%) | 99 (22.7) | 80 (22.9) | 19 (22.1) | 0.888 |
| Third heart sound (S3 gallop), n (%) | 53 (12.2) | 44 (12.6) | 9 (10.5) | 0.714 |
| Six minute walk test distance, m | 270.8 (106.83) | 275 (106.4) | 253.6 (107.43) | 0.096 |
| Quality of life | ||||
| KCCQ overall score | 59 (20.05) | 58.4 (20.16) | 61.7 (19.48) | 0.173 |
| EQ‐5D health score | 54.2 (16.27) | 54.2 (16.25) | 54 (16.49) | 0.922 |
| Laboratory measurements | ||||
| Haemoglobin, g/dL | 11.9 (1.29) | 12.2 (1.18) | 10.9 (1.14) | <0.001 |
| Haematocrit | 0.4 (0.04) | 0.4 (0.04) | 0.3 (0.03) | <0.001 |
| Serum ferritin, μg/L | 53.8 (57.8) | 54.5 (59.31) | 51.1 (51.39) | 0.624 |
| Transferrin saturation, % | 17.4 (11.25) | 17.7 (9.92) | 16.1 (15.55) | 0.242 |
| C‐reactive protein, mg/L | 5 (4.33) | 4.9 (4.33) | 5.2 (4.34) | 0.577 |
| Sodium, mmol/L | 140.7 (2.83) | 140.8 (2.87) | 140.4 (2.63) | 0.221 |
| Serum albumin, g/L | 43.1 (2.78) | 43.3 (2.72) | 42.2 (2.83) | 0.001 |
| Alanine aminotransferase, U/L | 19.8 (11.14) | 20.2 (11.31) | 18.5 (10.42) | 0.231 |
| Aspartate amiontransferase, U/L | 22.8 (9.58) | 22.7 (9.67) | 23.1 (9.29) | 0.752 |
| Creatinine, mg/dL | 1.2 (0.59) | 1.1 (0.43) | 1.5 (0.96) | <0.001 |
| GFR (MDRD), mL/min/1.73 m2 | 63.7 (22.57) | 65.1 (21.47) | 58.1 (25.98) | 0.010 |
| Blood urea nitrogen, mg/dL | 24.5 (12.87) | 23.4 (11.81) | 28.8 (15.86) | 0.001 |
| Calculated plasma volumes | ||||
| Ideal plasma volume, mL | 3037 (573) | 3153 (550) | 2563 (395) | <0.001 |
| Actual plasma volume, mL | 2843 (441) | 2878 (445) | 2698 (394) | 0.001 |
| Plasma volume status (PVS), % | −5.5 (7.71) | −8.2 (5.6) | 5.5 (4.71) | — |
| Medications, % | ||||
| ACE inhibitor or ARB, n (%) | 402 (92.2) | 326 (93.1) | 76 (88.4) | 0.1751 |
| β‐blocker, n (%) | 373 (85.6) | 299 (85.4) | 74 (86.0) | 1.000 |
| Aldosterone antagonist, n (%) | 211 (48.4) | 167 (47.7) | 44 (51.2) | 0.6303 |
| Cardiac glycosides, n (%) | 69 (15.8) | 52 (14.9) | 17 (19.8) | 0.3217 |
| Diuretic, n (%) | 402 (92.2) | 323 (92.3) | 79 (91.9) | 1.000 |
Data are means (SD) or numbers (frequency). ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease equation; PTCA, percutaneous coronary angioplasty; PVS, plasma volume status.
Figure 1.
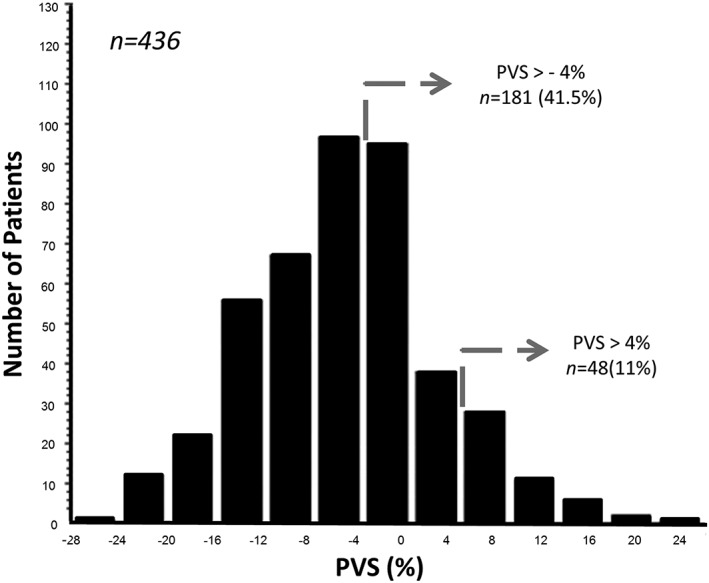
Distribution of plasma volume status (PVS) in FAIR‐HF.
On stratification, patients who were estimated to have a relatively increased PV (i.e. a PVS > 0%) tended to be older and female with biochemical markers of potentially greater fluid retention such as a lower serum albumin, higher blood urea nitrogen, and a lower estimated glomerular filtration rate than those with a PVS ≤ 0% (Table 1). They also had poorer haemodynamics and less premorbid hypertension. In a multivariable model incorporating age, gender, systolic blood pressure, albumin, estimated glomerular filtration rate, LVEF, and the use of diuretics, ACE inhibitors and β‐blockers, only age (odds ratio 0.97, 0.02), systolic blood pressure (odds ratio 1.02, 0.01), and serum albumin (odds ratio 1.13, 0.01) independently predicted PVS.
Impact of ferric carboxymaltose on calculated plasma volume status and clinical congestion
At baseline, mean PVS was similar in the FCM (−5.3% ± 7.7%) and placebo (−6% ± 7.8%) groups. Over time, treatment with FCM was associated with significant reductions from baseline in PVS at week 4 (−2.5 ± 5.9% vs. 0.6 ± 4.3%), 12 (−3.9 ± 6.8% vs. 0.2 ± 5.3%), and 24 (−4.1 ± 7.4% vs. −2.0 ± 5.5%), compared with placebo [Figure 2 A]. The treatment effect was maximal at week 12. Treatment with FCM was also associated with significant increases from baseline in Hct at week 4 (0.02 ± 0.04% vs. −0.004 ± 0.03%), 12 (0.03 ± 0.04% vs. −0.002 ± 0.04%), and 24 [0.03 ± 0.05% vs. 0.01 ± 0.04%; Figure 2 B]. In contrast, FCM treatment was associated with a significant reduction from baseline in weight at week 4 (−0.2 ± 2.4 kg vs. 0.3 ± 1.8 kg), but not at week 12 (0.2 ± 3.0 kg vs. 0.6 ± 3.3 kg), or 24 [0.3 ± 3.2 kg vs. 0.6 ± 3.7 kg; Figure 2 C]. Similar to the effect on PVS, there was an improvement in peripheral oedema with FCM treatment compared with placebo [Figure 2 D] that did not reach statistical significance. Similar improvements in jugular venous distension (0.54), pulmonary oedema (0.35), or the presence of an S3 gallop rhythm (1.00) with FCM treatment were not evident.
Figure 2.
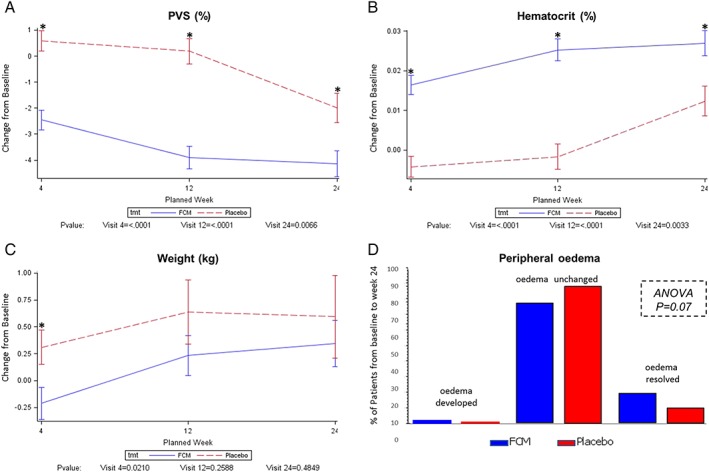
Change in markers of congestion in FAIR‐HF. Alterations from baseline to week 24 in PVS (A), Hct (B), weight (C), and peripheral oedema (D) are shown. A total of 372, 371, and 309 patients had data for analysis at weeks 4, 12, and 24, respectively. ANOVA, analysis of variance; FCM, ferric carboxymaltose; PVS, plasma volume status.
Clinical correlates of change in calculated plasma volume status
A total of 309 (70.9%) patients survived to week 24 and had sufficient data to calculate change in PVS. From baseline to week 24, mean change in PVS (∆PVS) in this cohort was −3.5 ± 6.9%, with volume expansion (∆PVS > 0%) evident in 93 (30.1%) patients. Compared with those with volume expansion, patients with decreasing volume status were more likely to improve their NYHA status by at least one class (33.3% vs. 15.1%, 0.005) despite adjustment for treatment and the interaction term for PVS change and treatment (0.23 for interaction). In the subgroup of patients who received FCM, NYHA class improved in 36.3% in those with ∆PVS ≤ 0% compared with 21.4% in those with ∆PVS > 0%. Similarly, in the placebo‐treated subgroup, NYHA class improved in 25% and 5.4% of those with ∆PVS ≤ 0% and >0%, respectively. In contrast, while improvements in exercise capacity, as reflected by ∆6MWD, was greater in those with ∆PVS ≤ 0% (43.5 m vs. 26.8 m, 0.02), this became non‐significant (0.13) after adjustment for treatment and the interaction term for PVS change and treatment (0.65 for interaction).
Calculated plasma volume status and outcomes in FAIR‐HF
Safety endpoints are listed in Table 2. During the study period, 55 (13%) patients experienced the key safety endpoint of all‐cause death or hospitalization, 9 (2%) died from any cause, 8 (2%) experienced a cardiovascular death, 3 (1%) experienced a CHF death, 46 (11%) were hospitalized for any cause, and 25 (6%) died or were hospitalized for worsening CHF.
Table 2.
Safety endpoints stratified by baseline PVS cut‐off
| PVS ≤ −4% (n = 255) | PVS > −4% (n = 181) | |||||
|---|---|---|---|---|---|---|
| Endpoint or event | Number of events | Incidence/ (100 patient‐year at risk) | Number of events | Incidence/ (100 patient‐year at risk) | Hazard ratio 95% CI | P value |
| Any hospitalization or deatha | 23 | 20 (16.7) | 32 | 25 (31.6) | 1.89 (1.05–3.40) | 0.031 |
| Any death | 3 | 3 (2.4) | 6 | 6 (7.1) | 2.94 (0.74–11.76) | 0.11 |
| Cardiovascular death | 3 | 3 (2.4) | 5 | 5 (5.9) | 2.45 (0.58–10.25) | 0.21 |
| Death due to worsening CHF | 1 | 1 (0.8) | 2 | 2 (2.4) | 2.93 (0.27–32.35) | 0.36 |
| Any hospitalization | 20 | 17 (14.2) | 26 | 21 (26.5) | 1.86 (0.98–3.53) | 0.053 |
| Any hospitalization for any cardiovascular reason | 15 | 14 (11.6) | 18 | 14 (17.3) | 1.48 (0.71–3.11) | 0.30 |
| Any hospitalization for worsening CHF | 7 | 6 (4.9) | 9 | 7 (8.4) | 1.74 (0.58–5.18) | 0.31 |
| Any hospitalization for any cardiovascular reason or death | 18 | 17 (14.1) | 24 | 18 (22.2) | 1.57 (0.81–3.05) | 0.18 |
| Any hospitalization for worsening CHF or death | 10 | 9 (7.3) | 15 | 12 (14.4) | 1.98 (0.84–4.71) | 0.11 |
| Any hospitalization or death for any cardiovascular reason | 18 | 17 (14.1) | 23 | 17 (21.0) | 1.48 (0.76–2.91) | 0.25 |
| Any hospitalization or death for worsening CHF | 8 | 7 (5.7) | 11 | 8 (9.6) | 1.70 (0.62–4.69) | 0.30 |
Only unplanned hospitalizations and deaths are summarized. CHF, chronic heart failure; PVS, plasma volume status.
Key endpoint.
A PVS cut‐off of −4% was previously shown to be optimally predictive of outcomes in a large CHF cohort.11 In this FAIR‐HF population, patients with a baseline PVS > −4% experienced a higher incidence of all safety endpoints than those with a PVS ≤ −4%, with the difference in incidence statistically significant for the key safety endpoint of all‐cause death or hospitalization. In Cox analyses, a PVS > −4% was associated with an 89% (unadjusted HR 1.89, 95% CI 1.05 to 3.40, 0.03) greater risk of the key endpoint (Figure 3) even after bivariate adjustment for treatment assignment (adjusted HR 1.88, 95% CI 1.01 to 3.51, 0.046), JVP (adjusted HR 2.18, 95% CI 1.05 to 4.51, 0.04), or the presence of pulmonary (adjusted HR 1.88, 95% CI 1.01 to 3.50, 0.047) or peripheral oedema (adjusted HR 1.89, 95% CI 1.01 to 3.52, 0.045). As a continuous variable, higher PVS values were also associated with a greater incidence of all‐cause death or hospitalization (unadjusted HR 1.07, 95% CI 1.03 to 1.12, P < 0.001).
Figure 3.
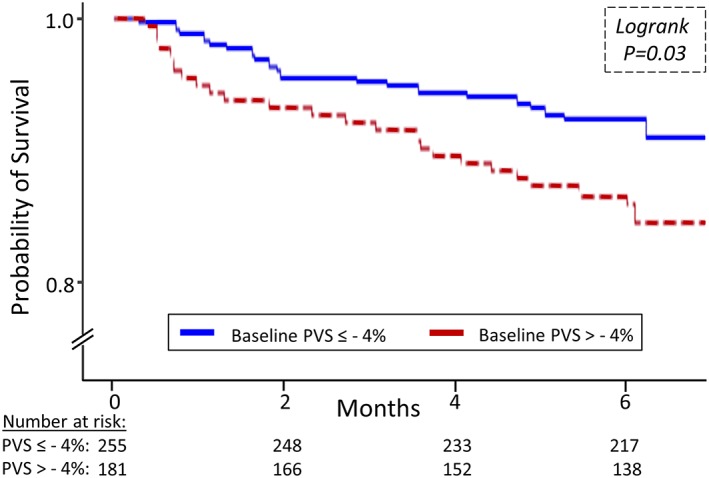
Baseline PVS categories (≤−4% vs. >−4%) and survival. PVS, plasma volume status.
Discussion
Iron deficiency escalates symptoms and exercise intolerance in CHF and might do so by promoting fluid retention. We tested this hypothesis and the prognostic utility of PVS, a novel index of congestion, in the large FAIR‐HF data set and made several observations. First, volume status, as estimated by the PVS equation, was mildly contracted in this stable population and related to age, gender, and biochemical markers of fluid overload. Second, compared with placebo, IV iron therapy with FCM was associated with greater reductions in PVS and other measures of congestion such as weight. The impact of FCM on PVS and weight was evident early at 4 weeks supporting a potential direct decongestive effect of iron repletion in CHF. Third, an increase in PVS over time was associated with worsening symptoms and exercise capacity irrespective of treatment allocation. Fourth, higher PVS values predicted an increased risk of death or hospitalization over the coming 6 months.
Plasma volume status was marginally contracted in the FAIR‐HF cohort to a degree comparable with other stable CHF patients. Using tracer dilution techniques, Bonfils et al. and Adlbrecht et al. reported actual PV values of 37 ± 6 mL/kg and 37 ± 4 mL/kg, respectively,14, 15 in line with our calculated value of 37 ± 3 mL/kg. This contrasts to the higher PV levels measured in decompensated CHF patients (58 ± 3 mL/kg) and healthy controls (43 ± 3 mL/kg)16 and reflects the impact of optimal medical and device‐based therapies on volume status. Consistent with the aforementioned PV levels, and the fact that the ideal PV equation was originally derived in normal individuals, it is unsurprising that patients in FAIR‐HF had mean PV levels that were ~5% lower than their estimated ideal. This contraction of PV in CHF is important as it likely allows the failing myocardium to continue to operate on a reasonable portion of the Frank–Starling curve.
Concordant with its potential utility as a surrogate of volume overload, calculated PVS was higher in patients with features of greater disease severity such as poorer haemodynamics and correlated to key biochemical markers of congestion. This included serum albumin that is known to modulate plasma oncotic pressure.17 These associations, together with our prior demonstration of a good correlation between calculated and measured PV levels,11 supported our use of the PVS equation to gauge changes in intravascular filing with IV FCM.
Treatment with FCM was associated with reductions in PVS at 4 weeks and the fact that this was paralleled by attenuations in weight, which is a clinical barometer of congestion, suggests that FCM might have had a true and early decongestive effect. This is consistent with the FERRIC‐HF trial that showed a 4.6 kg relative reduction in weight with IV iron sucrose compared with control over 16 weeks.8 It also accords with Toblli et al. who reported a 1.5 kg/m2 relative reduction in body mass index with IV iron sucrose vs. saline over 6 months.18 In contrast, no meaningful changes in other physical markers of congestion were seen with FCM. This likely reflects the subjective nature of physical examinations, especially in the setting of multicentre trials. While definitive answers cannot be given, several mechanisms might explain the reduction in PVS seen with FCM.
Treatment with FCM might have been linked to attenuations in PVS for a number of reasons. First, it could be argued that decrements in PVS merely reflect the increments in Hct seen with FCM and are not truly indicative of a change in congestion. However, FCM treatment was also accompanied by reductions in weight. Given that weight is a clinically accepted gauge of short‐term PV changes and that no evidence exists to suggest that FCM triggers non‐oedematous weight loss, it is likely that FCM truly diminished congestion. This diminution might have been indirect by virtue of iron repletion improving myocardial function leading to downstream improvements in global clinical status and congestion. Alternatively, FCM might have improved renal function19 and/or directly modulated key aspects of the CHF syndrome such as the neurohormonal axis to trigger decongestion. This is plausible given that ID enhances circulating catecholamine levels that promote congestion partly by activating the renin–angiotensin–aldosterone cascade.6, 7, 20 This direct mode of action appears more credible given that 4 weeks is conventionally too short for any agent to significantly remodel the failing myocardium, and IV iron repletion in CHF has yet to be shown to improve LVEF on echocardiography.2, 8 Irrespective of the underlying mechanism(s), changes in PVS over time correlated to alterations in other indices of CHF status.
Temporal increases in PVS were accompanied by worsening symptoms. This likely reflects the combination of impaired tissue oxygenation because of lower haemoglobin levels, decreased effort tolerance because of weight gain, and impaired lung and skeletal muscle function as a result of tissue congestion. That changes in PVS were linked to changes in key markers of survival suggested that it too might also be related to mortality.
Similar to the findings in the larger VaL‐HeFT population,11 calculated PVS as a continuous or dichotomized (≤−4% vs. >−4%) variable forecasted deaths and hospitalizations in the FAIR‐HF cohort. Moreover, it did so after adjustment for treatment allocation implying that the associations elucidated here were not simply driven by FCM. This is consistent with the ominous nature of other surrogates of congestion such as elevated pulmonary capillary wedge pressures, ultrasound indices of raised intracardiac filling, jugular venous distension, a third heart sound, congestive symptoms, and natriuretic peptides.21, 22, 23, 24, 25 Calculated PVS might be prognostic because it truly reflects fluid retention that could be a marker or mediator of CHF progression. Alternatively, it may only be mirroring the prognostic implications of weight and Hct or other factors in CHF that modulate these variables.
Our study has limitations. First, it is a retrospective post hoc analysis of the FAIR‐HF trial with all the inherent weaknesses this entails including the inability to establish a causal relation between correlated variables. Second, like all surrogates of congestion, the PVS equation is not perfect, underestimates true volume status, and has so far only shown modest correlation with invasively determined PV in CHF patients in a retrospective study.11 Additional refinements of this equation are likely needed but, ultimately, the true utility of PVS can only be gauged via prospective interventional studies. Third, we did not quantify PV directly, and further studies are needed to investigate the impact of IV iron repletion on volume status as measured using radioisotope techniques. For now, the results presented in this analysis should be considered hypothesis generating.
In summary, IV iron therapy with FCM is associated with reductions in PVS and weight even as early as 4 weeks after treatment initiation. This supports a potential decongestive effect of IV iron therapy in CHF that might be one mechanism via which iron repletion improves symptoms, quality of life, and exercise performance in this population. Calculated PVS, a simple novel marker of fluid overload, predicted deaths and hospitalizations in the FAIR‐HF cohort irrespective of treatment assignment.
Conflict of interest
The FAIR‐HF trial was supported by Vifor Pharma, Glattbrugg, Switzerland. All authors had full access to the study data, decided to submit the report for publication, and assume responsibility for the completeness and accuracy of the data, and the content and integrity of the report. C.M. and S.C. are employees of Vifor. D.O.O. has received speaker's fees and research support from Vifor and Pharmacosmos. S.D.A. has received fees for consulting and/or speaking from Bayer, Boehringer Ingelheim, Novartis, Servier, and Vifor. S.D.A. has received research grants from Abbott Vascular and Vifor. G.P. is a member of Committees in trials and registries sponsored by Vifor, Novartis, Bayer, and Servier. P.P. has received consulting and speaker's fees and research grants from Vifor.
Funding
The FAIR‐HF trial was supported by Vifor Pharma, Glattbrugg, Switzerland. D.O.O. is supported by the British Heart Foundation (grant number FS/14/77/30913), the NIHR Biomedical Research Centre at Guy's and St Thomas' NHS foundation trust and King's College London.
Acknowledgements
None to declare.
Okonko, D. O. , Jouhra, F. , Abu‐Own, H. , Filippatos, G. , Colet, J. C. , Suki, C. , Mori, C. , Ponikowski, P. , Anker, S. D. , and for the FAIR‐HF Investigators (2019) Effect of ferric carboxymaltose on calculated plasma volume status and clinical congestion: a FAIR‐HF substudy. ESC Heart Failure, 6: 621–628. 10.1002/ehf2.12462.
References
- 1. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors and relation to anemia, exercise capacity and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 2. Jankowska EA, Tkaczyszyn M, Suchocki T, Droz D, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomised controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 3. Nunez J, Comin‐Colet J, Minana G, Nunez E, Santas E, Mollar A, Valero E, Garcia‐Blas S, Cardells I, Bodi V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for heart failure. Eur J Heart Fail 2016; 18: 798–802. [DOI] [PubMed] [Google Scholar]
- 4. Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep 2011; 8: 233–241. [DOI] [PubMed] [Google Scholar]
- 5. Klein L. Treating hemodynamic congestion is the key to prevent heart failure hospitalizations. JACC Heart Fail 2016; 4: 345–347. [DOI] [PubMed] [Google Scholar]
- 6. Dillmann E, Johnson DG, Martin J, Mackler B, Finch C. Catecholamine elevation in iron deficiency. Am J Phys 1979; 237: R297–R300. [DOI] [PubMed] [Google Scholar]
- 7. Webb TE, Krill CE Jr, Oski FA, Tsou KC. Relationship of iron status to urinary norepinephrine excretion in children 7‐12 years of age. J Pediatr Gastroenterol Nutr 1982; 1: 207–209. [DOI] [PubMed] [Google Scholar]
- 8. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and non‐anemic patients with symptomatic chronic heart failure and iron deficiency (FERRIC‐HF): a randomised, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 9. Hakim RM. Plasmapheresis In Daugirdas J. T., Blake P. G., Ing T. S., eds. Handbook of Dialysis, Vol. 236, third ed. Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 10. Longo D. Table 218: body fluids and other mass data In Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine, 18th ed. McGraw‐Hill; 2011. p A‐1. [Google Scholar]
- 11. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, Papalia F, Weissert S, Coats CJ, Thomas M, Kuskowski M, Cohn JN, Woldman S, Anand IS, Okonko DO. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015; 17: 35–43. [DOI] [PubMed] [Google Scholar]
- 12. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhardt Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 1–13. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 14. Bonfils PK, Damgaard M, Taskiran M, Goetze JP, Norsk P, Gadsboll N. Impact of diuretic treatment and sodium intake on pasma volume in patients with compensated systolic heart failure. Eur Heart J 2010; 12: 995–1001. [DOI] [PubMed] [Google Scholar]
- 15. Adlbrecht C, Kommata S, Hulsmann M, Szekeres T, Bieglmayer C, Strunk G, Karanikas G, Berger R, Mortl D, Kletter K, Maurer G, Lang IM, Pacher R. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J 2008; 29: 2343–2350. [DOI] [PubMed] [Google Scholar]
- 16. Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole‐Wilson PA, Harris PC. Oedema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation 1989; 80: 299–305. [DOI] [PubMed] [Google Scholar]
- 17. Jabbour R, Ling HZ, Norrington K, Amaral N, Zaman N, Aggarwal S, Aung N, Barron A, Manisty C, Baruah R, Cole GD, Missouris CG, Mayet J, Francis DP, Cheng AS, Thomas M, Woldman S, Okonko DO. Serum albumin changes and multivariate dynamic risk modelling in chronic heart failure. Int J Cardiol 2014; 176: 437–443. [DOI] [PubMed] [Google Scholar]
- 18. Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 19. Ponikowski P, Filippatos G, Colet JC, Willenheimer R, Dickstein K, Lüscher T, Gaudesius G, von Eisenhart Rothe B, Mori C, Greenlaw N, Ford I, Macdougall I, Anker SD, FAIR‐HF Trial Investigators . The impact of intravenous ferric carboxymaltose on renal function: an analysis of the FAIR‐HF study. Eur J Heart Fail 2015; 17: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension 2011; 57: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 22. Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Pinkstaff S, Arena R. Echocardiography with tissue doppler imaging and cardiopulmonary exercise testing in patients with heart failure: a correlative and prognosis analysis. Int J Cardiol 2010; 143: 323–329. [DOI] [PubMed] [Google Scholar]
- 23. Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017; 19: 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001; 345: 574–581. [DOI] [PubMed] [Google Scholar]
- 25. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017; 19: 627–634. [DOI] [PubMed] [Google Scholar]


