Abstract
Background
Chikungunya virus is an alpha virus with high similarity to Dengue and Zika viruses, both in transmission cycle and in clinical presentation. Chikungunya is a re-emerging mosquito-borne infection known to cause small to very large outbreaks/epidemics at frequent intervals. In 2016, India witnessed a large outbreak of Chikungunya infection affecting more than 58,000 people. This study was undertaken to look at the genotypic phylogeny to know the relatedness with previously reported strains.
Methods
During the 2016 outbreak, samples from all patients clinically suspected to have Chikungunya were collected and subjected to testing for IgM antibody by ELISA and viral RNA detection by RT-PCR. Sequencing of the E1 gene segment was done to create a phylogenetic tree comparison with other strains.
Results
Serum samples were collected from 142 patients of clinically suspected Chikungunya infection. Majority of the patients were in the age group of 31–50 years accounting for more than 35% of the total cases. Twenty eight samples were positive for IgM antibody. Thirty seven samples were positive for viral RNA by RT-PCR. Only 06 cases were positive by both tests. Mutations in the amino acids K211E, M269V and D284E in the E1 gene segment of the Chikungunya virus were observed in the seven strains that were sequenced. On phylogeny tree, all the strains were found to belong to the ECSA genotype.
Conclusion
Actively searching for the potential epidemic causing mutations and reporting of novel mutations may help in better understanding and probably forecasting of future CHIKV outbreaks and its nature.
Keywords: Chikungunya, Outbreak, Phylogeny, Mutations
Introduction
Chikungunya virus (CHIKV) is an alphavirus, from the Togaviridae family possessing very high similarity with Dengue and Zika virus, both in transmission cycle, in clinical presentation and in the propensity to cause large outbreaks. CHIKV is mainly transmitted by Aedes aegypti and Aedes albopictus mosquitoes.1 Strains with adaptive mutations like A226V in E1 gene are transmitted faster by Ae. albopictus. Ae. aegypti is the predominant vector in India. Human-mosquito-human transmission is the main epidemic cycle.2 Persistent arthralgia, sometimes for many years is common in the chronic stage of the disease.3 Reports of death by CHIKV was thought to be rare, thought to kill only those who are young, old or immunocompromised, but in the 2005–2006 outbreaks, a case fatality as high as 1/1000 patients was observed in La Reunion Island.4, 5
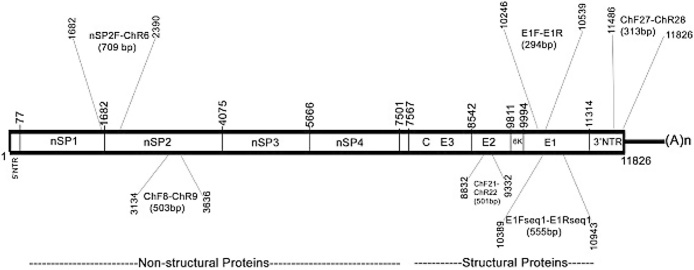
CHIKV is a single stranded positive sense RNA virus. The genomic organization of CHIKV is depicted in Fig. 1. The whole genome is 11,805 bp long with two third of the genome coding for non-structural proteins (nsP1, nsP2, nsP3 and nsP4) while one third codes for the structural proteins (E1, E2, E3, 6K and C). Mutations in any of these regions can influence the virulence as well as transmission capabilities. CHIK viruses have been grouped into three genotypes based on the E1 gene sequences. They are Asian, East/Central/South African (ECSA) and west African genotypes.6, 7 India has been affected by ECSA genotypes in majority of the outbreaks except for the first two major outbreaks in the year 1963 and 1973 when Asian genotype was the causative agent. The prototype strains of the ECSA genotype, S27 and Ross strains were both isolated from the 1952 Tanzania outbreak. Their complete genome sequences were recently determined.8
Fig. 1.
Structure of the chikungunya virus genome. NTR – Non Translated Region, nSP – non Structural Proteins, E1–E3 – Structural Proteins, E1Fseq/E1Rseq – primer pair used to amplify the 555 bp segment.
CHIKV is a re-emerging mosquito-borne infection known to cause small to very large outbreaks/epidemics at frequent intervals and for its very high attack rate during epidemics.4 India is one of the worst affected country witnessing many large scale outbreaks. Calcutta in India witnessed its first CHIKV outbreak in 1963. This was followed by many epidemics in Indian regions like Chennai, Pondicherry, and Vellore in 1964; Visakhapatnam, Rajamundhry, Kakinada and Nagpur in 1965; and Barsi in 1973.9 After a time gap of 32 years India witnessed a massive outbreak in 2005–2006.10 Countries other than India who also have witnessed epidemic resurgence of CHIKV are the Democratic Republic of Congo (DRC) in 2000 and Indonesia in 2001–2003 after gaps of 39 and 20 years respectively.11, 12
In 2016, India again had a large outbreak of Chikungunya virus affecting more than 58,000 persons. This is the highest number of cases reported by India after the 2005–2006 outbreak. Cases of CHIKV in 2016 were seen to be double of the numbers reported in 2015. Karnataka and Delhi were the two most severely affected stated having more than 13,000 and 12,000 cases respectively.13 Of the 29 Indian states, 27 of them reported cases of Chikungunya in varying numbers. Only Lakshadweep and Dadra & Nagar Haveli did not report any case of Chikungunya in 2016.
In the present study, we carried out molecular detection of Chikungunya virus, in seropositive as well as seronegative patients, clinically suspected of suffering from this viral infection. Sequencing was done to detect the presence of previously published mutations as well as any novel mutation in the present season, which may be responsible for the severity of the outbreak and the disease.14 Phylogenetic analysis was done to see the relatedness of the present virus with that of the previously reported chikungunya viruses from India and from around the globe.
Materials and methods
Samples
During the outbreak of Chikungunya in 2016, samples from patients reporting to our centre with fever, arthralgia with or without rash, during the monsoon season, clinically suspected to have Chikungunya were collected. During the study period of June to September 2016, a total of 142 samples were collected.
CHIKV IgM antibody ELISA
All the samples were tested for the presence of IgM antibody against CHIKV by IgM antibody capture ELISA kit provided by the National Institute of Virology, Pune as per the protocols supplied along with the kit. Briefly, IgM antibodies in the patients’ serum were captured by anti-human IgM coated on to the surface of the wells of the microtiter plate. In the next step, CHIKV antigen was added, which bound to the captured human IgM in the sample with unbound antigen being removed during the washing step. Further, biotinylated anti CHIKV monoclonal antibody was added followed by Avidin-HRP. Then, chromogenic substrate (TMB/H2O2) was added and the reaction was subsequently stopped by 1N H2SO4. The optical density was measured at 450 nm on an ELISA reader (Readwell TOUCH Automatic ELISA Plate analyser, Robonik India).
RNA extraction
Viral RNA was extracted using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. Briefly 560 μl of buffer AVL was added to 140 μl of serum and vortexed for 15 s and the mixture was incubated at room temperature (15–25 °C) for 10 min. Ethanol (560 μl) was added to this mixture and vortexed. This mixture was then transferred to the spin column and centrifuged at 6000 × g (Remi, India) for 1 min spinning down a volume of 630 μl each time. The lysate was then washed first with Buffer AW1 and then Buffer AW2 centrifuging each time at 6000 × g. The RNA was eluted in 70 μl of elution buffer in a collection tube by centrifuging at 6000 × g and stored at −80 °C until use.
cDNA synthesis
The RNA extracted in the above step was used as the template to generate complementary DNA by reverse transcription using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) following the manufacturer's instructions. A reaction volume of 20 μl was used for the reaction comprising 10 μl 2× Reverse Transcription Master Mix (10X RT Buffer, 25X dNTP mix, 10X Random primers, RT Enzyme) and 10 μl of the extracted nucleic acid template. The reaction was carried out in a thermal cycler (GeneAmp 9700, Applied Biosystems, USA) with the following cycling conditions: 25 °C for 10 min, 37 °C for 120 min, 85 °C for 05 min and finally at 4 °C.
PCR
A 555 bp segment of the E1 gene of the Chikungunya virus genome was amplified by polymerase chain reaction using forward primer 5′-GCTCCGCGTCCTTTACC-3′ and reverse primer 5′-ATGGCGACGCCCCCAAAGTC-3′ (Fig. 2).15 The amplification was performed in a volume of 25 μl containing 12.5 μl of the PCR enzyme/dNTP mix [DreamTaq Green PCR Master Mix (2×), Thermo Fisher scientific, USA], 5 pmol each of the forward and reverse primer, 5 μl of the cDNA and nuclease free water. The cycling parameters were set at the following temperatures: initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C for 1 min followed by a final extension of 10 min at 72 °C. The amplified products were electrophoresed on a 1% agarose gel in at 100 V for 20 min and photographed on a UV transilluminator.
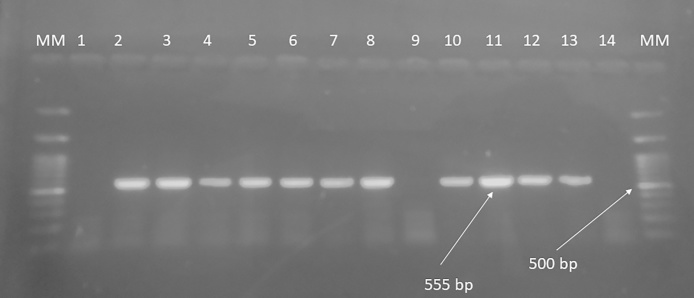
Fig. 2.
Representative image of the Gel electrophoresis of the amplicons. Lane 1 Negative control, Lane 2–8 and 10–13 show a 555-base pair band (arrow).15 Lane 9 and 14 are negative samples. MM – 100 bp molecular marker. 500 bp band is marked for reference.
Nucleotide sequencing
Samples that showed high intensity amplicons on PCR were chosen at random, for nucleotide sequencing. The sequencing of the purified PCR products was performed using ABI PRISM Big Dye terminator v3.1 cycle sequencing ready reaction kit (Applied Biosystems, USA) with the same set of primers as used for the RT-PCR. The nucleotide sequence obtained was then compared with published GenBank sequences using the BLAST analysis on the website of the National Centre for Biotechnology Information (NCBI) http://www.ncbi.nlm.nih.gov/BLAST.
Phylogenetic analysis
MEGA 6 software was used for performing the Phylogenetic analysis.16 The viral sequences obtained, were aligned using Clustal W software embedded in the MEGA 6 software. The phylogenetic tree was constructed using Neighbor-Joining method with the Kimura two-parameter distance model along with Bootstrap test with 1000 replications for evaluating the reliability of the analysis. The details of the sequences used for the Phylogenetic analysis are given in Table 1.
Table 1.
Published sequences used in this study for sequence and phylogeny tree analysis (accessed from the NCBI website: www.ncbi.nlm.nih.gov/nucleotide/).
| S No | GenBank accession no | Isolate/strain | Host | Country/continent | Year |
|---|---|---|---|---|---|
| 1 | AF192891 | PM 2951 | Ae. aegypti | Senegal | 1966 |
| 2 | AF192892 | 37997 | Ae. furcifer | Senegal | 1983 |
| 3 | AF192893 | IBH35 | Human | Nigeria | 1964 |
| 4 | AF192894 | RSU1 | Human | Indonesia | 1985 |
| 5 | AF192898 | 1455/75 | Human | Thailand | 1975 |
| 6 | AF192900 | SV-0451/96 | Human | Thailand | 1996 |
| 7 | AF192906 | CAR 256 | Unknown | Central Africa | Unknown |
| 8 | AF369024 | S27-African prototype | Human | Central East/South Africa | 2001 |
| 9 | AF192907 | UG AG41855 | Human | Uganda | 1982 |
| 10 | AY424803 | 653496 | Human | Nagpur (India) | 2003 |
| 11 | AY549575 | Chik007 | Human | Democratic Republic of the Congo | 2000 |
| 12 | AY549579 | Chik1719 | Human | Democratic Republic of the Congo | 2000 |
| 13 | AY549580 | Chik1720 | Human | Democratic Republic of the Congo | 2000 |
| 14 | AY549583 | Chik1730 | Human | Democratic Republic of the Congo | 2000 |
| 15 | DQ462748 | IMT/6382 | Human | Reunion_Island | 2005 |
| 16 | DQ462749 | IMT/AAI72 | Human | Reunion_Island | 2005 |
| 17 | EF027139 | IND-00-MH4 | Mosquito | Yawat, India | 2000 |
| 18 | EF027140 | IND-63-WB1 | Human | India | 1963 |
| 19 | EF027141 | IND-73-MH5 | Human | India | 1973 |
| 20 | EF051584 | Chik Cam 7079 | Human | Cameroon | 2006 |
| 21 | EF210157 | DRDE-06 | Human | India | 2006 |
| 22 | EF452493 | AF15561 | Human | Thailand | 2007 |
| 23 | EF452494 | TSI-GSD-218-VR1 | Human | USA | 2007 |
| 24 | EU192143 | 0706aTw | Human | Indonesia | 2007 |
| 25 | EU727248 | SGPGI/2007/04 | Human | India | 2007 |
| 26 | HM045797 | RSU1 | Human | Indonesia | 1985 |
| 27 | HM045811 | Ross low-psg | Human | Tanzania | 1953 |
| 28 | HM045816 | SH 3013 | Human | Senegal | 1966 |
| 29 | HM045817 | HD 180760 | Human | Senegal | 2005 |
| 30 | KY039475 | AIIMS_CHIK_6905 | Human | India | 2016 |
| 31 | EU037962 | Wuerzburg | Human | Mauritius | 2006 |
| 32 | M20303 | O’Nyong-nyong virus (Gulu strain) | Anopheles gambiae | California | 1988 |
| 33 | MF448547 | CHIKV/AHRR/2016/17 | Human | India (Present study) | 2016 |
| 34 | MF448548 | CHIKV/AHRR/2016/25 | Human | India (Present study) | 2016 |
| 35 | MF448549 | CHIKV/AHRR/2016/34 | Human | India (Present study) | 2016 |
| 36 | MF448550 | CHIKV/AHRR/2016/71 | Human | India (Present study) | 2016 |
| 37 | MF448551 | CHIKV/AHRR/2016/89 | Human | India (Present study) | 2016 |
| 38 | MF448552 | CHIKV/AHRR/2016/108 | Human | India (Present study) | 2016 |
| 39 | MF448553 | CHIKV/AHRR/2016/112 | Human | India (Present study) | 2016 |
Results
A total of 142 samples from patients clinically suspected to be suffering from Chikungunya were received in our laboratory during the outbreak in the year 2016. Seventy-six samples were from female patients while 66 were from male patients. The minimum age of patients was as low as 05 years and the maximum age was 92 years. Majority of the patients were in the age group of 31–40 years (n = 27) followed by 41–50 years of age (n = 23) accounting for more than 35% of the total cases.
CHIKV IgM ELISA
Of the total samples received, 28 samples were found to be positive and remaining 114 samples were negative for IgM antibody.
Two step reverse transcriptase PCR
All 142 samples, irrespective of their ELISA result, were tested for presence of chikungunya viral RNA by two step RT-PCR. A total of 37 cases out of the 142 samples were positive for viral RNA by RT-PCR. Of these only 06 cases were IgM antibody positive, whereas the remaining 31 RT-PCR positive samples were negative for IgM antibody.
Sequencing
Sequences of seven strains were deciphered and submitted to GenBank (GenBank Accession no MF448547–MF448553). These sequences were aligned using MEGA 6 gene alignment software16 and were compared with the prototype AF369024 S27 and HM045811 Ross strains belonging to the ECSA genotype for the presence of mutations. On nucleotide comparison, all the seven stains showed mutations as compared to the prototype strains (Table 2). The present study strains differed from the S27 and Ross strain by 2.7 and 2.9% respectively. On comparison with the different genotypes, the strains in our study were found to be 97.27 ± 1.48%, 94.06 ± 0.43% and 86.18 ± 0.23% similar with ECSA, Asian and West African Genotype strains respectively (Table 3).
Table 2.
Comparison of amino acid substitution between isolates reported in present study with that of the ECSA prototype strain S27 & Ross strain.
| Amino acid residue position | AF369024/S27 | HM045811/Ross Tanzania | CHIKV/AHRR/2016/17 | CHIKV/AHRR/2016/25 | CHIKV/AHRR/2016/34 | CHIKV/AHRR/2016/71 | CHIKV/AHRR/2016/89 | CHIKV/AHRR/2016/108 | CHIKV/AHRR/2016/112 |
|---|---|---|---|---|---|---|---|---|---|
| 145 | T | T | . | . | . | . | . | . | A |
| 149 | N | N | . | . | . | K | . | . | . |
| 150 | G | G | . | . | . | D | . | . | . |
| 211 | K | K | E | E | E | E | E | E | E |
| 269 | M | M | V | V | V | V | V | V | V |
| 284 | D | D | E | E | E | E | E | E | E |
| 314 | G | G | A | . | A | . | A | . | . |
Table 3.
Percent identity of CHIKV strains isolated in the present study with that of ECSA, Asian and West African genotype strains.
| Genotype | Nucleotide level | Amino acid level |
|---|---|---|
| ECSA | 97.27 ± 1.48 | 89.53 ± 3.52 |
| Asian | 94.06 ± 0.43 | 81.53 ± 1.16 |
| West African | 86.18 ± 0.23 | 62.48 ± 0.61 |
Phylogenetic analysis
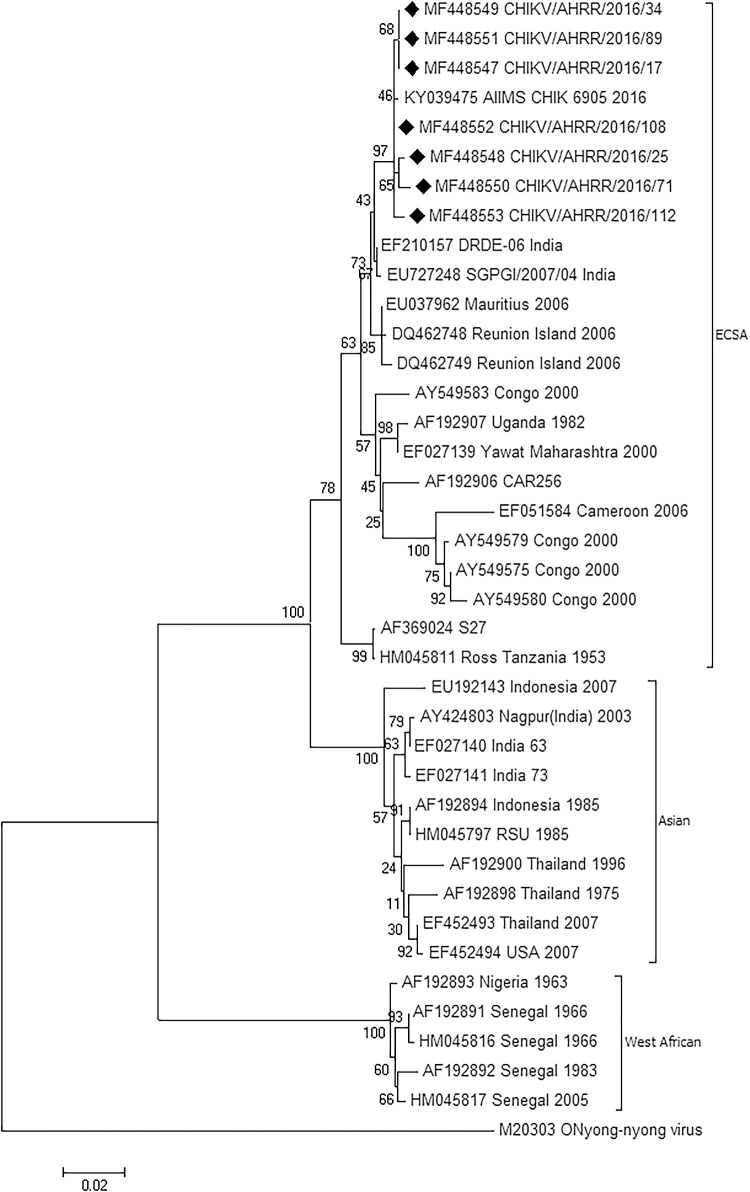
On the phylogeny tree, all the seven strains were found to belong to the ECSA genotype along with the S27 and Ross prototype strains (Fig. 3). The study isolates were more closely related to the Mauritius and Reunion Island isolates as compared to the S27 isolates which is the ECSA prototype strain. One recently submitted strain sequence from AIIMS, Delhi (GenBank Accn No KY039475) of the 2016 monsoon season was also found to be similar to the strains found in this study. The old Indian strains reported in the year 1963 (EF027140) and 1973 (EF027141) were grouped in the Asian genotype along with one recent strain of 2003 isolated in Nagpur in India (AY424803). Isolates from Nigeria and Senegal were grouped in the west African genotype.
Fig. 3.
Phylogeny tree constructed using Neighbor-Joining method of MEGA 6 software. The sequences reported in this study are indicated by “♦”. ECSA – East Central And South African Genotype, Asian – Asian Genotype, West African – West African Genotype.
Discussion
Chikungunya is a re-emerging viral infection, a significant public health problem, mainly in the tropical and subtropical regions. After the resurgence of CHIKV in 2005–2006, Chikungunya made a huge come back in India in 2016 affecting thousands of people across the whole country. Once the presence of CHIKV infection was felt in the early monsoon season of the year 2016, there was an increase in the number of samples being sent by the clinicians for testing for CHIKV at an early stage of the disease. It is very important to pick up cases early in the development stage both for the specific patient management and for the early implementation of adequate control measure to halt the rapid spread of these vector borne diseases.
Mean incubation period for onset of fever is 03 days.17, 18, 19 Onset of fever coincides with viraemia. This acute infection phase lasts for about 01 week until when the viraemia ends and IgM appears.20, 21 CHIKV IgM antibody response is slow in onset, and in some cases, it may take as long as six to twelve weeks for the IgM antibodies to appear in sufficient concentration to be picked up in ELISA. As per WHO guidelines, it is not advisable to go for the antibody test in the first week.22 On the contrary, during the first week of CHIKV infection, viraemia can reach very high levels (viral loads of 3.3 × 109 copies/ml).23 So, it becomes very necessary to screen these samples for presence of viral RNA by a molecular method, so as to assess the actual number of true positive cases.
Of all the samples collected, only six samples had presence of both IgM antibody and RNA. 22 samples were only IgM positive whereas 31 samples were only RT-PCR positive. These can be explained by the fact that chikungunya was not clinically suspected at the very beginning of this outbreak. So, in the early phase of the outbreak, the testing for the CHIKV was requested late in the disease stage when the viraemia had already disappeared. Later on, when the CHIKV outbreak was confirmed and the number of cases increased, the CHIKV testing was done at a much earlier stage than before which resulted in more cases of PCR positivity in the later part of the outbreak. Hence testing for IgM ELISA is not recommended as a routine in the first week as this may result in false negatives.
India experienced chikungunya outbreaks earlier in 1963 and 1973 caused by Asian genotypes. Thereafter, Asian genotype was replaced by the ECSA genotype. ECSA genotype was first reported in India from a mosquito in the year 2000 (Yawat strain). Although India did not suffer any major outbreak by this genotype in 2000, the same genotype caused one of the largest outbreaks in India in the year 2005–2006 appearing after a gap of 32 years. Subsequently, all the subsequent outbreaks and sporadic cases reported in India belong to the ECSA genotype.
All of the Indian strains of this study and the previously reported strains starting from Yawat in Maharashtra in the year 2000 to the 2005–2006 outbreak and subsequent sporadic cases belonged to the ECSA genotype. Only the first two outbreaks of 1963 and 1973 belonged to the Asian genotype. As India has witnessed a shift of CHIKV genotype from Asian in 1963 and 1973 to ECSA in 2005–2006, vigilance is required to keep watching for any possible shift in future which may have epidemic potential. The shift to a ECSA genotype may be attributed to some un-identified mutations in ECSA lineage responsible for adaptation of ECSA to Ae. albopictus mosquitoes. These mutations might be the plausible reason for the displacement of Asian lineage by ECSA lineage in India where both genotypes exist.24 This would require a comparative whole genome analysis of the various strains isolated all over India in order to arrive at a firm conclusion.
Three mutations K211E, M269V and D284E observed in all the isolates of this study were also reported in all of the nine isolates isolated between 2011 and 2014 in Karnataka and also in all the 14 strains sequenced in the year 2010 from Delhi.25 Two of the mutations (M269V & D284E) seen in this study were also reported among the cases from India reported during the year 2006–2008 and among the 2005–2006 outbreak cases.26, 27 D284E was not observed in the year 2000 in the Yawat strain from Maharashtra in India.27
K211E has been reported in many reports from different parts of India. It was observed in one of the 2005–2006 strain from Karnataka, in all the Delhi strain in 2010, in all the Bangalore strains from the year 2011 to 2014.24, 25, 27 In the present study also, all the strains have showed K211E mutation. K211E is an established positively selected site with a posterior probability of >75%.27, 28
As per literature, A226V, M269V and A316V have the potential to cause epidemics.26 A226V in E1 gene is the mutation responsible for increase in midgut infectivity and dissemination to the salivary glands among Ae. albopictus mosquitoes and their subsequent enhanced transmission capabilities to vertebrate species.14 It is the mutation with the highest epidemic potential. This association of A226V mutation and increased transmissibility was originally made for Reunion Island (RU) strains, but this mutation though detected in Indian studies earlier, was not found in any of the strains in the present study.15, 29, 30 This mutation was also absent in 2005–2006 Indian outbreak when India witnessed the re-emergence of this virus after 32 years affecting as many as 1.3 million patients of 13 different states. We suggest that these should be actively looked for in each CHIKV season to predict the potential epidemic at the earliest.
Few unique mutations were observed in some of the strains in this study which were not found published in literature. They are T145A in CHIKV/AHRR/2016/112 isolate, N149K in CHIKV/AHRR/2016/71, G150D in CHIKV/AHRR/2016/71 and G314A among CHIKV/AHRR/2016/17, CHIKV/AHRR/2016/34 and CHIKV/AHRR/2016/89 isolates (Table 4). These mutations will require to be studied further to establish whether any of these had a causal relationship with the present large outbreak.
Table 4.
Amino acid substitution reported in E1 gene among Indian strains and the effect of some of the mutations on virulence as reported.
| Author | Reference | Mutations reported | Effect of mutations |
|---|---|---|---|
| Schuffenecker et al. 2006 | 6 | A226V and D284E | A226V: Leads to increased fitness, midgut infectivity dissemination to the salivary glands and transmissibility of the virus by Aedes albopictus mosquitoes |
| Arankalle et al. 2007 | 27 | V19I, K211N, V213I, M269V, D284E, A377T | M269V: Increased transmissibility of the virus |
| Santhosh et al. 2008 | 29 | V14A and A226V | A226V: as above |
| Niyas et al. 2010 | 15 | A226V and V291I | A226V: as above |
| Sreekumar et al. 2010 | 30 | V04A, E127G, D184G, Y195H, M197V, A226V, G248E, D284E, P304L, T396A, K411R, C433R | A226V: as above |
| Shrinet et al. 2012 | 25 | K211E, M269V, D284E, V179A, S234P, R196K and R247C | M269V: Increased transmissibility of the virus |
| Mudurangaplar et al. 2016 | 24 | K211E, M269V and D284E | -do- |
| Present study | M269V, D284E, K211E, T145A, N149K, G150D and G314A | M269V: increased transmissibility of the virus. Further studies will be required to determine the effect of the new mutations |
As there is no vaccine available against this vector borne viral disease, protection from the mosquito bite is the only way left to keep one protected from this disease. The mosquito involved in CHIKV transmission bites throughout the day with peak time during dawn and a large part of the evening. All measures to prevent mosquito breeding should be taken. Steps to prevent mosquito bite like wearing full sleeved clothing, applying insect repellants, installing window/door netting and use of mosquito nets should be followed strictly to protect oneself from this viral infection.
Conclusion
Actively searching for the potential epidemic causing mutations and reporting of novel mutations may help in better understanding and probably forecasting of future CHIKV outbreaks and its nature.
Conflicts of interest
The authors have none to declare.
Acknowledgement
Technical assistance rendered by K.S. Sreelesh is gratefully acknowledged.
References
- 1.Kuan G., Ramirez S., Gresh L. Seroprevalence of anti-Chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first Chikungunya epidemic, 2014–2015. PLoS Negl Trop Dis. 2016;10(6):2014–2015. doi: 10.1371/journal.pntd.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver S.C., Forrester N.L. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Schilte C., Staikovsky F., Couderc T. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 5.Josseran L.L., Paquet C., Zehgnoun A. Chikungunya disease outbreak, Reunion Island. Emerg Infect Dis. 2006;12(12):1994. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuffenecker I., Iteman I., Michault A. Genome microevolution of Chikungunya viruses causing the Indian ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81(2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 8.Khan A.H., Morita K., del Carmen Parquet M., Hasebe F., Mathenge E.G.M., Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83(12):3075–3084. doi: 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- 9.Jupp P.G., McIntosh B.M. Chikungunya virus disease. Arboviruses Epidemiol Ecol. 1988;2:137–157. [Google Scholar]
- 10.Yergolkar P.N., Tandale B.V., Arankalle V.A. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12(10):1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastorino B., Muyembe-Tamfum J.J., Bessaud M. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004;74(2):277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 12.Laras K., Sukri N.C., Larasati R.P. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99(2):128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Directorate General of Health Services. National Vector Borne Disease Control Programme [Internet]. p. 1. Cited 24.03.17.
- 14.Tsetsarkin K.A., Vanlandingham D.L., Mcgee C.E., Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niyas K.P., Abraham R., Unnikrishnan R.N., Mathew T., Nair S. Molecular characterization of Chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, South India. Virol J. 2010;7(189):1–8. doi: 10.1186/1743-422X-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph K.E., Lessler J., Moloney R.M., Kmush B., Cummings D.A.T. Incubation periods of mosquito-borne viral infections: a systematic review. Am J Trop Med Hyg. 2014;90(5):882–891. doi: 10.4269/ajtmh.13-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suhrbier A., Jaffar-Bandjee M.-C., Gasque P. Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol. 2012;8(7):420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 19.Brouard C., Bernillon P., Quatresous I. Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. 2008;48(7):1333–1341. doi: 10.1111/j.1537-2995.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 20.Thiberville S., Boisson V., Gaudart J., Simon F., Flahault A., De Lamballerie X. Chikungunya fever: a clinical and virological investigation of outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl Trop Dis. 2013;7(1):e2004. doi: 10.1371/journal.pntd.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staikowsky F., Talarmin F., Grivard P. Prospective study of Chikungunya virus acute infection in the Island of La Reunion during the 2005–2006 outbreak. PLoS ONE. 2009;4(10):e7603. doi: 10.1371/journal.pone.0007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . 2008. Guidelines on Clinical Management of Chikungunya Fever. [Google Scholar]
- 23.Parola P., De Lamballerie X., Jourdan J. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12(10):1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudurangaplar B., Peerapur B.V. Molecular characterisation of clinical isolates of chikungunya virus: a study from Tertiary Care Hospitals in Southern India. J Clin Diagnostic Res. 2016;10(3):DC14. doi: 10.7860/JCDR/2016/18370.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrinet J., Jain S., Sharma A. Genetic characterization of Chikungunya virus from New Delhi reveal emergence of a new molecular signature in Indian isolates. Virol J. 2012;9(1):100. doi: 10.1186/1743-422X-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh R.K., Tiwari S., Mishra V.K., Tiwari R., Dhole T.N. Molecular epidemiology of Chikungunya virus: mutation in E1 gene region. J Virol Methods. 2012;185(2):213–220. doi: 10.1016/j.jviromet.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Arankalle V.A., Shrivastava S., Cherian S. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol. 2007;88(7):1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 28.Sumathy K., Ella K.M. Genetic diversity of Chikungunya virus, India 2006–2010: evolutionary dynamics and serotype analyses. J Med Virol. 2012;84(3):462–470. doi: 10.1002/jmv.23187. [DOI] [PubMed] [Google Scholar]
- 29.Santhosh S.R., Dash P.K., Parida M.M., Khan M., Tiwari M., Rao P.V.L. Comparative full genome analysis revealed E1: A226V shift in 2007 Indian Chikungunya virus isolates. Virus Res. 2008;135:36–41. doi: 10.1016/j.virusres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Sreekumar E., Issac A., Nair S. Genetic characterization of 2006–2008 isolates of Chikungunya virus from Kerala, South India, by whole genome sequence analysis. Virus Genes. 2010;40(1):14–27. doi: 10.1007/s11262-009-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]