Abstract
Aims
Uptitrating angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers (ACE‐I/ARBs), beta‐blockers, and mineralocorticoid receptor antagonists (MRAs) to optimal doses in heart failure with reduced ejection fraction (HFrEF) is associated with improved outcomes and recommended in guidelines. Studies of ambulatory patients found that a minority are prescribed optimal doses. However, dose at hospital discharge has rarely been reported. This information may guide quality improvement initiatives during and following discharge.
Methods and results
We assessed 370 consecutive patients with HFrEF hospitalized at two centres in Vancouver, Canada. Of those without contraindications, 86.4%, 93.4%, and 44.7% were prescribed an ACE‐I/ARB/sacubitril–valsartan, beta‐blocker, or MRA, respectively. The proportion of eligible patients prescribed target dose was respectively 28.6%, 31.7%, and 4.1%. Forty‐two of 248 eligible patients (16.9%) were prescribed ≥50% of target dose, and only three patients received target dosing of all three medication classes. In multivariate regression models, cardiologist involvement in care was independently associated with increased dose and prescription of ≥50% of target dose for all medications, whereas a history of HF was only predictive for beta‐blockers.
Conclusions
In a single‐region experience of hospitalized HFrEF patients, a high proportion of eligible patients were discharged on ACE‐I/ARB or beta‐blocker. Less than half were prescribed MRAs, and few were prescribed ≥50% or target dosing of all medications. Further exploration into barriers to medication uptitration, and improvement in processes of care, is needed.
Keywords: Acute heart failure, Systolic heart failure, HFrEF, Guideline‐directed medical therapy, Guideline adherence
Introduction
Guideline‐directed medical therapy (GDMT) in heart failure with reduced ejection fraction (HFrEF) includes angiotensin‐converting enzyme inhibitors (ACE‐I) or angiotensin receptor blockers (ARBs), beta‐blockers, and mineralocorticoid receptor antagonists (MRAs) for all patients.1, 2, 3 Assessment of appropriate use requires patient‐level knowledge of indications, contraindications, and tolerability. While early cohorts and administrative data suggested major underutilization,4, 5 recent studies such as the ESC‐HF and CHAMP‐HF registries indicate lower true non‐adherence rates of approximately 5%.6, 7, 8 Maximum tolerated doses were utilized in the landmark clinical trials, are recommended in guidelines,1, 2, 3 and associated with improved outcomes but not excess discontinuation rates.9, 10, 11, 12, 13, 14, 15, 16 Dose therefore has potential to be a more rigorous quality indicator in ambulatory care, but assessment requires knowledge of the individual drug and target dose that may be limited. Prescription of GDMT of any dose at hospital discharge is also associated with improved outcomes.17, 18, 19 However, the relevance of dose in the hospital setting is less certain, as rapid titration (particularly of beta‐blockers) has potential to increase adverse effects despite no clear demonstration of harm.20 Nevertheless, hospitalization often provides access to specialist care and an opportunity for uptitration that may otherwise not occur in the community. Moreover, medication dose in patients with pre‐existing HF is a potential surrogate marker of care in the region served by a hospital. The Guidelines Adherence Indicator 50+ (GAI50+) is a potential quality metric that quantifies HF medication guideline adherence, contraindications, and dose.21 High GAI50+ scores have been associated with reduced mortality and symptom classification among ambulatory patients.21 We examined current practice during HF hospitalization in terms of (i) guideline adherence, (ii) target dosing, and (iii) extension of the GAI50+ to triple therapy.
Methods
Data sources
A hospital‐based quality assurance programme was analysed, which links electronic hospital information and laboratory systems to the Discharge Abstract Database from the Canadian Institute of Health Information. The Discharge Abstract Database is a population‐based administrative database that captures demographics, admission and discharge dates, and diagnostic and procedural codes. This information was supplemented by chart review by two trained clinical care analysts using an electronic data abstraction form which incorporates detailed mandatory data fields with standardized coding of reasons for non‐prescription. Research ethics approval was granted by the University of British Columbia—Providence Healthcare Research Institute Review Board.
Patient population
All adults age ≥18 with an unplanned admission to St. Paul's or Mt. St. Joseph's Hospitals in Vancouver, British Columbia, Canada, between 1 April 2015 and 31 December 2017 with a most responsible diagnosis of HF were included. In order to meet criteria for inclusion, participants needed a primary or secondary diagnosis of HF based on the following International Classification of Disease, Tenth Revision, Canadian Enhancement (ICD‐10‐CA) codes, in keeping with the American Heart Association's Get With the Guidelines—HF definition22: I11.0, hypertensive heart disease with additional code for heart failure; I25.5, ischaemic cardiomyopathy; I42.0, dilated cardiomyopathy; I42.6, alcoholic cardiomyopathy; I42.7, cardiomyopathy due to drugs and other external agents; I42.8, other cardiomyopathies; I42.9, cardiomyopathy, unspecified; I43, cardiomyopathy in diseases classified elsewhere; I50.0, congestive heart failure; I50.1, left ventricular failure; and I50.9, heart failure, unspecified.
We only included patients with a documented left ventricular ejection fraction (LVEF) of ≤40%. For patients with multiple admissions during the study period, only the index admission was included. Patients were excluded if they were admitted for a planned intervention, had a ventricular assist device, died during hospitalization, left hospital against medical advice, or had an incomplete chart.
Data collection and statistical analysis
Baseline characteristics were stratified by de novo or pre‐existing diagnosis of HFrEF and compared using two‐sample t‐tests for continuous variables and the Mann–Whitney U‐test for non‐normal distributions. For categorical variables, Pearson's χ 2 or Fisher's exact test was used. We described the proportion of patients: (i) on any, ≥50% of target dose, or target dosing of each medication class; and (ii) receiving indicated prescriptions of all three medication classes concurrently at any, ≥50% of target doses as summarized by the GAI50+,21 or target dose according to guidelines.1, 2, 3 The GAI50+ has been previously applied to HF patients,21 and its algorithm is described in the Supporting Information. Ordinal multivariable logistic regression models were created for each medication class to define variables independently associated with increasing dosing level, stratified as no prescription, <50% of target dose, ≥50% to 99% of target dose, and target dose. Where there was evidence of non‐proportionality (e.g. age), a partial proportional odds model was created with different response variables for each dosing level.23 Separate logistic regression models were also created to determinate variables associated with ≥50% and target dosing. The following covariates were forced into initial models based on previous associations with optimal dosing21, 24, 25: age, sex, co‐morbidity burden as determined by the Charlson co‐morbidity index,26 LVEF, prior myocardial infarction, pre‐existing HF, and admission under a cardiologist (all of which were board certified in general cardiology or had additional subspecialty training)27, 28; systolic blood pressure (SBP), estimated glomerular filtration rate (eGFR), and serum potassium for ACE‐I/ARB and MRA; and diagnosis of atrial fibrillation for beta‐blockers. Variables were also included in initial models if P < 0.20 on univariable modelling. A backward stepwise approach was used, where predictors with P ≤ 0.05 were considered statistically significant and retained in final multivariable models.
Missing data were excluded from analyses. For all statistical tests, a P value <0.05 was considered significant. All analyses were performed in SAS version 9.4.
Results
One thousand eighty‐six patients were hospitalized with a primary diagnosis of HF and discharged alive (Figure 1). After excluding repeat hospitalizations (n = 259), patients with no LV function evaluation (n = 108), and those with LVEF >40% (n = 349), 370 patients with HFrEF were included as the final study population.
Figure 1.
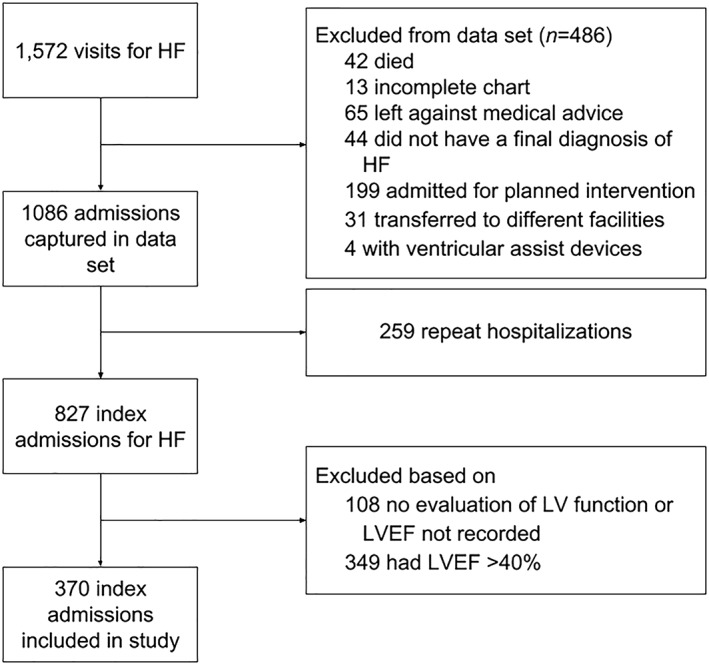
Flow diagram of patients into study. HF, heart failure; LVEF, left ventricular ejection fraction.
The mean age was 66.2 years, and 70% were male (Table 1). Cardiovascular disease, risk factors, and non‐cardiac co‐morbidities were common: existing HF (59%), prior myocardial infarction (22%), atrial fibrillation (48%), hypertension (57%), diabetes mellitus (33%), chronic obstructive pulmonary disease (17%), and chronic kidney disease (28%). The mean LVEF was 26.6%. Half (50%) of patients were cared for by a cardiologist. With respect to factors that may influence uptitration of medication, a significantly higher proportion of those with pre‐existing HF had prior chronic kidney disease (37% vs. 14%, P < 0.0001) but significantly lower mean SBP (122.9 vs. 134.6 mmHg, P < 0.0001) and mean diastolic BP (76.2 vs. 83.1 mmHg, P = 0.001). The proportion of those with prior myocardial infarction (24% vs. 17%, P = 0.09), atrial fibrillation (50% vs. 44%, P = 0.22), co‐morbidity burden by the Charlson co‐morbidity index (P = 0.06), or mean heart rate on electrocardiogram was not significantly different (83.2 vs. 83.0, P = 0.94).
Table 1.
Baseline characteristics of enrolled patients
| All (n = 370) | Pre‐existing HF (n = 219) | De novo HF (n = 151) | P‐value | |
|---|---|---|---|---|
| Age | 66.2 ± 16.2 | 67.5 ± 16.2 | 65.2 ± 16.1 | 0.18 |
| Male sex | 260 (71) | 159 (73) | 101 (67) | 0.28 |
| Current smoker | 82 (22) | 48 (22) | 34 (23) | 0.89 |
| Cardiovascular disease | ||||
| Prior myocardial infarction | 80 (22) | 54 (25) | 26 (17) | 0.09 |
| Prior CABG or PCI | 66 (18) | 49 (22) | 17 (11) | 0.006* |
| Atrial fibrillation | 176 (48) | 110 (50) | 66 (44) | 0.22 |
| Prior Stroke or TIA | 42 (11) | 27 (12) | 15 (10) | 0.48 |
| Peripheral arterial disease | 20 (5) | 12 (6) | 8 (5) | 0.94 |
| Hypertension | 209 (57) | 134 (61) | 75 (50) | 0.03* |
| Diabetes mellitus | 121 (33) | 77 (35) | 44 (29) | 0.23 |
| Non‐cardiovascular disease | ||||
| COPD | 61 (17) | 42 (19) | 19 (13) | 0.09 |
| Malignancy | 34 (9) | 24 (11) | 10 (7) | 0.16 |
| Liver disease | 33 (9) | 28 (13) | 5 (3) | 0.002* |
| Chronic kidney disease | 103 (28) | 82 (37) | 21 (14) | <0.0001* |
| Charlson co‐morbidity index | 0.06 | |||
| ≤2 | 230 (62) | 126 (58) | 104 (69) | |
| 3–4 | 117 (32) | 76 (35) | 41 (27) | |
| ≥5 | 23 (6) | 17 (8) | 6 (4) | |
| Medications and treatments | ||||
| Digoxin | 37 (10) | 27 (12) | 10 (7) | 0.07 |
| Hydralazine | 56 (15) | 41 (19) | 15 (27) | 0.03* |
| Nitrates | 100 (27) | 74 (34) | 26 (17) | <0.001* |
| Loop diuretic | 285 (77) | 172 (79) | 113 (75) | 0.41 |
| Acetylsalicylic acid (ASA) | 153 (41) | 89 (41) | 64 (42) | 0.74 |
| Warfarin | 95 (26) | 66 (30) | 29 (19) | 0.02* |
| Direct oral anticoagulant | 68 (18) | 38 (17) | 30 (20) | 0.42 |
| Amiodarone | 30 (8) | 22 (10) | 8 (5) | 0.1 |
| Pacemaker | 43 (12) | 31 (14) | 12 (8) | 0.07 |
| ICD | 34 (9) | 31 (14) | 3 (2) | <0.0001* |
| LVEF | 26.6 ± 8.3 | 27.6 ± 8.2 | 26.0 ± 8.3 | 0.05* |
| Systolic blood pressure | 127.7 ± 27.7 | 122.9 ± 25.7 | 134.6 ± 26.7 | <0.0001* |
| Systolic BP <90 mmHg | 18 (5) | 14 (6) | 4 (3) | 0.14 |
| Diastolic blood pressure | 79.0 ± 17.1 | 76.2 ± 16.0 | 83.1 ± 17.8 | 0.0001* |
| Heart rate (on admission) | 95.0 ± 25.6 | 92.6 ± 25.6 | 98.3 ± 25.4 | 0.04* |
| Heart rate (on ECG) | 83.1 ± 20.0 | 83.2 ± 21.1 | 83 ± 18.4 | 0.94 |
| Haemoglobin | 126.7 ± 20.3 | 124.1 ± 21.3 | 130.5 ± 18.1 | 0.002* |
| eGFR (on admission) | 60.4 ± 26.4 | 56.7 ± 26.1 | 65.7 ± 26.1 | 0.001* |
| eGFR (on discharge) | 62.3 ± 25.9 | 58.6 ± 26.1 | 67.7 ± 24.6 | 0.009* |
| BNP | 1137 [582–2081] | 1259.5 [645.5–2104.5] | 900 [557–1910] | 0.11 |
| NT‐proBNP | 6182 [2829–12 852] | 6220 [2786–13 158] | 5881 [2829–12 852] | 0.39 |
| Cardiologist care | 183 (50) | 107 (49) | 76 (50) | 0.78 |
| Length of stay | 7.0 [4.0–11.0] | 8.0 [4.0–13.0] | 7.0 [4.0–11.0] | 0.27 |
BP, blood pressure; CABG, Coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, Implanted Cardioverter‐Defibrillator; LVEF, left ventricular ejection fraction; NT‐proBNP, N terminal pro brain natriuretic peptide; PCI, percutaneous coronary intervention; TIA, Transient Ischemic Attack.
Values are means ± standard deviation, n (%), or median [interquartile range].
P‐value <0.05.
Overall prescription rates, ≥50% of target, and target dosing
Among all 370 patients, 66%, 88%, and 38% were prescribed an ACE‐I/ARB, beta‐blocker, and MRA, respectively (Figure 2). Among eligible patients without contraindications (Figure 3), 86%, 93%, and 48% were prescribed an ACE‐I/ARB/angiotensin receptor‐neprilysin inhibitor (ARNI), beta‐blocker, and MRA, respectively, while 60%, 59%, and 27% were prescribed ≥50% of target dose. The percentage of eligible patients at target dose was respectively 29%, 32%, and 4%. Frequent contraindications to therapy included renal dysfunction (n = 71 and 40 for ACE‐I/ARB/ARNI and MRA, respectively), hyperkalaemia (n = 1 and 7 for ACE‐I/ARB/ARNI and MRA, respectively), hypotension (n = 6, 5, and 3 for ACE‐I/ARB/ARNI, beta‐blocker, and MRA, respectively), and bradycardia (n = 3 for beta‐blockers).
Figure 2.
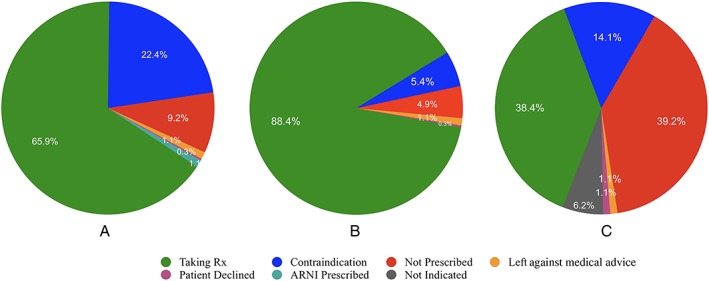
Number and percentage of patients taking each medication class and reasons for non‐prescription: (A) angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker/ARNI, (B) beta‐blockers, and (C) mineralocorticoid receptor antagonist.
Figure 3.
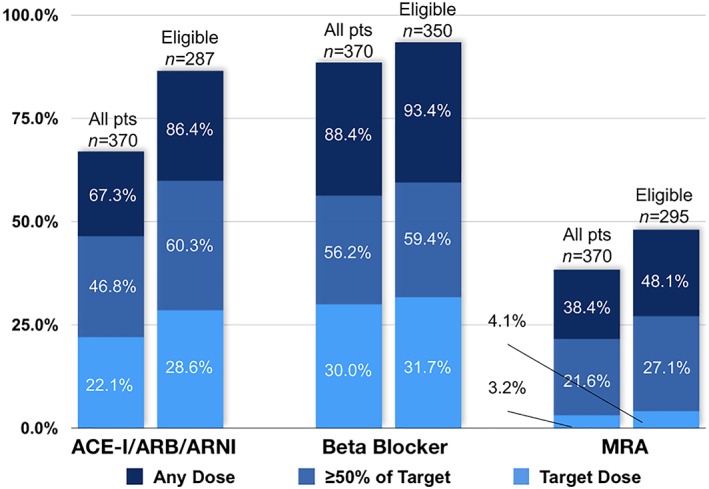
Percentage of patients at each dosing level among all patients (left of paired bar graphs) and eligible patients (right of paired bar graphs). ACE‐I/ARB, angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Among the 248 eligible patients without contraindication to any component of triple therapy, 111 (44%) received all three medication classes concurrently (Table 2). Forty‐two of 248 eligible patients (17%) were prescribed ≥50% of target dose of all medication classes, which represents the ‘high’ GAI50+ rating for this study population. Only three patients received target dosing of all medication classes. There was no significant difference between those with pre‐existing and de novo HF in the proportion prescribed any, ≥50%, or targeting dosing of all three medications classes concurrently.
Table 2.
No. and percentage of patients at various doses of all three medication classes
| All without contraindication (n = 248) | Pre‐existing HF (n = 139) | De novo HF (n = 109) | P‐value | |
|---|---|---|---|---|
| No. on any dose of all 3 medications | 109 (44.0%) | 68 (48.9%) | 41 (37.6%) | 0.08 |
| No. at GAI50+ of all 3 medications | 42 (16.9%) | 24 (17.3%) | 18 (16.5%) | 0.88 |
| No. at target doses of all 3 medications | 3 (1.2%) | 2 (1.4%) | 1 (0.9%) | 0.58 |
GAI50+, Guidelines Adherence Indicator 50+; HF, heart failure.
Predictors of appropriate prescribing and dose
Tables 3 and 4 list the predictors of increased dosing level and ≥50% of target dosing, respectively, of each medication class in either univariate or multivariate modelling, with predictors of target dosing for ACE‐I/ARB and beta‐blockers listed in Supporting Information, Table S1 . Increasing age was independently associated with reduced dose of all medications. SBP was independently associated with increased dose and prescription of ≥50% of target for ACE‐I/ARB. However, eGFR and serum potassium were not independent predictors of dose after multivariable adjustment. Pre‐existing HF was independently associated with increased dose, ≥50% of target, and target dose for beta‐blockers. Cardiologist involvement in care was the most consistent independent predictor of dose among all medication classes, whether assessed by level of dose, prescription of ≥50% of target, or maximum dose. The characteristics of patients admitted under a cardiologist vs. a non‐cardiologist are defined in Supporting Information, Table S2 .
Table 3.
Predictors of increased dosing level among eligible patients
| Covariate | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| ACE‐I/ARB | ||
| Agea (per 10 year increase, from 0% to <50% of target dose) | 0.83 (0.67–1.04), P = 0.10 | 0.83 (0.66–1.04), P = 0.10 |
| Agea (per 10 year increase, from <50% to 50–99% of target dose) | 0.70 (0.60–0.83), P < 0.001 | 0.69 (0.58–0.82), P < 0.0001* |
| Agea (per 10 year increase, from 50% to 99% of target dose) | 0.82 (0.70–0.96), P < 0.001 | 0.85 (0.72–1.00), P = 0.0.05 |
| Female sex | 0.98 (0.62–1.55), P = 0.92 | P = 0.96 |
| History of heart failure | 0.71 (0.47–1.08), P = 0.11 | P = 0.90 |
| History of myocardial infarction | 0.71 (0.42–1.17), P = 0.18 | P = 0.95 |
| LVEF (per 10% increase) | 1.00 (0.78–1.29), P = 0.99 | P = 0.19 |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.85 (0.54–1.36), P = 0.51 | P = 0.99 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.33 (0.10–1.09), P = 0.69 | P = 0.99 |
| Systolic BP (per 10 mmHg increase) | 1.05 (0.97–1.14), P = 0.22 | 1.10 (1.01–1.20), P = 0.04* |
| eGFR (per 10 mL/min/1.73 m increase) | 1.12 (1.02–1.22), P = 0.02 | P = 0.78 |
| Serum potassium (per mmol/L increase) | 2.10 (1.29–3.44), P = 0.003 | P = 0.80 |
| Cardiologist care | 2.64 (1.72–4.06), P < 0.001 | 2.35 (1.50–3.70), P < 0.001* |
| Beta‐blocker | ||
| Agea (per 10 year increase, from 0% to <50% of target dose) | 0.81 (0.63–1.03), P = 0.08 | 0.87 (0.68–1.10), P = 0.24 |
| Agea (per 10 year increase, from <50% to 50–99% of target dose) | 0.81 (0.71–0.93), P = 0.002 | 0.80 (0.69–0.93), P = 0.003* |
| Agea (per 10 year increase, from 50% to 99% of target dose) | 0.97 (0.94–1.12), P = 0.64 | 0.94 (0.80–1.11), P = 0.46 |
| Female sex | 0.99 (0.65–1.50), P = 0.9 | P = 0.42 |
| History of heart failure | 2.25 (1.51–3.34), P < 0.0001 | 1.98 (1.32–2.98), P = 0.001* |
| History of myocardial infarction | 0.80 (0.50–1.28), P = 0.35 | P = 0.33 |
| History of atrial fibrillation | 1.41 (0.96–2.06), P = 0.08 | 1.81 (1.19–2.75), P = 0.006* |
| LVEF (per 10% increase) | 0.98 (0.78–1.23), P = 0.87 | P = 0.65 |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.88 (0.58–1.33), P = 0.53 | P = 0.83 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.60 (0.25–1.45), P = 0.26 | P = 0.83 |
| Systolic BP (per 10 mmHg increase) | 0.94 (0.87–1.01), P = 0.08 | P = 0.84 |
| Heart rate (per 10 bpm increase) | 0.97 (0.88–1.06), P = 0.48 | P = 0.30 |
| Cardiologist care | 1.86 (1.27–2.74), P = 0.002 | 1.87 (1.24–2.84), P = 0.003* |
| MRA | ||
| Age (per 10 year increase) | 0.73 (0.64–0.83), P < 0.001 | 0.85 (0.72–0.99), P = 0.04* |
| Female sex | 0.57 (0.35–0.93), P = 0.02 | P = 0.31 |
| History of heart failure | 1.82 (1.18–2.83), P = 0.007 | 1.66 (1.04–2.65), P = 0.03* |
| History of myocardial infarction | 1.56 (0.94–2.60), P = 0.09 | 1.81 (1.04–3.14), P = 0.04* |
| LVEF (per 10% increase) | 0.58 (0.45–0.75), P < 0.001 | 0.62 (0.46–0.82), P = 0.001* |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.64 (0.40–1.05), P = 0.08 | P = 0.15 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.57 (0.21–1.55), P = 0.27 | P = 0.15 |
| Systolic BP (per 10 mmHg increase) | 0.87 (0.80–0.95), P = 0.002 | P = 0.39 |
| eGFR (per 10 mL/min/1.73 m increase) | 1.08 (0.99–1.17), P = 0.08 | P = 0.99 |
| Serum potassium (per mmol/L increase) | 1.59 (0.96–2.64), P = 0.07 | P = 0.86 |
| Cardiologist care | 3.48 (2.22–5.44), P < 0.001 | 2.99 (1.86–4.81), P < 0.0001* |
ACE‐I/ARB, angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
Variable violated proportional odds assumption; effect displayed by each dosing level.
P < 0.05 and included in final model.
Table 4.
Predictors of prescription of ≥50% of target dose among eligible patients
| Covariate | Unadjusted odds ratio (95% CI) | Adjusted odds ratio |
|---|---|---|
| ACE‐I/ARB | ||
| Age (per 10 year increase) | 0.72 (0.61–0.84), P < 0.0001 | 0.71 (0.59–0.85), P < 0.001* |
| Female sex | 0.90 (0.53–1.51), P = 0.69 | P = 0.88 |
| History of heart failure | 0.61 (0.37–0.98), P = 0.04 | 0.56 (0.32–0.96), P = 0.04* |
| History of myocardial infarction | 0.65 (0.37–1.16), P = 0.14 | P = 0.15 |
| LVEF (per 10% increase) | 1.03 (0.89–1.19), P = 0.70 | P = 0.22 |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.96 (0.57–1.63), P = 0.89 | P = 0.22 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.50 (0.13–1.93), P = 0.32 | P = 0.22 |
| Systolic BP (per 10 mmHg increase) | 1.06 (0.96–1.16), P = 0.24 | 1.16 (1.03–1.29), P = 0.01* |
| eGFR (per 10 mL/min/1.73 m increase) | 1.15 (1.04–1.28), P = 0.008 | P = 0.82 |
| Serum potassium (per mmol/L increase) | 2.20 (1.24–3.90), P = 0.007 | 1.95 (1.05–3.63), P = 0.04* |
| Cardiologist care | 2.71 (1.66–4.43), P < 0.0001 | 2.40 (1.40–4.13), P = 0.002* |
| Beta‐blocker | ||
| Age (per 10 year increase) | 0.81 (0.71–0.93), P = 0.003 | 0.85 (0.74–0.99), P = 0.04* |
| Female sex | 0.96 (0.60–1.53), P = 0.85 | P = 0.47 |
| History of heart failure | 2.56 (1.64–3.98), P < 0.0001 | 2.57 (1.63–4.05), P < 0.0001* |
| History of myocardial infarction | 0.73 (0.44–1.23), P = 0.24 | P = 0.23 |
| History of atrial fibrillation | 1.21 (0.79–1.86), P = 0.38 | P = 0.06 |
| LVEF (per 10% increase) | 0.97 (0.75–1.25), P = 0.80 | P = 0.59 |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.86 (0.54–1.38), P = 0.54 | P = 0.85 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.62 (0.24–1.63), P = 0.33 | P = 0.85 |
| Systolic BP (per 10 mmHg increase) | 0.94 (0.87–1.02), P = 0.13 | P = 0.93 |
| Heart rate (per 10 bpm increase) | 0.98 (0.88–1.10), P = 0.77 | P = 0.50 |
| Cardiologist care | 1.74 (1.13–2.69), P = 0.01 | 1.64 (1.02–2.62), P = 0.04* |
| MRA | ||
| Age (per 10 year increase) | 0.72 (0.62–0.85), P < 0.0001 | P = 0.20 |
| Female sex | 0.59 (0.33–1.09), P = 0.09 | P = 0.60 |
| History of heart failure | 1.63 (0.96–2.77), P = 0.07 | P = 0.08 |
| History of myocardial infarction | 1.15 (0.62–2.12), P = 0.66 | P = 0.29 |
| LVEF (per 10% increase) | 0.54 (0.39–0.75), P < 0.001 | 0.51 (0.37–0.72), P < 0.001* |
| Charlson co‐morbidity score 3–4 vs. ≤2 | 0.66 (0.36–1.20), P = 0.17 | P = 0.47 |
| Charlson co‐morbidity score ≥5 vs. ≤2 | 0.56 (0.15–2.00), P = 0.37 | P = 0.47 |
| Systolic BP (per 10 mmHg increase) | 0.90 (0.81–0.99), P = 0.04 | P = 0.83 |
| eGFR (per 10 mL/min/1.73 m increase) | 1.10 (0.99–1.21), P = 0.08 | P = 0.75 |
| Serum potassium (per mmol/L increase) | 1.08 (0.59–1.96), P = 0.80 | P = 0.24 |
| Cardiologist care | 3.14 (1.82–5.43), P < 0.0001 | 2.95 (1.68–5.18), P < 0.001* |
ACE‐I/ARB, angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
P < 0.05 and included in final model.
Discussion
We report several key findings in this detailed analysis of medical therapy dosing. Most eligible patients hospitalized with HFrEF were prescribed an ACE‐I/ARB or beta‐blocker, but less than half were prescribed an MRA. Among eligible patients, approximately 60% on ACE‐I/ARB/ARNI and beta‐blockers were prescribed ≥50% of target dose but only about 30% prescribed target dose. Few patients were prescribed MRA target dose. Overall, less than half of patients were prescribed all three medication classes concurrently, and less than a fifth were prescribed ≥50% of triple therapy target dosing. Cardiologist involvement in care was most consistently associated with increased dose and prescription of ≥50% or target dosing of medications.
Overall prescription rates
In Danish, Dutch, or multinational registries that restricted analyses to HFrEF outpatients such as the Hjerteplus (n = 8792),29 QUALIFY (n = 7092),30 ESC‐HF (n = 4792),6 CHAMP‐HF (n = 3518),8 and CHECK‐HF registries (n = 5701),31 prescription rates ranged from 85% to 94% for ACE‐I/ARBs, 81% to 93% for beta‐blockers, and 32% to 69% for MRAs. This suggests slightly lower rates of ACE‐I/ARB and MRA prescription in our cohort compared with ambulatory HFrEF patients, which can be explained in part by higher rates of renal dysfunction at baseline among hospitalized HF patients.6 However, our cohort had similar rates of beta‐blocker and MRA prescription, but lower ACE‐I/ARB prescription, at discharge compared with inpatients in the Get With the Guidelines Registry.32
Target dosing
Several large cohorts and trials have reported dose, including a Swedish multicentre cohort (n = 2093)33; the BIOSTAT‐CHF study (n = 2100)16; the ESC‐HF,6 CHAMP‐HF,8 CHECK‐HF,31 and PINNACLE (n = 1421)34 registries; and the GUIDE‐IT trial.35 The proportions prescribed ≥50% and target dose for each class are ACE‐I/ARB 40% to 76% for ≥50% and 14% to 44% target; beta‐blockers 31% to 55% for ≥50% and 6% to 28% target; and MRA 49% to 98% for ≥50% and 31% to 77% target. We found similar ACE‐I/ARB doses, higher beta‐blocker, but lower MRA doses. The reason for such high beta‐blocker uptake and dosing was unclear, as there were similar or lower percentages of patients with other indications for beta‐blockers (such as atrial fibrillation or prior myocardial infarction) compared with previous studies.6, 16, 21, 33 However, the majority of patients had a pre‐existing diagnosis of HF, which might have allowed for prior beta‐blocker uptitration.
A substantially lower proportion were prescribed ≥50% or target dosing of MRAs. One potential explanation is that 94% of prescribed MRAs were spironolactone, which has variable recommendations for uptitration. In the RALES trial, spironolactone dose was increased from 25 to 50 mg only if patients ‘showed signs or symptoms of progression of [HF] without evidence of hyperkalemia’.36 As a result, while the CCS and ESC guidelines recommend a target spironolactone dose of 50 mg daily,2, 3 the 2013 ACCF/AHA guidelines recommend 25 mg once or twice daily.1 The differences between guideline recommendations have led to variable target dose definitions between studies,6, 8, 35 with the ACCF/AHA guidelines likely influencing practice most during our study period.
Neurohormonal blockade is titrated gradually in the outpatient setting based on randomized controlled trial protocols. Rapid titration, particularly while in hospital, has potential to cause adverse effects, and HF exacerbations can be a significant barrier to medication titration.24 However, there is limited evidence to define the optimal rate of titration. Surprisingly, the highest rates of prescription of any or target dose in our cohort were beta‐blockers, the class associated with worse outcomes when uptitrated in a volume overloaded state.
The Guidelines Adherence Indicator 50+ metric
The GAI was initially defined as the proportion of patients prescribed individual GDMT.37 Subsequent refinements have considered eligibility and contraindications,38, 39, 40, 41 combined dual therapy (ACE‐I/ARB with beta‐blocker), and recommended target doses.21, 39 In the HIR Austria registry of 1014 ambulatory HFrEF patients, 64.4% of patients received ≥50% of ACE‐I/ARB and beta‐blockers after 1 year follow‐up.21 Among 661 hospitalized HFrEF patients in France, 35% were prescribed ≥50% target dose of dual therapy at discharge, which increased to 53% at first outpatient consultation.42 The proportion was much lower (20%) in a study of Swedish primary care, which was unable to account for indication without LVEF information.33 The dual GAI50+ is associated with decreased New York Heart Association class, N terminal pro brain natriuretic peptide, and mortality in ambulatory HFrEF patients.21
Our study extends these studies in two important ways. First, by combining detailed electronic data with trained chart abstraction, we define indications, objective contraindications according to guidelines (renal function, heart rate, and BP), and also more subtle reasons for non‐prescription such as patient preference and previous intolerance in a standardized manner. The observed dual therapy GAI50+ was 49%, which lies between the two aforementioned studies. Second, we extend the GAI50+ to triple therapy, which is now recommended for all patients with HFrEF in contemporary guidelines. The proportion of patients achieving triple GAI50+ was just 17%, in part due to the aforementioned low MRA dosing.
Predictors of appropriate prescribing and dose
Relatively few studies have assessed predictors of medication dose.12, 21, 24 Younger age was associated with increased or target dosing of beta‐blockers in an Australian outpatient disease management program25 and a registry of community HF patients.43 However, increased age in our cohort was associated with decreased dosing level among all medications classes. Similar to our findings, pre‐existing HF also predicted target dosing of beta‐blockers and MRAs in the aforementioned Australian cohort24 and guideline adherence in the HIR Austrian registry.21 While lower eGFR was also associated with decreased guideline adherence in the Austrian registry,21 it did not independently predict dose in our cohort, nor that from Australia.24 Medications were solely uptitrated by physicians in this study, and the efficacy and safety of dose adjustment by specialized nurses in conjunction with physicians are under investigation.44 Our study is the only to apply ordinal logistic regression to dosing level and to examine predictors of MRA dosing level among patients not yet at target.
In ambulatory patients, cardiologist care has been associated with improved uptake of GDMT, target doses of ACE‐I and beta‐blockers, and better outcomes.24, 45, 46, 47 In hospitalized patients, a similar association with overall ACE‐I/ARB and beta‐blocker prescription was reported in the UK National HF Audit, multiple analyses of administrative data in Ontario, US Medicare and Medicaid inpatients, and smaller cohorts.27, 28, 48, 49, 50 The UK audit found a similar association for MRA prescription.28 Cardiologist care has also been associated with target dosing of ACE‐I and beta‐blockers in 236 Scottish51 and 2454 US inpatients, respectively.52 We extend this extensive body of literature in several ways. First, we demonstrate the consistency of cardiology care as a predictor of all three medication classes in hospitalized patients. Second, we observed an independent relationship across the dose spectrum using ordinal logistic regression, as opposed to simply an association with target dosing. However, our findings must be interpreted with caution. Although we adjusted for age, BP, renal function, and co‐morbidity burden, patients attended by cardiology were younger, probably with less co‐morbidity and frailty that may be incompletely accounted for by statistical models.27, 48, 49, 50, 52 Further, we were unable to capture whether cardiologists had additional HF training or expertise.
Several additional limitations merit consideration. BP was only captured from admission, whereas inpatient or discharge BP may be more relevant to titration decisions. The audit only assessed discharge medication dose. Comparison with admission dose would more accurately characterize the opportunity for titration. Lastly, our study did not include long‐term clinical outcomes, such as re‐hospitalization or mortality.
Conclusion
In this single‐region experience of hospitalized HFrEF patients, a high proportion of eligible patients were discharged on ACE‐I/ARB/ARNI or beta‐blockers. Fewer were prescribed MRAs, and very few were prescribed target dosing of all medication classes. We report triple GAI50+ for the first time as a metric combining both uptake and dose, with potential to serve as a marker of care processes at the geographic or organizational level. Further research is needed to understand the distribution of this metric in ambulatory and hospitalized populations, the association with outcomes, and utility in guiding quality improvement. Hospitalization provides specialist care and an opportunity to optimize therapy—how much to optimize safely remains to be determined.
Conflict of interest
None declared.
Funding
None declared.
Supporting information
Table S1. Predictors of target dose prescription among eligible patients
Table S2. Characteristics of patients at baseline treated by a cardiologist vs. a non‐cardiologist
Diamant M. J., Virani S. A., MacKenzie W. J., Ignaszewski A., Toma M., and Hawkins N. M. (2019) Medical therapy doses at hospital discharge in patients with existing and de novo heart failure, ESC Heart Failure, 6, 774–783. 10.1002/ehf2.12454.
Site of Research: St. Paul's Hospital and Mount St. Joseph's Hospital (affiliated with University of British Columbia), Vancouver, British Columbia, Canada.
References
- 1. WRITING COMMITTEE MEMBERS , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, Howlett JG, Koshman SL, Lepage S, McKelvie RS, Moe GW, Rajda M, Swiggum E, Virani SA, Zieroth S, Al‐Hesayen A, Cohen‐Solal A, D'Astous M, De S, Estrella‐Holder E, Fremes S, Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, LeBlanc MH, Masoudi FA, Ross HJ, Roussin A, Sussex B. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017; 33: 1342–1433. [DOI] [PubMed] [Google Scholar]
- 4. Jain A, Mills P, Nunn LM, Butler J, Luddington L, Ross V, Cliffe P, Ranjadayalan K, Timmis AD. Success of a multidisciplinary heart failure clinic for initiation and up‐titration of key therapeutic agents. Eur J Heart Fail 2005; 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 5. Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N. Evaluation of the management of heart failure in primary care. Fam Pract 2009; 26: 145–153. [DOI] [PubMed] [Google Scholar]
- 6. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L, on behalf of the Heart Failure Association of the ESC (HFA) . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 7. Crespo‐Leiro MG, Segovia‐Cubero J, González‐Costello J, Bayes‐Genis A, López‐Fernández S, Roig E, Sanz‐Julve M, Fernández‐Vivancos C, de Mora‐Martín M, García‐Pinilla JM, Varela‐Román A, Almenar‐Bonet L, Lara‐Padrón A, de la Fuente‐Galán L, Delgado‐Jiménez J. Adherence to the ESC heart failure treatment guidelines in Spain: ESC heart failure long‐term registry. Rev Esp Cardiol 2015; 68: 785–793. [DOI] [PubMed] [Google Scholar]
- 8. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Poole‐Wilson PA, Armstrong PW, Cleland JGF, Horowitz JD, Massie BM, Ryden L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin‐converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation 1999; 100: 2312–2318. [DOI] [PubMed] [Google Scholar]
- 10. Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996; 94: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 11. Sargento L, Simões AV, Longo S, Lousada N, dos Reis RP. Treatment with optimal dose angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers has a positive effect on long‐term survival in older individuals (aged >70 years) and octogenarians with systolic heart failure. Drugs Aging 2016; 33: 675–683. [DOI] [PubMed] [Google Scholar]
- 12. Mantziari L, Guha K, Khalique Z, McDonagh T, Sharma R. Relation of dosing of the renin–angiotensin system inhibitors after cardiac resynchronization therapy to long‐term prognosis. Am J Cardiol 2012; 109: 1619–1625. [DOI] [PubMed] [Google Scholar]
- 13. Fiuzat M, Wojdyla D, Kitzman D, Fleg J, Keteyian SJ, Kraus WE, Piña IL, Whellan D, O'Connor CM. Relationship of beta‐blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF‐ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol 2012; 60: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt S, Hurlimann D, Starck CT, Hindricks G, Luscher TF, Ruschitzka F, Steffel J. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J 2014; 35: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 15. Khan MS, Fonarow GC, Ahmed A, Greene SJ, Vaduganathan M, Khan H, Marti C, Gheorghiade M, Butler J. Dose of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers and outcomes in heart failure: a meta‐analysis. Circ Heart Fail 2017; 10: e003956. [DOI] [PubMed] [Google Scholar]
- 16. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, ter Maaten JM, Ng LL, Ponikowski P, Samani N, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J 2017; 38: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 17. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Coordinators . Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program. J Am Coll Cardiol 2008; 52: 190–199. [DOI] [PubMed] [Google Scholar]
- 18. Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, Smith EE, Heidenreich P, Hernandez AF, Yancy CW, Bhatt DL. Initiation, continuation, or withdrawal of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc 2017; 6: e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, Spinar J, Müller C, Harjola V‐P, Lassus J, Miró Ò, Maggioni AP, AlHabib KF, Choi D‐J, Park JJ, Zhang Y, Zhang J, Januzzi JL, Kajimoto K, Cohen‐Solal A, Mebazaa A, on behalf of the GREAT Network . Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity‐score matched study: heart failure oral therapies and outcome in AHF. Eur J Heart Fail 2018; 20: 345–354. [DOI] [PubMed] [Google Scholar]
- 20. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta‐blocker withdrawal in acute decompensated heart failure: a systematic review and meta‐analysis. JACC Heart Fail 2015; 3: 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poelzl G, Altenberger J, Pacher R, Ebner CH, Wieser M, Winter A, Fruhwald F, Dornaus C, Ehmsen U, Reiter S, Steinacher R, Huelsmann M, Eder V, Boehmer A, Pilgersdorfer L, Ablasser K, Keroe D, Groebner H, Auer J, Jakl G, Hallas A, Ess M, Ulmer H. Dose matters! Optimisation of guideline adherence is associated with lower mortality in stable patients with chronic heart failure. Int J Cardiol 2014; 175: 83–89. [DOI] [PubMed] [Google Scholar]
- 22. Get With The Guidelines®–Heart Failure. American Heart Association; 2016.
- 23. Peterson B, Harrell FE. Partial proportional odds models for ordinal response variables. Appl Stat 1990; 39: 205. [Google Scholar]
- 24. Carroll R, Mudge A, Suna J, Denaro C, Atherton J. Prescribing and up‐titration in recently hospitalized heart failure patients attending a disease management program. Int J Cardiol 2016; 216: 121–127. [DOI] [PubMed] [Google Scholar]
- 25. Hickey A, Suna J, Marquart L, Denaro C, Javorsky G, Munns A, Mudge A, Atherton JJ. Improving medication titration in heart failure by embedding a structured medication titration plan. Int J Cardiol 2016; 224: 99–106. [DOI] [PubMed] [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 27. Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation 2003; 108: 184–191. [DOI] [PubMed] [Google Scholar]
- 28. Donkor A, McDonagh T, Hardman S, Shote A, National Heart Failure Audit Steering Group Committee . British Heart Failure Audit, 2014‐2015.pdf. National Institute for Cardiovascular Outcomes Research; 2016.
- 29. Gjesing A, Schou M, Torp‐Pedersen C, Køber L, Gustafsson F, Hildebrandt P, Videbaek L, Wiggers H, Demant M, Charlot M, Gislason GH. Patient adherence to evidence‐based pharmacotherapy in systolic heart failure and the transition of follow‐up from specialized heart failure outpatient clinics to primary care. Eur J Heart Fail 2013; 15: 671–678. [DOI] [PubMed] [Google Scholar]
- 30. Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L, on behalf of the QUALIFY Investigators . Physicians' adherence to guideline‐recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey: adherence to heart failure guidelines. Eur J Heart Fail 2016; 18: 514–522. [DOI] [PubMed] [Google Scholar]
- 31. Brunner‐La Rocca H‐P, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma H‐J, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ. Contemporary drug treatment of chronic heart failure with reduced ejection fraction. JACC Heart Fail 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 32. Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC. Patterns and predictors of evidence‐based medication continuation among hospitalized heart failure patients (from Get With the Guidelines–Heart Failure). Am J Cardiol 2011; 107: 1818–1823. [DOI] [PubMed] [Google Scholar]
- 33. Dahlstrom U, Hakansson J, Swedberg K, Waldenstrom A. Adequacy of diagnosis and treatment of chronic heart failure in primary health care in Sweden. Eur J Heart Fail 2009; 11: 92–98. [DOI] [PubMed] [Google Scholar]
- 34. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, Givertz MM. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019; 73: 935–944. [DOI] [PubMed] [Google Scholar]
- 35. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Januzzi JL, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne‐Nickens P, O'Connor CM. Effect of natriuretic peptide–guided therapy on hospitalization or cardiovascular mortality in high‐risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 37. Komajda M, Lapuerta P, Hermans N, Gonzalez‐Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole‐Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J 2005; 26: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 38. Störk S, Hense HW, Zentgraf C, Uebelacker I, Jahns R, Ertl G, Angermann CE. Pharmacotherapy according to treatment guidelines is associated with lower mortality in a community‐based sample of patients with chronic heart failure: a prospective cohort study. Eur J Heart Fail 2008; 10: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 39. Peters‐Klimm F, Müller‐Tasch T, Schellberg D, Remppis A, Barth A, Holzapfel N, Jünger J, Herzog W, Szecsenyi J. Guideline adherence for pharmacotherapy of chronic systolic heart failure in general practice: a closer look on evidence‐based therapy. Clin Res Cardiol 2008; 97: 244–252. [DOI] [PubMed] [Google Scholar]
- 40. Frankenstein L, Remppis A, Fluegel A, Doesch A, Katus HA, Senges J, Zugck C. The association between long‐term longitudinal trends in guideline adherence and mortality in relation to age and sex. Eur J Heart Fail 2010; 12: 574–580. [DOI] [PubMed] [Google Scholar]
- 41. Zugck C, Franke J, Gelbrich G, Frankenstein L, Scheffold T, Pankuweit S, Duengen HD, Regitz‐Zagrosek V, Pieske B, Neumann T, Rauchhaus M, Angermann CE, Katus HA, Ertl GE, Störk S. Implementation of pharmacotherapy guidelines in heart failure: experience from the German Competence Network Heart Failure. Clin Res Cardiol 2012; 101: 263–272. [DOI] [PubMed] [Google Scholar]
- 42. Cohen Solal A, Leurs I, Assyag P, Beauvais F, Clerson P, Contre C, Thebaut J‐F, Genoun M. Optimization of heart FailUre medical Treatment after hospital discharge according to left ventricUlaR Ejection fraction: the FUTURE survey. Arch Cardiovasc Dis 2012; 105: 355–365. [DOI] [PubMed] [Google Scholar]
- 43. Fowler MB, Lottes SR, Nelson JJ, Lukas MA, Gilbert EM, Greenberg B, Massie BM, Abraham WT, Franciosa JA. β‐Blocker dosing in community‐based treatment of heart failure. Am Heart J 2007; 153: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 44. Oyanguren J, García‐Garrido L, Nebot Margalef M, Lekuona I, Comin‐Colet J, Manito N, Roure J, Ruiz Rodriguez P, Enjuanes C, Latorre P, Torcal Laguna J, García‐Gutiérrez S, on behalf of the ETIFIC7 research group . Design of a multicentre randomized controlled trial to assess the safety and efficacy of dose titration by specialized nurses in patients with heart failure. ETIFIC study protocol: ETIFIC study protocol. ESC Heart Fail 2017; 4: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker DW, Hayes RP, Massie BM, Craig CA. Variations in family physicians' and cardiologists' care for patients with heart failure. Am Heart J 1999; 138: 826–834. [DOI] [PubMed] [Google Scholar]
- 46. Pereira Barretto AC, Cucê Nobre MR, Lancarotte I, Scipioni AR, Franchini Ramires JA. Do cardiologists at a university hospital adopt the guidelines for the treatment of heart failure? Arq Bras Cardiol 2001; 77: 30–36. [DOI] [PubMed] [Google Scholar]
- 47. McKee SP, Leslie SJ, LeMaitre JP, Webb DJ, Denvir MA. Management of chronic heart failure due to systolic left ventricular dysfunction by cardiologist and non‐cardiologist physicians. Eur J Heart Fail 2003; 5: 549–555. [DOI] [PubMed] [Google Scholar]
- 48. Foody JM, Rathore SS, Wang Y, Herrin J, Masoudi FA, Havranek EP, Krumholz HM. Physician specialty and mortality among elderly patients hospitalized with heart failure. Am J Med 2005; 118: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 49. Boom NK, Lee DS, Tu JV. Comparison of processes of care and clinical outcomes for patients newly hospitalized for heart failure attended by different physician specialists. Am Heart J 2012; 163: 252–259. [DOI] [PubMed] [Google Scholar]
- 50. Bellotti P, Badano LP, Acquarone N, Griffo R, Lo Pinto G, Maggioni AP, Mattiauda C, Menardo G, Mombelloni P, OSCUR Investigators . Specialty‐related differences in the epidemiology, clinical profile, management and outcome of patients hospitalized for heart failure; the OSCUR study. Oucome dello Scompenso Cardiaco in relazione all'Utilizzo delle Risore. Eur Heart J 2001; 22: 596–604. [DOI] [PubMed] [Google Scholar]
- 51. Davie AP, McMurray JJ. ACE inhibitors and heart failure in hospital: any difference between cardiologists and general physicians? Postgrad Med J 1999; 75: 219–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Philbin EF, Weil HFC, Erb TA, Jenkins PL. Cardiology or primary care for heart failure in the community setting. Chest 1999; 116: 346–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors of target dose prescription among eligible patients
Table S2. Characteristics of patients at baseline treated by a cardiologist vs. a non‐cardiologist


